Abstract
Here, we report, for the first time, a microsporidian infection in laboratory-reared larvae of the damselfly Ischnura elegans. Infected larvae originated from field-collected adult females, which were caught in southern Poland in August 2020 (the second half of the flight season). Higher rearing temperatures and the presence of predator cues from the invasive alien signal crayfish (Pacifastacus leniusculus) increased the number of infected larvae. Infected larvae had distorted wing development, and all individuals died before emergence. Hence, microsporidian infection in I. elegans larvae impacted damselfly morphology and life history. We propose that warming temperature and stress caused by non-consumptive effects triggered by invasive alien predators are possible factors that produce negative fitness consequences following microsporidian infection in a key amphibious ectotherm.
1. Introduction
Parasitism is one of the most common and important interspecific interactions. It occurs in the kingdoms of all living organisms and takes different forms, from occasional ectoparasitism to close obligatory endoparasitism. Parasitic infections have various outcomes for the hosts, ranging from temporary mild sickness to death [1]. Parasites can affect host physiology, immunology (e.g., by causing tissue damage or increasing metabolism) [1,2,3], behaviour (e.g., changes in attraction to light) [2,4] and life history (e.g., decreases in fecundity) [5]. A high prevalence of parasitic infection may change host population density or even lead to population extinction [6], especially if host individuals are weakly resistant to a particular parasite or if a parasite is highly transmissible [2,6].
Researchers have focused on parasite–host interactions and have claimed that insects—taxonomically the most numerous and diverse group of animals on Earth [7]—are probably infected by equal numbers of parasites and parasitoids [8,9,10]. An important group among these invertebrates are dragonflies (Odonata), which are amphibious and hemimetabolic insects that have aquatic larvae and terrestrial imago. Both stages of odonates can be infected by parasites belonging to several systematic groups, including ectoparasitic water mites (arachnids) and endoparasitic gregarines (protists) [11,12]. Water mites decrease the survival of infected adults [13]. Gregarines invade both larval and adult odonate stages [14]. Gregarine infection leads to a shorter imago lifespan [15] or lower adult fat content, which negatively affects host reproduction [16]. Nematodes (Nematoda) and plathelminths (Digenea and Cestoda) are other endoparasites that infect odonates [11]. Odonates have also been recorded as hosting endoparasitic microsporidia fungal-related protists [17,18,19]. In such infections, there is a scarcity of information regarding parasitic effects on odonate fitness-related traits [17,18,20].
Microsporidia, which constitute approximately 1500 named species, are unicellular parasites that reproduce through spores. As parasites, they are limited to animal hosts, including insects, crustaceans, fishes and humans [21]. Microsporidia can be host- and tissue-specific. These species are transferred vertically and horizontally and often have multiple spore types and sometimes intermediate hosts. Alternatively, microsporidia can be opportunistic (towards hosts and tissues) in situations with horizontal transmission and one host [22]. In extreme cases, parasites can take over host cells and change host metabolism and reproduction [23,24]. In odonates, infection with microsporidia occurs in the fat body, where the parasite can be found at various developmental stages. The infected odonate larvae are often whiter or paler than noninfected ones [18,19]. However, there is no information on whether and to what extent microsporidia affect odonate life history. Data from other groups of insects indicate that infections caused by microsporidia might negatively affect host fitness-related traits, e.g., fecundity [5,8,25].
Here, we present the first record of microsporidian infection in laboratory-reared damselfly Ischnura elegans (Odonata: Zygoptera) larvae, a model species for eco-evolutionary studies [26,27,28,29]. This endoparasite affected larval survival, larval size and emergence success in the host.
2. Materials and Methods
Some of the data from our long-term experiment on damselflies are presented, in which we reared individuals from the egg stage through the larval stage until emergence or pre-emergence death.
Adult female I. elegans in copula were collected from two ponds in the city of Kraków, Poland, at two time points during summer: 5 July 2020 (Staw Płaszowski, 50.042908, 19.967240, and Staw Dąbski, 50.064735, 19.987438) and 8 August 2020 (Staw Płaszowski). After collection, females were placed in plastic containers equipped with wet filter paper for egg laying. The plastic containers were placed in a Styrofoam box with ice packs to keep the temperature low. Under such conditions, females were transported by car to the Institute of Nature Conservation at the Polish Academy of Sciences (PAS), where the laboratory experiment was run. Females were kept in containers in a room with a natural photoperiod and temperature until they laid eggs.
