Review of the Genus Orphnus Macleay, 1819 (Coleoptera: Scarabaeidae: Orphninae) from Kenya, with Description of New Species †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collections
- ARCL—Andreas Reichenbach collection, Leipzig, Germany (Andreas Reichenbach);
- BMNH—The Natural History Museum, London, United Kingdom (Maxwell Barclay);
- EBCT—Enrico Barbero collection, Torino, Italy (Enrico Barbero);
- GDOR—Museo Civico di Storia Naturale Giacomo Doria, Genova, Italy (Roberto Poggi);
- HNHM—Hungarian Natural History Museum (Termeszettudomanyi Muzeum), Budapest, Hungary (†Otto Merkl);
- MCSNC—Museo Civico di Storia Naturale di Carmagnola, Carmagnola, Italy (Enrico Barbero);
- MHNG—Muséum d’histoire naturelle de la Ville de Genève, Geneva, Switzerland (Giulio Cuccodoro);
- MNHB—Museum of Natural History, Leibniz Institute for Evolution and Biodiversity Science, Berlin, Germany (Bernd Jaeger);
- MNHN—Museum national d’histoire naturelle, Paris, France (Olivier Montreuil);
- NHMB—Naturhistorisches Museum, Basel, Switzerland (Eva Sprecher-Uebersax);
- NMNHW—National Museum of Natural History, Smithsonian Institution, Washington, U.S.A. (David Furth);
