Abstract
Scallops are bivalve filter-feeding mollusks that can attain a large size and have great importance in terms of their ecological roles and commercial value. Overfishing has led to a decrease in scallop stocks worldwide, leading to intense development of the aquaculture industry. The latter is well-established in Asian countries in the East Sea and Sea of Japan. In this paper, we summarized information regarding the biology, stock dynamics, and fishery of yesso scallops inhabiting Russian waters of the Sea of Japan. This species has relatively rapid growth rates and reaches a marketable size of 100 mm in shell height at age 3–5 years. In Russian waters, the total commercial stock of yesso scallops in 2021 was estimated at 2784 t. Commercial fisheries have been banned since 2020 due to the depletion of natural stocks as a result of illegal fishing and overexploitation. The total allowable catch for yesso scallops has been set at 3 t for monitoring and recreational purposes. The cultivation of the scallops includes spat collection, spat intermediate culture, and final grow-out on the seafloor or in suspended cages. In the past decade, this industry has demonstrated rapid growth and currently accounts for >16,000 t per year. Environmental fluctuations and epizootics seem to be the most important challenges for the scallop aquaculture sector in the Sea of Japan.
1. Introduction
Scallops are bivalve mollusks belonging to the superfamily Pectinoidea. This group includes the extant families Cyclochlamydidae, Pectinidae, Propeamussiidae, and Spondylidae [1]. Scallops are considered a diverse group in terms of their morphology, behavior, and ecology [2]. As with other large bivalves, scallops are widely distributed in marine habitats from shallow to deep water. Although most species of scallops are small in size, some species (for example, weathervane scallop Patinopecten caurinus and rock scallop Crassadoma gigantea) can reach shell heights as large as 250 mm and solid weights as large as 1 kg. Large scallop species have significant economic importance and support commercial fisheries and aquaculture worldwide [3]. Scallops represent an important component of benthic marine communities [4,5], being ecological engineers whose activity keep the water clean from microbes, pathogens, suspended particles, and contaminants [6,7] and create new habitats for infauna [8]. Scallops are prey organisms for various predators including starfish, fish, and crustaceans [9]. More than 400 scallop species occur in all the seas of the world [1,2] including the North Pacific.
The Sea of Japan is a marginal sea of the western Pacific Ocean with a total area of 978,000 km2 and a mean depth of 1752 m. The sea is bounded by the Russian Federation and Korea to the west and by Japan and Sakhalin Island to the east [10]. It has a relatively warm climate with the prevailing northwest monsoon wind from December to March and the southerly tropical monsoon blows from the North Pacific onto the Asian mainland in summer [11]. Because the Sea of Japan is a semi-closed area, the effects of local winds are greater than that of swell, and, especially in winter, the wave height distribution is driven by the seasonal wind speed and the fetch [12]. The northern part of the area freezes in winter. A counterclockwise pattern determines the water circulation in the sea. In summer, a distinct thermocline separates two water masses: the surface water and the middle water with relatively high temperature and salinity. The deep water with temperatures ranging from 0 to 0.5 °C forms a third water mass [10]. The concentration of dissolved oxygen is usually high, providing a good basis for diverse flora (>800 species) and fauna (>3500 species). Due to favorable environmental conditions, there are many abundant stocks of commercially important species including saury, mackerel, Jack mackerels, sardines, anchovies, herring, sea bream, salmon, trout, squid, crabs, mollusks, sea urchins, and sea cucumbers with fishing grounds being mostly located on the continental shelves and in adjacent waters [11].
Scallops have historically been considered a delicacy, especially in the local markets of the USA as well as European and Asian countries where the fresh product is consumed. Its high demand guarantees a continued high value in the mentioned markets [13]. Currently, the growing demand for scallops has led to overfishing of many natural stocks [14,15] and the development of their aquaculture [16]. Asian countries, Japan and China, and Latin American countries, Peru and Chile, have achieved considerable success in scallop production under cultivation conditions, providing as high as 90% of the global scallop supply [3,16,17,18]. Scallops continue to bring high prices from both commercial fisheries and aquaculture ventures. For example, world prices for the adductor muscles of yesso scallops vary from USD 110 to 129 per kg. In Russia, they account for RUB 1600–2400 per kg (USD 23–34 per kg).
Among scallops inhabiting Russian waters of the Sea of Japan, the yesso scallop (Mizuhopecten yessoensis) is the most important species in terms of its abundance, wide distribution, and potential for fisheries and aquaculture [19]. Populations of these bivalve mollusks in the region have been explored by native inhabitants of the coasts of Sakhalin, the Kurils, Japan, Primorye, and Korea since the Palaeolithic age [19]. In Peter the Great Bay, large-scale fishing of scallops occurred between 1920 and 1937 when motorboats equipped with dredges were introduced. As a result, local stocks rapidly depleted. After World War II, industrial fishing was renewed for a short period but then totally banned in 1960. At coastal sites in northern Primorye, southern Sakhalin, Kuril Islands, and the Bering Sea, yesso scallops and other scallop species were harvested by small seiners equipped with steel 1.5–3.0 m wide dredges towed for 5–30 min. In Peter the Great Bay, scallops are harvested by divers [19].
In Russia, the marine fisheries and aquaculture sectors are of high priority for further development of coastal communities, infrastructure, and human well-being, and new techniques and scientific data are required to supply farming of cultured species [20,21,22]. In the past decade, there has been significant development of the scallop aquaculture industry, and new data concerning the biology of yesso scallops have been obtained. We combined the new biological data with those previously published and reviewed by Ivin et al. [19] instead of simple reference to the mentioned paper for the convenience of the reader. Thus, the aim of our paper is to summarize information regarding the main biological aspects and current status of the fishery and aquaculture of Mizuhopecten yessoensis in the Russian part of the Sea of Japan.
2. Biological Aspects
2.1. Distribution
The yesso scallop, Mizuhopecten yessoensis Jay, also called the common scallop, Japanese scallop, Russian scallop, Primorsky scallop, or giant scallop, is the most abundant and important scallop species in Russian waters of the Sea of Japan. This cold-water mollusk is widely distributed at coastal sub-Arctic sites in the North Pacific Ocean, southern part of the Sea of Okhotsk, and the Sea of Japan. It occurs along the coasts of the southern Kuril Islands, Sakhalin, Hokkaido, northern Honshu, and North Korea [19,23,24].
2.2. Life Cycle
Mizuhopecten yessoensis is a predominantly gonochoric species, i.e., both sexes are separate and invariable. The sex ratio (males:females) varies from 0.83:1 to 1.52:1 depending on the population age structure [25]. The events that occur in the reproductive cycle of a scallop population include activation, growth and gametogenesis, ripening of gametes, pre-spawning, spawning, and an inactive or ‘resting’ period [26]. The gonadosomatic index (GSI), which expresses gonadal weight as a proportion of the total body weight, is used extensively as an indicator to define the stages of gametogenic cycles in scallops [26]. In yesso scallops from Peter the Great Bay, different stages of the reproductive cycle strongly correlate with water temperature (Table 1).

Table 1.
Gonadosomatic index (GSI) at different stages of the yesso scallop gametogenic cycle in the Sea of Japan in relation to water temperature [27].
The life cycle (Figure 1) starts when demersal eggs of Mizuhopecten yessoensis (average diameter 60–70 µm) are fertilized in the sea.
