Contribution of Sheep Grazing to Plant Diversity in Natural Grasslands
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Acquisition and Processing
2.2.1. GPS Trajectory Data Acquisition
2.2.2. Grassland Community Characterization Survey
2.2.3. Faeces Collection
2.2.4. Seed Collection and Simulated Ingestion Experiment
2.3. Data Analysis
3. Results
3.1. Sheep Grazing Area and Moving Trajectory in the Spring and Autumn
3.2. Quantitative Characteristics of the Species and Communities in the Grazing Trajectory Surface
Community Types and Species Distribution in Grazing Trajectory Surface
3.3. Environmental Interpretation of Community Formation in Grazed Grassland
3.4. The Source of Exotic Species within the Community
4. Discussion
4.1. Effects of the Different Seasons on the Trajectory of the Grazing Sheep
4.2. Quantitative Characteristics of Species and Communities in the Grazing Trajectory Surface
4.3. The Source of Exotic Species within the Community
4.3.1. Species and Quantity Characteristics of Germinating Plant Seedlings in the Sheep Feces
4.3.2. Effects of the Digestive Tract on the Seed Germination Characteristics of the Exotic Plant Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, W.; Wan, J.; Yang, J. Morphological and Physiological Characteristics of Seriphidium Transiliense Seed and Response of Endozoochorous by Sheep. Highlights Sci. Online 2015, 8, 500–509. [Google Scholar]
- Wang, S. Response of Morphology and Germinability of Heteromorphic Seeds of Four Chenopodiaceae Pecies to Sheep Rumen Digestion. Chin. J. Ecol. 2018, 37, 119–127. [Google Scholar] [CrossRef]
- Jensen, T.S.; Nielsen, O.F. Rodents as Seed Dispersers in a Heath? Oak Wood Succession. Oecologia 1986, 70, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Janzen, D.H. Dispersal of Small Seeds by Big Herbivores: Foliage Is the Fruit. Am. Nat. 1984, 123, 338–353. [Google Scholar] [CrossRef]
- Couvreur, M.; Cosyns, E.; Hermy, M.; Hoffmann, M. Complementarity of Epi- and Endozoochory of Plant Seeds by Free Ranging Donkeys. Ecography 2005, 28, 37–48. [Google Scholar] [CrossRef]