- NMPC—National Museum, Prague, Czech Republic (Jiří Hájek);
- RMCA—Musee royal de l’Afrique Centrale, Tervuren, Belgium (Marc De Meyer);
- SMTFD—Staatliches Museum für Tierkunde, Dresden, Germany (Olaf Jaeger);
- TMSA—Ditsong National Museum of Natural History, Pretoria, South Africa (Ruth Müller);
- ZFMAKB—Zoologisches Forschungsinstitut und Museum A. Konig, Bonn, Germany (Dirk Ahrens);
- ZIN—Zoological Institute, Russian Academy of Sciences, St. Petersburg, Russia (Andrey Frolov);
- ZMUKK—Zoologisk Museum, Universitet Københavns, Copenhagen, Denmark (Alexey Solodovnikov).
2.2. Terminology and Format
2.3. Preparation of Specimens
2.4. Digital Images
2.5. Locality Maps
3. Results/Taxonomy
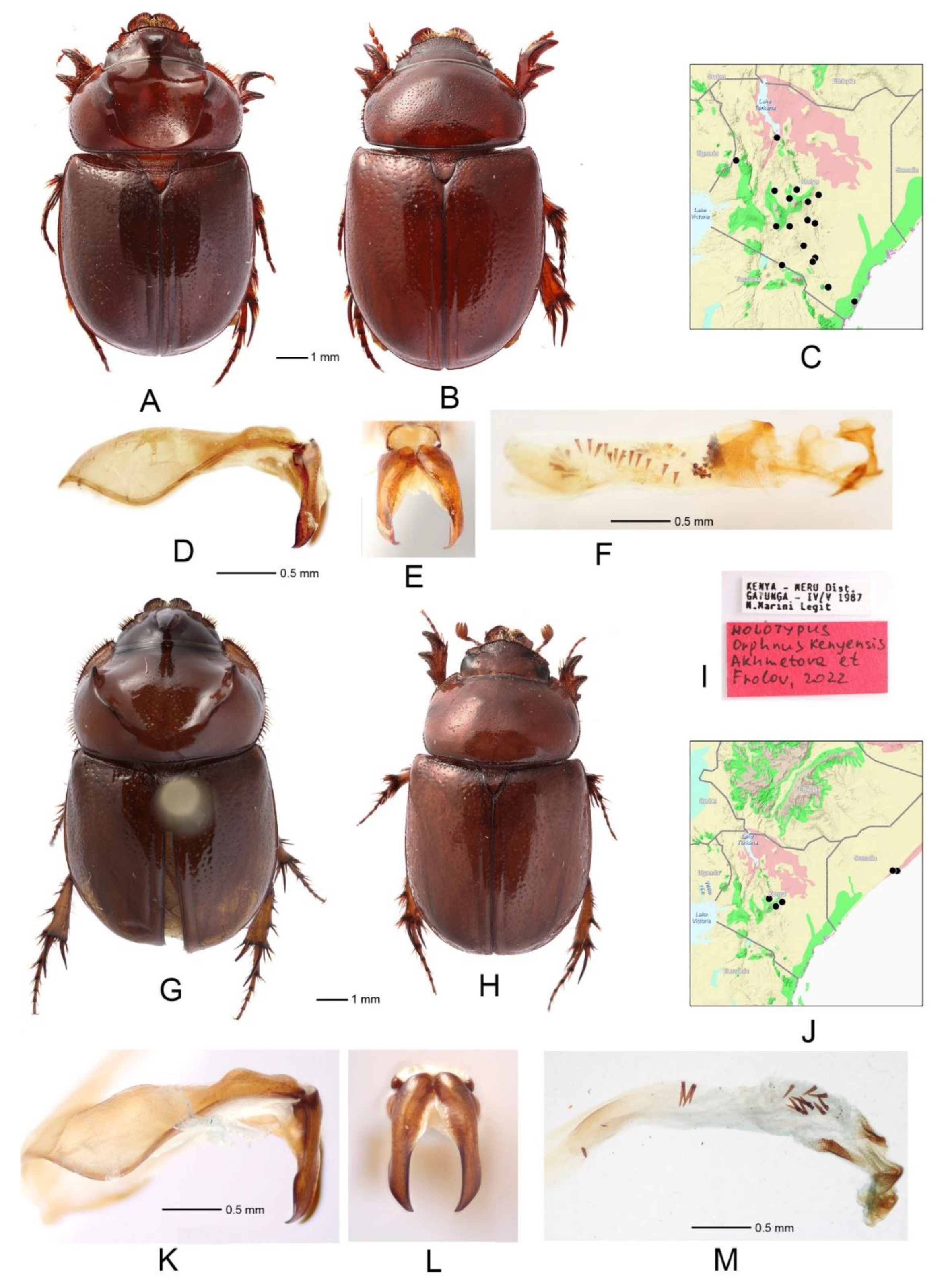
3.1. Orphnus (Orphnus) rufithorax Benderitter (Figure 1A–G)
3.1.1. Differential Diagnosis
3.1.2. Type Material Examined
3.1.3. Additional Material Examined
3.1.4. Distribution
3.1.5. Remarks
3.2. Orphnus (Orphnus) thoracicus Linell (Figure 1H–M)
3.2.1. Differential Diagnosis
3.2.2. Type Material Examined
3.2.3. Additional Material Examined
3.2.4. Distribution
