Abstract
Ethnobotany has been, for too long, a descriptive discipline. However, ethnobotanists are increasingly calling for a paradigm shift towards the formulation of unifying theories and hypothesis-driven research in ethnobotany. Here, we formulated a theory, termed time-since-introduction theory, to explain the integration of alien plants into local pharmacopoeias in their recipient environment. This theory suggests that the factor time is paramount in determining which alien plants are more likely to be included in the medicinal flora of the areas they are introduced in. The theory relies on three hypotheses, the availability and versatility hypotheses alongside the residence time hypothesis newly proposed in the present study. We tested this theory by fitting a structural equation model to ethnobotanical data collected on South Africa’s alien woody flora. Although residence time is a direct predictor of the medicinal status of alien plants, it is a better predictor when mediated through plant versatility. These findings are in support of the theory, and we consequently proposed a framework that can be used to understand different paths linking all three hypotheses. Collectively, our study shows the value of time in the development of ethnobotanical knowledge and fully responds to the pressing call for a paradigm shift in ethnobotany.
1. Introduction
Originally, ethnobotany referred to the study of the traditional utilitarian relationship between humans and plant resources, focusing on how and which plants are used for and by humans [1]. Ethnobotany has now branched out of this restrictive view to include a broader human–plant interaction beyond a utilitarian relationship, e.g., the symbolic, ecological, and cognitive relationship [2,3]. Most ethnobotanical studies are interested in documenting people’s knowledge of useful plants to humans and what these plants are used for [4,5]. Increasingly, more authors are now calling for the vast wealth of ethnobotanical knowledge documented over centuries to be used to drive hypothesis-inspired and theory-driven studies [6,7,8,9,10,11,12,13,14,15,16], a common practice in any scientific discipline. Some authors even questioned whether ethnobotany, as a scientific discipline, has a unifying theory since the discipline has remained, for too long, largely descriptive without clearly defined theoretical frameworks [16,17,18,19]. Consequently, there has been repeated calls for a paradigm shift [15].
While a hypothesis is a preliminary proposed explanation for an observation, a theory is an “integrated and hierarchical set of empirical hypotheses that together explain a significant fraction of a scientific observation” [20]. Here, we proposed a theory, termed time-since-introduction theory to explain the integration of alien plants into local medicinal flora. This theory integrates three hypotheses, two of which are already known in ethnobotany—the availability [18,19] and versatility hypotheses [21,22]—and a third hypothesis newly defined in the present study, that is, the residence time hypothesis.
The availability hypothesis suggests that easily accessible or locally abundant alien plants are more likely to be used for medicine in their invaded regions [18,19], and evidence for this has also been shown for native species (e.g., [10,23]). The versatility hypothesis predicts that alien plants that have multiple non-medicinal uses for humans (food, ornamental, etc.) are more likely to be eventually used for medicine in their invaded regions [22,24]. Although these two hypotheses were supported in many studies [15,23], they did not account explicitly for the factor time in the development of the medicinal knowledge of alien species, although the development of medicinal knowledge is time-dependent, i.e., it follows a dynamic process over time (e.g., [25]) shaped by the environment [8]. Consequently, we propose a new hypothesis—termed the residence time hypothesis—that explicitly highlights the factor time in the development of ethnobotanical knowledge. We formulate this hypothesis as follows: the residence time hypothesis predicts that the longer the residence time of an alien plant in a new environment, the more likely it is for the plant to be integrated into local medicinal flora. Although reference [25] did not explicitly define the residence time hypothesis, their study already evoked the value of the factor time in the development of traditional medicinal knowledge: the longer people are surrounded by certain plants, the more time for trial, error, and communication with other people, the more likely they are to use the plant as medicine, and the more likely the medicine is effective.
The aim of the present study is to propose a theoretical framework that integrates several hypotheses to shed light on how medicinal knowledge is developed on alien plants in their invaded environment. Alien plants are one of the major threats driving the ongoing biodiversity crisis [26], and global efforts are devoted to fighting these threats, particularly in South Africa where tremendous financial resources have been spent on alien species control, unfortunately, with mixed success [27]. However, since humans, in general, select and move these alien species around, there is a social dimension to biodiversity conservation [28], including the dynamic of alien species populations and their control that cannot be ignored if we are to be successful. It is impossible for socially driven environmental problems to be solved effectively if provided solutions are not understood, adopted, and implemented by and with local people [29]. The question now is: how could ethnobotanical knowledge be valuable in conservation science [30] if we have a poor or limited understanding of the mechanisms driving plant selection and use by local people? These mechanisms behind plant selection and use can be understood only if ethnobotanists use the wealth of descriptive plant use data documented over centuries to conduct hypothesis- and theory-driven studies [15,16,19].