- Baraza, E.; Valiente-Banuet, A. Seed Dispersal by Domestic Goats in a Semiarid Thornscrub of Mexico. J. Arid Environ. 2008, 72, 1973–1976. [Google Scholar] [CrossRef]
- Doucette, K.M.; Wittenberg, K.M.; McCaughey, W.P. Seed Recovery and Germination of Reseeded Species Fed to Cattle. J. Range Manag. 2001, 54, 575. [Google Scholar] [CrossRef]
- Yu, X.; Xu, C.; Wang, F.; Shang, Z.; Long, R. Recovery and Germinability of Seeds Ingested by Yaks and Tibetan Sheep Could Have Important Effects on the Population Dynamics of Alpine Meadow Plants on the Qinghai-Tibetan Plateau. Rangel. J. 2012, 34, 249. [Google Scholar] [CrossRef]
- Shiponeni, N.N.; Milton, S.J. Seed Dispersal in the Dung of Large Herbivores: Implications for Restoration of Renosterveld Shrubland Old Fields. Biodivers. Conserv. 2006, 15, 3161–3175. [Google Scholar] [CrossRef]
- Will, H.; Tackenberg, Q. A Mechanistic Simulation Model of Seed Dispersal by Animals. J. Ecol. 2008, 96, 1011–1022. [Google Scholar] [CrossRef]
- Zheng, J.; Sang, W.; Ma, K. Advances in Model Construction of Anemochoric Seed Long-Distance Dispersal. Chin. J. Plant Ecol. 2004, 28, 414–425. [Google Scholar]
- Morales, J.M.; Carlo, T.A. The Effects of Plant Distribution and Frugivore Density on the Scale and Shape of Dispersal Kernels. Ecology 2006, 87, 1489–1496. [Google Scholar] [CrossRef]
- Timothy, H.; Karl, V.; Gerhard, K.; Rajanathan, R. Using GPS Technology to Understand Spatial and Temporal Activity of Kangaroos in a Peri-Urban Environment. Animals 2018, 8, 97. [Google Scholar] [CrossRef]
- Peco, B.; Lopez-Merino, L.; Alvir, M. Survival and Germination of Mediterranean Grassland Species after Simulated Sheep Ingestion: Ecological Correlates with Seed Traits. Acta Oecologica 2006, 30, 269–275. [Google Scholar] [CrossRef]
- Warner, A. Rate of Passage of Digesta through the Gut of Mammals and Birds. Nutr. Abstr. Rev. Serise 1981, 51, 789–820. [Google Scholar]
- Wang, S.; Lu, W.; Waly, N.; Ma, C.; Zhang, Q.; Wang, C. Recovery and Germination of Seeds after Passage through the Gut of Kazakh Sheep on the North Slope of the Tianshan Mountains. Seed Sci. Res. 2017, 27, 43–49. [Google Scholar] [CrossRef]
