Abstract
Small-scale fisheries (SSFs) in the Mediterranean and Black seas play a significant social and economic role, representing 84% of the fishing fleet (70,000 vessels), 26% of total revenue (USD 633 million) and 60% of total employment (150,000 people), with the Food and Agriculture Organization (FAO) recently taking important initiatives to sustain livelihoods. Effective management of important natural fisheries resources that sustain livelihoods requires a holistic approach accounting for all parts of the catch. Quantitative data on seasonal catch dynamics together with classification of bycatch species to IUCN vulnerability status and associated revenues from retained incidental catch were used to reveal the effect of a small-scale shrimp trap fishery on bycatch. We use three main quantitative variables (i.e., density, biomass and number of bycatch species) and show a positive correlation between bycatch and the seasonal catch dynamics of the target species during late spring and summer. On the contrary, discards were proportionally lower during winter, with the majority of discarded fish species not considered endangered. Six retained species in spring–summer and five discarded species in winter were found to modulate the structure of species’ assemblage. Out of 55 bycatch species, 26 were retained and 29 discarded. Only four species were considered threatened, all of which were caught in very low numbers (<2 individuals), while the majority of the retained species was not considered endangered. The rapid sorting time (<1 min/per trap) pointed towards a decreased effect on certain discarded crustacean species and a significant reduction in total bycatch with timely haul time (from 71 to 47%). The results of this study can be used when considering future mitigation measures for this fishery, while the methodology used can provide insights into the management of similar trap fisheries worldwide when taking into account the sustainability of SSFs and the regional vulnerability status of bycatch species.
Keywords:
management; natural resources; biodiversity; incidental catch; discards; revenues; fisheries 1. Introduction
There is widespread national and international recognition that discards in many world fisheries constitutes an unnecessary catch and raises ecological and economic considerations that require the urgent attention of fisheries and environmental management organizations [1,2,3,4,5,6]. Incidentally, caught species are important components in fisheries management, as they constitute an economic conundrum for the sector and the discards as an unnecessary biomass to fishers [7,8]. This pattern is even more evident in small-scale fisheries (SSFs) due to the nature of fleets characterized as a low income and with limited possibility for economic diversification in certain partially isolated areas.
Approximately 10 million tons are discarded annually around the world, the majority of which (93% averaged over the period 1950–2014) are carried out by industrial large-scale fisheries [9]. Among various types of fishing, shrimp trawling is the biggest contributor to incidental catches, with the greatest ratio of incidental catch to shrimp catch. Ratios of 5:1 in temperate and subtropical waters and 10:1 in tropical waters have been presented, owing sometimes to the poor selectivity of the fine-meshed nets [10].
The high rate of discard in global fisheries (e.g., trawl fisheries) may have an ecological footprint on benthos, fish and bird species, thus inducing changes to trophic interactions which, in return, may alter ecosystem structure and function [11,12]. The significance of the apparent waste of fish arising from discarding has also increased with the realization that the majority of the world’s fisheries resources are either fully or overexploited [10,13,14]. An important component for effective governance is that all sources of fishing mortality are considered including both incidental and discarded catch [15,16,17,18]. The call upon an ecosystem-based fisheries management has been difficult to implement and, as such, single species stock assessments are still the main driving management option for European fisheries [1,10,19].
Small-scale fisheries (SSFs) are of great importance in terms of job opportunities and contributions to the economy of coastal communities. SSFs have been estimated to generate approximately 53% of direct employment within the EU fishing sector, representing approximately 83% of fishing vessels and a quarter of the catch value [20]. To date, most studies carried out in the Mediterranean Sea concern discards from trawl and purse seine fisheries, and information from SSFs is currently lacking, although they constitute the major part of the fleet [7,21]. A representative case of a SSF in the Mediterranean Sea is the one targeting narwal shrimp, Plesionika narval, (locally called “Symi shrimp”) in the southeastern Aegean Sea using traps [22,23,24]. At present, investigations of bycatch are currently unknown for this specific fishing method, area, and the eastern Mediterranean Sea. The economic importance of the narwal shrimp as a target species to local economies has not been extensively studied on a Mediterranean level [25]. In the studied area of the Aegean Sea, the narwal shrimp is marketed frozen (in 1 kg bags) and sold at prices ranging between euros (EUR) 25 and 30 per kg, while prices at restaurants reach EUR 120 per kg. Fishery is mainly targeting the narwal shrimp, but catches also include other demersal species that are of commercial importance or discarded [26]. Until recently (i.e., December 2021), the studied fishery was neither regulated by means of quotas nor by fish markets. Shrimps and the retained part of bycatch were distributed based on market demands, either directly by the fishermen to the restaurants or through individual middleman fishmongers and at fish markets. Soak time in this fishery varies and is mainly regulated by cost/profit motives. Traps are either set during sunset and hauled before sunrise or set for 24 h with the motive of reducing costs of transport to/from fishing areas. In April 2019, a national management plan was adopted and introduced for all vessels, regardless of vessel length, an electronic reporting system (ERS), a vessel monitoring system (VMS), permit between 1 May to 31 August, a minimum mesh size of 12 mm and a ban to fish at depths less than 40 m, which followed from the results of the research project “Plesionika Manage” that was funded by the European Marine Fisheries Fund, EMFF-Greece, which showed high concentrations of egg-bearing females at these depths [22,23,24,27,28,29].
Quantitative data were used to reveal the temporal bycatch dynamics over an annual fisheries cycle and provide insights into the sustainable management of this important natural resource. The aspects considered in this study were the bycatch to target species density, biomass sand market value, seasonal variations in densities of bycatch species, correlations to regional vulnerability status of bycatch species and the effect of soak time on bycatch. The results of this study are discussed in relation to the viability of SSFs with the aim to provide insights into the sustainable management of an important resource within an area of limited potential for economic diversification for fishers.
2. Materials and Methods
2.1. Definitions
To reflect upon the difference between the part retained and the part discarded, we used the FAO definition of bycatch: incidental catch + discards (i.e., incidental catch is defined as the catch retained and the remaining catch discarded for whatever reason) [30].
2.2. Sampling
A total of 150 commercial circular shrimp traps (FPO) were deployed monthly at each of the five selected locations (i.e., 1: Halki; 2: Symi; 3: Tilos; 4: Nisyros; 5: Karpathos) in the southeastern Aegean Sea during an annual cycle (11 months between November 2014 to October 2015; Figure 1). Due to the rough weather conditions, sampling in February was not possible. The total monthly and annual effort was 750 and 8250 traps, respectively. Part of the study was performed under the framework of the “Plesionika Manage” project aimed at studying aspects of spatial and temporal distribution [23], diet [28], reproduction [29], selectivity of gear [24], fishery [22] and management scenarios [31] for P. narval as well as inferring the status of the soldier striped shrimp, Plesionika edwardsii [26]. The locations and depths studied were randomly selected after personal interviews with all active commercial fishermen and logbooks provided by the fishery department of the South Aegean District Prefecture in Greece [22,23].

Figure 1.
Study area and locations of the samplings in the southeastern Aegean Sea (i.e., 1: Halki; 2: Symi; 3: Tilos; 4: Nisyros; 5: Karpathos). Marked in dark grey is the effective fishing area presented.
The SSF fleet consisted of 42 vessels with lengths from 4.5 to 14 m, usually operated by 1 to 2 fishermen [22]. Fishing is undertaken by means of bottom shrimp traps at depths from 5 to 300 m. Two replicate lines with 25 traps were deployed at each of three depth strata (10–30, 70–90 and 150–170 m) and at each location. Traps were covered with a polyamide-based net with a mesh size of 12 mm (knot to knot) and with an upper trap opening of 13 cm (Figure 2). Targeted shrimps were attracted to the traps with bait consisting of a mixture of oily fish (e.g., Sardina pilchardus and Scomber scomber) and stabilized with flour and water. The total length of the line was adjusted to the fishing depth and 25 traps were attached to the ground line with a distance of 35 m between the traps along the bottom, following common fishery practice in the studied area. Each trap was attached to the ground line with another 2 m side rope. Equidistantly, floats were placed to hinder the line/rope from entangling, while weights were used to keep the traps close to the bottom (see Kalogirou et al. [24] for further details of fishing practice). The fishing survey was overnight (20.00–07.00 h). After a soak time of 12 h, the traps were lifted, and all bycatch species were sorted into incidental catch and discards. The bycatch was counted (i.e., number of specimens), weighted (i.e., g wet weight) without information on the depth due to the rapid sorting during hauling (<1 min per trap) and kept on ice until transported to the lab for further identification and measurements.

