Co-Evaluation of Plant Leaf Nutrient Concentrations and Resorption in Response to Fertilization under Different Nutrient-Limited Conditions
Abstract
:1. Introduction
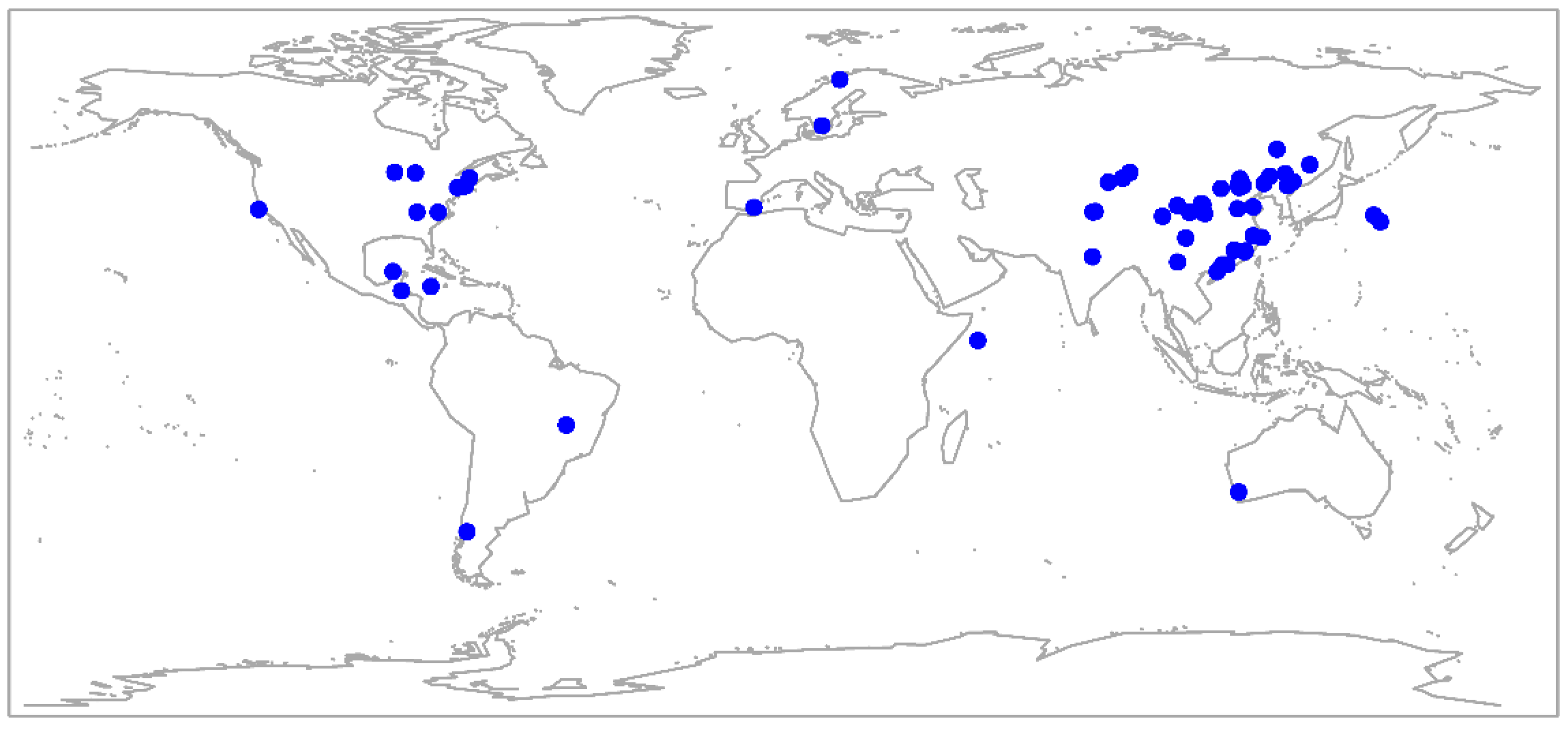
2. Materials and Methods
3. Results
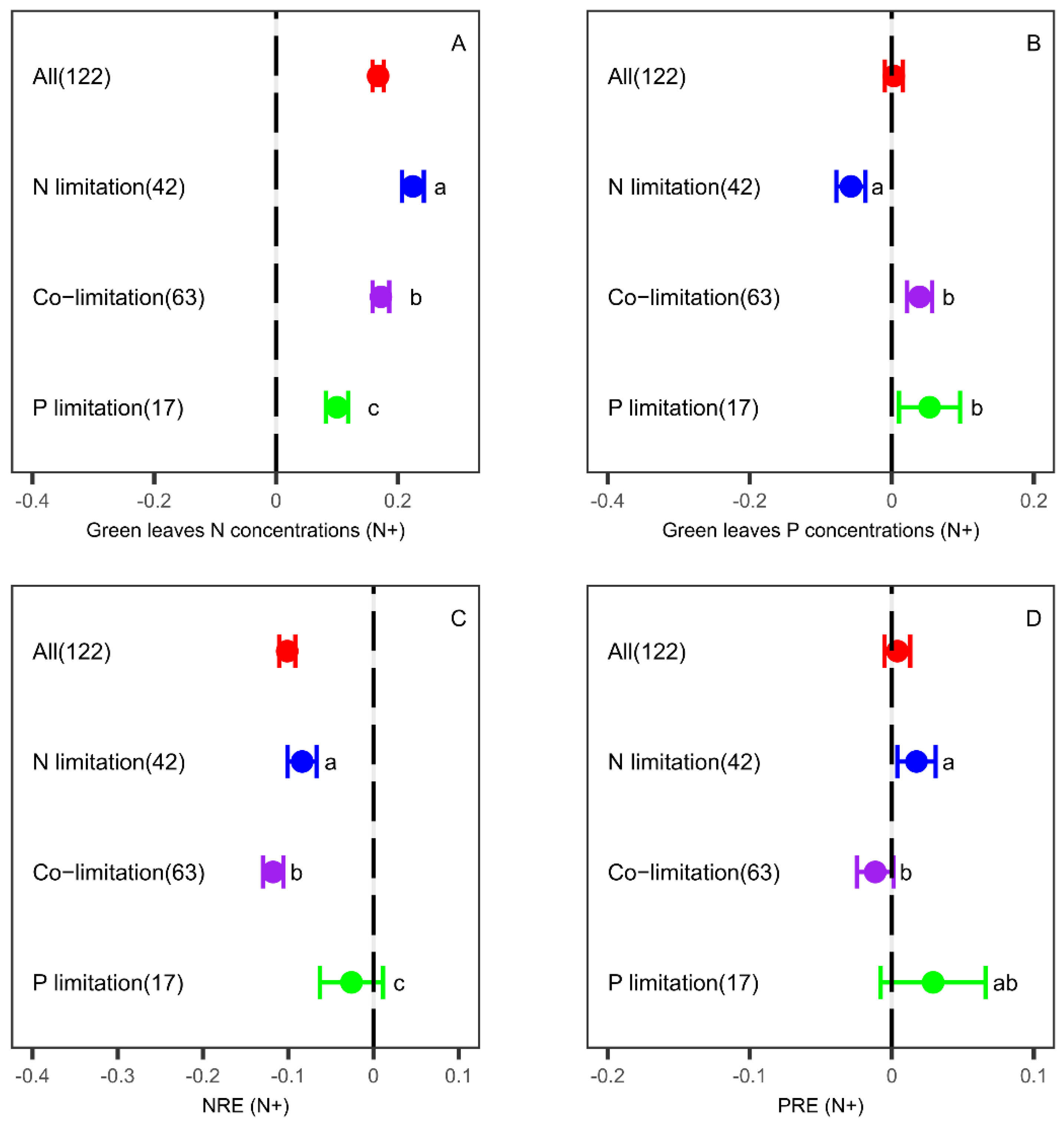
3.1. Effects of N Fertilization on Leaf Nutrient Concentrations and Resorption
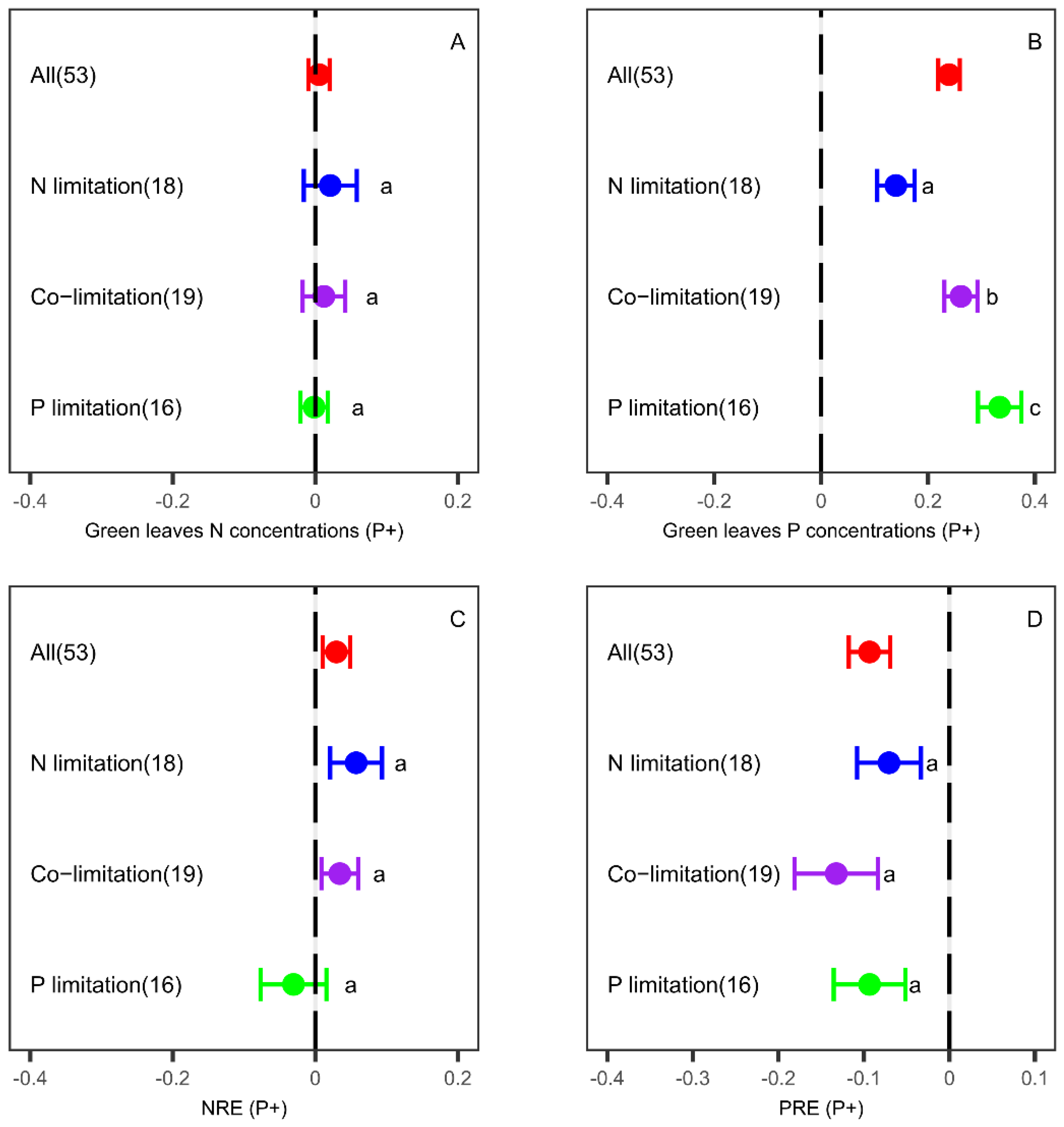
3.2. Effects of P Fertilization on Leaf Nutrient Concentrations and Resorption
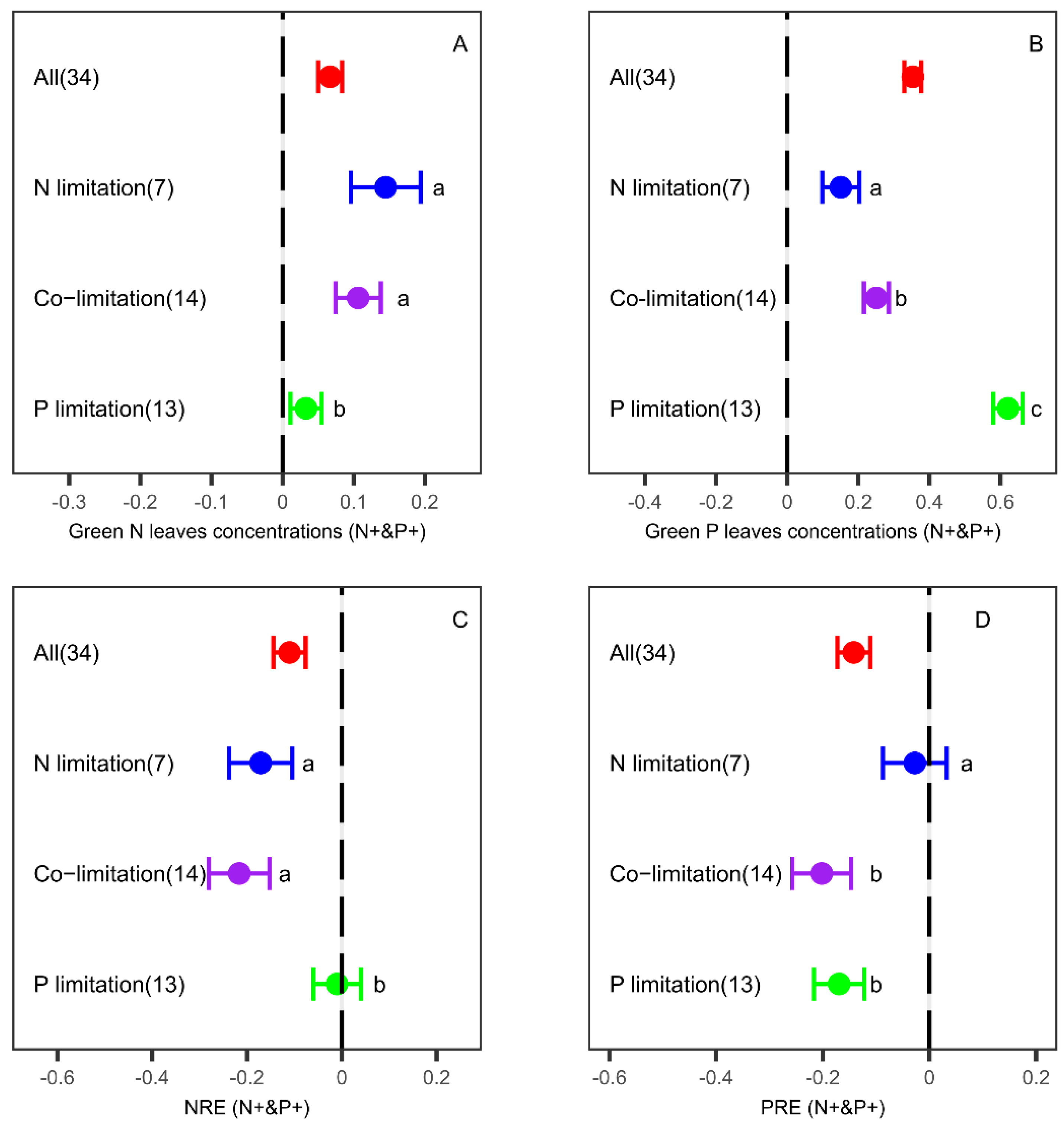
3.3. Effects of N and P Co-Fertilization on Leaf Nutrient Concentrations and Resorption
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- See, C.R.; Yanai, R.D.; Fisk, M.C.; Vadeboncoeur, M.A.; Quintero, B.A.; Fahey, T.J. Soil nitrogen affects phosphorus recycling: Foliar resorption and plant-soil feedbacks in a northern hardwood forest. Ecology 2015, 96, 2488–2498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobe, R.K.; Lepczyk, C.A.; Iyer, M. Resorption Efficiency Decreases with Increasing Green Leaf Nutrients in a Global Data Set. Ecology 2005, 86, 2780–2792. [Google Scholar] [CrossRef]
- Lü, X.T.; Reed, S.C.; Yu, Q.; Han, X.G. Nutrient resorption helps drive intra-specific coupling of foliar nitrogen and phosphorus under nutrient-enriched conditions. Plant Soil 2016, 398, 111–120. [Google Scholar] [CrossRef]
- Grime, J.P. Benefits of plant diversity to ecosystems] immediate, filter and founder effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Güsewell, S. Nutrient resorption of wetland graminoids is related to the type of nutrient limitation. Funct. Ecol. 2005, 19, 344–354. [Google Scholar] [CrossRef]
- Han, W.; Tang, L.; Chen, Y.; Fang, J. Relationship between the relative limitation and resorption efficiency of nitrogen vs phosphorus in woody plants. PLoS ONE 2013, 8, e83366. [Google Scholar] [CrossRef]
- Manzoni, S.; Trofymow, J.A.; Jackson, R.B.; Porporato, A. Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol. Monogr. 2010, 80, 89–106. [Google Scholar] [CrossRef]
- Vergutz, L.; Manzoni, S.; Porporato, A.; Novais, R.F.; Jackson, R.B. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol. Monogr. 2012, 82, 205–220. [Google Scholar] [CrossRef] [Green Version]