- Henkin, Z.; Ungar, E.D.; Dolev, A. Foraging Behaviour of Beef Cattle in the Hilly Terrain of a Mediterranean Grassland. Rangel. J. 2012, 34, 163. [Google Scholar] [CrossRef]
- Anar, A. The Response of Sheep Feeding Behavior and the Weight Gain of Grazing Pressure. Master’s Thesis, Xinjiang University, Xinjiang, China, 2014. [Google Scholar]
- Wang, W.Q. Research for Grazing Behavior Based on Grazing Spatio-Temporal Trajectory Data. Master’s Thesis, Shihezi University, Xinjiang, China, 2017. [Google Scholar]
- Augustine, D.; Derner, J. Assessing Herbivore Foraging Behavior with GPS Collars in a Semiaridgrassland. Sensors 2013, 13, 3711–3723. [Google Scholar] [CrossRef]
- Lin, L.; Dickhoefer, U.; Müller, K.; Wurina Susenbeth, A. Grazing Behavior of Sheep at Different Stocking Rates in the Inner Mongolian Steppe, China. Appl. Anim. Behav. Sci. 2011, 129, 36–42. [Google Scholar] [CrossRef]
- Liu, P.; Ding, L.; Chen, J. Study on the Grazing Behavior of Yak and Cattle-Yak in Autumn Pasture of Oilian Mountain by GPS Tracking and Positioning System. Acta Ecol. Anim. Domastici 2015, 36, 56–60. [Google Scholar]
- Wang, S. Analysis of Sheep Grazing Behavior and Interaction with Grassland Environment in Desert Steppes Using 3S. Chin. J. Eco-Agric. 2015, 23, 860–867. [Google Scholar] [CrossRef]
- Yoshitoshi, R.; Watanabe, N.; Kawamura, K.; Sakanoue, S.; Mizoguchi, R.; Lee, H.J.; Kurokawa, Y. Distinguishing Cattle Foraging Activities Using an Accelerometry-Based Activity Monitor. Angeland Ecol. Manag. 2013, 66, 382–386. [Google Scholar] [CrossRef]
- Yang, R.; Wang, X. The Vertical Distriersity and Dynamic Chbution of Vegetation Patterns and Soil Properties at the Northern Slope of Ili River Valley. Res. Soil Water Conserv. 2016, 23, 32–39. [Google Scholar] [CrossRef]
- Li, Y. Species Divaracteristics of Plant Communities in Kanas Nature Reserve of Xinjiang. Master’s Thesis, Northwest University, Shaanxi, China, 2010. [Google Scholar]
- Li, Z.S.; Tang, J.W.; Zheng, Z.; Li, Q.J.; Luo, C.K.; Liu, Z.A.; Li, Z.N.; Duan, W.Y.; Guo, X.M. A Study on Plant Diversity of Tropical Montane Rainforests in Xishuangbanna, YunNan. Chin. J. Plant Ecol. 2004, 28, 833–843. [Google Scholar] [CrossRef]