Figure 2.
Commercial shrimp traps covered with polyamide-based nets of a mesh size of 12 mm (knot to knot) with an upper trap opening of 13 cm.
2.3. Revenues and Economic Value of Catch and Bycatch
For the targeted narwal shrimp, P. narval, and the incidentally caught soldier striped shrimp, Plesionika edwardsii, the cephalothorax was removed and discarded, while the abdomen and tail were retained, packed into 1 kg plastic bags and stored in a freezer until landed. To reveal the marketed wet weight of these two species, the abdomen/tail wet weight ratio (ARWW, in g) was calculated monthly from a random subsample of 150 uncut individuals:
where ARWW is the abdomen wet weight ratio; WWc is the wet weight of the carapace; WWa is the wet weight of the abdomen. All weights were calculated irrespective of sex and/or presence of eggs.
Revenues deriving from incidental catch were monthly calculated as:
where A is the regional market price for each species (EUR/kg), and B is each species biomass (g) (Supplementary Materials Table S1).
For the targeted species and because market prices vary among seasons and quantities sold, the ARWW was multiplied by the average market price of 25 EUR/kg.
2.4. Data Analysis
The main quantitative variables considered were species diversity (i.e., number of species), density (i.e., number of individuals) and biomass (in g). All parameters accounted for an effort of 150 traps, i.e., location level. Seasonality was classified as winter: January to March; spring: April to June; summer: July to September; autumn: October to December following CTD measurements (conductivity, temperature, depth; SeaBat sensor 7125) [23].
Species were grouped into four major Phyla (Chordata, Arthropoda, Echinodermata and Mollusca) and further categorized into six regional vulnerability categories based on classification by IUCN [32]: LC, least concern; EN, endangered; NT, near threatened; VU, vulnerable; DD, data deficient; NE, not evaluated. None of the species recorded were classified as CR, critically endangered.
To reveal the effect of season (fixed factor, 4 levels) and location (random factor, 5 levels) on the number of species, biomass and density for each of the catch part (i.e., target species, incidental catch and discards) and taxonomic level (i.e., Chordata, Echinodermata, Arthropoda and Mollusca), we used linear mixed-effects models (LMMs) based on the restricted maximum likelihood method (RELM) [33].
The same approach as LMMs was also used to reveal the effect of season and location for the:
- Contribution of incidental catch biomass, expressed as the ratio of incidental catch to target species;
- Revenues from incidental catch.
2.5. Assemblage Structure
To reveal the effect of season on the assemblage structure of incidental catches and discards, a multivariate one-way PERMANOVA was used based on species densities and biomass [34]. In addition, the densities of incidentally caught and discarded species were pooled into groups of species according to IUCN vulnerability status.
To reveal the effect of season on vulnerability status of incidentally caught and discarded species, we used a matrix (i.e., pooled density within a vulnerability grouping of species by location) in a multivariate one-way PERMANOVA. When less than 150 permutations were available, p value was obtained by Monte Carlo simulations for all PERMANOVAs [34]. For graphical representation of the multivariate analysis, a principal coordinate analysis (PCoA) was employed. To reduce the effect of the most dominant species in density and biomass, a fourth root transformation of the Bray–Curtis similarity matrix was applied.
A Spearman’s rank order correlation was used to reveal the relationship between species densities and biomass and with the resulting multivariate patterns as depicted in the PCoA. Species that exhibited correlation values greater than 0.6 were added to the PCoA plot as vectors. To reveal significant relationships between targeted, incidentally caught, and discarded species, the Spearman’s rank order correlation was employed for both density and biomass.
Univariate and multivariate analyses were performed using the PRIMER-E v6 [35], PERMANOVA + [34] and complemented with the SPSS software.
2.6. Haul Time
To reveal the effect of soak time on bycatch, a supplementary monthly sampling with identical effort was performed at two locations off Karpathos Island during the main period of fisheries interest (1 May to 31 August) at which traps were hauled before 07:00 am. A t-test was used to reveal the differences in the densities of incidental caught and discarded individuals between soak hours (before and after 07:00 am).
3. Results
3.1. Catch and Revenues of Incidental Catch
The mean ARWW was 0.62 for the targeted narwal shrimp and 0.67 for the soldier striped shrimp. This indicates a similar retained weight per individual for both species, after the carapace was removed.
The total catch in the fishery survey was 1.61 tons, distributed among the target decapod species with 999 kg (commercial value of EUR 14,252), incidental catch at 480 kg (commercial value of EUR 4737) and discards with 133 kg. Incidental catch biomass included teleosts (68%), mollusks (17%) and crustaceans (15%). The congeneric incidentally caught species, P. edwardsii, amounted to 70 kg of the total catch, with a commercial value of EUR 703.50. Species that were caught in the fishery and discarded were represented by crustaceans, echinoderms and teleosts and had no commercial value (Table 1 and Table 2). The mean revenue from the incidental ranged from EUR 4.70 to 145.00 per effort (mean: EUR 55.50). The revenue from incidental catches varied significantly among seasons with lower values during winter (mean EUR 50.83 ± 39.89 SD) and the greatest during spring (EUR 79.42 ± 29.34 SD).

Table 1.
Landings (kg) and value (EUR) of total catch (i.e., target species, incidental catch and discards) for a total effort of 8250 traps during an annual cycle in the small-scale shrimp-trap fishery of the southeastern Aegean Sea (eastern Mediterranean Sea).

Table 2.
Species list of incidental catch (i) and discards (d) in the number of individuals and total biomass (kg; in brackets) for each of the sampling locations (i.e., Karpathos, Nisyros, Symi, Tilos and Halki) during an annual cycle from 2014 to 2015.
3.2. Variation in the Species Diversity of the Bycatch
In total, 55 species were caught in this SSF, 26 of which were classified as incidental catch and 29 as discards. Among the fish, 22 species were incidental and 12 discarded. The dominant incidentally caught fish species were Phycis phycis, Serranus cabrilla, Serranus scriba, Pagrus pagrus, Muraena helena and Conger conger, contributing to 83% to the total fish biomass (Table 2). The only species within Mollusca that was fresquently present and that contributed considerably to the total incidental catch biomass was Octopus vulgaris (Table 2). The dominant species among Crustacea were the decapod crabs, Homola barbata, and Macropodia rostrata and the anomurans Dardanus arrosor and Dardanus callidus that occurred at all locations (Table 2). All crustacean species, except the congeneric P. edwardsii, Palinurus elephas and Scyllarides latus, were discarded due to the lack of commercial value (Table 2). Similarly, all Echinodermata were discarded and had a low contribution (<1‰) to the total catch biomass (Table 2). Within Echinodermata, Stylocidaris affinis was the most dominant species, and none of the brachyurans was of economic interest (Table 2). The number of incidentally caught and discarded species varied significantly among the seasons with a significantly lower number of species during winter (Figure 3c; Table 3; Appendix A, Table A1).

Figure 3.
Seasonal variation expressed as the mean ± standard deviation in (a) biomass, (b) density, and the (c) number of targeted species, bycatch and discards. Seasonal variations represented as the mean ± standard deviation in (d) biomass, (e), density and (f) number of species for the four major Phyla (i.e., Arthropoda, Chordata, Echinodermata and Mollusca).

Table 3.
Summary of the linear mixed model effects in the number of species, with season (fixed factor) and location (random factor) as predictors.
A significant seasonal within-Phylum difference in the number of species was found for Chordata and Echinodermata, with a higher number of species during spring and summer for Chordata and during winter for Echinodermata (Table 3; Figure 3f; Appendix A, Table A1).