The temperatures at which eggs and larvae were reared were then changed once a week to follow seasonal changes in the mean weekly temperatures in shallow-water habitats (optimal for damselfly larvae [11]). As in previous laboratory experiments of damselflies’ life histories [30,31], the temperature was derived from lake model FLake [32]. The experiment consisted of two temperature treatments: a reference temperature that mimicked the actual temperature at the collection site and an elevated temperature treatment, where the temperature was elevated by 4 °C to mimic the predicted temperature change by the end of the 21st century [33]. Except for the overwintering conditions (see below), we followed weekly seasonal changes in daylight (photoperiod) according to civil twilight length in Kraków. In the wild, I. elegans overwinter in the larval stage, so that individuals experienced winter diapause. During the experiment, we simulated winter conditions. We programmed a constant temperature of 6 °C in the reference temperature treatment and 10 °C in the elevated-temperature treatment. No light was provided during the overwintering conditions. For experimental temperature and photoperiod distributions and ranges, see the Supporting Information (Supplementary Material Table S1).
Every egg clutch laid by individual females was divided into six and placed in separate plastic containers (15 cm × 11 cm × 7.5 cm) filled with 600 mL of water. The water consisted of ¾ dechlorinated tap water and ¼ dechlorinated tap water with or without predator cues originating from European perch (Perca fluviatilis) and signal crayfish (P. leniusculus). Eggs from every female were present in every treatment: the three predation cues (none, perch, or crayfish) and the two temperatures (reference or elevated). Perch and crayfish cues were not mixed; hence, damselflies from different predator treatment groups experienced these predator cues independently. At hatching, every predator treatment group during the egg stage was further divided into two new groups: a group that experienced predator cues during the egg stage and the larval stage and a control group that did not experience predator cues during the larval stage (only the predator effects from the egg stage) (Figure 1). The water was refilled every other day to keep the predator cue approximately constant, considering the length of cue biodegradation [34]. Additionally, previous experiments have demonstrated that predator cue refill every other day affects damselfly life history traits [28,29]. Hatching occurred two to three weeks after the eggs had been laid. After hatching, the larvae were transferred to other containers (19 cm × 12 cm × 9 cm) filled with 1 l of water and kept in groups of 15–20 individuals for the first 14 days. Keeping individuals in groups at this stage increases larval survival. Fourteen days after hatching, every larva was individually placed in a plastic cup (height = 9 cm, diameter = 4 cm, volume = 200 mL) filled with 100 mL of water. The water refill in every container that held 15–20 larvae and the water refill in cups holding individual larvae were analogous (¾ dechlorinated tap water and ¼ of dechlorinated tap water with or without predator cue, ¼ changed every second day) to that previously described. The larvae were fed twice a day (morning and afternoon) during weekdays and once a day during weekends with 1 mL of Artemia sp. Nauplii solution (mean = 198.5, SD = 92.4 nauplii/1 mL, N = 38). During the first 14 days, larvae kept in groups received 10 mL of solution. During the winter, the larvae were fed once a day. When the larvae reached the prefinal instar before emergence (F-1), they were additionally provided with one live Chironomidae larvae three times a week (Monday, Wednesday and Friday).
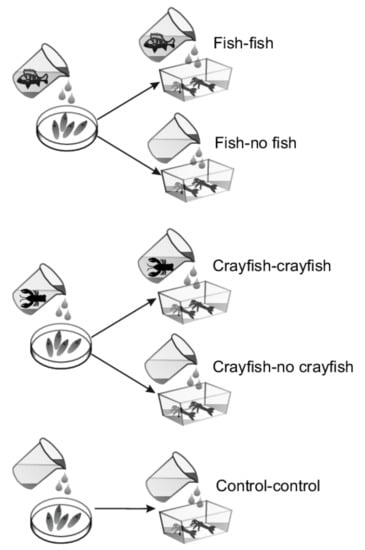
Figure 1.
Experimental design. Initially, I. elegans eggs were assigned to groups receiving perch cues, crayfish cues or no predator cues. At hatching, the perch cues egg group was divided into perch-cues and no-perch-cues larval groups, and the crayfish cues egg group was divided into crayfish cues and no crayfish cues larval groups. The control group experienced no perch or crayfish cues in either the egg or larval stage.