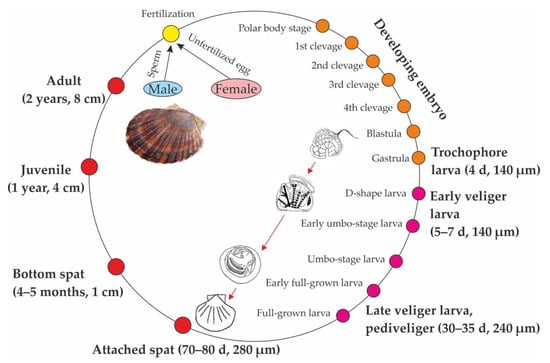
Figure 1.
Life cycle of Mizuhopecten yessoensis in the Sea of Japan (modified from [3]).
Usually, female fecundity varies from 25 to 30 million eggs but can reach 180 million eggs depending on size and age [28]. The initiation of spawning has been observed to occur with increasing water temperature and the temperature-driven progression of the plankton community [3]. As has been established by Maru [29], yesso scallops start to spawn when the cumulative water temperature over 2.2 °C reaches 285 degree-days. In Russian waters of the Sea of Japan, spawning occurs from mid-May to June at temperatures of 7–9 °C [30]. The normal embryonic development of Mizuhopecten yessoensis requires a salinity range between 14 and 21.5 psu [31]. After 4 d, fertilized eggs develop to the trochophore stage. A day or two later, the early veliger larval stage (D-veliger, 100–150 µm) forms a fully formed prodissoconch shell. Then, the growth of the umbones of the larvae lasts for 25–30 days. D-veligers of yesso scallops have only 2 teeth per shell valve at each end of the hinge line [32]. Veligers of Mizuhopecten yessoensis can filter 3–45 µL h–1 of seawater and are capable of ingesting 50–700 algal cells h–1 depending on larval size [33]. As a rule, veligers occur near the water surface during night hours [34].
The next stage called “pediveliger” (240 µm) attaches to the substratum with byssal threads 40 days after fertilization [3]. Pediveligers have 6 teeth per shell valve [32]. On settlement, juvenile scallops (250–280 µm) are positively geotropic or geotactic, or negatively photokinetic [35]. Suitable substrata for Mizuhopecten yessoensis include both natural and artificial surfaces at depths from 1 to 3 m [35].
The survival rate from fertilized eggs to attached juveniles is estimated to be 5–8% [36]. The main factor driving the total spat density of Mizuhopecten yessoensis is the concentration of food available for their parents [37]. Rapid changes in the shell morphology of the dissoconch (spat shell) and growth of internal organs start immediately after settlement, resulting in the adult scallop form with a shell height of 10–30 mm.
From 4 to 5 months after attachment when the spat reach 1 cm in shell length, young mollusks start to fall off the substratum, and the free spat then inhabit the seafloor [3]. At this stage, the survival rate of mollusks is 5–10% or even 0% under unfavorable conditions [28]. In general, only 4% of juvenile scallops survive as long as 6 months [35]. By the next spring, the bottom-dwelling animals develop into juveniles with 2–5 cm shell lengths depending on temperature, food quality and availability, water exchange, and other environmental conditions [38].
2.3. Feeding, Growth, and Mortality
Yesso scallops are filter-feeders and, according to Mikulich and Tsikhon-Lukanina [39], adult mollusks are capable of ingesting relatively large particles (up to 950 μm). On the sandy bottom, the scallops due to feeding on phytoplankton and detritus can establish shallow depressions. The most abundant food items are detritus (60–70%), the diatom Cocconeis scutellum, the dinoflagellates Dinophysis acuta and Protoceratium areolatum, the tintinnids Helicostomella longa and H. subulata, the cladoceran Evadne nordmanni, small copepods, Copepoda nauplii, and larvae of bivalve mollusks [39]. The feeding and growth of Mizuhopecten yessoensis have been established to be obstructed when the flow rate surpasses 20 cm s–1 [40]. Yesso scallops grow at temperatures ranging from –2 to 26 °C with the optimum at 4–6 °C [19]. In the Russian part of the Sea of Japan, this temperature optimum occurs in May–June and September–October [41]. Three-year-old scallops reach 90–110 mm in shell height. The largest mollusks (190–195 mm of shell height, age 16 years) usually occur at a 20 m depth in sandy habitats with good water exchange and relatively constant temperatures. In coastal waters of the southern Sea of Japan (Primorye) where the average water temperature is 8–9 °C, yesso scallops grow faster than in the northern part of the sea (Tatar Strait) where the average water temperature is 4–5 °C [42]. Size-at-age data for yesso scallops are summarized in Figure 2.
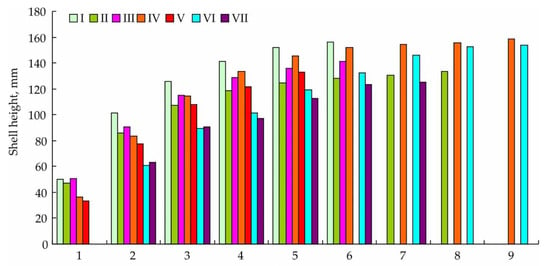
Figure 2.
Age of different-sized Mizuhopecten yessoensis in Russian waters of the Sea of Japan (modified from [42,43]). I—Furugelm Island (42°30′ N, 130°56′ E), II—Andreev Inlet (43°06′ N, 132°19′ E), III—Putyatin Island (42°51′ N, 132°25′ E), IV—Olga Bay (43°42′ N, 135°15′ E), V—Vladimir Bay (43°55′ N, 135°29′ E), VI—Sovetskaya Gavan Bay (48°58′ N, 140°18′ E), VII—Chikhachev Bay (51°27′ N, 140°50′ E).
The level of pollution can also affect the growth rate in Mizuhopecten yessoensis. Indeed, in 4-year-old mollusks at the active gametogenic stage, the total weight of somatic tissues was found to be 60 g during the period of a heavy pollution load and 90 g during the period of a low load of contaminants [44].
Mortality rates of Mizuhopecten yessoensis are dependent on their age being high at age 0–2, 6–7, and 9–10 and low in 2–5-year-old individuals [45]. In Russian waters, the major natural predators of yesso scallops are the starfish Asterias amurensis and Distolasterias nipon. Small mollusks are prey for the gastropods Boreotrophon candelabrum, Tritonia japonica, and Nucella heyseana, octopuses, king crabs, and hermit crabs [19,46].
2.4. Epibionts
Shellfish species are known to be hosts for a variety of epibiotic species [47,48,49]. In the Sea of Japan, a list of epibiotic organisms living on the shells of yesso scallops includes some 100 species [49,50,51], among which the highest density and biomass are found for barnacles (Hesperibalanus hesperius and Balanus rostratus), polydorid polychaetes (Dipolydora bidentata and Polydora brevipalpa), red algae (Polysiphonia spp. and Gelidium spp.), brown algae (Sphacelaria furcigera), green algae (Ulva fenestrata, Codium fragile, and Cladophora stimpsonii), and small gastropods (Odostomia culta and O. fujitanii). Although the majority of epibiotic species are commensals, negative effects for hosts (reduced water flow and food accessibility, increased shell weight) are registered when the epibionts occur at high densities. Moreover, shell-dwelling organisms (Polydora, Dipolydora, and Odostomia) can exacerbate the erosion effect demonstrating evidence for parasitism [52]. The prevalence of epibionts and incidence of bioerosion as well as the associated scallop mortality were found to be closely correlated with environmental pollution being high when a substantial pollution load was registered within scallop beds [53].
2.5. Toxins
Yesso scallops can accumulate biotoxins produced by harmful microalgal species such as Dinophysis spp., Pseudo-nitzschia spp., Alexandrium spp., and Gymnodinium catenatum.