3.3. Variation in the Density and Biomass of Bycatch
The density of incidental catches and discards varied significantly among the seasons, with incidental catches posing significantly higher values during the main fishery period (i.e., spring and summer) than in winter (Table 4; Appendix A, Table A2; Figure 3b). Target species densities ranged from 1014 to 23,741 individuals per sampling day and presented a statistically significant seasonal variation (Table 4; Appendix A, Table A2). Pairwise comparisons revealed significantly higher densities of the target species during spring (pairwise tests, p < 0.05) compared to all other seasons. The density of discarded species was significantly lower during winter compared to all other seasons (Figure 3b; Table 4).

Table 4.
Summary of the linear mixed model effects based on the species’ densities per category, with season (fixed factor) and location (random factor) as predictors.
The highest total densities were found for Arthropoda (14,704 individuals) followed by fish species (4154 individuals), Mollusca (57 individuals) and Echinodermata (130 individuals) (Table 2). The densities of fish and echinoderms varied significantly among the seasons (Table 4). Fish species presented significantly higher densities during spring and summer, while echinoderms displayed higher densities during winter (pairwise tests, p < 0.05) (Figure 3e, Table 4).
Target species biomass varied significantly between seasons ranging between 3.04 and 51.28 kg, with the greatest values observed during spring (pairwise comparisons, p < 0.05) Table 5; Figure 3a; Appendix A, Table A3). Similarly, the biomass of incidental catches varied significantly seasonally with lower values during winter (pairwise comparisons, p < 0.05) (Figure 3a; Table 5; Appendix A, Table A3). In contrast, the biomass of discards did not vary significantly seasonally (Figure 3a; Table 5; Appendix A, Table A3).

Table 5.
Summary of Linear Mixed Model Effects for species biomass with season (fixed factor) and location (random factor) as predictors.
Among the Phyla, Chordata presented the greatest total biomass (224.91 kg), followed by Arthropoda (87.72 kg), Mollusca (41.45 kg), and Echinodermata (1.06 kg; Table 2). Significant seasonal variations in biomass were found for Chordata, Echinodermata and Arthropoda (Table 5). The highest values for Chordata and Arthropoda were found during spring and the lowest during winter (pairwise comparisons, p < 0.05), while Echinodermata revealed the significantly greatest values during winter (pairwise comparisons, p < 0.05) (Figure 3d; Table 5).
Bycatch biomass was significantly correlated to target species biomass (incidental catch: rho = 0.38, p = 0.004; discards: rho = 0.41, p = 0.002), while at the Phyla-level, only the biomass of Chordata and Arthropoda were correlated to target species biomass, rho = 0.55, p = 0.001; rho = 0.92, p = 0.0001, respectively. Similarly, the densities of bycatch were significantly correlated to target species densities (incidental catch: rho = 0.38, p = 0.003; discards: rho = 0.34, p = 0.011) (Figure 3).
The mean ratio of bycatch to target species biomass did not vary significantly among seasons (Table 6; Appendix A, Table A4). The mean ratio of 0.35 ± 0.34 SD (i.e., one-third) was indicative throughout the year (ranging from 0.02 to 1.85) (Appendix A, Table A5). Revenues were found to be significantly different among seasons with winter significantly different from spring, summer, and autumn. A significant difference was also found between spring and autumn (Table 6; Appendix A, Table A4 and Table A5).

Table 6.
Summary of Linear Mixed Model for the biomass ratio between incidental catch and target species, and revenues from incidental catch, with season (fixed factor) and location (random factor) as predictors.
3.4. Assemblage Structure of Incidental Catches and Discards
The assemblage structure of incidental catches and discards based on density and biomass varied significantly between seasons (PERMANOVA and PCoA; Pseudo-F = 4.90, df = 3 and p = 0.001 and Pseudo-F = 5.52, df = 3 and p = 0.001, respectively). Pairwise comparisons revealed significant differences between all pairs of seasons (p < 0.05) (Figure 4).
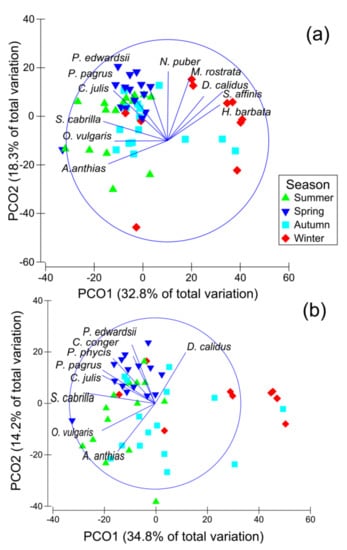
Figure 4.
Seasonal principal coordinate analysis (PCoA) plot of bycatch species based on density (a) and biomass (b). Two axes explained 51.1% of the total variation in density and 49% for biomass. Species with a Spearman rank order correlation higher than 0.6 are shown as the overlying vectors.
Densities of the Arthropoda species (i.e., Homola barbata, Dardanus calidus and Macropodia rostrata) showed a strong positive relationship with PCo1, indicating an association with the winter season, whereas the fish species P. pagrus and Coris julis, Serranus cabrilla and Anthias anthias exhibited a strong negative relationship with PCo1, signifying a strong relationship with the spring and summer seasons. In terms of biomass, P. edwardsii, Conger conger, Phycis phycis, P. pagrus and S. cabrilla presented the strongest relationships with spring and summer, while Octopus vulgaris and Anthias anthias exhibited a pronounced relationship with summer and autumn (Figure 4).
Target species biomass was correlated to bycatch biomass and discards biomass. A similar pattern was also found for the correlation between target species and the two Phyla of Chordata and Arthropoda (Figure 5).
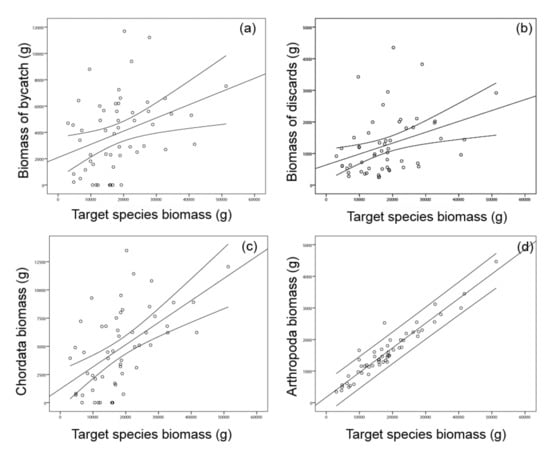
Figure 5.
Scatter plots depicting the relationship between target species’ biomass to the biomass of (a) incidental catch, (b) discards, Chordata (c) and Arthropoda (d). The linear regression and 95% confidence intervals are shown.
3.5. Vulnerability Status
In total, 55 species were considered either as incidental catch or discards: 22 species were classified as not evaluated (NE: 4 as bycatch and 18 as discards), 27 of least concern (LC: 17 as incidental catch and 10 as discards), 4 vulnerable (VU: 3 as bycatch and 1 as discard), 1 data deficient (DD: as discard) and 1 as nearly threatened (NT: as bycatch) (Table 2) [32]. The only endangered incidentally caught species, Epinephelus marginatus, was present in very low quantities, i.e., three individuals (Table 3). In addition, the three vulnerable species, P. elephans, Balistes capriscus and Labrus viridis, and the only nearly threatened species, Epinephelus aeneus, were also observed in very low quantities, i.e., four, one, one, and one, respectively (Table 2).
A significant seasonal effect was found in the assemblage structure of bycatch species when classified in vulnerability status (Pseudo-F = 8.09, df = 3 and p = 0.001), resulting in statistically dissimilar assemblages between all the compared seasons (pairwise comparisons; p < 0.05 in all cases) (Figure 6). Since the majority of species fell into two vulnerability statuses, LC and NE, those were also the driving observed pattern as also revealed by Spearman’s rank order correlation vectors (rho > 0.6).