When the larvae reached the prefinal and final instars (F-1 and F-0), we noticed that some individuals had white structures inside of their body. For these individuals, we recorded the length of larval development (in days) between hatching and emergence or between hatching and death. We photographed the larvae to measure larval head width and wing pad length. In odonates, head width reflects overall body size and is used together with wing pad length to identify the larval instar. Live larvae were photographed with a binocular microscope (SMZ 745T; Nikon, Tokyo, Japan) with a camera (DFK 23UP031; Nikon). Larvae were placed in a drop of water on a Petri dish and the photographer took a picture. We photographed larvae on 1 April 2021, when the vast majority of noninfected larvae had already emerged, and on 22 April 2021, to estimate their growth. The head widths and wing pads lengths were measured using ImageJ software (NIH, Bethesda, MD, USA).
2.1. Histology
Three randomly selected larvae were chosen for microscopic analyses. Damselflies were cut into two halves, and both were used to prepare fresh smears and fixed in Bouin’s solution histological slides. Following fixation, the samples were dehydrated in a graded ethyl alcohol series (Linegal Chemicals, Warszawa, Poland), cleared with isopropyl alcohol (Leica, Wetzlar, Germany) and Clearene (Leica), and embedded in Paraplast Plus (Leica). Serial cross-sections (4–6 μm thick) were cut with a rotary microtome (Hyrax M55, Zeiss, Oberkochen, Germany). Histological slides were deparaffinized in Clearene (Leica), rehydrated in a graded ethyl alcohol series (Linegal Chemicals), and stained with Ehrlich haematoxylin (Carl Roth, Karlsruhe, Germany) and with a 1% ethanol solution of eosin Y (Analab, Warszawa, Poland). Then, the slides were dehydrated in 96% ethanol (Linegal Chemicals) and isopropyl alcohol (Leica), cleared in Clearene (Leica), and embedded in CV Ultra (Leica). Dried and nonstained fresh smears as well as fixed and stained tissue cross-sections were analysed under a light microscope (Eclipse 80i, Nikon) in a bright field and photographed using a digital camera (Axio Cam 305 colour, Zeiss) and image acquisition software (ZEN 3.3. blue edition, Zeiss).
2.2. Statistical Analysis
We found infections only in larvae originating from one population (Staw Płaszowski, later collection date) and only in individuals who were reared in the elevated temperature treatment. Therefore, we limited our analyses to this group.
As we noticed the infected larvae in the middle of the experiment, it was possible that some of them would die before being classified as infected. Thus, comparisons of the larval development duration of infected and noninfected individuals would be biased. Therefore, we reported only raw values for larval development duration.
All the analyses were performed in R software [35] with the following packages: emmeans [36], lme4 [37] and lmerTest [38]; ggplot2 [39] was used for graph preparation.
We analysed the data on the presence of infection among predator cue treatments using a generalized linear model with a binomial distribution and a logit link function. We plotted the distribution of larval head widths and wing pad lengths, and for comparison, we added head width and wing pad length ranges for F-1 instars based on observation of the larvae originating from females collected at the same site [40]. In independent tests, we compared the head widths and wing pad lengths between two measurement dates via a general linear mixed model. Head widths or wing pad lengths were response variables, measurement date was a fixed predictor, and individual ID was a random factor. We used Tukey HSD tests for pairwise level comparisons.
3. Results
3.1. General View
Infected larvae had white structures along the long axis of their bodies (thorax and abdomen) that were visible through the body shell. In the analysed subset, where infections were present (Staw Płaszowski, later collection date), there were 182 larvae in total, of which 33 were classified as infected, 87 as not infected that emerged with success and 62 as not infected that died before emergence. Larval infection was significantly affected by predator cue treatment (χ2 =15.12, p = 0.001). Pairwise comparisons revealed significant differences between the perch (egg and larva) vs. signal crayfish (egg and larva) groups (p = 0.04) and the control vs. signal crayfish (egg and larva) groups (p = 0.05). In both cases, infected larvae were more abundant in the signal crayfish group. A higher number of infected larvae was also found in the signal crayfish (egg)–control (larva) group, but the difference was not significant (the lowest p = 0.2 from the pairwise comparison with the perch (egg and larva) group) (Figure 2).
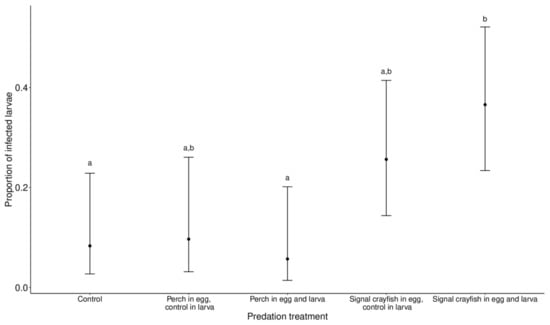
Figure 2.