In the present study, we aim to demonstrate how the three hypotheses (availability, versatility, and residence time hypotheses) could be integrated into a single theoretical framework termed time-since-introduction theory to explain the integration of alien plants, outside their native ranges, into local pharmacopoeias. To illustrate our theoretical framework, we used the alien woody flora of South Africa, a country well-known for the incredible richness of its native flora (24,000 vascular plants and three biodiversity hotspots [31]) as a model. A complete list of alien woody plants as well as their years of introduction into South Africa was compiled (Table S1), allowing us to determine their residence time from the year of introduction of the plant species into South Africa to the year 2018 where the statistical analysis reported in the present study was conducted. In addition, several variables pertaining to ethnobotanical knowledge were also collected (Table S1): total uses recorded for each alien plant (medicinal and non-medicinal uses), its medicinal status (a binary variable defined as non-medicinal versus medicinal), number of recipes reported for the alien plants, number of plant organs used medicinally, and the availability of the alien plant.
2. Materials and Methods
2.1. Study Area
The test of time-since-introduction theory was based on South Africa’s alien flora. South Africa is found at the southern tip of Africa stretching from 22° S to 35° S in latitude and 17° E to 33° E in longitude. Its neighboring countries are Namibia, Zimbabwe, Mozambique, and Botswana (Figure 1). South Africa is well-known for its megadiverse flora as well as its diverse cultural groups with an incredible wealth of medicinal knowledge [31].
Figure 1.
Location of South Africa in relation to its neighboring countries with a highlight of the diversity of regional biomes.
2.2. Data Collection
2.2.1. South Africa’s Alien Woody Flora and Residence Time
Our dataset of alien woody plants and their years of introduction to South Africa was obtained from a previously published study by reference [32]. The dataset represents the most comprehensive checklist of alien woody species, combining datasets of references [33,34] and reference [35] with local experts’ knowledge from all major research institutions and academic centers working on alien plants in South Africa [32]. In total, our dataset represents 210 alien woody plants (Table S1) used for various hypothesis testings in our research group (e.g., [23,36]).
2.2.2. Availability, Versatility, and Medicinal Use Data of Alien Woody Plants
The occurrence of each alien species was documented using various resources, but mainly the Global Biodiversity Information Facility [37] and regional and online local flora databases and ethnobotanical publications [38]; Flora of Botswana, http://www.botswanaflora.com/ accessed on 27 January 2017; Flora of Mozambique, http://www.mozambiqueflora.com, accessed on 27 January 2017; Flora of Southern Africa, http://posa.sanbi.org.www57.cpt1.host-h.net/flora/browse.php?src=FloraSA accessed on 5 February 2018; Flora of Zimbabwe, http://www.zimbabweflora.co.zw/, accessed on 27 January 2017; Trees Atlas of Namibia, http://treeatlas.biodiversity.org.na/ accessed on 27 January 2017; Prelude Database for Medicinal Plants in Africa, http://www.africamuseum.be/en/research/collections_libraries/biology/prelude, accessed on 27 January 2017. This information is available in Table S1.
We documented through an intensive literature search the different services these species provide to humans in southern Africa. First, we used the Web of Science (WoS) to retrieve existing scientific ethnobotanical studies in the region. Second, we performed search for each species by using combinations of keywords such as “scientific name of species”, “South Africa”, “uses”, “usages”, “utilisation” and “benefit”. We also made use of Google and Google Scholar for scientific and grey literature using similar keywords to retrieve online resources such as regional and country-specific journals, proceedings, technical reports, herbarium and commercial websites informing on the uses of woody plants in our dataset. The Southern African Plant Invaders Atlas (http://www.agis.agric.za/wip/, accessed on 1 March 2017) was also consulted. In addition, we consulted key books on the regional flora such as Trees of Southern Africa, Field Guide to Trees of Southern Africa, and Guide to Trees Introduced into Southern Africa [35,38,39]. Additionally, the Prelude Database for Medicinal Plants in Africa (http://www.africamuseum.be/collections/external/prelude; accessed on 10 February 2017) was also consulted. All the different uses retrieved from this wide and intensive literature search were grouped into 12 distinct categories of services ranging from medicinal use to ornamental and spiritual use (Table S1). From all these sources, further information on the number of recipes and organs used to treat specific ailments were retrieved. Documented information on the number of recipes and organs used by identified medicinal alien woody plants is found in Table S1. Number of organs are total numbers of organs or parts of the plant used medicinally. For analysis purpose, the medicinal status was coded as 0 (non-medicinal) and 1 (medicinal).