- Güsewell, S. N: P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.Y.; Chen, H.Y.H. Global-scale patterns of nutrient resorption associated with latitude, temperature and precipitation. Glob. Ecol. 2009, 18, 11–18. [Google Scholar] [CrossRef]
- Sun, X.; Kang, H.; Chen, H.Y.; Bjorn, B.; Samuel, B.F.; Liu, C. Biogeographic patterns of nutrient resorption from Quercus variabilis Blume leaves across China. Plant. Biol. 2016, 18, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Y.; Chen, H.Y.H. Decoupling of nitrogen and phosphorus in terrestrial plants associated with global changes. Nat. Clim. Chang. 2015, 5, 465–469. [Google Scholar] [CrossRef]
- Gerdol, R.; Iacumin, P.; Brancaleoni, L. Differential effects of soil chemistry on the foliar resorption of nitrogen and phosphorus across altitudinal gradients. Funct. Ecol. 2019, 33, 1351–1361. [Google Scholar] [CrossRef]
- Wright, I.J.; Westoby, M. Nutrient concentration, resorption and lifespan: Leaf traits of Australian sclerophyll species. Funct. Ecol. 2003, 17, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Li, N.; Wang, W.; Liu, X.; Du, F.; Yao, D. Nutrients resorption and stoichiometry characteristics of different-aged plantations of Larix kaempferi in the Qinling Mountains, central China. PLoS ONE 2017, 12, e0189424. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.-S.; Niklas, K.J.; Liu, Y.; Fang, X.-M.; Wan, S.-Z.; Wang, H. Nitrogen and phosphorus additions alter nutrient dynamics but not resorption efficiencies of Chinese fir leaves and twigs differing in age. Tree Physiol. 2015, 35, 1106–1117. [Google Scholar] [CrossRef] [Green Version]
- Feller, I.C.; Lovelock, C.E.; Piou, C. Growth and nutrient conservation in rhizophora mangle in response to fertilization along latitudinal and tidal gradients. Smithson. Contrib. Mar. Sci. 2009, 38, 345–358. [Google Scholar]
- Gonzales, K.; Yanai, R. Nitrogen–phosphorous interactions in young northern hardwoods indicate P limitation: Foliar concentrations and resorption in a factorial N by P addition experiment. Oecologia 2019, 189, 829–840. [Google Scholar] [CrossRef]