The proportion of microsporidian infections in I. elegans larvae in the different predator treatment groups. Ischnura elegans eggs and larvae were exposed to perch or signal crayfish cues either during egg or egg and larval stages. The control group was not exposed to predator cues during either stage. Data are displayed as the marginal means ±95% CI. Different letters at the top of the error bars indicate significant differences between experimental groups.
3.2. Histological Description
Histological analysis showed that larvae were infected with microsporidia (Figure 3A). These parasites filled the thorax and abdomen (Figure 3B). On the cross-sections of the body, hypertrophied cells of the fat body lobes were filled in by the microsporidia and surrounding organs (Figure 3B–F). Germ gonads with active gametogenesis were visible (Figure 3F).
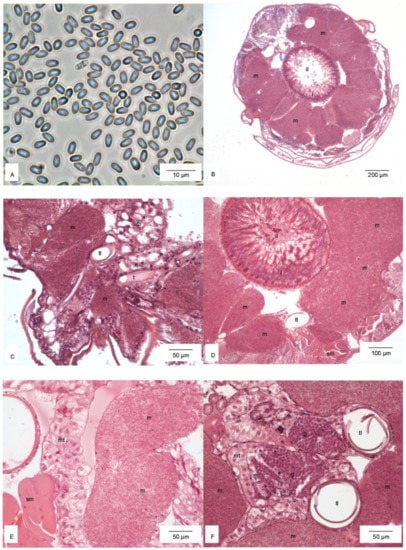
Figure 3.
Microsporidia (A). Cross-section through the thorax (B). Hypertrophied cells of the fat body filled in by microsporidia and the surrounding noninfected fat body (C), intestine (D), Malpighian tubules (E), and germ gonads (F). Abbreviations: fb, noninfected fat body; g, germ gonads; i, intestine; il, intestinal lumen; m, hypertrophied cells of the fat body with microsporidia; mt, Malpighian tubules; sm, striated muscles; t, tracheal tube; tl, tracheal lumen.
3.3. Life History
Infected larvae did not metamorphose, and so did not emerge. The longest development duration between hatching and emergence of noninfected larvae was 231 days. The longest development duration of noninfected larvae that died before emergence was 251 days. Six infected larvae lived longer than 251 days; of these, the longest larval lifespan was 261 days.
In infected larvae, the range of head widths overlapped with the head widths of reference larvae, for both F-1 (first measurement date) and F-0 (second measurement date). The head widths increased significantly between the two measurement dates (t = 4.46, p = 0.003), as did the wing pad lengths (t = 2.26, p = 0.04). However, after removing two outliers from the data from the second measurement date, the wing pad length did not differ between measurement dates (t = 1.04, p = 0.32).
4. Discussion
We report, for the first time, the negative effects of endoparasitic microsporidian infection on life history in a damselfly model for eco-evolutionary research, I. elegans. Other insects [8,22,25,41], including odonates (suborder Anisoptera: Aeschna viridis [17], Orthetrum albistylum speciosum [20], Aeschna grandis and Libellula quadrimaculata [18,42], Tholymis tillarga [43], Tramea limbata [44]; suborder Zygoptera: Calopteryx virgo [45], Coenagrion pulchellum [46] C. hastulatum [19]) were reported to carry this pathogen; however, these results were based mainly on observational, not experimental, studies. Hence, previous results could not directly answer the question of whether parasites or other factors caused reductions in host fitness. The results of our experimental approach provide insights into pathogenic effects on fitness-related traits in the studied organism and how these pathogenic effects might modify damselfly population dynamics.
We could not identify the microsporidia to the species level, which would otherwise extend the list of parasites in odonates (and I. elegans, in particular). However, our identification at the phylum level indicates that microsporidia are likely as common in odonates as in other groups of insects [25].