- Li, G.; Wang, H.; Zhu, Z. Effects of Ecological Factors on Plant Communities of Ziwuling Mountain, Shaanxi Province, China. Acta Ecol. Sin. 2008, 28, 2463–2471. [Google Scholar] [CrossRef]
- Yan, J.; Liang, C.; Fu, X.; Wang, W.; Wang, L.; Jia, C. The Responses of Annual Plant Traits to Rainfall Variation in Steppe and Desert Regions. Acta Prataculturae Sin. 2013, 22, 68–76. [Google Scholar]
- Yan, K. Characteristics of Spring and Autunm Pasture Plant Community under Different Utilization Modes in Xinjiang. Pratacultural. Sci. 2011, 28, 1339–1344. [Google Scholar]
- Zhu, G.; Wei, Z.; Yang, J.; Yang, S. The Effects of Grazing Systems on the Above-Ground Bio-Mass of the Plants in Stipa Breviflora Community. Grassl. China 2002, 24, 15–19, 51. [Google Scholar]
- Wang, S.; Peng, F.; Lu, W.; Chen, Y.; Jing, P. Seed Morphology and Effects of Sheep Rumen Digestion on Seed Germination of 28 Gramineae Plants. J. Appl. Ecol. 2017, 28, 3908–3916. [Google Scholar] [CrossRef]
- Guo, L.Z. Biological Characteristics of Weed Polygonum Aviculare in Rapeseed Fields in Sichuan Water Area of Qinghai Province. Gansu Agric. Sci. Technol. 2004, 3, 52–53. [Google Scholar]
- Peng, J.; Zhu, J. Studies on Burgeon Characteristic of Convolvulus Arvensisseed and Investigation on Rule of Seedling. Xinjiang Agric. Sci. 2005, 4, 253–256. [Google Scholar]
- Wang, S.; Lu, W.; Chen, Y.; Jing, P. Effect of Sheep Digestive Tract on the Recovery and Germination of Seeds of Fifteen Leguminous Plants in the Northern Xinjiang Region, China. Chin. J. Plan Ecol. 2018, 42, 185–194. [Google Scholar] [CrossRef][Green Version]
- Grande, D.; Mancilla-Leytón, J.M.; Delgado-Pertiñez, M.; Martín-Vicente, A. Endozoochorus Seed Dispersal by Goats: Recovery, Germinability and Emergence of Five Mediterranean Shrub Species. Ournal. Agric. Res. 2013, 11, 347. [Google Scholar] [CrossRef]
- Calviño-Cancela, M.; Martín-Herrero, J. Effectiveness of a Varied Assemblage of Seed Dispersers of a Fleshy-Fruited Plant. Ecology 2009, 90, 3503–3515. [Google Scholar] [CrossRef] [PubMed]

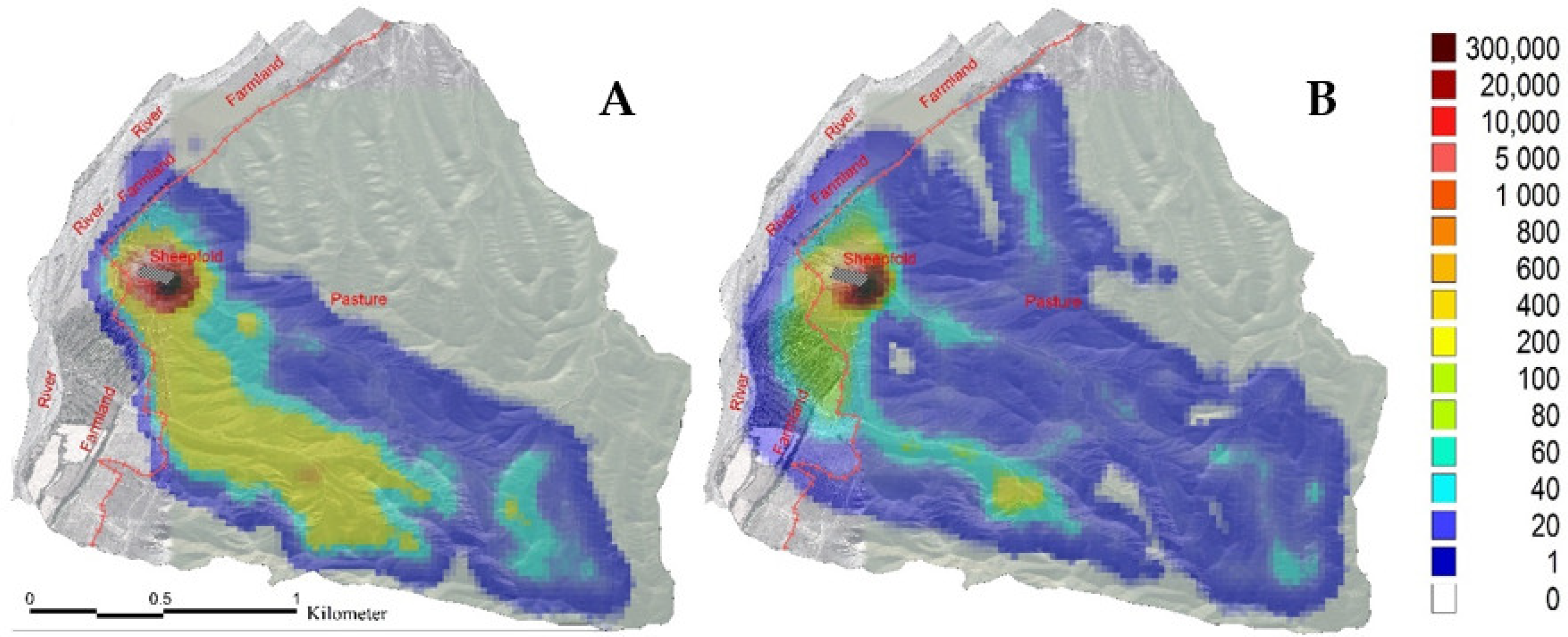

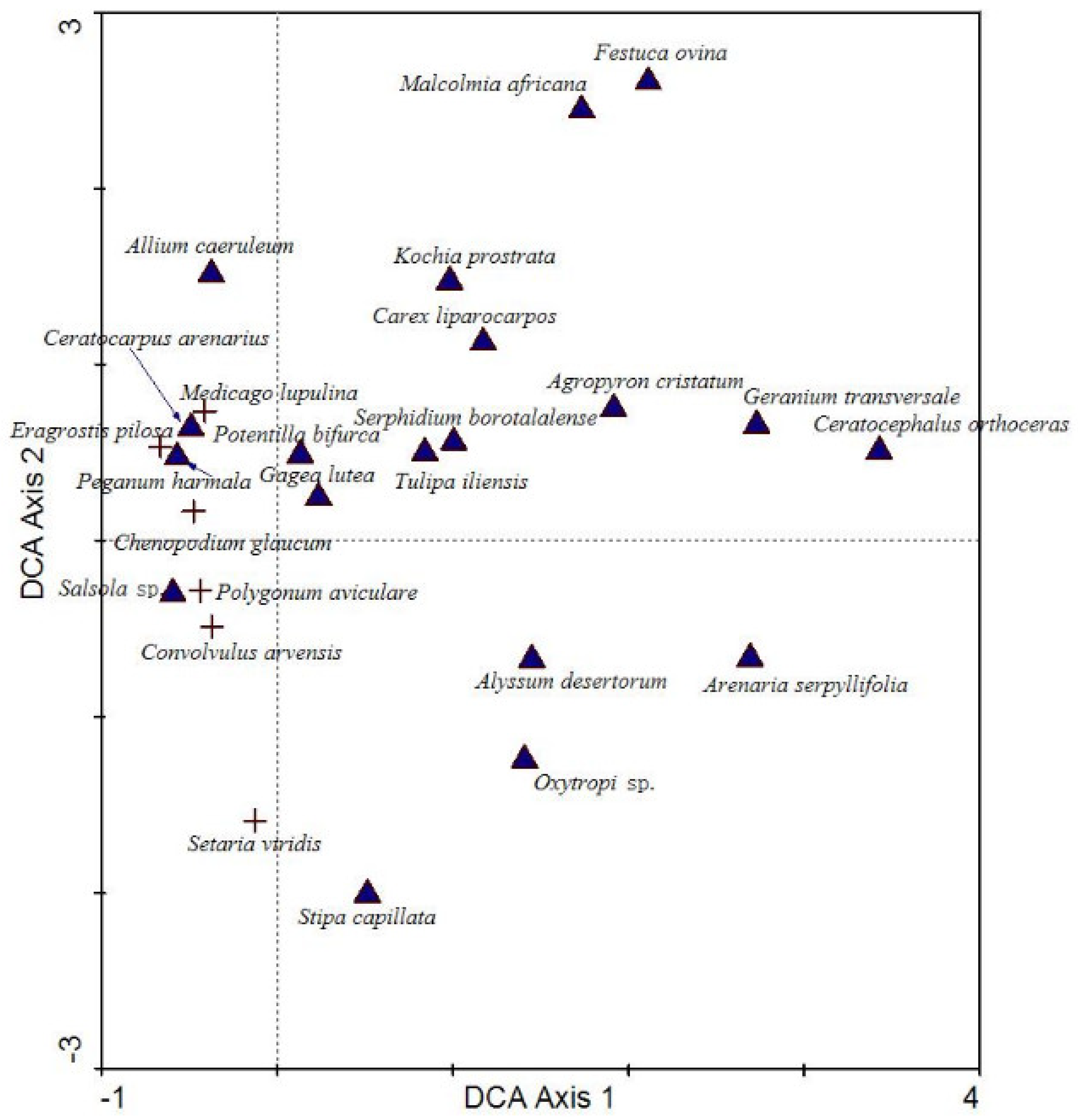
| Project | Spring (T = 5 d, N = 5 Sheep) | Autumn (T = 5 d, N = 5 Sheep) |