- Huang, G.; Su, Y.-G.; Mu, X.-H.; Li, Y. Foliar nutrient resorption responses of three life-form plants to water and nitrogen additions in a temperate desert. Plant Soil 2018, 424, 479–489. [Google Scholar] [CrossRef]
- Huang, J.; Wang, P.; Niu, Y.; Yu, H.; Ma, F.; Xiao, G.; Xu, X. Changes in C:N:P stoichiometry modify N and P conservation strategies of a desert steppe species Glycyrrhiza uralensis. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Yu, H.; Wang, B.; Li, L.; Xiao, G.; Yuan, Z. Nutrient resorption based on different estimations of five perennial herbaceous species from the grassland in inner Mongolia, China. J. Arid Environ. 2012, 76, 1–8. [Google Scholar] [CrossRef]
- Kang, J.; Han, G.; Ren, H.; Zhu, Y.; Zhang, X.; Wang, Y. Responses of plant nutrient contents and resorption to warming and nitrogen addition under different precipitation conditions in a desert grassland. Acta Bot. Boreali-Occident. Sin. 2019, 39, 1651–1660. [Google Scholar]
- Kong, M.; Kang, J.; Han, C.-L.; Gu, Y.-J.; Siddique, K.H.; Li, F.-M. Nitrogen, Phosphorus, and Potassium Resorption Responses of Alfalfa to Increasing Soil Water and P Availability in a Semi-Arid Environment. Agronomy 2020, 10, 310. [Google Scholar] [CrossRef] [Green Version]
- Kou, L.; Wang, H.; Gao, W.; Chen, W.; Yang, H.; Li, S. Nitrogen addition regulates tradeoff between root capture and foliar resorption of nitrogen and phosphorus in a subtropical pine plantation. Trees 2016, 31, 77–91. [Google Scholar] [CrossRef]
- Kozovits, A.R.; Bustamante, M.M.C.; Garofalo, C.R.; Bucci, S.; Franco, A.C.; Goldstein, G.; Meinzer, F.C. Nutrient resorption and patterns of litter production and decomposition in a Neotropical Savanna. Funct. Ecol. 2007, 21, 1034–1043. [Google Scholar] [CrossRef]
- Li, L.; Gao, X.; Li, X.; Lin, L.; Zeng, F.; Gui, D.; Lu, Y. Nitrogen (N) and phosphorus (P) resorption of two dominant alpine perennial grass species in response to contrasting N and P availability. Environ. Exp. Bot. 2016, 127, 37–44. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Liu, B.; Lei, J.; Yue, Z.; Li, C. Imbalanced stoichiometric patterns in foliar nutrient resorption response to N and P addition in grazing alpine grassland. Acta Oecologica 2019, 102, 103505. [Google Scholar] [CrossRef]