None of the infected I. elegans larvae emerged, indicating that microsporidian infection has a lethal effect on the premature damselfly. Similar negative effects on life history traits have been reported in other insects. For example, in the mosquito Aedes aegypti, microsporidian infection led to a reduction in the number of eggs laid by adult females and lower offspring hatching success [5]. A similar infection effect on the host was found in Muscidifurax raptor, a parasitoid wasp of the house fly (Musca domestica) [8]. In Drosophila melanogaster, artificial microsporidian infection of adults led to death caused by the parasites’ use of the fat storage [41]. If the pathogen is highly transmissible, it might negatively affect the host population. However, we think it is possible that damselfly larvae carrying fewer endoparasites, which could not be identified as infected ‘by eye’, successfully emerged. In such situations, it would be interesting to study delayed or carry-over pathogenic effects from the larval to imago stage.
The considerable increase in head width in larvae carrying microsporidia could suggest that these individuals had reached the minimal size for emergence; however, minimal wet mass for emergence is also important [40]. During the experiment, we did not weigh the larvae. Nonetheless, the largest infected larvae, estimated ‘by eye’, reached a body size similar to that of the noninfected individuals that eventually successfully emerged. Distorted wing development during last instars would likely prevent the damselflies from unfolding their wings and flying if they emerged. Notably, in mosquitoes, microsporidia did not affect wing development [8]. Individuals with head widths that indicated that they were F-1 or F-0 instars tended to have wing lengths that would classify them as previous instars. Moreover, hardly any wing development occurred between the two measurement dates. Over the same period, head width significantly increased. We suggest that delayed wing development led to prolonged larval development and finally larval death. Nonetheless, measuring a larger number of morphological traits would probably show other aspects of abnormal development in the infected larvae.
We found infections only in animals originating from one population and at one time, sampled later in the season. The larvae from the first collection date, which included both collection sites, were held in the same climatic chamber. This suggests that the parasites did not transfer between the cups, or that earlier-collected larvae were more resistant to infection and did not show symptoms. The infection of individuals from just one population and one sampling date indicates that, in our laboratory, microsporidia were transferred horizontally. Some microsporidia genera can also be transferred vertically [47]. The higher experimental temperatures in the elevated temperature condition probably increased pathogen development and, thus, damselfly sickness because only larvae grown at higher temperatures showed signs of infection. This might also explain the presence of parasites only in larvae whose mothers were field-sampled later in the summer, when the ambient temperature was higher than during the earlier sampling point.
According to pathogen transfer, microsporidian spores could be disseminated among damselflies through water exchange between the aquaria that housed predators and cups holding damselfly eggs and larvae. However, this explanation is less likely since infected damselfly larvae were reported, although with lower frequency, in all experimental groups.
Infections were generally more frequent in the signal crayfish cue group than in the perch and control groups. This suggests that chemicals released by the crayfish promoted the infection. Animals experiencing predator cues are more stressed [48,49]. Stress can cause changes in a number of traits, including physiological traits such as metabolic rate [48] and immune function, in potential prey [50,51,52]. Reduced immune function can, in turn, increase the exposure of host animals to pathogens. The results from previous studies on I. elegans indicated that exposing damselfly egg and larval stages to predator cues delayed egg and larval development [28,29,40], thus indicating that this damselfly is sensitive to predator-risk cues. Hence, a possible explanation for the highest microsporidian infection experienced by the crayfish cue group could be stress reactions in the host. Interestingly, larval infection was only elevated in the signal crayfish cue group, indicating that a stronger stress is imposed by alien invasive allopatric predators (the signal crayfish cue group) than native sympatric predators (the perch cue group). This discrepancy is in accordance with our previous results, where we showed prolonged egg development in groups treated with signal crayfish cues, but not perch cues, when compared to the control group [29].
5. Conclusions
Our results broaden our knowledge of the effects of microsporidian infection on life history traits in a key amphibious organism. We underline the importance of possible microsporidian infection in damselflies that are used as model organisms in eco-evolutionary studies. Overlooking this factor may impact the experimental results. Future studies should focus on the mechanism of infection and the impact of microsporidia on different host traits and conditions that increase the risk of parasitism. Such studies would shed light on the association between microsporidian infection and hosts exposed to ecologically challenging conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14060428/s1; Table S1. Experimental temperature and photoperiod distributions and ranges.