2.3. Data Analysis
The relationships between all variables were tested by fitting a structural equation model (SEM) using the function psem implemented in the R package piecewise-SEM [40]. An SEM is a multivariate and powerful technique used to test and evaluate a theoretical framework, that is, a multivariate causal relationship [41]. The benefit of an SEM is that various causal relationships can be defined and tested simultaneously.
Five statistical models were included in the SEM, and each of them was grounded on the following rationale. First, the variable availability was modelled as a function of residence time, assuming that early introduced alien species (longer residence time) would be more available to local people than those recently introduced. Second, the variable number of recipes was modelled as a function of medicinal status (i.e., for a species to have a medicinal recipe, it has to be first and foremost medicinal), availability (i.e., available species are more likely to be used for medicine as predicted by availability hypothesis), residence time (i.e., the longer the residence time of an alien plant, the more medicinal recipes will be developed for that species by local people). Third, we modelled “medicinal status” as a function of residence time (residence time hypothesis), availability (availability hypothesis), and total uses (i.e., versatility hypothesis). Fourth, the variable “total uses” was modelled as a function of residence time and availability, assuming that the longer the residence time, the more available the alien species and the more uses (e.g., medicinal, food, horticultural uses, etc.) will be established by the local people. Finally, the fifth model included in the SEM was developed for the variable “number organs used” as a function of medicinal status, the number of recipes, residence time, and total uses. This fifth model is based on the assumption that the use of several different organs medicinally means (i) the plant is medicinal (medicinal status), (ii) it has more recipes that involve several organs (number of recipes), (iii) the plant has been around for a long enough time (residence time) so that multiple uses have been established for it in the local community (total uses).
The adequacy of the SEM fitted was assessed using its overall Goodness-of-fit (C value) and the p-value. The parameter C is indicative of whether the SEM is good enough to explain the data such that the lower the C value the better the SEM for the data. The p-value here is interpreted as follows: p < 0.05 means the SEM departs significantly from the best fit and therefore should be rejected; p > 0.05, on the contrary, means the SEM is no different from the best fit and therefore can be used to explain the data [40,42].
Each of these five models that form the SEM was fitted using the R function glmer.nb (negative binomial generalised linear mixed effect models), except for the model of “medicinal status” for which the R function glmer was used instead. For four of the response variables that are “count data” (availability, number of recipes, total uses, and number of organs used), the negative binomial distribution was used to account for over-dispersion [43], and the binomial error family was used for “medicinal status” since it is a binary variable (medicinal versus non-medicinal). In addition, the shared evolutionary history among South Africa’s alien species [37] justified our use of mixed effect model (GLMER). The R script used for this analysis is provided in the Supplementary Information.
3. Results and Discussion
Our analysis shows that the fitted SEM is an appropriate meta-model (theoretical framework) that explains how medicinal knowledge could relate to residence time and other ethnobotanical variables such as plant availability and versatility (Figure 2; Fisher C = 0.54; df = 4, p = 0.96).

Figure 2.
Theoretical framework (Structural Equation Model) proposed and tested in this study to explain the time-since-introduction theory. Thick black arrows indicate significant relationships, while grey arrows are non-significant relationships. Numbers on arrows are path coefficients. * Indicates level of significance of a relationship at alpha = 0.05; when a relationship is not significant but close to significance level, its value is rather mentioned instead of stars (*). The number of stars (*) indicates the significant levels of a relationship. NS = non-significant.