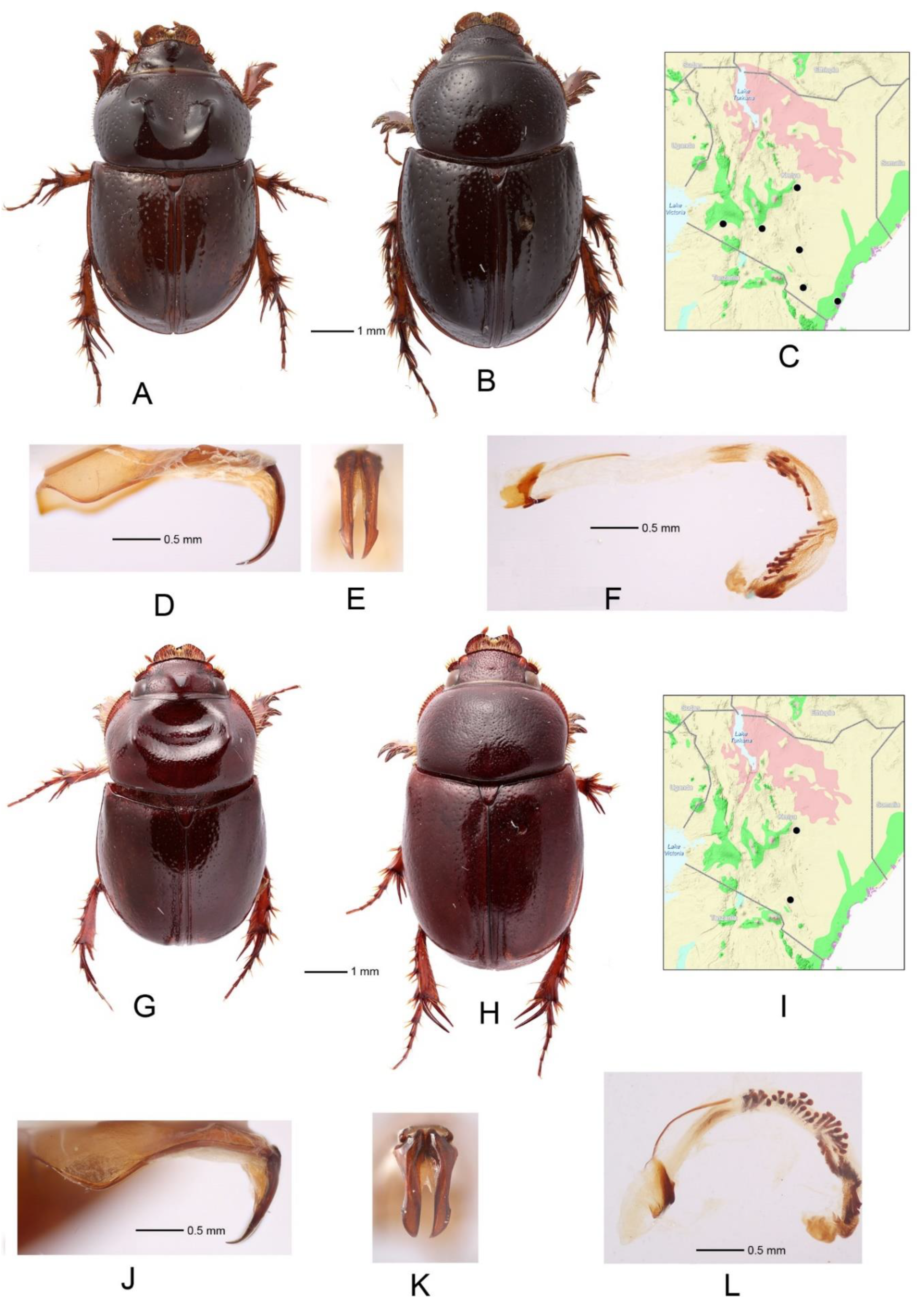
3.3. Orphnus (Orphnus) macleayi Laporte de Castelnau (Figure 2A–F)
3.3.1. Differential Diagnosis
3.3.2. Material Examined
3.3.3. Distribution
3.4. Orphnus (Orphnus) kenyensis Akhmetova et Frolov, sp. nov. (Figure 2G–M)
3.4.1. Differential Diagnosis
3.4.2. Etymology
3.4.3. Type Material
3.4.4. Description
3.4.5. Paratypes and Variability
3.4.6. Distribution
3.5. Orphnus (Orphnus) mombasaensis Benderitter (Figure 3A–F)
3.5.1. Differential Diagnosis
3.5.2. Type Material Examined
3.5.3. Other Material Examined
3.5.4. Distribution
3.5.5. Remarks
3.6. Orphnus (Orphnus) tristis Pic (Figure 3G–L)
3.6.1. Differential Diagnosis
3.6.2. Material Examined
3.6.3. Distribution
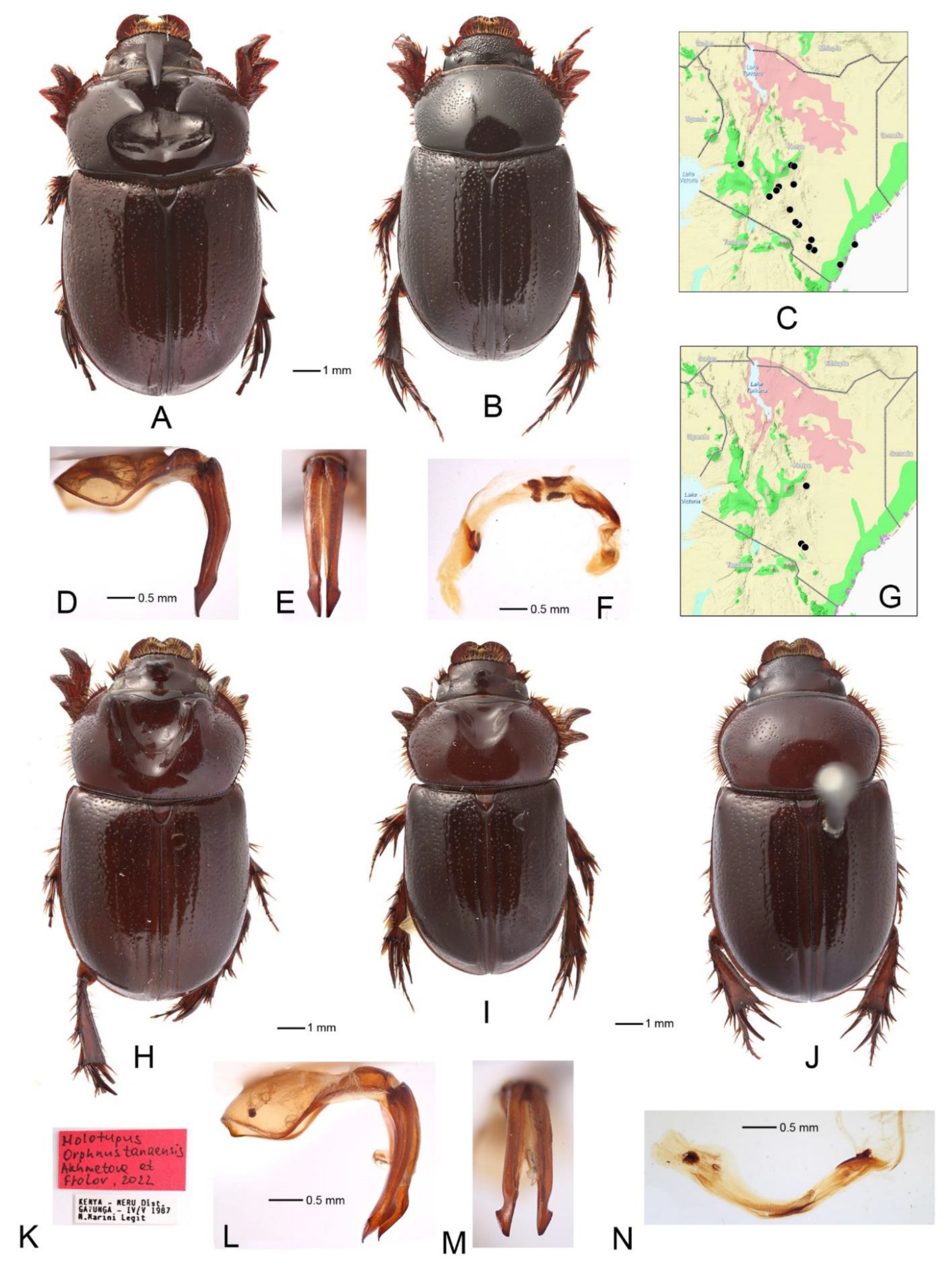
3.7. Orphnus (Orphnus) jeanneli Benderitter (Figure 4A–F)
3.7.1. Differential Diagnosis
3.7.2. Type Material Examined
3.7.3. Additional Material Examined
3.7.4. Distribution
3.7.5. Remarks
3.8. Orphnus (Orphnus) chappuisi Paulian (Figure 4G,H)
3.8.1. Type Material Examined
3.8.2. Remarks
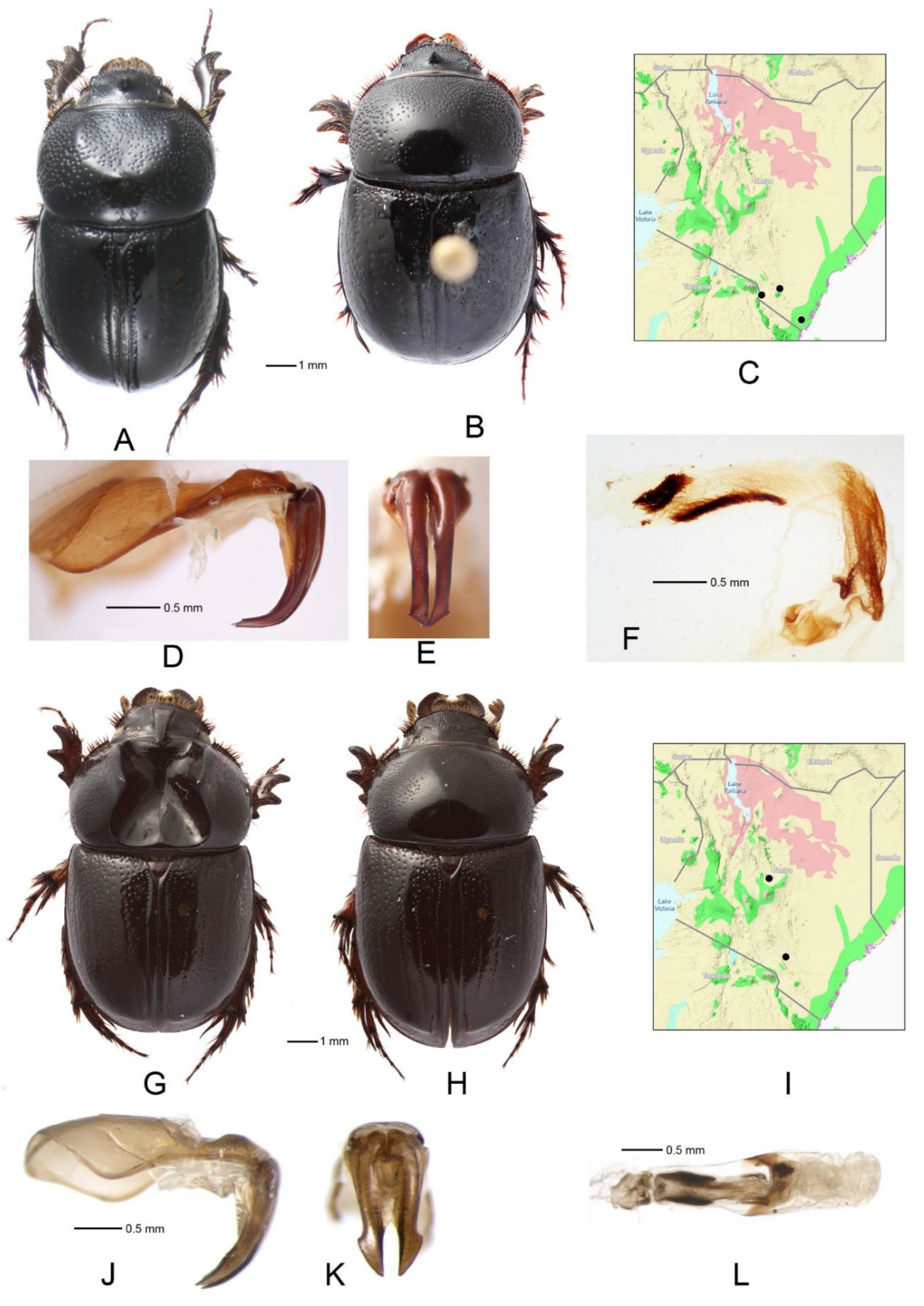
3.9. Orphnus (Orphnus) sansibaricus Kolbe (Figure 5A–F)