Author Contributions
Conceptualization, A.A. and S.S.; methodology, A.A., A.M.L. and S.S.; software, A.A., A.M.L. and S.S.; validation, A.A., A.M.L., J.I.R.L. and S.S.; formal analysis, A.A. and S.S.; investigation, A.A., A.M.L. and S.S.; resources, S.S. and A.M.L.; data curation, A.A.; writing— A.A., A.M.L., J.I.R.L. and S.S.; writing—original draft preparation, A.A., A.M.L., J.I.R.L. and S.S.; writing—review and editing, A.A., A.M.L., J.I.R.L. and S.S.; visualization, A.A., A.M.L. and S.S.; supervision, A.A. and S.S.; project administration, S.S.; funding acquisition, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research leading to these results was funded by the Norwegian Financial Mechanism 2014–2021, project no. 2019/34/H/NZ8/00683 (ECOPOND). A.A. and S.S. were supported by the National Science Centre, Poland (grant 2019/33/B/NZ8/00521) and the Institute of Nature Conservation. A.M.L. was supported by the Institute of Environmental Sciences, Jagiellonian University (N18/DBS/000003).
Institutional Review Board Statement
The animal study protocol was approved by the Local Ethical Committee (ref. 394/2020), Regional Directorate for Environmental Protection in Białystok (ref. WPN.6205.21.2020.ML) and Regional Directorate for Environmental Protection in Kraków (ref. OP-158 I.672.8.2020.MK1).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in open repository https://zenodo.org (accessed on 1 May 2021).
Acknowledgments
We would like to thank E. Sieńko, M. Nowicki, and P. Laskowski for catching the crayfish and U. Norling for help in identifying the parasites we found in our culture.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gunn, A.; Pitt, S.J. Parasitology: An Integrated Approach; John Willey & Sons: Hoboken, NJ, USA, 2012; ISBN 9780470684245. [Google Scholar]
- Goater, T.M.; Goater, C.P.; Esch, G.W. Parasitism, the Diveristy and Ecology of the Animal Parasites; Cambridge University Press: Cambridge, UK, 2014; ISBN 9788578110796. [Google Scholar]
- Sadd, B.M.; Siva-Jothy, M.T. Self-harm caused by an insect’s innate immunity. Proc. R. Soc. B Biol. Sci. 2006, 273, 2571–2574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bethel, W.M.; Holmes, J.C. Altered evasive behavior and responses to light in amphipods harboring acanthocephalan cystacanths. J. Parasitol. 1973, 59, 945–956. [Google Scholar] [CrossRef]
- Becnel, J.J.; Garcia, J.J.; Johnson, M.A. Edhazardia aedis (Microspora: Culicosporidae) effects on the reproductive capacity of Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 1995, 32, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D.; Lipsitch, M.; Mangin, K.L. The Effect of Parasites on Host Population Density and Extinction: Experimental Epidemiology with Daphnia and Six Microparasites. Am. Nat. 2000, 156, 459. [Google Scholar] [CrossRef]
- Grimaldi, D.; Engel, M.S. Evolution of the Insects; Cambridge University Press: Cambridge, UK, 2005; ISBN 9780521821490. [Google Scholar]
- Geden, C.J.; Long, S.J.; Rutz, D.A.; Becnel, J.J. Nosema disease of the parasitoid muscidifurax raptor (Hymenoptera: Pteromalidae): Prevalence, patterns of transmission, management, and impact. Biol. Control 1995, 5, 607–614. [Google Scholar] [CrossRef]
- Fredensborg, B.L.; Fossdal Í Kálvalíð, I.; Johannesen, T.B.; Stensvold, C.R.; Nielsen, H.V.; Kapel, C.M.O. Parasites modulate the gut-microbiome in insects: A proof-of-concept study. PLoS ONE 2020, 15, e0227561. [Google Scholar] [CrossRef] [Green Version]
- Humber, R.A. Fungal Pathogens and Parasites of Insects. In Applied Microbial Systematics; Priest, F.G., Goodfellow, M., Eds.; Springer Science+Business Media: Dordrecht, The Netherlands, 2000; pp. 203–231. ISBN 1599051494. [Google Scholar]
- Corbet, P.S. Dragonflies—Behaviour and Ecology of Odonata; Harley Books: Colchester, UK, 1999. [Google Scholar]
- Forbes, M.R.; Robb, T. Testing hypotheses about parasite-mediated selection using odonate hosts. In Dragonflies and Damselflies: Model Organisms for Ecological and Evolutionary Research; Córdoba-Aguilar, A., Ed.; Oxford University Press: Oxford, UK, 2008; pp. 175–188. [Google Scholar]