Specifically, our SEM reveals different paths through which residence time predicts the medicinal status of alien plants (Table 1). Firstly, there is a direct path with a positive significant relationship such that early introduced alien plants tend to be medicinal than not (Figure 3a; β1 = 0.007 ± 0.002, p = 0.014), supporting the residence time hypothesis. Secondly, residence time also predicts medicinal status, but this relationship is mediated through plant availability (residence time → availability (β2.1 = 0.0009 ± 0.001) → medicinal status (β2.2 = 6.6288 ± 0.002)) such that the coefficient for this indirect path is β2 = β1.2 × β2.2 = 0.005965. This availability-mediated relationship between residence time and medicinal status is in support of the availability hypothesis, which predicts that alien species that are more accessible to people or more abundant stand a better chance of being integrated into local medicinal flora than species that are rare or are of a restricted distribution range [21,44]. This hypothesis has been employed to explain the medicinal uses of not only native plants [10] but also the incorporation of alien plants into local pharmacopoeias [11,45]. The metrics of availability of a given plant have been variously defined, including the distance from people’s home to the location of the plants in the wild or the price of the plant in the traditional medicinal market [46]. Recently, the presence/absence of a plant in administratively delimited regions has also been used to define its availability [11]. This recent definition of availability was used in the present study.

Table 1.
Coefficients of all relationships tested in the Structural Equation Model (SEM) that illustrates the time-since-introduction theory. p-values in bold correspond to statistically significant relationships. Two of the relationships were only marginally significant (ms; p < 0.10), but the remaining significant relationships were more so at p < 0.05). The number of stars (*) indicates the significant level of a relationship.
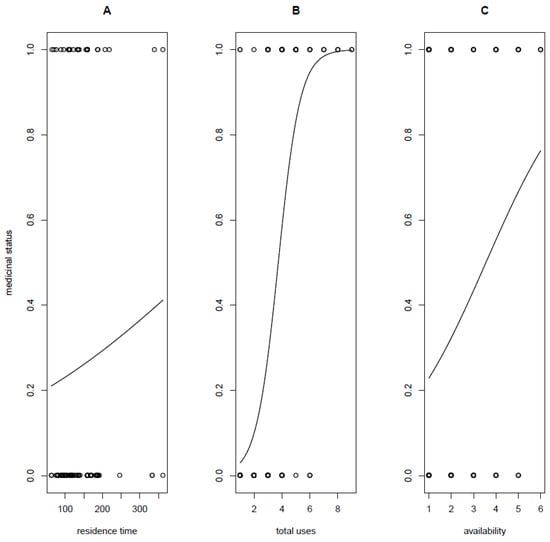
Figure 3.
Significant relationships in the theoretical framework defining the time-since-introduction theory. Relationships between medicinal status and residence time (A), total use (B), and availability (C).
Thirdly, residence time also predicts indirectly the medicinal status, but this relationship is mediated by total uses (residence time → total uses (β3.1 = 0.0016 ± 0.0009)→ medicinal status (Figure 3B; β3.2 = 20.0485 ± 0.0014)) such that the coefficient for this indirect path is β3 = β3.1 × β3.2 = 0.032. This relationship mediated by the total number of uses matches the prediction of the versatility hypothesis, which suggests that alien plants that have multiple uses are more likely to be integrated into local medicinal flora in their new environment [22,23,24]: alien plants can first be introduced into a new geographic region primarily for diverse services including food, construction materials, ornamental, etc., and then used at a later stage for medicinal purposes when the need to fill some therapeutic gaps in local pharmacopoeias arises [11,24,47].
The last path linking residence time to medicinal status is mediated through both availability (Figure 3C) and total uses (residence time → availability (β4.1 = 0.0009 ± 0.001; Figure 4A) → total uses (β4.2 = 0.1155 ± 0.0392; Figure 4B) → medicinal status (β4.3 = 20.0485 ± 0.0014)), but this path shows the lowest coefficient β4 = β4.1 × β4.2 × β4.3= 0.00208. It is therefore clear that the path with the highest coefficient is the indirect path mediated through total uses (β3 > β1 > β2 > β4), suggesting that residence time may directly predict the medicinal status of alien plants but that the most important predictive power of residence time is mediated by plant versatility (see reference [23]).
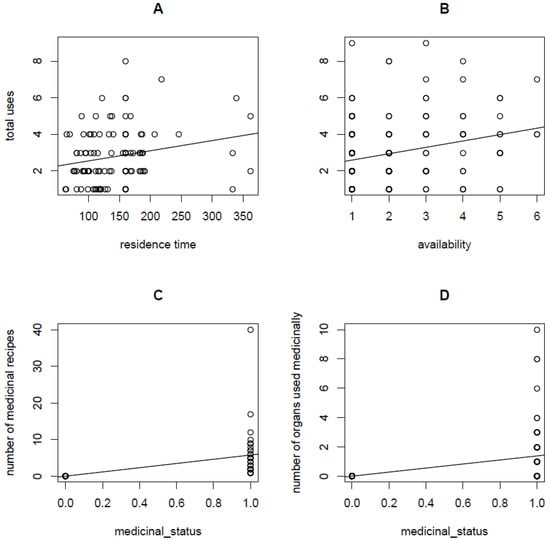
Figure 4.