Diarrhetic shellfish poisoning effects may be caused by dinophysistoxins, pectenotoxins, yessotoxins, and okadaic acid. The main producers of these toxins are Dinophysis spp., among which D. acuminata, D. fortii, and D. norvegica are the most abundant (concentrations of 500,000, 5000, and 3000 cells L–1, respectively) [19,54,55]. In general, the concentrations of diarrhetic shellfish poisoning toxins in yesso scallops are below the permissible level of 16 µg per 100 g [56,57]. In the Sea of Japan, the dinoflagellates Alexandrium acatenella (mean concentration 400,000 cells L–1), Alexandrium tamarense (700,000 cells L–1), and Gymnodinium catenatum (30 cysts cm–3) are the most common producers of saxitoxin, which is responsible for paralytic shellfish poisoning at concentrations higher than the permissible level of 80 µg per 100 g. However, there were only a few occasions when this level was exceeded without known human poisonings [19]. Amnesic shellfish poisoning is caused by domoic acid producers from the genus Pseudo-nitzschia. In the Sea of Japan, the highest abundance (11,000,000 cells L–1) is registered for P. pungens and P. multiseries [58]. Concentrations of domoic acid (permissible level = 20 mg kg–1) in tissues of yesso scallops do not exceed 0.1 mg g–1 [56]. The highest level of this toxin is usually registered in January [59].
2.6. Meat Yield and Chemical Composition
In Peter the Great Bay, the yesso scallop meat yield (muscle weight) accounts for 10–18% [30,60]. Muscles of Mizuhopecten yessoensis contain a high percentage of moisture and a low percentage of fat. The muscle is richer in total protein than the mantle but the latter contains higher levels of moisture, fat, and ash [61]. The most common macroelements in the muscle and mantle of Mizuhopecten yessoensis are Ca, K, Na, and Mg. The mantle was found to contain higher proportions of these elements in comparison to the muscle [61]. Muscles and mantles of yesso scallops are rich in vitamins B12, B2 (riboflavin), and B1 (thiamine) with higher levels in scallop muscles (Table 2).

Table 2.
Biochemical composition and microelement and vitamin content in muscles and mantles of wild yesso scallops from the Sea of Japan [61].
2.7. Heavy Metals
Concentrations of metals in muscles, gonads, and mantles of 3-year-old yesso scallops account for 1.8, 1.4, and 0.7 μg g–1 d.w. for Cd, 0.04, 0.03, and 0.06 μg g–1 d.w. for Pb, and 0.5, 0.1, and 0.3 μg g–1 d.w. for As, respectively. These levels are much lower than the permissible levels (2 μg g–1 d.w. for Cd, 10 μg g–1 d.w. for Pb, and 5 μg g–1 d.w. for As) [62]. According to Zhad’ko et al. [63], concentrations of Cd, Pb, and As in muscles of 1- and 2-year-lod scallops are 0.29, 5.6, and 0.41 μg g–1 w.w. and 0.21, 1.1, and 1.4 μg g–1 w.w., respectively.
3. Stocks and Fishery
In Peter the Great Bay, the most important scallop beds of Mizuhopecten yessoensis are located along the coast at a low density (0.01–0.2 ind. m–2) and biomass (1–35 g m–2) [64,65]. The highest stock is registered in the mouth of the Tumannaya River, Amursky Bay, and near Russky Island (Figure 3a).
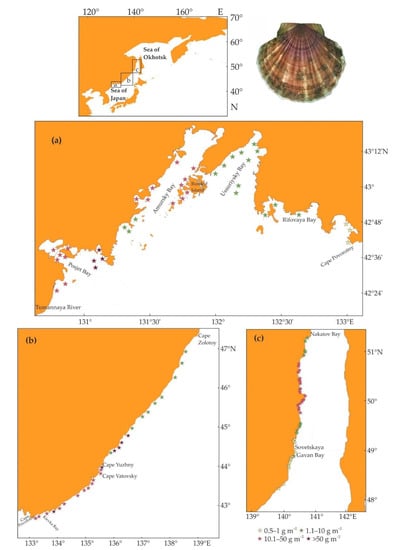
Figure 3.
Location of major Mizuhopecten yessoensis beds in Russian waters of the Sea of Japan. (a) Peter the Great Bay, (b) coastal zone between Cape Povorotny and Cape Zolotoy, (c) Tatar Straight (modified from [66,67,68,69]).
The highest proportions of commercially sized scallops (120 mm shell height) are found in the mouth of the Tumannaya River and in Rifovaya Bay (96–99%). In Peter the Great Bay, the total stock is estimated to be 721 t and the commercial stock is 414 t [66,67,68].
At coastal sites between Cape Povorotny and Cape Zolotoy, there are a lot of scallop beds, but they have low abundance (0.01–0.6 ind. m–2) and biomass (2–127 g m–2). The most abundant beds are found in Kievka Bay (Figure 3b) where the majority of scallops belong to the 105–140 mm size class (76.7%), and in the area between Cape Vatovsky and Cape Yuzhny (Figure 3b) where scallops are most abundant in the 135–140 mm size class (48%). The total and commercial stocks of yesso scallops across this area are 1637 and 756 t, respectively [68]. In the areas located to the south of Cape Zolotoy, commercial fishing of Mizuhopecten yessoensis is not allowed but a total allowable catch of 2 t is set for scientific and recreational purposes [68].
In the northwestern part of the Tatar Strait, scallop beds occur along the coast of the continental part at depths ranging from 10 to 45 m with high-density aggregations being located in the northern part of the area from Sovetskaya Gavan Bay to Nakatov Bay (Figure 3c). There is a trend to decrease in the mean density from 0.09–0.2 ind. m–2 in 2003 to 0.002–0.04 m–2 in 2018 [70]. Currently, the local population consists of specimens with 104–207 m shell heights, 105–940 g body weights, and 3–11 yr ages. The proportion of commercial yesso scallops is 95%. In the area with a total square of 140 km2, the frequency of occurrence of Mizuhopecten yessoensis is 19%. The mean density is estimated to be 0.03 ind. m–2 and the total stock is 382 t or 804,000 ind. [69]. In this area, the growth rate of Mizuhopecten yessoensis is lower than in Peter the Great Bay, and the life span does not exceed 8 years. Mollusks reach a marketable size of 120 mm in the 5th year of life [71].
In the past decade, the total catch of Mizuhopecten yessoensis varied considerably with a peak of 75 t in 2014 (Figure 4). The official catch levels are lower than the total allowable catch.
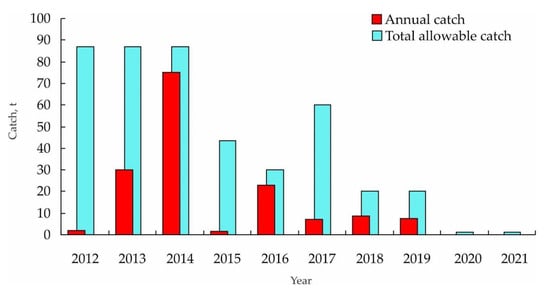
Figure 4.
Total annual catch of Mizuhopecten yessoensis in Russian waters of the Sea of Japan (Primorye) [69,70].
Since 2020, this fishery has been banned due to the high rate of illegal fishing which accounted for 100 t per year in 2003–2008, and due to the use of dredges [69,70]. The application of these gears is a reaction to a depletion of shallow-water beds where SCUBA diving has become unprofitable. In recent years, the total allowable catch for yesso scallops has been set at 1 t for scientific surveys.