3.9.1. Differential Diagnosis
3.9.2. Material Examined
3.9.3. Distribution
3.10. Orphnus (Orphnus) tanaensis Akhmetova et Frolov, sp. nov. (Figure 5G–N)
3.10.1. Differential Diagnosis
3.10.2. Etymology
3.10.3. Type Material Examined
3.10.4. Description
3.10.5. Paratypes and Variability
3.10.6. Distribution
3.10.7. Remarks
3.11. Orphnus (Phornus) compactus Petrovitz (Figure 6A–F)
3.11.1. Differential Diagnosis
3.11.2. Material Examined
3.11.3. Distribution
3.12. Orphnus (Orphnus) compactilis Quedenfeldt (Figure 6G–L)
3.12.1. Differential Diagnosis
3.12.2. Material Examined
3.12.3. Distribution
- 1.
- Sides of pronotum with long setae (Figure 1A,B) .............................. Orphnus rufithorax
- –
- Sides of pronotum without setae, only margins setose ..................................................... 2
- 2.
- –
- 3.
- Pronotum with more or less developed posterio-medial bulge (Figure 1H) ................................................................................................................................. Orphnus thoracicus
- –
- 4.
- Tarsomeres of anterior legs widened, somewhat moniliform (Figure 2B) ....................... Orphnus macleaya
- –
- Tarsomeres of anterior legs slender, cylindrical (Figure 2H) ......................................... 5
- 5.
- Prothoracic lateral ridges bimodal (Figure 2G) ................... Orphnus kenyensis sp. nov.
- –
- Prothoracic lateral ridges unimodal (Figure 3A,G) ........................................................ 6
- 6.
- –
- Apices of parameres wider, less tapering apically (Figure 3J,K); pronotal ridges widely separated; lateral margins of pronotum slightly sinuate posteriorly (Figure 3G) .......................................................................................................................... Orphnus tristis
- 7.
- Endophallus with a long curved sclerite, without distinct clusters or fields of spinules (Figure 4F) ................................................................................................... Orphnus jeanneli
- –
- 8.
- Apices of parameres curved upward (Figure 5D,L) ....................................................... 9
- –
- Apices of parameres evenly curved downward (Figure 6D,J) ...................................... 10
- 9.