Additional significant relationships in the theoretical framework defining the time-since-introduction theory. Relationship between total uses and residence time (A), total uses and availability (B), and between number of recipes and medicinal status (C) as well as between number of organs used medicinally and medicinal status (D).
In addition, our SEM analysis indicates that medicinal status correlates with both the number of recipes (i.e., medicinal plants are more likely to have some recipes than not, β = 23.18 ± 2.08, p < 0.001, Figure 4C) and number of organs medicinally useful (β = 23.66 ± 2.95, p < 0.001, Figure 4D), while medicinal status itself is predicted by the total number of uses (versatility hypothesis, β = 20.04 ± 0.001, p < 0.001; [23]). Interestingly, early introduced alien plants to South Africa (longer residence time) tend to have a higher number of uses than late introduced species (shorter residence time), although the relationship is only marginally significant (β = 0.0016 ± 0.0009, p = 0.09). These direct and indirect relationships provide clear evidence of how multiple hypotheses can be integrated to form a unifying theory in ethnobotany, a theory termed, in this study, as time-since-introduction theory.
In response to early calls to formulate testable theories in ethnobotany [18,48], several studies instead developed methodological rigor [49] and ethnobotanical indices [50]. In addition, other studies repeated the call for “less quantification” and more theory-inspired and hypothesis-driven research in ethnobotany [15,51,52,53]. Current challenges and critics faced by ethnobotany define a critical period in the evolution of the discipline, a lengthy trajectory that other sister disciplines went through. In the 1990s, ecology, which is now well-known with several theories, was also questioned as the discipline had no general laws or a unifying theory at the time [54,55,56,57]. Increasingly, ethnobotanists are taking the same trajectory, making use of the wonderful and unique descriptive ethnobotanical data documented for centuries across different geographic regions of the world to formulate and/or test various ethnobotanical hypotheses [6,7,8,9,10,11,12,13,14,19,58,59,60]. It has not been possible to test widely the residence time hypothesis of the introduction of alien plants into local medicinal flora simply because of the lack of data on the times of introduction of these species into their recipient environments. Fortunately, such historical data exist in South Africa and allow us to fill the knowledge gap in the present study. In the context of multiple calls for a paradigm shift, the present study proposes and tests an ethnobotanical theory that integrates multiple hypotheses to explain the integration of alien plants into local medicinal flora. Here, the theory was tested on alien woody plants. We suggest that future studies test the theory on different life forms in different geographical contexts.
4. Conclusions
Ethnobotany is the discipline interested in the interactions of humans with plants. Most ethnobotanical studies have generated tremendous datasets on plant uses across the globe. However, calls to use existing datasets for more hypothesis- and theory-driven studies are now increasing. Here, we formulate, test, and provide evidence for the role the factor time plays in the process of including alien plants into local medicinal flora. We then provide a theoretical framework, termed time-since-introduction theory, that integrates three ethnobotanical hypotheses in support of the theory and call for more studies to test the theory with different datasets across different geographies. The main challenges reside in the fact that data on the introduction dates of alien plants into a new environment are not always documented. This will make the test of the theory less frequent unless we start documenting such data from now on.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14040286/s1, Table S1: All variables collected and analyzed in this study; R script used to analyze the data in Table S1.
Author Contributions
K.Y. conceived and designed the study; A.E.A. collected the data; K.Y. analyzed the data and wrote the paper; H.O.E., A.M.E.-S., A.E.A. and S.S. provided comments on the first draft and further editorial works. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by King Saud University (RSP-2021/118).
Institutional Review Board Statement
Not applicable since data were collected from the literature.
Data Availability Statement
All data analysed in this study are presented in Supplementary Information files.