4. Aquaculture
4.1. Methods
Cultivation of scallops includes spat collection, spat intermediate culture, and final grow-out (Figure 5).
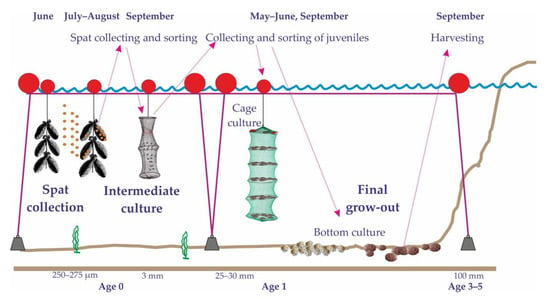
Figure 5.
Scheme showing the cultivation process of yesso scallops in the Sea of Japan [19,72].
The main growing areas are situated in Peter the Great Bay. The most important farming sites are Posjet Bay, Amursky Bay, Ussuriysky Bay, and Nakhodka Bay. The most important farms in the coastal zone between Cape Povorotny and Cape Zolotoy are Kievka Bay and Vladimir Bay.
Russian scallop farmers use either conical plates of perforated plastic (diameter 250 mm) covered by mesh stockings (size 7–12 mm) or commercial onion bags with mesh/monofilament filling (nylon or polyethylene) as collectors for scallop spat. These collectors are usually placed at 5–10 m depths in semi-closed bays and inlets and at 15–20 m depths in open waters in early June for the development of the primary bacterial film, which is known to promote better spat attachment [72]. According to Gabaev [73], the optimal timing of the deployment of Mizuhopecten yessoensis spat collectors for the establishment of biofilm depends on the length of the sea ice coverage period in the region. If this period is ≤103 d, the collectors should be placed 25–35 d after the spawning starts at 14–16 m depths at an angle of 90–120° to the prevailing coastal current. In the case of the 103–108 d period, these characteristics are 15–20 d, 12–14 m, and 120–150°. In the case of the ≥108 d period, the collectors should be placed into the sea in autumn of the previous year at 10–12 m depths at an angle of 150–180°.
In the Sea of Japan, yesso scallop settlement starts in mid-June, but in warmer and colder years, it starts in early June and early July, respectively [74]. Immediately after settlement, the larvae have a height of 250–275 µm. It increases to 3 mm in August. In strong years, more than 1000 larvae can be collected. The minimum level providing a profitable scallop yield is 200 larvae per collector [19].
Intermediate culture, i.e., rearing of spat from the collector to a shell length of 25–30 mm, is conducted in suspended cages. Spat collecting and sorting are conducted in autumn. Young mollusks are scraped off into multi-tier cages (0.12 m–2) attached to horizontal long-lines. Sorting the collectors is a labor-intensive technique, which requires scallops to be hand-picked or sorted away from the rest of the settled organisms such as bivalves, echinoderms, and polychaete worms. A raft with long lines is placed into the sea until April–May of the next year. At this stage, the mortality rate of Mizuhopecten yessoensis is approximately 10% [19].
Afterward, 1-year-old mollusks are used for final grow-out, which takes place either in prepared bottoms where the scallops enjoy a lower density than in native habitats (10–20 ind. m–2) for faster growth rates and better weight increments or in suspended cages at densities of 20–25 ind. per cage for 1-year-old mollusks and 5–7 ind. per cage for 2-year-old individuals [75]. The latter method provides better results because yesso scallops can utilize a much greater proportion of suspended particles and, therefore, attain optimal growth performance. Moreover, scallops cultivated on the seafloor usually have a 10–15 mm smaller shell height than native scallops [19] due to shell breakage during transportation and stress associated with the adaptation to new environmental conditions. Scallops inhabiting silted sand demonstrate better growth performance than their conspecifics from muddy grounds because of higher concentrations of suspended particles and lower oxygen levels [76,77].
The average size and weight data for yesso scallops in suspended culture are presented in Table 3.

Table 3.
Size and weight of Mizuhopecten yessoensis cultivated in suspended cages in the Sea of Japan [78].
Bottom grow-out lasts for 2–4 years mainly depending on temperature and habitat conditions [72]. The survival rate is 5–20% and the production rate is 5–15 t per ha. Commercially sized scallops are collected by divers. Sea cage cultivation lasts for 3 years and yields a much higher survival rate (70–90%) [19,79]. Commercial scallops are harvested manually after cage retrieval. Despite the lower yield, bottom cultivation is considered less expensive than sea cage culture [80].
4.2. Production
Modern scallop aquaculture for Mizuhopecten yessoensis has been developed since 1968 and went through a period of rapid expansion since the late 1980s. In the mid-1980s, the USSR produced over 10 million spat per year. From 1972 to 2002, more than 128,000,000 young scallops were transferred from sea farms to nearshore locations to maintain natural populations. As a result, overexploited scallop beds in Peter the Great Bay were fully restored [81]. The disintegration of the USSR resulted in the collapse of the industry in the 1990s, but a trend of its recovery has persisted since 1997. Indeed, the number of molluscan farms increased from 20 in 2000 to 34 in 2010 and to 114 in 2021 [82,83,84].
In the Sea of Japan, the Russian aquaculture production for yesso scallops have fluctuated around 150 t between 1991 and 1995 and peaked in value at 1000 t in 1994 (Figure 6a).
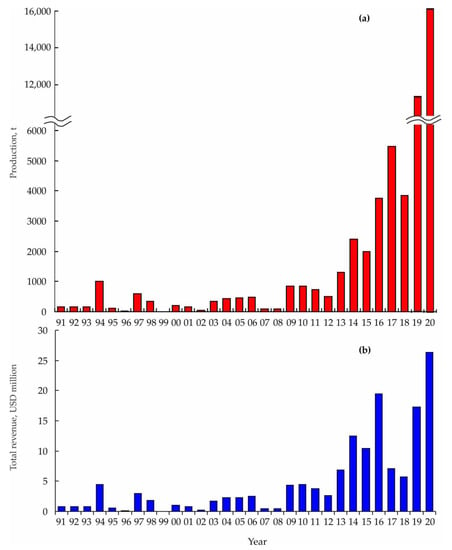
Figure 6.
Total annual aquaculture production (a) and revenue (b) of Mizuhopecten yessoensis in Russian waters of the Sea of Japan [85].
The total amount of scallops dropped dramatically in the next year (26 t) but increased again to 600 t in 1997. In 2000–2002, the total annual production did not exceed 200 t. In the subsequent four years, scallop farms demonstrated a stable production rate of 350–450 t per year. A fourfold reduction was observed in 2007 and 2008 while a recovery to 850 t per year was registered in 2009 and 2010 [86,87]. Since then, a stable trend to increase in the total production has begun and, in 2019 and 2020, its level was 11,531 and 16,259 t, respectively [85]. Over the past decade, there has been an increase in the total revenue (Figure 6b) with maximal values in 2016 (USD 19.5 million), 2019 (USD 17.3 million), and 2020 (USD 26.3 million) [85].
4.3. Problems
Despite relatively high production levels, this industry faces significant problems with selling scallop products. For example, only 10% of the total production was sold in 2019 [84]. This was mainly due to these shellfish not being a traditional product in Russia and their export being limited.