- Li, L.; Zeng, D.; Mao, R.; Yu, Z. Nitrogen and phosphorus resorption of Artemisia scoparia, Chenopodium acuminatum, Cannabis sativa, and Phragmites communis under nitrogen and phosphorus additions in a semiarid grassland, China. Plant Soil Environ. 2012, 58, 446–451. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Lv, J.; Peng, C.; Xiang, W.; Xiao, W.; Song, X. Nitrogen -addition accelerates phosphorus cycling and changes phosphorus use strategy in a subtropical moso bamboo forest. Environ. Res. Lett. 2021, 16, 024023. [Google Scholar] [CrossRef]
- Liu, G.; Xing, Y.; Wang, Q.; Wang, L.; Feng, Y.; Yin, Z.; Wang, X.; Liu, T. Long-term nitrogen addition regulates root nutrient capture and leaf nutrient resorption in Larix gmelinii in a boreal forest. Forstwiss. Centralblatt 2021, 140, 763–776. [Google Scholar] [CrossRef]
- Lu, J.; Yang, M.; Liu, M.; Lu, Y.; Yang, H. Nitrogen and phosphorus fertilizations alter nitrogen, phosphorus and potassium resorption of alfalfa in the Loess Plateau of China. J. Plant Nutr. 2019, 42, 2234–2246. [Google Scholar] [CrossRef]
- Lü, X.-T.; Han, X.-G. Nutrient resorption responses to water and nitrogen amendment in semi-arid grassland of Inner Mongolia, China. Plant Soil 2009, 327, 481–491. [Google Scholar] [CrossRef]
- Lu, X.; Kong, D.-L.; Pan, Q.-M.; Simmons, M.E.; Han, X.-G. Nitrogen and water availability interact to affect leaf stoichiometry in a semi-arid grassland. Oecologia 2011, 168, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Lü, X.-T.; Reed, S.; Yu, Q.; He, N.-P.; Wang, Z.-W.; Han, X.-G. Convergent responses of nitrogen and phosphorus resorption to nitrogen inputs in a semiarid grassland. Glob. Chang. Biol. 2013, 19, 2775–2784. [Google Scholar] [CrossRef]
- Mao, R.; Song, C.-C.; Zhang, X.-H.; Wang, X.-W.; Zhang, Z.-H. Response of leaf, sheath and stem nutrient resorption to 7 years of N addition in freshwater wetland of Northeast China. Plant Soil 2012, 364, 385–394. [Google Scholar] [CrossRef]