Acknowledgments
We thank Bezeng S. Bezeng for sharing his PhD data with us on alien invasive species. We also thank three anonymous reviewers for their contributions to improving an earlier draft. The authors extend their deep appreciation to the Researchers Supporting Project (RSP-2021/118), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Harshberger, J.W. The Purposes of Ethno-Botany. Bot. Gaz. 1896, 21, 146–154. [Google Scholar] [CrossRef]
- Alexiades, M.N. Selected Guidelines for Ethnobotanical Research: A Field Manual; The New York Botanical Garden: Bronx, NY, USA, 1996; Volume 10. [Google Scholar]
- Soejarto, D.D.; Fong, H.H.S.; Tan, G.T.; Zhang, H.J.; Ma, C.Y.; Franzblau, S.G.; Gyllenhaal, C.; Riley, M.C.; Kadushin, M.R.; Pezzuto, J.M.; et al. Ethnobotany/ethnopharmacology and mass bioprospecting: Issues on intellectual property and benefit-sharin. J. Ethnopharmacol. 2005, 100, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Balick, M. Transforming ethnobotany for the new millennium. Ann. Mol. Bot. Gard. 1996, 83, 58–66. [Google Scholar] [CrossRef]
- Etkin, N.L. Ethnopharmacology: Biobehavioral approaches in the anthropological study of indigenous medicines. Annu. Rev. Anthropol. 1988, 17, 23–42. [Google Scholar] [CrossRef]
- Hurrell, J.A.; Albuquerque, U.P. Is ethnobotany an ecological science? Steps towards a complex ethnobotany. Ethnobiol. Conserv. 2012, 1, 1–16. [Google Scholar]
- Soldati, G.T.; Albuquerque, U.P. A new application for the optimal foraging theory: The extraction of medicinal plants. Evid.-Based Complementary Altern. Med. 2012, 2012, 364564. [Google Scholar] [CrossRef]
- Saslis-Lagoudakis, C.H.; Hawkins, J.A.; Greenhill, S.J.; Pendry, C.A.; Watson, M.F.; Tuladhar-Douglas, W.; Baral, S.R.; Savolainen, V. The evolution of traditional knowledge: Environment shapes medicinal plant use in Nepal. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132768. [Google Scholar] [CrossRef]
- Yessoufou, K.; Daru, B.H.; Muasya, A.M. Phylogenetic exploration of commonly used medicinal plants in South Africa. Mol. Ecol. Resour. 2015, 15, 405–413. [Google Scholar] [CrossRef]
- Cámara-Leret, R.; Faurby, S.; Macía, M.J.; Balslev, H.; Göldel, B.; Svenning, J.-C.; Kissling, W.D.; Rønsted, N.; Saslis-Lagoudakis, C.H. Fundamental species traits explain provisioning services of tropical American palms. Nat. Plants 2017, 3, 16220. [Google Scholar] [CrossRef]
- Hart, G.; Gaoue, O.G.; de la Torre, L.; Navarrete, H.; Muriel, P.; Macía, M.J.; Balslev, H.; León-Yánez, S.; Jørgensen, P.; Duffy, D.C. Availability, diversification and versatility explain human selection of introduced plants in Ecuadorian traditional medicine. PLoS ONE 2017, 12, e0184369. [Google Scholar] [CrossRef]
- Teixidor-Toneu, I.; Jordan, F.M.; Hawkins, J.A. Comparative phylogenetic methods and the cultural evolution of medicinal plant use. Nat. Plants 2018, 4, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, U.P.; de Medeiros, P.M.; Ferreira Júnior, W.S.; da Silva, T.C.; da Silva, R.R.V.; Gonçalves-Souza, T. Socialecological theory of maximization: Basic concepts and two initial models. Biol. Theory 2019, 14, 73–85. [Google Scholar] [CrossRef]
- Seyler, B.C.; Gaoue, O.G.; Tang, Y.; Duffy, D.C. Understanding knowledge threatened by declining wild orchid populations in an urbanizing China (Sichuan). Environ. Conserv. 2019, 46, 318–325. [Google Scholar] [CrossRef]
- Gaoue, O.G.; Coe, M.A.; Bond, M.; Hart, G.; Seyler, B.C.; McMillen, H. Theories and Major Hypotheses in Ethnobotany. Econ. Bot. 2017, 71, 269–287. [Google Scholar] [CrossRef]
- Gaoue, O.G.; Moutouama, J.K.; Coe, M.A.; Bond, M.O.; Green, E.; Sero, N.B.; Bezeng, B.S.; Yessoufou, K. Methodological advances for hypothesis driven Ethnobiology. Biol. Rev. 2021, 96, 2281–2303. [Google Scholar] [CrossRef]
- Ford, R.I. The Nature and Status of Ethnobotany; University of Michigan: Ann Arbor, MI, USA, 1978. [Google Scholar]