Financial problems of protracted payback periods in aquaculture and the necessity of significant investment in equipment remain major concerns. A possible solution is the stimulation of potential investors. Over recent years, there has been a significant increase in the total investment including foreign sources. Thus, the large aquaculture producer, Dalian Shangpintang (China), was a co-investor of a farm for scallops and sea cucumbers in the Hasan Area. In 2020, a total of 9,000,000 spat were collected for further bottom grow-out [88].
Cultivated scallops are hosts for numerous species of bacterial contaminants including 29 species among which Gram-negative bacteria are most prevalent. Some species such as Aeromonas, Vibrio, and Pseudomonas are potentially pathogenic, especially under poor environmental conditions [19]. Diseases and pathogens seem to have a negative impact on cultured mollusks [89]. Mass scallop deaths caused by bacterial and prokaryotic infections were not recorded until 2019 when an epizootic outbreak of an unidentified pathogen caused a mortality rate as high as 80% of cultured scallops. According to Zhurba and Leskova [79], Brovkina and Kostina [90], and Gavrilova et al. [91], the main agents responsible for the high mortality of scallop spat are alveolates of the genus Perkinsus and ciliates Trichodina spp. Older mollusks suffer from epibionts including macroalgae, sponges, ascidians, shellfish, bivalves, gastropods, polychaetes, and barnacles, and pathogens including fungi, flagellates, ciliates, turbellaria, alveolates, and bacteria. These organisms have been shown to cause detachment of the scallop soft tissue and abnormal shape of their shells.
Other important problems experienced by scallop farmers are storms and fluctuations in water temperature and salinity, which can influence the scallop distribution on the seafloor and their mortality and growth [84,92]. The mass mortality of spat may be associated with local freshening events as has been shown for a scallop farm located in Moryak-Rybolov Bay (eastern part of Primorye) [93].
5. Conclusions
Russian waters of the Sea of Japan are characterized by favorable environmental conditions for many bivalve species among which yesso scallops are widely distributed, predominantly at shallow-water sites, and have excellent potential for harvesting and farming. Temperature, salinity, food supply, and water pollution appear to be the most important environmental variables regulating the growth and reproduction of the adults as well as the survival and growth of the embryos and larvae. The high market value of yesso scallops from the Sea of Japan combined with their rapid growth and short life span has led to the depletion of their stocks due to overfishing. Currently, the most significant and persistent commercial stocks of scallops occur at coastal sites between Cape Povorotny and Cape Zolotoy. Commercial fisheries for this scallop species are forbidden except for a quota of 3 t per year for scientific and recreational purposes. Growing demand for scallop muscles has led to the development and refinement of hatchery, nursery, and grow-out techniques. This industry demonstrated significant success until the early 1990s when early releases of juvenile scallops into the natural environment were used to rehabilitate depleted grounds, a collapse until the mid-1990s when the majority of farms were closed, and a rapid growth since the 2000s when the industry gave high priority. Bottom cultivation of juvenile mollusks and their rearing in suspended cages are currently used for grow-out of juveniles after spat cultivation. Unstable environmental conditions including storms and water temperature fluctuations as well as epizootic events are the main problems of scallop aquaculture in the Russian Far East.
Author Contributions
Conceptualization, A.G.D.; methodology, A.G.D.; validation, A.G.D. and V.G.D.; investigation, A.G.D. and V.G.D.; visualization V.G.D.; writing—original draft, A.G.D.; writing—review and editing, A.G.D. and V.G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Ministry of Science and Higher Education of the Russian Federation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to anonymous reviewers for valuable comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Waller, T.R. Evolutionary relationships among commercial scallops (Mollusca: Bivalvia: Pectinidae). In Scallops: Biology, Ecology and Aquaculture; Shumway, S.E., Ed.; Elsevier: New York, NY, USA, 1991; pp. 1–73. [Google Scholar]
- Serb, J.M. Reconciling morphological and molecular approaches in developing a phylogeny for the Pectinidae (Mollusca: Bivalvia). In Scallops Biology, Ecology, Aquaculture, and Fisheries, 3rd ed.; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–29. [Google Scholar]
- Kosaka, Y. Scallop fisheries and aquaculture in Japan. In Scallops Biology, Ecology, Aquaculture, and Fisheries, 3rd ed.; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 891–936. [Google Scholar]
- Schiaparelli, S.; Linse, K. A reassessment of the distribution of the common Antarctic scallop Adamussium colbecki (Smith, 1902). Deep Sea Res. II 2006, 53, 912–920. [Google Scholar] [CrossRef]
- Duncan, P.F.; Brand, A.R.; Strand, Ø.; Foucher, E. The European scallop fisheries for Pecten maximus, Aequipecten opercularis, Chlamys islandica, and Mimachlamys varia. In Scallops Biology, Ecology, Aquaculture, and Fisheries, 3rd ed.; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 781–858. [Google Scholar]
- Al-Sid-Cheikh, M.; Rowland, S.J.; Stevenson, K.; Rouleau, C.; Henry, T.B.; Thompson, R.C. Uptake, whole-body distribution, and depuration of nanoplastics by the scallop Pecten maximus at environmentally realistic concentrations. Environ. Sci. Technol. 2018, 52, 14480–14486. [Google Scholar] [CrossRef]
- Matrosova, I.V.; Kovaleva, T.S. Some biological characteristics of the Primorsky scallop cultivated in the area of the oil terminal in Kozmino Bay (Nakhodka Bay, Sea of Japan). E3S Web Conf. 2021, 320, 01002. [Google Scholar] [CrossRef]
- Bremec, C.-S.; Schejter, L. Chaetopterus antarcticus (Polychaeta: Chaetopteridae) in Argentinian shelf scallop beds: From infaunal to epifaunal life habits. Rev. De Biol. Trop. 2019, 67, 39–50. [Google Scholar] [CrossRef]
- Marsden, I.D.; Cranford, P.J. Scallops and marine contaminants. In Scallops Biology, Ecology, Aquaculture, and Fisheries, 3rd ed.; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 567–584. [Google Scholar]
- Dobrovolskiy, A.D.; Zalogin, B.S. The Seas of the USSR; Moscow State University Press: Moscow, Russian, 1982. (In Russian) [Google Scholar]
- Uda, M.; Morgan, J.R. Sea of Japan. Encyclopedia Britannica. 16 February 2016. Available online: https://www.britannica.com/place/Sea-of-Japan (accessed on 31 March 2022).