- Mao, R.; Zeng, D.-H.; Zhang, X.-H.; Song, C.-C. Responses of plant nutrient resorption to phosphorus addition in freshwater marsh of Northeast China. Sci. Rep. 2015, 5, 8097. [Google Scholar] [CrossRef]
- Mo, Q.; Zou, B.; Li, Y.; Chen, Y.; Zhang, W.; Mao, R.; Ding, Y.; Wang, J.; Lu, X.; Li, X.; et al. Response of plant nutrient stoichiometry to fertilization varied with plant tissues in a tropical forest. Sci. Rep. 2015, 5, 14605. [Google Scholar] [CrossRef]
- Mo, Q.; Chen, Y.; Wang, F.; Zou, B.; Li, Y.; Yu, S.; Li, X.; Li, Z. Nitrogen to phosphorus ratios of two understory plant species in response to nitrogen and phosphorus addition in tropical forest of southern china. Chin. J. Appl. Environ. Biol. 2015, 21, 919–925. [Google Scholar]
- Rejmánková, E.; Snyder, J.M. Emergent macrophytes in phosphorus limited marshes: Do phosphorus usage strategies change after nutrient addition? Plant Soil 2008, 313, 141–153. [Google Scholar] [CrossRef]
- Ren, H.; Kang, J.; Yuan, Z.; Xu, Z.; Han, G. Responses of nutrient resorption to warming and nitrogen fertilization in contrasting wet and dry years in a desert grassland. Plant Soil 2018, 432, 65–73. [Google Scholar] [CrossRef]
- Ren, H.; Xu, Z.; Huang, J.; Lü, X.; Zeng, D.-H.; Yuan, Z.; Han, X.; Fang, Y. Increased precipitation induces a positive plant-soil feedback in a semi-arid grassland. Plant Soil 2014, 389, 211–223. [Google Scholar] [CrossRef]
- Servais, S.; Kominoski, J.S.; Davis, S.E.; Gaiser, E.E.; Pachόn, J.; Troxler, T.G. Effects of Nutrient-Limitation on Disturbance Recovery in Experimental Mangrove Wetlands. Wetlands 2018, 39, 337–347. [Google Scholar] [CrossRef]
- Shen, F.-F.; Li, Y.-Y.; Liu, W.-F.; Duan, H.-L.; Fan, H.-B.; Hu, L.; Meng, Q.-Y. Responses of nitrogen and phosphorus resorption from leaves and branches to long-term nitrogen deposition in a Chinese fir plantation. Chin. J. Plant Ecol. 2018, 42, 926–937. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Yang, X.; Sun, X.; Chen, W.; Yang, G.; Liu, N.; Chen, J.; Zhang, Y. Increased precipitation modulates the influence of nitrogen and litter inputs on the nutrient resorption proficiency rather than efficiency of Leymus chinensis. Plant Ecol. 2017, 219, 217–230. [Google Scholar] [CrossRef]