- Phillips, O.; Gentry, A.H. The useful plants of Tambopata, Peru: II. Additional hypothesis testing in quantitative ethnobotany. Econ. Bot. 1993, 47, 33–43. [Google Scholar] [CrossRef]
- Gaoue, O.G.; Yessoufou, K.; Mankga, L.; Vodouhe, F. Phylogeny reveals non-random medicinal plant organs selection by local people in Benin. Plants People Planet 2021, 3, 710–720. [Google Scholar] [CrossRef]
- Krebs, C.J. Hypothesis testing in ecology. In Research Techniques in Animal Ecology; Columbia University Press: New York, NY, USA, 2000; pp. 1–14. [Google Scholar]
- Voeks, R.A. Disturbance pharmacopoeias: Medicine and myth from the humid tropics. Ann. Assoc. Am. Geogr. 2004, 94, 868–888. [Google Scholar]
- Alencar, N.L.; de Sousa Araújo, T.A.; Amorim, E.L.C.; Albuquerque, U.P. The inclusion and selection of medicinal plants in traditional pharmacopoeias—Evidence in support of the diversification hypothesis. Econ. Bot. 2010, 64, 68–79. [Google Scholar] [CrossRef]
- Yessoufou, K.; Ambani, A.E.; Elansary, H.O.; Gaoue, O.G. Alien woody plants are more versatile than native, but both share similar therapeutic redundancy in South Africa. PLoS ONE 2021, 16, e0260390. [Google Scholar] [CrossRef]
- Bennett, B.C.; Prance, G.T. Introduced plants in the indigenous pharmacopoeia of northern South America. Econ. Bot. 2000, 1, 90–102. [Google Scholar] [CrossRef]
- Fabricant, D.S.; Farnsworth, N.R. The Value of Plants Used in Traditional Medicine for Drug Discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar] [PubMed]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; Garcia, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human-induced Species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.R.; Ivey, P.; Manyama, P.; Nanni, I. A new national unit for invasive species detection, assessment and eradication planning. S. Afr. J. Sci. 2013, 109, 111. [Google Scholar] [CrossRef]
- Poe, M.R.; Norman, K.C.; Levin, P.S. Cultural dimensions of socioecological systems: Key connections and guiding principles for conservation in coastal environments. Conserv. Lett. 2014, 7, 166–175. [Google Scholar] [CrossRef]
- Winter, K.B.; Ticktin TQuazi, S.A. Biocultural restoration in Hawai’I also achieves core conservation goals. Ecol. Soc. 2020, 25, 26. [Google Scholar]
- Bennett, N.J.; Roth, R.; Klain, S.C.; Chan, K.; Christie, P.; Clark, D.A.; Cullman, G.; Curran, D.; Durbin, T.J.; Epstein, G.; et al. Conservation social science: Understanding and integrating human dimensions to improve conservation. Biol. Conserv. 2017, 205, 93–108. [Google Scholar] [CrossRef]
- Germishuizen, G.; Meyer, N.L. Plants of Southern Africa: An Annotated Checklist; National Botanical Institute: Pretoria, South Africa, 2003; Volume 14, pp. 192–196. [Google Scholar]
- Bezeng, S.B.; Morales-Castilla, I.; Van der Bank, M.; Yessoufou KDaru, B.H.; Davies, J. Climate change may reduce the spread of non-native species. Ecosphere 2017, 8, e01694. [Google Scholar] [CrossRef]
- Henderson, L. Alien weeds and invasive plants. In Plant Protection Research Institute Handbook No. 12; Agricultural Research Council: Pretoria, South Africa, 2001. [Google Scholar]
- Henderson, L. Invasive, naturalized and casual alien plants in southern Africa: A summary based on the Southern African Plant Invaders Atlas (SAPIA). Bothalia 2007, 37, 215–248. [Google Scholar] [CrossRef]
- Coates-Palgrave, M.; Coates-Palgrave, K. Trees of Southern Africa, 3rd ed.; Struik Nature: Cape Town, South Africa, 2002. [Google Scholar]
- Yessoufou, K.; Ambani, A.E. Are Introduced Alien Species More Predisposed to Invasion in Recipient Environments If They Provide a Wider Range of Services to Humans? Diversity 2021, 13, 553. [Google Scholar] [CrossRef]
- Global Biodiversity Information Facility. 2017. Available online: https://www.gbif.org/occurrence/search?taxon_key=3880247 (accessed on 25 June 2017).