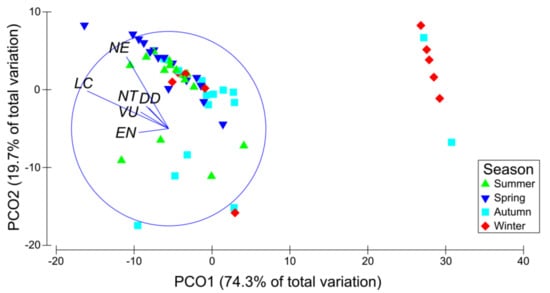
Figure 6.
Principal coordinate analysis plot (PCoA) of bycatch and discards species assemblage by season according to IUCN conservation status. The first two axes explained 94% of the total variation. Conservation status categories with a Spearman rank order correlation higher than 0.6 are shown as overlying vectors.
3.6. Haul Time
For all samplings, a later haul time resulted in higher densities of bycatch species. A significant negative correlation was found for densities between both incidentally caught and discarded species with haul time (t-test; df = 1 and p < 0.05; Figure 7) reaching a total monthly reduction from 47 to 71% with earlier haul time (before 07.00 a.m.).
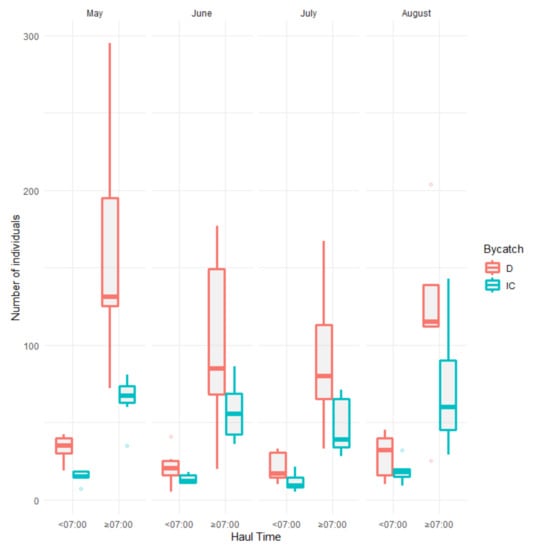
Figure 7.
Number of individuals caught as incidental catch (IC) and discarded (D) before and after 07:00 a.m. during the main fishery period (1 May to 31 August).
4. Discussion
This study provides novel information on the interaction between a small-scale shrimp trap fishery and bycatch species from a remote area in the eastern Mediterranean Sea. The results revealed a lower bycatch to target shrimp ratio compared to global estimates from shrimp trawling [10], with no seasonality over the year. Incidentally caught species contributed importantly to total biomass (30%), income (24%) and number of individuals, while discarded species contributed with a lower fraction to total biomass (8%) but with a high contribution to the number of individuals (80%). Among discards, fish comprised the vast majority of incidental catch biomass with an important contribution to the number of individuals but of low conservation concern. In addition, the majority of discarded Arthropoda, Echinodermata and Mollusca were mainly represented by species that still have not been evaluated by IUCN, pointing towards an increased need for regional assessment of species and use in fisheries management.
Seasonal variations, both in density and biomass of Arthropoda, were strongly related to the winter season, pointing towards a strengthened measure to ban fisheries during winter when accounting for sustaining revenues and reducing the total impact on discards [27]. This is in agreement with winter as a season where discarded species were proportionally lower in terms of contribution to total bycatch and no commercial interest in fisheries during this season. Densities of bycatch species were significantly reduced from 71% to 47% with earlier haul time, thus providing an important aspect for complemented management measures that minimize unwanted discards and increase biodiversity conservation. These results may be of direct use to similar SSFs in the Mediterranean Sea and elsewhere when accounting for spatiotemporal measures to manage similar fisheries [36,37,38,39].
Similar to the studied fishery, many coastal communities remain dependent on fisheries for their income [40,41]. The studied area of the Dodecanese archipelago in the southeastern Aegean Sea consists of small islands and islets, the majority of which are inhabited by small fishing communities (e.g., Symi, Chalki and Kastellorizo) with limited potential for economic diversification. This is mainly attributed to irregular communication with other more economically independent surrounding islands and the capital as well as seasonality and dependence on tourism. The temporal dynamics of P. narval, mainly dominant during late spring and summer [23], activates fishers during this period with a significant effect on effort and revenues for this SSF [22]. The biomass and value of incidental catch for the SSF studied was low compared to incidental catches in other fishing gears (e.g., trawls) but still considered a significant complementary income for these small-scale coastal fishers.
The results of this study revealed similar discard ratios to other similar trap fisheries in the Mediterranean [42] and lower than similar trap fisheries in the North Atlantic [37]. Quantitative information on other global small-scale fisheries is almost non-existent [10,43], thus signifying the importance of such studies from SSFs. This is further reflected by the recent GFCM initiative to map all small-scale fishery projects performed in the Mediterranean area [44]. The results of this study revealed lower discards ratios (8%) than similar trap fisheries in deeper waters (15%, depths from 100 to 500 m) of the southern Tyrrhenian Sea [42]. The crustacean and echinoderm parts of the discards were assumed to have an increased survival due to the significantly shorter sorting time (less than one minute per trap) in the trap fishery compared to trawl fisheries (10 to 280 min) [45] and clyde fishing boats (45 to 300 min) [46]. This implies a lower impact on biodiversity and function of benthic communities. In addition, the low footprint and passive nature of traps were supportive for the survival of discarded echinoderms and crabs.
Assessment of species vulnerability can be used as a proxy for the sustainable use of marine resources taking into account biological and socioeconomic issues within the framework of ecosystem-based management [47,48]. Temporal variations in bycatch species density and biomass were strongly correlated to seasonal dynamics in target species, possibly implying an attraction to either the targeted shrimps trapped and/or to other bycatch and discarded species. This pattern was particularly evident for fish species during spring and summer with dietary studies confirming that the most dominant incidentally caught species in the studied fishery (i.e., P. phycis, P. pagrus, S. cabrilla and C. conger) prey upon the targeted narwal shrimp but also on several dominant discarded decapods, e.g., H. barbata and G. strigosa [49,50,51,52]. Similarly, the regularly observed, Octopus vulgaris, was highly probably attracted also to the discarded decapods, as these constitute an important part of their diet, e.g., M. squinado [53]. Both Arthropoda and Crustacea were strongly related to target species biomass with several species, such as N. puber, M. rostrata, D. calidus and H. barbata, revealing a strong association with the winter season. The winter season showed a strong relationship with Echinodermata, among which S. affinis contributed most to this seasonal pattern.
Trap fisheries are fairly selectively characterized as low footprint with minimal habitat impacts that deliver catches of high quality compared to global trawl fisheries [39,43,54,55]. Safeguarding trap fisheries in the Mediterranean Sea, an area characterized by overexploitation of most of its fisheries resources [14,56], could help reduce the use of less sustainable fishing gears and practices by other SSFs in the area [14]. This fishery is still not considered optimized due to the lack of justified studies on discarded species survivability at haulback. The lack of seasonal variations in discarded species and moderate high-value incidental catch not considered endangered together with a potential fishery during late spring and summer, in line with fishers’ interest [31], would therefore account for a complementary income retaining incidentally caught species not considered endangered, while a ban during winter would reduce the total impact of bycatch. In addition, the constant bycatch-to-shrimp ratio over season, with the majority of species not considered endangered, would therefore account for safeguarding this SSF sustainability.
The results should be interpreted with caution considering the limitations of our study due to the lack of evaluation of conservation status for the discarded species actively involved in the formulation of the observed seasonal variation. A measure to only allow nocturnal activity for this fishery will significantly reduce incidental catches of species, allowing other diurnal SSFs to perform their activity (e.g., set netting). To further elucidate the ecological footprint of this SSF on the ecosystem, complementary studies on discarded species vulnerability and survivability/mortality caused by various fishing gears, food availability and predator–prey relationships are recommended [57]. The findings of this study could prove beneficial in developing adapted management plans for this fishery and region by effectively retaining an important income, safeguarding small-scale fisheries in accordance with the recent Malta MedFish4Ever declaration and FAO guidelines on SSFs [57,58] while, at the same time, minimizing the overall effect on vulnerable species and sustaining biodiversity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14040292/s1, Table S1: Market price (€/kg) of commercially important bycatch species in the south-eastern Aegean Sea in 2016.