- Shi, B.; Ling, X.; Cui, H.; Song, W.; Gao, Y.; Sun, W. Response of nutrient resorption ofleymus chinensisto nitrogen and phosphorus addition in a meadow steppe of northeast china. Plant Biol. 2020, 22, 1123–1132. [Google Scholar] [CrossRef]
- Su, Y.; Luo, Y.; Geng, F.; Han, W.; Zhu, Y.; Li, K.; Liu, X. Response of stoichiometric characteristics of nitrogen and phosphorus in plant leaves in an alpine grasslands to nitrogen deposition in the tianshan mountains. Arid. Zone Res. 2019, 36, 430–436. [Google Scholar]
- Su, Y.; Ma, X.; Le, J.; Li, K.; Han, W.; Liu, X. Decoupling of nitrogen and phosphorus in dominant grass species in response to long-term nitrogen addition in an Alpine Grassland in Central Asia. Plant Ecol. 2021, 222, 261–274. [Google Scholar] [CrossRef]
- Wan, X.B.; Wang, Q.G.; Yan, G.-Y.; Xing, Y.J. Response of ecological stoichiometric characteristics and photosynthetic characteristics of plant leaves to long-term n deposition in natural secondary forest. Bull. Bot. Res. 2019, 39, 407–420. [Google Scholar]
- Wang, B.; Huang, G.; Ma, J.; Li, Y. Responses of nutrients resorption of five desert ephemeral plants to water and nitrogen additions. J. Desert Res. 2016, 36, 415–422. [Google Scholar]
- Wang, F.-C.; Fang, X.-M.; Wang, G.G.; Mao, R.; Lin, X.-F.; Wang, H.; Chen, F.-S. Effects of nutrient addition on foliar phosphorus fractions and their resorption in different-aged leaves of Chinese fir in subtropical China. Plant Soil 2019, 443, 41–54. [Google Scholar] [CrossRef]
- Yan, T.; Qu, T.; Song, H.; Ciais, P.; Piao, S.; Sun, Z.; Zeng, H. Contrasting effects of N addition on the N and P status of understory vegetation in plantations of sapling and matureLarix principis-rupprechtii. J. Plant Ecol. 2018, 11, 843–852. [Google Scholar] [CrossRef]