- Braune, P.; Rolff, J. Parasitism and survival in a damselfly: Does host sex matter? Proc. R. Soc. B Biol. Sci. 2001, 268, 1133–1137. [Google Scholar] [CrossRef] [Green Version]
- Hecker, K.R.; Forbes, M.R.; Léonard, N.J. Parasitism of damselflies (Enallagma boreale) by gregarines: Sex biases and relations to adult survivorship. Can. J. Zool. 2002, 80, 162–168. [Google Scholar] [CrossRef]
- Åbro, A. Gregarines: Their Effects on Damselflies (Odonata: Zygoptera). Entomol. Scandanavi 1971, 2, 294–300. [Google Scholar] [CrossRef]
- Siva-Jothy, M.T.; Plaistow, S.J. A fitness cost of eugregarine parasitism in a damselfly. Ecol. Entomol. 1999, 24, 465–470. [Google Scholar] [CrossRef]
- Sokolova, Y.Y.; Kryukova, N.A.; Glupov, V.V.; Fuxa, J.R. Systenostrema alba Larsson 1988 (Microsporidia, Thelohaniidae) in the dragonfly Aeshna viridis (Odonata, Aeshnidae) from South Siberia: Morphology and molecular characterization. J. Eukaryot. Microbiol. 2006, 53, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Larsson, J.I.R. On the taxonomy of the genus Systenostrema Hazard & Oldacre, 1975 (Microspora, Thelohaniidae), with description of two new species. Syst. Parasitol. 1988, 11, 3–17. [Google Scholar] [CrossRef]
- Larsson, J.I.R. On the Cytology and Taxonomic Position of Nudispora biformis N. G., N. Sp. (Microspora, Thelohaniidae), a Microsporidian Parasite of the Dragon Fly Coenagrion hastulatum in Sweden. J. Protozool. 1990, 37, 310–318. [Google Scholar] [CrossRef]
- Nakamura, H.; Kurimoto, N.; Imura, Y.; Hatakeyama, Y. The First Isolation of Microsporidia from Dragonflies in Japan. Jpn. J. Appl. Entomol. Zool. 2021, 65, 29–34. [Google Scholar] [CrossRef]
- Weiss, L.M.; Becnel, J.J. Microsporidia Pathogens of Opportunity; John Willey & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Becnel, J.J.; White, S.E.; Shapiro, A.M. Review of microsporidia-mosquito relationships: From the simple to the complex. Folia Parasitol. 2005, 52, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Senderskiy, I.V.; Timofeev, S.A.; Seliverstova, E.V.; Pavlova, O.A.; Dolgikh, V.V. Secretion of Antonospora (Paranosema) locustae proteins into infected cells suggests an active role of microsporidia in the control of host programs and metabolic processes. PLoS ONE 2014, 9, 93585. [Google Scholar] [CrossRef] [Green Version]
- Tamim El Jarkass, H.; Reinke, A.W. The ins and outs of host-microsporidia interactions during invasion, proliferation and exit. Cell. Microbiol. 2020, 22, e13247. [Google Scholar] [CrossRef]
- Becnel, J.J.; Andreadis, T.G. Microsporidia in Insects. In Microsporidia: Pathogens of Opportunity; Weiss, L.M., Becnel, J.J., Eds.; John Willey & Sons: Hoboken, NJ, USA, 2014; pp. 521–571. ISBN 9781118395264. [Google Scholar]
- Córdoba-Aguilar, A. Dragonflies and Damselflies: Model Organisms for Ecological and Evolutionary Research; Córdoba-Aguilar, A., Ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2008; ISBN 9789896540821. [Google Scholar]
- Stoks, R.; Córdoba-Aguilar, A. Evolution ary Ecology of Odonata: A Complex Life Cycle Perspective. Annu. Rev. Entomol. 2012, 57, 249–265. [Google Scholar] [CrossRef]
- Sniegula, S.; d’Amour Nsanzimana, J.; Johansson, F. Predation risk affects egg mortality and carry over effects in the larval stages in damselflies. Freshw. Biol. 2019, 64, 778–786. [Google Scholar] [CrossRef]
- Antoł, A.; Sniegula, S. Damselfly eggs alter their development rate in the presence of an invasive alien cue but not a native predator cue. Ecol. Evol. 2021, 11, 9361–9369. [Google Scholar] [CrossRef]
- Sniegula, S.; Golab, M.J.; Johansson, F. Cannibalism and activity rate in larval damselflies increase along a latitudinal gradient as a consequence of time constraints. BMC Evol. Biol. 2017, 17, 167. [Google Scholar] [CrossRef] [PubMed]
- Johansson, F.; Watts, P.C.; Sniegula, S.; Berger, D. Natural selection mediated by seasonal time constraints increases the alignment between evolvability and developmental plasticity. Evolution 2021, 75, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Lake Model Flake FLake. Available online: http://www.flake.igb-berlin.de/ (accessed on 26 March 2022).