Author Contributions
Conceptualization, S.K.; methodology, S.K.; software, S.K. and C.D.M.; validation, S.K., C.D.M. and D.P.; formal analysis, S.K. and C.D.M.; investigation, S.K.; data curation, S.K.; writing—original draft preparation, S.K.; writing—review and editing, S.K., C.D.M., D.P., C.D., M.M., H.M., L.P.; visualization, S.K.; project administration, S.K.; funding acquisition, S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the European Marine Fisheries Fund (EMFF) 2007–2013—Greece—Operational programme for Fisheries (OPF), 3.5 Pilot projects—Plesionika manage.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We are kindly thankful to Ilias Santorinios (HCMR-Rhodes) for the laboratory analysis and species identification and the crew of the fishing vessel.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Linear Mixed Model effects regarding number of species with season (fixed factor) and location (random factor) as predictors.
Table A1.
Linear Mixed Model effects regarding number of species with season (fixed factor) and location (random factor) as predictors.
| Number of incidentally caught species | F | p value | Pair-wise LSD | ||
| Fixed effects | Intercept | 317.03 | 0.001 | ||
| Season | 12.98 | 0.001 | A vs. S; A vs. W; Sp vs. W; S vs. W | ||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 0.03 | 0.24 | 0.12 | 0.901 |
| Number of Chordata species | F | pvalue | Pair-wise LSD | ||
| Fixed effects | Intercept | 268.64 | 0.001 | ||
| Season | 15.49 | 0.001 | A vs. S; A vs. W; Sp vs. W; S vs. W; Sp vs. A | ||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 0.01 | 0.001 | 5.05 | 0.680 |
| Number of Echinodermata species | F | pvalue | Pair-wise LSD | ||
| Fixed effects | Intercept | 152.03 | 0.001 | ||
| Season | 8.90 | 0.001 | A vs. W; Sp vs. S; Sp vs. W; S vs. W | ||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 0.06 | 0.07 | 0.81 | 0.418 |
| Number of Arthropoda species | F | pvalue | Pair-wise LSD | ||
| Fixed effects | Intercept | 71.83 | 0.001 | ||
| Season | 1.71 | 0.178 | |||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 0.89 | 0.74 | 1.13 | 0.251 |
| Number of Mollusca species | F | pvalue | Pair-wise LSD | ||
| Fixed effects | Intercept | 57.28 | 0.001 | ||
| Season | 1.62 | 0.195 | |||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 0.30 | 0.06 | 5.05 | 0.344 |
| Number of Discarded species | F | pvalue | Pair-wise LSD | ||
| Fixed effects | Intercept | 140.15 | 0.001 | ||
| Season | 3.43 | 0.024 | |||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 1.14 | 1.08 | 1.05 | 0.290 |
Pair—wise comparisons among the fixed factor levels are based on estimated marginal means and least significant difference (LSD) (A: autumn, W: winter, Sp: spring and S: summer). Only statistically significant pair-wise differences at a p value < 0.05 are shown.

Table A2.
Linear Mixed Model Effects results regarding species density with season (fixed factor) and location (random factor) as predictors.
Table A2.
Linear Mixed Model Effects results regarding species density with season (fixed factor) and location (random factor) as predictors.
| Density of incidental catches | F | p value | Pair-wise LSD | ||
| Fixed effects | Intercept | 120.20 | 0.001 | ||
| Season | 7.18 | 0.001 | AvsW;SpvsW;SvsW | ||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 0.01 | 0 | 0.50 | 0.720 |
| Density of discards | F | pvalue | Pair-wise LSD | ||
| Fixed effects | Intercept | 49.79 | 0.002 | ||
| Season | 3.59 | 0.020 | AvsSp | ||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 617.39 | 573.08 | 1.07 | 0.284 |
| Density of targeted P. narval | F | pvalue | Pair-wise LSD | ||
| Fixed effects | Intercept | 36.15 | 0.004 | ||
| Season | 14.55 | 0.001 | SpvsA;SpvsS;SpvsW | ||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 8157215 | 6287164 | 1.29 | 0.194 |
| Density of Chordata | F | pvalue | Pair-wise LSD | ||
| Fixed effects | Intercept | 10.26 | 0.033 | ||
| Season | 7.85 | 0.001 | AvsSp;AvsS;SpvsA;SpvsW;SvsW | ||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 15946.01 | 12425.47 | 1.28 | 0.199 |
| Density of Echinodermata | F | pvalue | Pair-wise LSD | ||
| Fixed effects | Intercept | 11.01 | 0.002 | ||
| Season | 6.63 | 0.001 | All comparisons were statistically significant | ||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 0.002 | 0 | 5.05 | 0.456 |
| Density of Arthropoda | F | pvalue | Pair-wise LSD | ||
| Fixed effects | Intercept | 16.531 | 0.015 | ||
| Season | 2.104 | 0.112 | |||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 3037.74 | 2890.31 | 1.05 | 0.293 |
| Density of Mollusca | F | pvalue | Pair-wise LSD | ||
| Fixed effects | Intercept | 4.20 | 0.106 | ||
| Season | 0.78 | 0.510 | |||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 75.020 | 294.52 | 0.255 | 0.799 |
Pairwise comparisons among the fixed factor levels are based on estimated marginal means and least significant difference (LSD) (A: autumn, W: winter, Sp: spring, S: summer). Only statistically significant pair-wise differences at a p value < 0.05 are shown.

Table A3.
Linear Mixed Model Effects results regarding species biomass with season (fixed factor) and location (random factor) as predictors.
Table A3.
Linear Mixed Model Effects results regarding species biomass with season (fixed factor) and location (random factor) as predictors.
| Biomass of incidental catch | F | p value | Pair-wise LSD | ||
| Fixed effects | Intercept | 103.83 | 0.0001 | ||
| Season | 4.36 | 0.008 | WvsA; WvsSp; WvsS | ||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 7054.81 | 1396.92 | 5.050 | 0.0001 |
| Biomass of discards | F | pvalue | Pair-wise LSD | ||
| Fixed effects | Intercept | 103.89 | 0.001 | ||
| Season | 2.189 | 0.101 | |||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 0.005 | 0.001 | 5.050 | 0.523 |
| Biomass of Chordata | F | pvalue | Pair-wise LSD | ||
| Fixed effects | Intercept | 61.61 | 0.001 | ||
| Season | 6.10 | 0.001 | SpvsA;SpvsW;SpvsS;WvsS | ||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 841589.48 | 1189576 | 0.707 | 0.479 |
| Biomass of Echinodermata | F | pvalue | Pair-wise LSD | ||
| Fixed effects | Intercept | 10.54 | 0.002 | ||
| Season | 5.90 | 0.002 | WvsA;WvsSp;WvsS | ||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 4463.2 | 838.8 | 5.050 | 0.254 |
| Biomass of Arthropoda | F | pvalue | Pair-wise LSD | ||
| Fixed effects | Intercept | 43.07 | 0.003 | ||
| Season | 11.89 | 0.001 | SpvsA;SpvsS;SpvsW | ||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 258835.17 | 201202.1 | 1.286 | 0.198 |
| Biomass of Mollusca | F | pvalue | Pair-wise LSD | ||
| Fixed effects | Intercept | 31.14 | 0.001 | ||
| Season | 1.96 | 0.131 | |||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 1146.18 | 226.97 | 5.040 | 0.524 |
| Biomass of targeted P. narval | F | pvalue | Pair-wise LSD | ||
| Fixed effects | Intercept | 37.54 | 0.004 | ||
| Season | 17.01 | 0.001 | AvsSp,AvsS;SpvsS;SpvsW;SvsA | ||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 39079083 | 2992340 | 1.306 | 0.192 |
Pair—wise comparisons among the fixed factor levels are based on estimated marginal means and least significant difference (LSD) (A: autumn, W: winter, Sp: spring, S: summer). Only statistically significant pair-wise differences at a p value < 0.05 are shown.