- Yang, H. Effects of nitrogen and phosphorus addition on leaf nutrient characteristics in a subtropical forest. Trees 2017, 32, 383–391. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, J.; Lyu, M.; Xiong, D.; Wang, J.; Chen, Y.; Li, Y.; Wang, M.; Yang, Y. Short-term effects of soil warming and nitrogen addition on the N:P stoichiometry of Cunninghamia lanceolata in subtropical regions. Plant Soil 2016, 411, 395–407. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, G.-S.; Shi, F.-X.; Mao, R. Biomass allocation between leaf and stem regulates community-level plant nutrient resorption efficiency response to nitrogen and phosphorus additions in a temperate wetland of Northeast China. J. Plant Ecol. 2020, 14, 58–66. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, X.; Hu, Y.; Zeng, D. Effects of nitrogen addition on nutrient allocation and nutrient resorption efficiency in larix gmelinii. Sci. Silvae Sin. 2010, 46, 14–19. [Google Scholar]
- Zheng, J.; She, W.; Zhang, Y.; Bai, Y.; Qin, S.; Wu, B. Nitrogen enrichment alters nutrient resorption and exacerbates phosphorus limitation in the desert shrub artemisia ordosica. Ecol. Evol. 2018, 8, 9998–10007. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.-L.; Zhao, Q.; Sun, Q.-Y.; Liu, L.; Zeng, D.-H. Nitrogen addition elevated autumn phosphorus retranslocation of living needles but not resorption in a nutrient-poor Pinus sylvestris var. Mongolica plantation. For. Ecol. Manag. 2020, 468, 118174. [Google Scholar] [CrossRef]
- Lin, L. Hybrid test for publication bias in meta-analysis. Stat. Methods Med. Res. 2020, 29, 2881–2899. [Google Scholar] [CrossRef]
- Peng, Y.; Guo, D.; Yang, Y. Global patterns of root dynamics under nitrogen enrichment. Glob. Ecol. Biogeogr. 2017, 26, 102–114. [Google Scholar] [CrossRef]
- Luo, Y.; Hui, D.; Zhang, D. Elevated carbon dioxide stimulates net accumulations of carbon and nitrogen in terrestrial ecosystems: A meta-analysis. Ecology 2006, 87, 53–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Z.Y.; Chen, H. Negative effects of fertilization on plant nutrient resorption. Ecology 2015, 96, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Penuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; van der Velde, M.; Bopp, L.; Boucher, O.; Godderis, Y.; Hinsinger, P.; Llusia, J.; et al. Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013, 4, 2934. [Google Scholar] [CrossRef] [Green Version]