- Ebuchi, N. Growth of wind waves with fetch in the Sea of Japan under winter monsoon investigated using data from satellite altimeters and scatterometer. J. Oceanogr. 1999, 55, 575–584. [Google Scholar] [CrossRef]
- Robinson, S.M.C.; Parsons, G.J.; Davidson, L.A.; Shumway, S.E.; Blake, N.J. Scallop aquaculture and fisheries in Eastern North America. In Scallops Biology, Ecology, Aquaculture, and Fisheries, 3rd ed.; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 737–779. [Google Scholar]
- Pezzuto, P.R.; Borzone, C.A. The collapse of the scallop Euvola ziczac (Linnaeus, 1758) (Bivalvia: Pectinidae) fishery in Brazil: Changes in distribution and relative abundance after 23 years of exploitation. Braz. J. Oceanogr. 2004, 52, 225–236. [Google Scholar] [CrossRef]
- Jonasson, J.P.; Thorarinsdottir, G.; Eiriksson, H.; Solmundsson, J.; Marteinsdottir, G. Collapse of the fishery for Iceland scallop (Chlamys islandica) in Breidafjordur, West Iceland. ICES J. Mari. Sci. 2007, 64, 298–308. [Google Scholar] [CrossRef]
- Kluger, L.C.; Taylor, M.H.; Wolff, M.; Stotz, W.; Mendo, J. From an open-access fishery to a regulated aquaculture business: The case of the most important Latin American bay scallop (Argopecten purpuratus). Rev. Aquacult. 2019, 11, 187–203. [Google Scholar] [CrossRef]
- Guo, X.; Luo, Y. Scallops and scallop aquaculture in China. In Scallops Biology, Ecology, Aquaculture, and Fisheries, 3rd ed.; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 937–952. [Google Scholar]
- Peng, D.; Yang, Q.; Mu, Y.; Zhang, H. The price difference and trend analysis of yesso scallop (Patinopecten yessoensis) in Changhai county, China. J. Mar. Sci. Eng. 2021, 9, 696. [Google Scholar] [CrossRef]
- Ivin, V.V.; Shevchenko, O.G.; Orlova, T.Y. Scallops of Northwestern Pacific Russian Federation. In Scallops Biology, Ecology, Aquaculture, and Fisheries, 3rd ed.; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 953–998. [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Aquaculture of green sea urchin in the Barents Sea: A brief review of Russian studies. Rev. Aquac. 2020, 12, 1280–1290. [Google Scholar] [CrossRef]
- Marinchenko, T.E. Aquaculture in the World and Russia: State and prospects. IOP Conf. Ser. Earth Environ. Sci. 2021, 715, 012052. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Cucumaria in Russian waters of the Barents Sea: Biological aspects and aquaculture potential. Front. Mar. Sci. 2021, 8, 613453. [Google Scholar] [CrossRef]
- Ivin, V.V.; Kalashnikov, V.Z. Scallops of the Russian waters of northwestern Pacific. Part 1. Biology and ecology. Bull. Russ. Far East Malacol. Soc. 2005, 9, 27–45. [Google Scholar]
- Brand, A.R. Scallop ecology: Distributions and behaviour. In Scallops Biology, Ecology, Aquaculture, and Fisheries, 3rd ed.; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 469–534. [Google Scholar]
- Silina, A.V. Sex change in scallop Patinopecten yessoensis: Response to population composition? PeerJ 2018, 6, e5240. [Google Scholar] [CrossRef]
- Barber, B.J.; Blake, N.J. Reproductive physiology. In Scallops Biology, Ecology, Aquaculture, and Fisheries, 3rd ed.; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 253–299. [Google Scholar]
- Sedova, L.G.; Viktorovskaya, G.I. Dependence of the intensity of metabolism on the reproductive activity of the scallop Mizuhopecten yessoensis (Jay). Izv. TINRO 2000, 127, 467–474. (In Russian) [Google Scholar]
- Yamamoto, G. Studies on the propagation of the scallop, Patinopecten yessoensis (Jay), in Mutsu Bay. Suisan Zouyoushoku Gyousho 1964, 6, 1–71. [Google Scholar]
- Maru, K. Ecological studies on the seed production of scallop, Patinopecten yessoensis (Jay). Sci. Rep. Hokkaido Fish. Exp. Stn. 1985, 27, 1–54. [Google Scholar]
- Belogrudov, E.A. Biological Bases of Cultivation of the Japanese Scallop Patinopecten yessoensis (Jay) (Molluska, Bivalvia) in Posjet Bay, Sea of Japan. Ph.D. Dissertation, Institute of Marine Biology, Vladivostok, Russian, 1981. (In Russian). [Google Scholar]
- Maru, K. Tolerance of a scallop, Patinopecten yessoensis (Jay) to temperature and specific gravity during early development stage. Sci. Rep. Hokkaido Fish. Exp. Stn. 1985, 27, 55–63. [Google Scholar]
- Maru, K. Morphological observations on the veliger larvae of a scallop, Patinopecten yessoensis (Jay). Sci. Rept. Hokkaido Fish. Exp. Stn. 1972, 14, 55–62. [Google Scholar]
- Macdonald, B.A. Physiological energetics of Japanese scallop Patinopecten yessoensis larvae. J. Exp. Mar. Biol. Ecol. 1988, 120, 155–170. [Google Scholar] [CrossRef]
- Maru, K.; Obara, A.; Kikuchi, K.; Okesaku, H. Studies on the ecology of the scallop Patinopecten yessoensis (Jay). 3. On the diurnal vertical distribution of scallop larvae. Sci. Rep. Hokkaido Fish. Exp. Stn. 1973, 15, 33–52. [Google Scholar]
- Golikov, A.N.; Scarlato, O.A. Abundance, dynamics and production of populations of edible bivalves Mizuhopecten yessoensis and Spisula sachalinensis related to the problem of organization of controllable submarine farms at the western shores of the Japan Sea. Helgol. Wiss. Meeresunt. 1970, 20, 493–513. [Google Scholar] [CrossRef][Green Version]
- Bregman, Y.E.; Guida, G.M. Ecology and development of the larvae of Yesso (Japanese) scallop in laboratory culture. In Mollusks: Systematics, Ecology and Regularities of Distribution, 7; Starobogatov, Y.I., Ed.; Nauka: Leningrad, Russian, 1983; pp. 191–192. (In Russian) [Google Scholar]
- Baba, K.; Sugawara, R.; Nitta, H.; Endou, K.; Miyazono, A. Relationship between spat density, food availability, and growth of spawners in cultured Mizuhopecten yessoensis in Funka Bay: Concurrence with El Nino Southern Oscillation. Can. J. Fish. Aquat. Sci. 2009, 66, 6–17. [Google Scholar] [CrossRef]
- Nan, X.; Wei, H.; Zhang, H.; Nie, H. Spatial difference in net growth rate of yesso scallop Patinopecten yessoensis revealed by an aquaculture ecosystem model. J. Ocean. Limnol. 2022, 40, 373–387. [Google Scholar] [CrossRef]
- Mikulich, L.V.; Tsikhon-Lukanina, A. Food of the scallop. Oceanology 1981, 21, 633–635. [Google Scholar]
- Miyazono, A. Influence of the intensity of water motion on growth and physiological conditions of scallops cultured in flow tanks. Sci. Rep. Hokkaido Fish. Exp. Stn. 2000, 58, 41–47. [Google Scholar]
- Luchin, V.A.; Grigoryeva, N.I. Effects of the water temperature on timing of spawning and the spat settling for yesso scallop (Mizuhopecten yessoensis Jay, 1857) in the Posyet Bay (Peter the Great Bay, Japan Sea). Izv. TINRO 2020, 200, 168–183. (In Russian) [Google Scholar] [CrossRef]