Table A4.
Linear Mixed Model Effects results regarding biomass ratio of bycatch to target species and revenues of the catch with season (fixed factor) and location (random factor) as predictors.
Table A4.
Linear Mixed Model Effects results regarding biomass ratio of bycatch to target species and revenues of the catch with season (fixed factor) and location (random factor) as predictors.
| Biomass ratio of bycatch to target species | F | p value | Pair-wise LSD | ||
| Fixed effects | Intercept | 54.74 | 0.001 | ||
| Season | 51.00 | 0.205 | |||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 0.11 | 0.02 | 5.04 | 0.351 |
| Revenus | F | pvalue | Pair-wise LSD | ||
| Fixed effects | Intercept | 149.03 | 0.001 | ||
| Season | 8.12 | 0.001 | SpvsA;SpvsW; WvsA; WvsS | ||
| Estimate | SE | Wald Z | pvalue | ||
| Random effects | Location | 9.79 | 69.52 | 0.14 | 0.888 |
Pair—wise comparisons among the fixed factor levels are based on estimated marginal means and least significant difference (LSD) (A: autumn, W: winter, Sp: spring, S: summer). Only statistically significant pair-wise differences at a p value < 0.05 are shown.

Table A5.
Estimates of fixed effects (winter was omitted by the method as redundant variable).
Table A5.
Estimates of fixed effects (winter was omitted by the method as redundant variable).
| Estimate | SE | p Value | 95% CI | |
| Number of incidentally caught species | ||||
| Intercept | 2.00 | 0.58 | 0.001 | 0.81, 3.18 |
| Autumn | 2.53 | 0.74 | 0.001 | 1.02, 4.03 |
| Spring | 4.33 | 0.74 | 0.001 | 2.82, 5.83 |
| Summer | 3.86 | 0.74 | 0.001 | 2.36, 5.37 |
| Number of discarded species | ||||
| Intercept | 6.60 | 0.80 | 0.001 | 4.89, 8.30 |
| Autumn | −0.60 | 0.83 | 0.477 | −2.28, 1.08 |
| Spring | 1.33 | 0.83 | 0.117 | −0.34, 3.01 |
| Summer | −0.86 | 0.83 | 0.305 | −2.54, 0.81 |
| Number of Chordata species | ||||
| Intercept | 1.60 | 0.68 | 0.023 | 0.23, 0.96 |
| Autumn | 2.73 | 0.87 | 0.003 | 0.96, 4.49 |
| Spring | 5.60 | 0.87 | 0.001 | 3.83, 7.36 |
| Summer | 4.60 | 0.87 | 0.001 | 2.83, 6.36 |
| Number of Echinodermata species | ||||
| Intercept | 2.70 | 0.25 | 0.001 | 2.18, 3.21 |
| Autumn | −1.16 | 0.28 | 0.001 | −1.74, −0.58 |
| Spring | −0.83 | 0.28 | 0.001 | −1.41, −0.25 |
| Summer | −1.43 | 0.28 | 0.001 | −2.01, −0.85 |
| Density of incidental catches | ||||
| Intercept | 8.10 | 6.24 | 0.200 | −4.43, 20.63 |
| Autumn | 20.30 | 8.06 | 0.015 | 4.11, 36.48 |
| Spring | 34.30 | 8.06 | 0.001 | 18.11, 50.48 |
| Summer | 31.56 | 8.06 | 0.001 | 15.38, 47.74 |
| Density of discards | ||||
| Intercept | 95.40 | 18.26 | 0.001 | 56.57, 134.22 |
| Autumn | −32.93 | 18.72 | 0.085 | −70.59, 4.72 |
| Spring | 21.33 | 18.72 | 0.260 | -16.32, 58.99 |
| Summer | −9.73 | 18.72 | 0.606 | −47.39, 27.92 |
| Density of targeted P. narval | ||||
| Intercept | 6440.30 | 1560.83 | 0.004 | 2791.92, 10088 |
| Autumn | −239.23 | 1158.14 | 0.837 | −2569, 2090.65 |
| Spring | 5809.70 | 1158.14 | 0.001 | 3479.81, 8139.58 |
| Summer | 779.23 | 1158.14 | 0.504 | −1550.65, 3109.11 |
| Density of Chordata | ||||
| Intercept | 103.10 | 70.50 | 0.182 | −59.87, 266.07 |
| Autumn | 5.43 | 54.49 | 0.921 | −104.19, 115.06 |
| Spring | 205.30 | 54.49 | 0.001 | 95.66, 314.93 |
| Summer | 137.36 | 54.49 | 0.015 | 27.73, 246.99 |
| Density of Echinodermata | ||||
| Intercept | 47.80 | 9.32 | 0.001 | 29.08, 66.51 |
| Autumn | −46.53 | 12.03 | 0.001 | −70.70, −22.36 |
| Spring | −43.93 | 12.03 | 0.001 | −68.10, −19.76 |
| Summer | −47.13 | 12.03 | 0.001 | −71.30, −22.96 |
| Density of Mollusca | ||||
| Intercept | 0.40 | 19.44 | 0.984 | −38.88, 39.68 |
| Autumn | 24.60 | 24.60 | 0.323 | −24.89, 74.09 |
| Spring | 13.29 | 24.60 | 0.592 | −36.23, 62.76 |
| Summer | 35.33 | 24.60 | 0.158 | −14.16, 84.83 |
| Biomass of incidental catches | ||||
| Intercept | 1385.40 | 839.88 | 0.105 | −300.75, 3071.54 |
| Autumn | 2871.40 | 1084.28 | 0.110 | 694.60, 5048.20 |
| Spring | 3862.20 | 1084.28 | 0.001 | 1685.39, 6039.00 |
| Summer | 2548.26 | 1084.28 | 0.023 | 371.46, 4725.06 |
| Biomass of Discards | ||||
| Intercept | 1166.51 | 284.66 | 0.001 | 595.03, 1737.99 |
| Autumn | −206.48 | 367.49 | 0.577 | −944.26, 531.29 |
| Spring | 588.96 | 367.49 | 0.115 | −148.80, 1326.74 |
| Summer | −22.94 | 367.49 | 0.950 | −760.71, 714.83 |
| Biomass of Chordata | ||||
| Intercept | 2182.84 | 1036.41 | 0.044 | 64.00, 4301.67 |
| Autumn | 1779.89 | 1228.70 | 0.154 | −691.94, 4251.73 |
| Spring | 5022.55 | 1228.70 | 0.001 | 2550.71, 7494.39 |
| Summer | 2724.02 | 1228.70 | 0.031 | 252.18, 5195.86 |
| Biomass of Echinodermata | ||||
| Intercept | 325.42 | 66.80 | 0.001 | 191.40, 459.64 |
| Autumn | −317.09 | 86.24 | 0.001 | −490.24, −143.94 |
| Spring | −287.46 | 86.24 | 0.002 | −460.61, −114.31 |
| Summer | −321.82 | 86.24 | 0.001 | −494.97, −148.67 |
| Biomass of Arthropoda | ||||
| Intercept | 1266.52 | 282.73 | 0.002 | 611.45, 1921,59 |
| Autumn | −31.25 | 216.68 | 0.886 | −467.15, 404.65 |
| Spring | 1006.07 | 216.68 | 0.001 | 570.17, 1441.98 |
| Summer | 229.19 | 216.68 | 0.296 | −207.71, 665.10 |
| Biomass of Mollusca | ||||
| Intercept | 395.00 | 338.55 | 0.249 | −284.67, 1074.67 |
| Autumn | 965.12 | 437.07 | 0.320 | −87.67, 1842.58 |
| Spring | 230.50 | 437.07 | 0.600 | −646.95, 1107.95 |
| Summer | 496.84 | 437.07 | 0.261 | −380.60, 1374.30 |
| Biomass ratio of bycatch to targeted P. narval | ||||
| Intercept | 0.22 | 0.10 | 0.041 | 0.09, 0.43 |
| Autumn | 0.27 | 0.13 | 0.054 | −0.05; 0.54 |
| Spring | 0.06 | 0.13 | 0.656 | −0.21; 0.33 |
| Summer | 0.13 | 0.13 | 0.315 | −0.13; 0.41 |
| Revenues | ||||
| Intercept | 20.62 | 9.46 | 0.035 | 1.51, 39.74 |
| Autumn | 30.21 | 12.08 | 0.016 | 5.88, 54.53 |
| Spring | 58.80 | 12.08 | 0.001 | 34.47, 83.12 |
| Summer | 39.33 | 12.25 | 0.002 | 14.66, 64.00 |
References
- Shester, G.G.; Micheli, F. Conservation challenges for small-scale fisheries: Bycatch and habitat impacts of traps and gillnets. Biol. Conserv. 2011, 144, 1673–1681. [Google Scholar] [CrossRef]
- Anger, K.; Moreira, G. Morphometric and reproductive traits of tropical Caridean shrimps. J. Crustacean Biol. 1998, 18, 823–838. [Google Scholar] [CrossRef]