|---|---|---|
| Average trajectory length (km) | 6.39 ± 0.442 b | 7.58 ± 0.071 a |
| Average velocity (km/h) | 0.61 | 0.63 |
| Trajectory length of the grassland Area (km) | 4.59 ± 0.421 b | 6.31 ± 0.052 a |
| Track length in the non-grassy areas (km) | 2.80 ± 0.552 a | 1.27 ± 0.015 b |
| Average length of residence in the grass area (h) | 6.02 ± 0.821 b | 7.13 ± 0.235 a |
| Stay time in the non-grassland areas (h) | 2.03 ± 0.552 a | 1.02 ± 0.102 b |
| Duration of the stay in the farmland (h) | 0.00 b | 2.21 ± 0.423 a |
| Trajectory length in the farmland (km) | 0.00 b | 1.06 ± 0.058 a |
| Community | Richness | Abundance | Simpson’s Index | Shannon–Wiener Index | Evenness |
|---|---|---|---|---|---|
| I | 16.27 ± 1.103 a | 135.27 ± 30.454 b | 0.83 ± 0.049 a | 3.07 ± 0.243 a | 0.76 ± 0.056 a |
| II | 12.67 ± 2.964 b | 115.25 ± 83.263 bc | 0.74 ± 0.034 a | 2.475 ± 0.270 b | 0.68 ± 0.059 b |
| III | 8.84 ± 2.340 c | 70.00 ± 14.921 c | 0.49 ± 0.112 b | 1.66 ± 0.398 c | 0.53 ± 0.088 c |
| IV | 12.25 ± 0.957 b | 302.75 ± 71.400 a | 0.60 ± 0.106 b | 1.85 ± 0.331 c | 0.51 ± 0.078 c |
| Marginal Effect | Conditional Effect | |||||
|---|---|---|---|---|---|---|
| Environmental Elements | Eigen Value | Environmental Elements | Eigen Value | p-Value | R-Value | F-Value |
| Soil organic matter | 0.48 | Soil organic matter | 0.48 | 0.002 ** | 0.23 | 8.46 |
| Exposure | 0.46 | Amount of waste | 0.31 | 0.002 ** | 0.15 | 5.89 |
| Soil compactness | 0.37 | Grazing intensity | 0.34 | 0.002 ** | 0.16 | 6.75 |
| Humidity | 0.34 | Litter | 0.29 | 0.002 ** | 0.14 | 5.26 |
| Amount of waste | 0.32 | Altitude | 0.23 | 0.002 ** | 0.12 | 4.41 |
| Litter | 0.31 | Exposure | 0.19 | 0.002 ** | 0.09 | 1.34 |
| Altitude | 0.25 | Soil compactness | 0.06 | 0.190 | 1.46 | |
| Grazing intensity | 0.23 | Humidity | 0.06 | 0.134 | 1.69 | |
| pH-Value | 0.22 | pH-Value | 0.07 | 0.070 | 1.69 |
| Plant Species | Autumn | Spring |
|---|---|---|
| Chenopodium album | 54.67 ± 8.96 a | 0 b |
| Setaria viridis | 2.67 ± 2.081 a | 1.60 ± 0.58 b |
| Semen Trigonellae | 9.67 ± 2.08 a | 1.21 ± 0.12 b |
| Echinochloacrus galli | 3.00 ± 1.00 a | 0 b |