- Dulenina, P.A.; Dulenin, A.A. The distribution, size and age compositions, and growth of the scallop Mizuhopecten yessoensis (Bivalvia: Pectinidae) in the northwestern Tatar Strait. Russ. J. Mar. Biol. 2012, 38, 310–317. [Google Scholar] [CrossRef]
- Silina, A.V.; Pozdnyakova, L.A. Linear growth of the light scallop Chlamys albidus (Pectinida, Pectinidae). Zool. Zhurn. 1986, 65, 741–746. (In Russian) [Google Scholar]
- Silina, A.V. Somatic and reproductive growth of the yesso scallop in polluted Amur Bay. Biol. Bull (Mosc.) 2020, 47, 291–298. [Google Scholar] [CrossRef]
- Silina, A.V. Mortality of late juvenile and adult stages of the scallop Mizuhopecten yessoensis (Jay). Aquaculture 1996, 141, 97–105. [Google Scholar] [CrossRef]
- Gabaev, D.D.; Kolotukhina, N.K. Influence of predatory gastropod Nucella (Thais) heyseana on population of scallop Mizuhopecten yessoensis (Jay). Russ. J. Ecol. 1999, 2, 153–156. [Google Scholar]
- Schejter, L.; Bremec, C. Epibionts on Flexopecten felipponei (Dall, 1922): An uncommon scallop from Argentina. Am. Malacol. Bull. 2007, 22, 75–82. [Google Scholar] [CrossRef]
- Mikac, B.; Tarullo, A.; Colangelo, M.A.; Abbiati, M.; Costantini, F. Shell infestation of the farmed Pacific oyster Magallana gigas by the endolith bivalve Rocellaria dubia. Diversity 2021, 13, 526. [Google Scholar] [CrossRef]
- Silina, A.V.; Zhukova, N.V. Association of the scallop Patinopecten yessoensis and epibiotic barnacle Balanus rostratus: Inter-specific interactions and trophic relationships determined by fatty acid analysis. Mar. Ecol. 2016, 37, 257–268. [Google Scholar] [CrossRef]
- Levenetz, I.R.; Ovsyannikova, I.I.; Lebedev, E.B. Composition of macroepibiosis of the scallop Mizuhopecten yessoensis in Peter the Great Bay, Sea of Japan. Bull. Russ. Far East Malacol. Soc. 2005, 9, 155–168. (In Russian) [Google Scholar]
- Levenets, I.R.; Lebedev, E.B.; Baranov, A.Y. Epibiotic macroalgae on scallops and oysters in shallow waters of southern Primorye. Inland Water Biol. 2021, 14, 528–535. [Google Scholar] [CrossRef]
- Gabaev, D.D. Effects of fouling on the Japanese scallop Mizuhopecten yessoensis (Jay) in Peter the Great Bay (Sea of Japan). Oceanology 2013, 53, 183–191. [Google Scholar] [CrossRef]
- Silina, A.V. A yesso scallop population exposed to climate-induced and anthropogenic habitat changes in Amur bay, Sea of Japan. Oceanology 2019, 59, 75–85. [Google Scholar] [CrossRef]
- Konovalova, G.V. Toxic and potentially toxic dinoflagellates from the Far East coastal waters of Russia. In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 275–279. [Google Scholar]
- Konovalova, G.V.; Selina, M.S. Dinophyta. In In Biota of the Russian Waters of the Sea of Japan, 8; Adrianov, A.V., Ed.; Dalnauka: Vladivostok, Russia; pp. 1–351. (In Russian)
- Orlova, T.Y. Diversity of potentially toxic microalgae on the east coast of Russia. In Marine Biodiversity and Ecosystem Dynamics of the Northwest Pacific Ocean; Song, S., Adrianov, A.V., Lutaenko, K.A., Xiao-Xia, S., Eds.; Science Press: Beijing, China, 2013; pp. 77–91. [Google Scholar]
- Kameneva, P.A.; Orlova, T.Y. Okadaic acid group toxins in hydrobionts of Russian seas: Producers, distribution, and safety regulation. Russ. J. Mar. Biol. 2017, 43, 331–341. [Google Scholar] [CrossRef]
- Orlova, T.Y.; Zhukova, N.V.; Stonik, I.V. Bloom-forming diatom Pseudo-nitzschia pungens in the Amurskiy Bay (the Sea of Japan): Morphology, ecology and biochemistry. In Harmful and Toxic Algal Blooms; Yasumoto, T., Oshima, Y., Fukuyo, Y., Eds.; Intergovernmental Oceanography Commission of UNESCO: Paris, France, 1996; pp. 147–150. [Google Scholar]
- Stonik, I.V.; Orlova, T.Y. The seasonal accumulation of amnesic toxin (domoic acid) in commercial bivalves Mytilus trossulus Gould, 1850 and Mizuhopecten yessoensis Jay, 1850 in Vostok Bay, Sea of Japan. Russ. J. Mar. Biol. 2020, 46, 56–58. [Google Scholar] [CrossRef]
- Afeichuk, L.S.; Didenko, E.M. Characteristic of natural and cultivated aggregations of the Japanese scallops Mizuhopecten yessoensis Jay in Kievka Bay (Sea of Japan). Izv. TINRO 2000, 127, 362–372. (In Russian) [Google Scholar]
- Klimova, E.Y.; Lapteva, E.P. Comparative analysis of technochemical characteristics of cultured and natural live yesso scallops. Sci. Work. Dalrybvtuz 2010, 22, 343–348. (In Russian) [Google Scholar]
- Vyaznikova, K.S.; Kovekovdova, L.T. Content of metals and arsenic in cultured scallop (Mizuhopecten yessoensis) and the impact of aquaculture facilities in the gulf of Peter the Great on the content of heavy metals in bottom sediments. Vestnik ASTU 2016, 3, 109–114. (In Russian) [Google Scholar]
- Zhad’ko, E.A.; Steblevskaya, N.I.; Polyakova, N.V.; Chusovitina, S.V. The content of some trace elements in the tissues of the annual and two-year coastal scallop of the Severnaya Bay of Peter the Great Gulf (Sea of Japan). Vestnik DVO RAN 2019, 6, 104–112. (In Russian) [Google Scholar]
- Sedova, L.G.; Sokolenko, D.A. Stock and distribution of the scallop Mizuhopecten yessoensis in the Southwestern part of Peter the Great Bay. Izv. TINRO 2008, 155, 76–87. (In Russian) [Google Scholar]
- Lysenko, V.N.; Zharikov, V.V.; Lebedev, A.M.; Sokolenko, D.A. Distribution of the yesso scallop, Mizuhopecten yessoensis (Jay, 1857) (Bivalvia: Pectinidae) in the Southern Part of the Far Eastern Marine Reserve. Russ. J. Mar. Biol. 2017, 43, 302–311. [Google Scholar] [CrossRef]
- Sedova, L.G.; Sokolenko, D.A. Population and size structure for the settlements of Mizuhopecten yessoensis in Peter the Great Bay (Japan Sea). Izv. TINRO 2014, 179, 226–235. (In Russian) [Google Scholar] [CrossRef]
- Sedova, L.G.; Sokolenko, D.A. Stock of yesso scallop in the coastal waters of Primorsky krai. In Natural Resources, Their Current State, Protection, Commercial and Technical Use: Materials of the VI All-Russian Scientific and Practical Conference (24–26 March 2015). Part I; Klochkova, N.G., Ed.; KamchatGTU: Petropavlovsk-Kamchatsky, Russia, 2015; pp. 136–140. (In Russian) [Google Scholar]
- Sokolenko, D.A.; Sedova, L.G. Primorye scallop (Mizuhopecten yessoensis). Region from the mouth of the Tumannaya River to the Cape Zolotoy. In Materials for the Total Allowable Catch in the Region of Fishery (Harvesting) of Aquatic Biological Resources in Inland Marine Waters of the Russian Federation, Territorial Sea of the Russian Federation, Continental Shelf of the Russian Federation, in Exclusive Economical Zone of the Russian Federation and in the Caspian Sea for 2003 (with Environmental Impact Assessment). Part 3. Invertebrate Animals and Algae; Anonymous, Ed.; Pacific Branch of VNIRO: Vladivostok, Russia, 2022; pp. 22–32. (In Russian) [Google Scholar]