- Ye, Y.; Alsaffar, A.H.; Mohammed, H.M.A. Bycatch and discards of the Kuwait shrimp fishery. Fish. Res. 2000, 45, 9–19. [Google Scholar] [CrossRef]
- Pauly, D.; Zeller, D. The best catch data that can possibly be? Rejoinder to Ye et al. “FAO’s statistic data and sustainability of fisheries and aquaculture. Mar. Policy 2017, 81, 406–410. [Google Scholar] [CrossRef]
- Pauly, D.; Zeller, D. Comments on FAOs State of World Fisheries and Aquaculture (SOFIA 2016). Mar. Policy 2017, 77, 176–181. [Google Scholar] [CrossRef]
- Popov, S.; Zeller, D. Reconstructed Russian Fisheries Catches in the Barents Sea: 1950–2014. Front. Mar. Sci. 2018, 5, 266. [Google Scholar] [CrossRef]
- Tzanatos, E.; Somarakis, S.; Tserpes, G.; Koutsikopoulos, C. Discarding practices in a Mediterranean small-scale fishing fleet (Patraikos Gulf, Greece). Fish. Manag. Ecol. 2007, 14, 277–285. [Google Scholar] [CrossRef]
- Tsagarakis, K.; Palialexis, A.; Vassilopoulou, V. Mediterranean fishery discards: Review of the existing knowledge. ICES J. Mar. Sci. 2013, 71, 1219–1234. [Google Scholar] [CrossRef]
- Zeller, D.; Cashion, T.; Palomares, M.; Pauly, D. Global marine fisheries discards: A synthesis of reconstructed data. Fish Fish. 2018, 19, 30–39. [Google Scholar] [CrossRef]
- Davies, R.W.D.; Cripps, S.J.; Nickson, A.; Porter, G. Defining and estimating global marine fisheries bycatch. Mar. Policy 2009, 33, 661–672. [Google Scholar] [CrossRef]
- Lewison, R.L.; Crowder, L.B.; Read, A.J.; Freeman, S.A. Understanding impacts of fisheries bycatch on marine megafauna. Trends Ecol. Evol. 2004, 19, 598–604. [Google Scholar] [CrossRef]
- Karris, G.; Ketsilis-Rinis, V.; Kalogeropoulou, A.; Xirouchakis, S.; Machias, A.; Maina, I.; Kavadas, S. The use of demersal trawling discards as a food source for two scavenging seabird species: A case study of an eastern Mediterranean oligotrophic marine ecosystem. Avian Res. 2018, 9, 26. [Google Scholar] [CrossRef]
- Pascoe, S. Bycatch Management and the Economics of Discarding; FAO: Rome, Italy, 1997; p. 137. [Google Scholar]
- Vasilakopoulos, P.; Maravelias, C.D.; Tserpes, G. The Alarming Decline of Mediterranean Fish Stocks. Curr. Biol. 2014, 24, 1643–1648. [Google Scholar] [CrossRef]
- Hall, S.J.; Mainprize, B.M. Managing by-catch and discards: How much progress are we making and how can we do better? Fish Fish. 2005, 6, 134–155. [Google Scholar] [CrossRef]
- FAO. Code of Conduct for Responsible Fisheries; FAO: Rome, Italy, 1995; 41p. [Google Scholar]
- Garcia, S.M.; Zerbi, A.; Aliaume, C.; Do Chi, T.; Lasserre, G. The Ecosystem Approach to Fisheries. Issues, Terminology, Principles, Institutional Foundations, Implementation and Outlook; FAO Fisheries Technical Paper; FAO: Rome, Italy, 2003; 71p. [Google Scholar]
- FAO. Report of the Technical Consultation to Develop International Guidelines on Bycatch Management and Reduction of Discards; FAO: Rome, Italy, 2010; 32p. [Google Scholar]
- Rochet, M.-J.; Catchpole, T.; Cadrin, S. Bycatch and discards: From improved knowledge to mitigation programmes. ICES J. Mar. Sci. 2014, 71, 1216–1218. [Google Scholar] [CrossRef]
- Guyader, O.; Berthou, P.; Koutsikopoulos, C.; Alban, F.; Demanèche, S.; Gaspar, M.B.; Eschbaum, R.; Fahy, E.; Tully, O.; Reynal, L.; et al. Small scale fisheries in Europe: A comparative analysis based on a selection of case studies. Fish. Res. 2013, 140, 1–13. [Google Scholar] [CrossRef]
- Lleonart, J.; Maynou, F. Fish stock assessments in the Mediterranean: State of the art. Sci. Mar. 2003, 67, 37–49. [Google Scholar] [CrossRef]
- Vasilakopoulos, P.; Maravelias, C.D.; Anastasopoulou, A.; Kapiris, K.; Smith, C.J.; Kalogirou, S. Premium small scale: The trap fishery for Plesionika narval (Decapoda, Pandalidae) in the eastern Mediterranean Sea. Hydrobiologia 2019, 826, 279–290. [Google Scholar] [CrossRef]
- Kalogirou, S.; Anastasopoulou, A.; Kapiris, K.; Maravelias, C.D.; Margaritis, M.; Smith, C.; Pihl, L. Spatial and temporal distribution of narwal shrimp Plesionika narval (Decapoda, Pandalidae) in the Aegean Sea (eastern Mediterranean Sea). Reg. Stud. Mar. Sci. 2017, 16, 240–248. [Google Scholar] [CrossRef]
- Kalogirou, S.; Pihl, L.; Maravelias, C.D.; Herrmann, B.; Smith, C.J.; Papadopoulou, N.; Notti, E.; Sala, A. Shrimp trap selectivity in a Mediterranean small-scale-fishery. Fish. Res. 2019, 211, 131–140. [Google Scholar] [CrossRef]
- González, J.A.; Tuset, V.M.; Lozano, I.J.; Santana, J.I. Biology of Plesionika narval (Crustacea, Decapoda, Pandalidae) Around the Canary Islands (Eastern Central Atlantic). Estuar. Coast. Shelf Sci. 1997, 44, 339–350. [Google Scholar] [CrossRef]
- Pantazi, V.; Mannini, A.; Vasilakopoulos, P.; Kapiris, K.; Megalofonou, P.; Kalogirou, S. That’s All I Know: Inferring the Status of Extremely Data-Limited Stocks. Front. Mar. Sci. 2020, 7, 904. [Google Scholar] [CrossRef]
- Bordbar, L.; Kapiris, K.; Kalogirou, S.; Anastasopoulou, A. First evidence of ingested plastics by a high commercial shrimp species (Plesionika narval) in the eastern Mediterranean. Mar. Pollut. Bull. 2018, 136, 472–476. [Google Scholar] [CrossRef]
- Bordbar, L.; Kapiris, K.; Anastasopoulou, A.; Maravelias, C.D.; Smith, C.J.; Voutsinas, E.; Kalogirou, S. Diet composition and temporal changes in the trophic patterns of Plesionika narval (Crustacea-Decapoda) in the Aegean Sea (Eastern Mediterranean Sea). Reg. Stud. Mar. Sci. 2019, 30, 100739. [Google Scholar] [CrossRef]
- Anastasopoulou, A.; Makantasi, P.; Kapiris, K.; Smith, C.J.; Maravelias, C.; Kalogirou, S. Reproductive biology of Plesionika narval in the SE Aegean Sea (Eastern Mediterranean). Mediterr. Mar. Sci. 2017, 18, 454–467. [Google Scholar] [CrossRef][Green Version]
- FAO. The State of Mediterranean and Black Sea Fisheries. General Fisheries Commission for the Mediterranean; FAO: Rome, Italy, 2018; 172p. [Google Scholar]
- Maravelias, C.D.; Vasilakopoulos, P.; Kalogirou, S. Participatory management in a high value small-scale fishery in the Mediterranean Sea. ICES J. Mar. Sci. 2018, 75, 2097–2106. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2018-2. 2019. Available online: https://www.iucnredlist.org/species/104651572/104651577 (accessed on 4 March 2019).