- –
- 10.
- –
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frolov, A.V.; Akhmetova, L.A. Description of a new brachypterous scarab species, Orphnus brevialatus (Coleoptera: Scarabaeidae: Orphninae) from East Africa, with notes on flightlessness in the orphnines. Zootaxa 2020, 4750, 425–431. [Google Scholar] [CrossRef]
- Petrovitz, R. Beitrag zur Kenntnis der Gattung Orphnus M’Leay (Orphninae, Scarabaeidae, Coleoptera). Rev. Zool. Bot. Afr. 1971, 84, 1–46. [Google Scholar]
- Paulian, R. Revision des Orphnus Africains (Coleoptera, Scarabaeidae). Ann. Soc. Entomol. Fr. 1948, 117, 1–75. [Google Scholar] [CrossRef]
- Benderitter, E. Ochodaeinae, Orphninae et Hybosorinae. Voyage de Ch. Alluaud et R. Jeannel en Afrique orientale 1911–1912. Résult. Sci. Coleopt. 1914, 5, 195–206. [Google Scholar]
- Frolov, A.V.; Montreuil, O.; Akhmetova, L.A. Review of the Madagascan Orphninae (Coleoptera: Scarabaeidae) with a revision of the genus Triodontus Westwood. Zootaxa 2016, 4207, 1–93. [Google Scholar] [CrossRef] [PubMed]
- Linell, M.L. List of Coleoptera collected on the Tana River, and on the Jombene Range, East Africa, by Mr. William Astor Chanler and Lieutenant Ludwig von Höhnel, with description of new genera and species. Proc. U. S. Natl. Mus. 1896, 18, 687–716. [Google Scholar] [CrossRef]
- Castelnau, F.L. Mémoire sur cinquante espèces nouvelles ou peu connues d’insectes. Ann. Soc. Entomol. Fr. 1832, 1, 386–415. [Google Scholar]
- Pic, M. Notes et descriptions. Mélanges Exot.-Entomol. 1928, 51, 1–36. [Google Scholar]
- Kolbe, H.J. Beiträge zur Kenntniss der Mistkäfer, Lamellicornia onthophila. Stettin. Entomol. Ztg. 1895, 56, 329–345. [Google Scholar]
- Gestro, R. Esplorazione del Giuba e dei suoi affluenti compiuta dal cap. V. Bottego durante durante gli anni 1892–1893 sotto gli auspici della Societa Geografica Italiana. Risultati zoologici. XVI. Coleotteri. Ann. Mus. Civ. Stor. Nat. Genova 1895, 15, 247–494. [Google Scholar] [CrossRef] [Green Version]
- Quedenfeldt, G. Verzeichniss der von Herrn Major a. D. von Mechow in Angola und am Quango-Strom 1878–1881 gesammelten Pectinicornen und Lamellicornen. Berl. Entomol. Z. 1884, 28, 265–340. [Google Scholar] [CrossRef]
- Gradinarov, D.; Petrova, Y.; Tasheva-Terzieva, E.; Frolov, A.V. Biology of the blind geobiont scarab beetle genus Chaetonyx Schaum (Scarabaeidae: Orphninae) with new distribution records of Ch. robustus Schaum from Bulgaria. ZooNotes 2015, 81, 1–14. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhmetova, L.A.; Frolov, A.V. Review of the Genus Orphnus Macleay, 1819 (Coleoptera: Scarabaeidae: Orphninae) from Kenya, with Description of New Species. Diversity 2022, 14, 373. https://doi.org/10.3390/d14050373
Akhmetova LA, Frolov AV. Review of the Genus Orphnus Macleay, 1819 (Coleoptera: Scarabaeidae: Orphninae) from Kenya, with Description of New Species. Diversity. 2022; 14(5):373. https://doi.org/10.3390/d14050373
Chicago/Turabian StyleAkhmetova, Lilia A., and Andrey V. Frolov. 2022. "Review of the Genus Orphnus Macleay, 1819 (Coleoptera: Scarabaeidae: Orphninae) from Kenya, with Description of New Species" Diversity 14, no. 5: 373. https://doi.org/10.3390/d14050373
APA StyleAkhmetova, L. A., & Frolov, A. V. (2022). Review of the Genus Orphnus Macleay, 1819 (Coleoptera: Scarabaeidae: Orphninae) from Kenya, with Description of New Species. Diversity, 14(5), 373. https://doi.org/10.3390/d14050373