- Dulenina, P.A. Primorye scallop (Mizuhopecten yessoensis) and Swift’s scallop (Chlamys swifti) in coastal waters of the northwestern part of the Tatar Straight within Khabarovsk territory (Primorye sub-zone to the north of the Cape Zolotoy). In Materials for the Total Allowable Catch in the Region of Fishery (Harvesting) of Aquatic Biological Resources in Inland Marine Waters of the Russian Federation, Territorial Sea of the Russian Federation, Continental Shelf of the Russian Federation, in Exclusive Economical Zone of the Russian Federation and in the Caspian Sea for 2003 (with Environmental Impact Assessment). Part 3. Invertebrate Animals and Algae; Anonymous, Ed.; Pacific Branch of VNIRO: Vladivostok, Russia, 2022; pp. 32–44. (In Russian) [Google Scholar]
- Dulenina, P.A.; Dulenin, A.A. Dynamics of stock for yesso scallop Mizuhopecten yessoensis (Jay, 1856) in the northwestern Tatar Strait from the beginning of its fishery to nowadays. Izv. TINRO 2021, 201, 533–546. (In Russian) [Google Scholar] [CrossRef]
- Silina, A.V.; Dulenina, P.A. Population of the Japanese scallop Mizuhopecten yessoensis (Pectinidae) near its northern distributional limit. Bull. Russ. Far East Malacol. Soc. 2012, 15–16, 170–175. (In Russian) [Google Scholar]
- Gavrilova, G.S.; Kucheryavenko, A.V.; Lyashenko, S.A. Modern state of the scallop Mizuhopecten yessoensis artificial cultivation in Primorye. Izv. TINRO 2005, 140, 376–382. (In Russian) [Google Scholar]
- Gabaev, D.D. About abundance forecasting juvenile of the Japanese scallop Patinopecten yessoensis, on plantation mariculture of Primorsky territory. Probl. Fish. 2020, 21, 313–330. (In Russian) [Google Scholar] [CrossRef]
- Grigoryeva, N.I. Study of the phenological dates of the Yesso scallop (Mizuhopecten yessoensis Jay, 1857) spawning and settling onsets in Minonosok Inlet (Posiet Bay, Peter the Great Bay, East Sea/Sea of Japan). Vestnik DVO RAN 2020, 5, 138–141. (In Russian) [Google Scholar]
- Lagunova, D.D.; Gerasimova, E.A.; Chernetsov, V.V. Efficiency of scallop cultivation combined method (combination of suspended and bottom). Sci. Work. Dalrybvtuz 2010, 22, 159–165. (In Russian) [Google Scholar]
- Silina, A.V.; Zhukova, N.V. Feeding and growth of Japanese scallop inhabiting different bottom sediment types. Biol. Bull. 2007, 34, 55–60. [Google Scholar] [CrossRef]
- Silina, A.V. The Yesso scallop on the bottom in Minonosok Bay of Posjeta Bay under mariculture condition. Biota Environ. 2018, 4, 92–108. (In Russian) [Google Scholar]
- Grigoryeva, N.I. Analysis of the size-weight mollusks characteristics in hanging culture in Possjet Bay (Sea of Japan) during 1970–2011. Bull. Russ. Far East Malacol. Soc. 2021, 25, 17–31. (In Russian) [Google Scholar]
- Zhurba, E.K.; Leskova, S.E. The experience of cultivating the seaside scallop (Mizuhopecten yessoensis Jay, 1857) in Severnaya Bay (Peter the Great Bay, Sea of Japan). In The State and Ways of Aquaculture Development in the Russian Federation in the Light of Import Substitution and Ensuring the Country’s Food Security: Proceedings of the II National Scientific and Practical Conference, St.Petersburg, 13–15 September 2017; Vasiliev, A.A., Kuznetsov, M.Y., Sivokhina, L.A., Poddubnaya, I.V., Eds.; OOO CeSAsin: Saratov, Russia, 2017; pp. 43–47. (In Russian) [Google Scholar]
- Brykov, V.A.; Kolotukhina, N.K. Biological concepts of Japanese scallop cultivation in Primorsky Krai coastal waters. Probl. Fish. 2010, 3, 564–586. (In Russian) [Google Scholar]
- Vyshkvartsev, D.I.; Regulev, V.N.; Reguleva, T.N.; Grigorjev, V.N.; Lebedev, E.B. The role of the oldest mariculture farm in restoration of stock of the Japanese scallop Mizuhopecten yessoensis (Jay, 1856) in Posyet Bay, Sea of Japan. Russ. J. Mar. Biol. 2005, 31, 181–186. [Google Scholar] [CrossRef]
- Ivin, V.V.; Kalashnikov, V.Z. Scallops of the Russian waters of northwestern Pacific. Part 2. Fishing and aquaculture. Bull. Russ. Far East Malacol. Soc. 2007, 11, 31–48. [Google Scholar]
- Markovtsev, V.G. Mariculture and ecological aspects of its development in Primorye. Far East Reg. Fish. 2008, 3, 4–9. (In Russian) [Google Scholar]
- Anonymous. A Maricultural Disaster. The Scallop Business Is Paralyzed. 2021. Available online: https://konkurent.ru/article/43829 (accessed on 24 April 2022). (In Russian).
- FAO. Fishery and Aquaculture Statistics. Global Aquaculture Production 1950–2020 (FishStatJ). FAO Fisheries and Aquaculture Division. Rome. Updated 2022. 2022. Available online: www.fao.org/fishery/statistics/software/fishstatj/en (accessed on 24 April 2022). (In Russian).
- Gavrilova, G.S.; Kim, L.N. Efficiency of scallop Mizuhopecten yessoensis cultivation in the Ussuri Bay (Japan Sea). Izv. TINRO 2016, 185, 240–250. (In Russian) [Google Scholar] [CrossRef]
- Gavrilova, G.S.; Kondratieva, E.S. Results of economic activity and problems of aquaculture development in the Possiet bay (Japan Sea) in 2000–2015. Izv. TINRO 2018, 195, 229–293. (In Russian) [Google Scholar] [CrossRef]
- Anonymous. In Primorye, Cultivation of Valuable Marine Hydrobionts Using Sea Ranching Has Began. 2020. Available online: https://kapital-rus.ru/uznai/news/v_primore_pristupili_k_vraschivaniu_cennh_morskih_gidrobiontov_pastbischnm_sposobom/ (accessed on 24 April 2022). (In Russian).
- Syasina, I.G. Histopathology of the Japanese scallop, Mizuhopecten yessoensis, cultured in the Experimental Marine Farm in Minonosok Bay (Russian Far East). Korean J. Malacol. 2007, 23, 173–180. [Google Scholar]
- Brovkina, E.P.; Kostina, E.A. The nature of the couts of epizootics during cage rearing of scallops in Primorye. Perkinsus is the likely cause of these diseases. Sci. Work. Dalrybvtuz 2020, 53, 41–52. (In Russian) [Google Scholar]
- Gavrilova, G.S.; Motora, Z.I.; Pozdnyakov, S.E. Results of examination the state of yesso scallop (Mizuhopecten yessoensis) on plantations of aquaculture in Primorye. Izv. TINRO 2021, 201, 895–909. (In Russian) [Google Scholar]
- Silina, A.V.; Latypov, Y.Y. Population dynamics of the Japanese scallop Mizuhopecten yessoensis (Bivalvia) under conditions of enhanced hydrodynamics. Russ. J. Mar. Biol. 2005, 31, 256–260. [Google Scholar] [CrossRef]
- Gavrilova, S.G.; Sukhin, I.Y.; Turabzhanova, I.S. First experience of cage cultivation of hatchery-produced juvenile scallop Mizuhopecten yessoensis at eastern coast of Primorye. Izv. TINRO 2019, 197, 208–218. (In Russian) [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).