| Polygonum aviculare | 2.33 ± 0.58 a | 0 b |
| Medicago lupulina | 6.78 ± 1.21 a | 0 b |
| Convolvulus arvensis | 1.23 ± 0.59 a | 0 b |
| Amaranthus retroflexus | 1.67 ± 1.15 a | 0 b |
| Bassia dasyphylla | 5.67 ± 1.53 a | 0 b |
| Carex liparocarpos | 1.67 ± 0.58 a | 0 b |
| Artemisia annua | 2.32 ± 0.58 a | 0 b |
| Festuca ovina | 3.58 ± 0.54 a | 0 b |
| Salsola collina | 3.65 ± 0.28 a | 0 b |
| Dysphania botrys | 6.33 ± 2.081 a | 0.67 ± 0.58 b |
| Galium verum | 2.33 ± 0.58 a | 0 b |
| Plant Species | 0 h | 8 h | 24 h | 36 h | 48 h |
|---|---|---|---|---|---|
| Chenopodium album | 40.67 ± 2.31 d | 80 ± 3.06 ab | 80.67 ± 5.03 ab | 76 ± 3.11 b | 64 ± 3.02 c |
| Setaria viridis | 76.67 ± 3.06 b | 92.67 ± 4.00 a | 87.33 ± 1.15 a | 72.67 ± 2 b | 71.33 ± 4.00 b |
| Convolvulus arvensis | 39.21 ± 2..50 d | 58.65 ± 3.24 c | 89.24 ± 10.26 a | 90.21 ± 9.58 a | 78.65 ± 14.23 b |
| Eragrostis pilosa | 8.25 ± 1.21 d | 25.26 ± 1.34 c | 77.01 ± 11.25 a | 45.10 ± 10.21 b | 25.14 ± 8.53 c |
| Polygonum aviculare | 89.00 ± 12.21 a | 56.32 ± 8.45 a | 13.50 ± 3.24 c | 9.32 ± 3.14 c | 8.52 ± 2.45 c |
| Medicago lupulina | 18.21 ± 2.35 d | 31.24 ± 4.58 c | 56.24 ± 8.25 b | 75.85 ± 6.89 a | 65.32 ± 8.24 b |
| Trigonella arcuata | 5.20 ± 0.28 c | 15.32 ± 1.69 b | 18.25 ± 1.25 b | 20.32 ± 2.69 a | 25.26 ± 2.34 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Cao, J.; Che, Z.; Guo, Y.; Ma, C.; Zhang, Q. Contribution of Sheep Grazing to Plant Diversity in Natural Grasslands. Diversity 2022, 14, 446. https://doi.org/10.3390/d14060446
Zhao J, Cao J, Che Z, Guo Y, Ma C, Zhang Q. Contribution of Sheep Grazing to Plant Diversity in Natural Grasslands. Diversity. 2022; 14(6):446. https://doi.org/10.3390/d14060446
Chicago/Turabian StyleZhao, Jiantao, Jiamin Cao, Zhaobi Che, Yaya Guo, Chunhui Ma, and Qianbing Zhang. 2022. "Contribution of Sheep Grazing to Plant Diversity in Natural Grasslands" Diversity 14, no. 6: 446. https://doi.org/10.3390/d14060446
APA StyleZhao, J., Cao, J., Che, Z., Guo, Y., Ma, C., & Zhang, Q. (2022). Contribution of Sheep Grazing to Plant Diversity in Natural Grasslands. Diversity, 14(6), 446. https://doi.org/10.3390/d14060446