- Parry, M.L.; Canziani, O.F.; Palutikof, J.P.; van der Linden, P.J.; Hanson, C.E. (Eds.) IPCC Climate Change Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Van Buskirk, J.; Krügel, A.; Kunz, J.; Miss, F.; Stamm, A. The Rate of Degradation of Chemical Cues Indicating Predation Risk: An Experiment and Review. Ethology 2014, 120, 942–949. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Lenth, R.V. Emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 2021, 163, 1–91. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. General rights lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Sniegula, S.; Raczyński, M.; Golab, M.J.; Johansson, F. Effects of predator cues carry over from egg and larval stage to adult life-history traits in a damselfly. Freshw. Sci. 2020, 39, 804–811. [Google Scholar] [CrossRef]
- Franchet, A.; Niehus, S.; Caravello, G.; Ferrandon, D. Phosphatidic acid as a limiting host metabolite for the proliferation of the microsporidium Tubulinosema ratisbonensis in Drosophila flies. Nat. Microbiol. 2019, 4, 645–655. [Google Scholar] [CrossRef]
- Larsson, J. Ultrastructural Investigation of 2 Microsporidia with Rod-Shaped Spores, with Descriptions of Cylindrospora-Fasciculata Sp-Nov and Resiomeria-Odonatae Gen Et Sp-Nov (microspora, Thelohaniidae). Protistologica 1986, 22, 379–398. [Google Scholar]
- Kalavati, C.; Narasimhamurti, C.C. New microsporidian parasite, Toxoglugea tillargi Sp-N from an odonate, Tholymis tillarga. Acta Protozool. 1978, 17, 279–283. [Google Scholar]
- Narasimhamurti, C.C.; Ahamed, S.N.; Kalavati, C. Two new species of microsporidia from the larvae ofTramea limbata (Odonata: Insecta). Proc. Anim. Sci. 1980, 89, 531–535. [Google Scholar] [CrossRef]
- Sprague, V. Classification and Phylogeny of the Microsporidia. In Comparative Pathobiology: Volume 2 Systematics of the Microsporidia; Bulla, L.A., Cheng, T.C., Eds.; Springer US: Boston, MA, USA, 1977; pp. 1–30. ISBN 978-1-4613-4205-2. [Google Scholar]
- Larsson, J. A revisionary study of the taxon Tuzetia Maurand, Fize, Fenqick and Michel, 1971, and related forms (Microspora, Tuzetiidae). Protistologica 1983, 19, 323–355. [Google Scholar]
- Cali, A.; Takvorian, P.M. Developmental Morphology and Life Cycles of the Microsporidia. In Microsporidia: Pathogens of Opportunity; Weiss, L.M., Becnel, J.J., Eds.; John Willey & Sons: Hoboken, NJ, USA, 2014; pp. 71–135. [Google Scholar]
- Antoł, A.; Kierat, J.; Czarnoleski, M. Sedentary prey facing an acute predation risk: Testing the hypothesis of inducible metabolite emission suppression in zebra mussels, Dreissena polymorpha. Hydrobiologia 2018, 810, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Jermacz, Ł.; Dzierżyńska-Białończyk, A.; Kobak, J. Predator diet, origin or both? Factors determining responses of omnivorous amphipods to predation cues. Hydrobiologia 2017, 785, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Mikolajewski, D.J.; Stoks, R.; Rolff, J.; Joop, G. Predators and cannibals modulate sex-specific plasticity in life-history and immune traits. Funct. Ecol. 2008, 22, 114–120. [Google Scholar] [CrossRef]
- Adamo, S.A.; Easy, R.H.; Kovalko, I.; MacDonald, J.; McKeen, A.; Swanburg, T.; Turnbull, K.F.; Reeve, C. Predator exposure-induced immunosuppression: Trade-off, immune redistribution or immune reconfiguration? J. Exp. Biol. 2017, 220, 868–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raczyński, M.; Stoks, R.; Johansson, F.; Sniegula, S. Size-mediated priority effects are trait-dependent and consistent across latitudes in a damselfly. Oikos 2021, 130, 1535–1547. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).