- Nakagawa, S.; Schielzeth, H. Repeatability for Gaussian and Non-Gaussian data: A practical guide for biologists. Biol. Rev. 2010, 85, 935–956. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational multivariate analysis of variance (PERMANOVA). In StatsRef: Statistics Reference Online; Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J.L., Eds.; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; PRIMER-E Ltd.: Plymouth, UK, 2006. [Google Scholar]
- Arrasate-López, M.; Tuset, V.M.; Santana, J.I.; Garcia-Mederos, A.; Ayza, O.; González, J.A. Fishing methods for sustainable shrimp fisheries in the Canary Islands (North-West Africa). Afr. J. Mar. Sci. 2012, 34, 331–339. [Google Scholar] [CrossRef]
- Pajuelo, J.G.; Triay-Portella, R.l.; Delgado, J.; Góis, A.R.; Correia, S.; Martins, A.; González, J.A. Changes in catch and bycatch composition and in species diversity of a semi-floating shrimp-trap fishery in three eastern Atlantic island ecosystems with different degrees of human alteration. Sci. Mar. 2018, 82, 107–114. [Google Scholar] [CrossRef]
- Lobo, A.S.; Balmford, A.; Arthur, R.; Manica, A. Commercializing bycatch can push a fishery beyond economic extinction. Conserv. Lett. 2010, 3, 277–285. [Google Scholar] [CrossRef]
- Sousa, R.; Pinho, M.R.; Delgado, J.; Biscoito, M.; Pinto, A.R.; Dellinger, T.; Gouveia, L.; Carvalho, D.; Henriques, P. Prospective study of the fishery of the shrimp Plesionika narval (Fabricius, 1787) in the Northeastern Atlantic. Braz. J. Biol. 2017, 77, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Horsten, M.B.; Kirkegaard, E. Bycatch from a Perspective of Sustainable Use; IUCN SSC European Sustainable Use Specialist Group: Fisheries Working Group; IUCN: Gland, Switzerland, 2003; pp. 1–16. [Google Scholar]
- Arculeo, M.; Brutto, S.L. Growth and reproduction data of Plesionika narval (Decapoda, Caridea, Pandalidae) off the Island of Ustica (southern Tyrrhenian Sea). Crustaceana 2011, 84, 1367–1375. [Google Scholar] [CrossRef]
- Castriota, L.; Falautano, M.; Romeo, T.; Florio, J.; Pelusi, P.; Finoia, M.G.; Andaloro, F. Crustacean fishery with bottom traps in an area of the southern Tyrrhenian Sea: Species composition, abundance and biomass. Mediterr. Mar. Sci. 2004, 5, 15–22. [Google Scholar] [CrossRef]
- Eichert, M.; Campos, A.; Fonseca, P.; Henriques, V.; Castro, M. Preliminary results on the use of semi-floating shrimp traps for the striped soldier shrimp, Plesionika edwardsii (Crustacea: Decapoda: Pandalidae), off the Algarve coast (southern Portugal). Sci. Mar. 2018, 82, 209–214. [Google Scholar] [CrossRef]
- FAO. Mapping of SSF Initiatives. Available online: https://maritime-spatial-planning.ec.europa.eu/events/mapping-small-scale-fisheries-initiatives (accessed on 1 September 2021).
- Chapman, C.J.; Shelton, P.M.J.; Shanks, A.M.; Gaten, E. Survival and growth of the Norway lobster Nephrops norvegicus in relation to light-induced eye damage. Mar. Biol. 2000, 136, 233–241. [Google Scholar] [CrossRef]
- Bergmann, M.; Moore, P.G. Survival of decapod crustaceans discarded in the Nephrops fishery of the Clyde Sea area, Scotland. ICES J. Mar. Sci. 2001, 58, 163–171. [Google Scholar] [CrossRef][Green Version]
- Donnan, D. Effects of fishing on non-target species and habitats: Biological, conservation and socio-economic issues. Aquat. Conserv. Mar. Freshw. Ecosyst. 2001, 11, 488. [Google Scholar] [CrossRef]
- Long, R.D.; Charles, A.; Stephenson, R.L. Key principles of marine ecosystem-based management. Mar. Policy 2015, 57, 53–60. [Google Scholar] [CrossRef]
- Morato, T.; Solà, E.; Grós, M.; Menezes, G. Diets of forkbeard (Phycis phycis) and conger eel (Conger conger) off the Azores during spring of 1996 and 1997. Arquipél 1999, 17, 51–64. [Google Scholar]
- Papaconstantinou, C.; Caragitsou, E. Feeding interaction between two sympatric species Pagrus pagrus and Phycis phycis around Kastellorizo Island (Dodecanese, Greece). Fish. Res. 1989, 7, 329–342. [Google Scholar] [CrossRef]
- Ajana, R.; Techetach, M.; Saoud, Y. Diet of Octopus vulgaris from the Moroccan Mediterranean Coast. Thalass. Int. J. Mar. Sci. 2018, 34, 415–420. [Google Scholar] [CrossRef]
- Silva, A.R.; Vieira, A.R.; Sequeira, V.; Paiva, R.B.; Gordo, L.S.; Neves, A. Diet and feeding strategy of the forkbeard Phycis phycis (Pisces: Phycidae) from the Portuguese continental coast. J. Mar. Biol. Assoc. U. K. 2017, 98, 1–9. [Google Scholar] [CrossRef]
- Ambrose, R.F.; Nelson, B.V. Predation by Octopus vulgaris in the Mediterranean. Mar. Ecol. 1983, 4, 251–261. [Google Scholar] [CrossRef]
- Suuronen, P.; Chopin, F.; Glass, C.; Løkkeborg, S.; Matsushita, Y.; Queirolo, D.; Rihan, D. Low impact and fuel efficient fishing—Looking beyond the horizon. Fish. Res. 2012, 119–120, 135–146. [Google Scholar] [CrossRef]
- Kroodsma, D.A.; Mayorga, J.; Hochberg, T.; Miller, N.A.; Boerder, K.; Ferretti, F.; Wilson, A.; Bergman, B.; White, T.D.; Block, B.A.; et al. Tracking the global footprint of fisheries. Science 2018, 359, 904–908. [Google Scholar] [CrossRef]
- Vasilakopoulos, P.; Maravelias, C.D. A tale of two seas: A meta-analysis of crustacean stocks in the NE Atlantic and the Mediterranean Sea. Fish Fish. 2016, 17, 617–636. [Google Scholar] [CrossRef]
- FAO. Voluntary Guidelines for Securing Sustainable Small-Scale Fisheries in the Context of Food Security and Poverty Eradication; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; 20p. [Google Scholar]
- Malta Medfish4Ever Declaration. Malta MedFish4Ever Ministerial Declaration Strengthening Fisheries Governance in the Mediterranean. Available online: https://ec.europa.eu/fisheries/sites/fisheries/files/2017–03–30-declaration-malta.pdf (accessed on 7 April 2017).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).