- Houlton, B.Z.; Wang, Y.P.; Vitousek, P.M.; Field, C.B. A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 2008, 454, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Reichert, T.; Rammig, A.; Fuchslueger, L.; Lugli, L.F.; Quesada, C.A.; Fleischer, K. Plant phosphorus-use and -acquisition strategies in Amazonia. New Phytol. 2022, 234, 1126–1143. [Google Scholar] [CrossRef] [PubMed]
- Marklein, A.R.; Houlton, B.Z. Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol. 2012, 193, 696–704. [Google Scholar] [CrossRef]
- Wen, Z.; Li, H.; Shen, J.; Rengel, Z. Maize responds to low shoot P concentration by altering root morphology rather than increasing root exudation. Plant. Soil 2017, 416, 377–389. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, Q.; Noll, L.; Hu, Y.; Wanek, W. Environmental effects on soil microbial nitrogen use efficiency are controlled by allocation of organic nitrogen to microbial growth and regulate gross N mineralization. Soil Biol. Biochem. 2019, 135, 304–315. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zhang, L.; Yao, X.; Li, J.; Deng, Q. Co-Evaluation of Plant Leaf Nutrient Concentrations and Resorption in Response to Fertilization under Different Nutrient-Limited Conditions. Diversity 2022, 14, 385. https://doi.org/10.3390/d14050385
Zhang M, Zhang L, Yao X, Li J, Deng Q. Co-Evaluation of Plant Leaf Nutrient Concentrations and Resorption in Response to Fertilization under Different Nutrient-Limited Conditions. Diversity. 2022; 14(5):385. https://doi.org/10.3390/d14050385
Chicago/Turabian StyleZhang, Meixia, Leiyi Zhang, Xianyu Yao, Jianling Li, and Qi Deng. 2022. "Co-Evaluation of Plant Leaf Nutrient Concentrations and Resorption in Response to Fertilization under Different Nutrient-Limited Conditions" Diversity 14, no. 5: 385. https://doi.org/10.3390/d14050385
APA StyleZhang, M., Zhang, L., Yao, X., Li, J., & Deng, Q. (2022). Co-Evaluation of Plant Leaf Nutrient Concentrations and Resorption in Response to Fertilization under Different Nutrient-Limited Conditions. Diversity, 14(5), 385. https://doi.org/10.3390/d14050385