- Van Wyk, B.; van Wyk, P. Field Guide to Trees of Southern Africa, 2nd ed.; Struik Publisher: Cape Town, South Africa, 2013. [Google Scholar]
- Glen, H.; Van Wyk, B. Guide to Trees Introduced into Southern Africa; Penguin Random House South Africa: Cape Town, South Africa, 2016. [Google Scholar]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol. Evol 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, J.; Shirkey, G.; John, R.; Wu, S.R.; Park, H.; Shao, C. Applications of structural equation modeling (SEM) in ecological studies: An updated review. Ecol. Processes 2016, 5, 19. [Google Scholar] [CrossRef]
- Schermelleh-Engel, K.; Moosbrugger, H.; Müller, H. Evaluating the Fit of Structural Equation Models: Tests of Significance and Descriptive Goodness-Of-Fit Measures. Methods Psychol. Res. 2003, 8, 23–74. [Google Scholar]
- O’Hara, R.B.; Kotze, D.J. Do not log-transform count data. Methods Ecol. Evol. 2010, 1, 118–122. [Google Scholar] [CrossRef]
- Albuquerque, U.P. Re-examining hypotheses concerning the use and knowledge of medicinal plants a study in the Caatinga vegetation of NE Brazil. J. Ethnobiol. Ethnomed. 2006, 2, 1–10. [Google Scholar] [CrossRef]
- Medeiros, P.M. Why is change feared? Exotic species in traditional pharmacopoeias. Ethnobiol. Conserv. 2013, 2, 1–5. [Google Scholar]
- Bletter, N.; Satdichan, M.; Sounthala, L.; Satdichan, D.; Sudmoon, R.; Noikotr, K.; Thani, T.; Yongvanit, S.; Chaveerach, A. Plants up for adoption: Why do Southeast Asians so readily accept introduced plants into use, especially those from the Neotropics? In Proceedings of the 2010 Southeast Asian Geography Association, Hanoi, Vietnam, 23–26 November 2010; pp. 1–17. [Google Scholar]
- Lozano, A.; Araújo, E.L.; Medeiros, M.F.T.; Albuquerque, U.P. The apparency hypothesis applied to a local pharmacopoeia in the Brazilian northeast. J. Ethnobiol. Ethnomed 2014, 10, 2. [Google Scholar] [CrossRef]
- Phillips, O.; Gentry, A.H. The useful plants of Tambopata, Peru: I. Statistical hypotheses tests with a new quantitative technique. Econ. Bot. 1993, 47, 15–32. [Google Scholar] [CrossRef]
- Albuquerque, U.P. Quantitative ethnobotany or quantification in ethnobotany? Ethnobot. Res. Appl. 2009, 7, 1–3. [Google Scholar] [CrossRef]
- Hoffman, B.; Gallaher, T. Importance indices in ethnobotany. Ethnobot. Res. Appl. 2007, 5, 201–218. [Google Scholar] [CrossRef]
- Bennett, B.C. Ethnobotany education, opportunities, and needs in the US. Ethnobot. Res. Appl. 2005, 3, 113–122. [Google Scholar] [CrossRef][Green Version]
- Martin, G.J. Ethnobotany: A Methods Manual; Earthscan: London, UK, 2007. [Google Scholar]
- Albuquerque, U.P.; Hanazaki, N. Commentary: Five problems in current ethnobotanical research and some suggestions for strengthening them. Hum. Ecol. 2009, 37, 653–661. [Google Scholar] [CrossRef]
- Weiner, J. On the practice of ecology. J. Ecol. 1995, 83, 153–158. [Google Scholar] [CrossRef]
- Aarssen, L.W. On the progress of ecology. Oikos 1997, 80, 177–178. [Google Scholar] [CrossRef]
- Lawton, J.H. Are there general laws in ecology? Oikos 1999, 84, 177–192. [Google Scholar] [CrossRef]
- Marquet, P.A.; Allen, A.P.; Brown, J.H.; Dunne, J.A.; Enquist, B.J.; Gillooly, J.F.; Gowaty, P.A.; Green, J.L.; Harte, J.; Hubbell, S.P.; et al. On Theory in Ecology. BioScience 2014, 64, 701–710. [Google Scholar] [CrossRef]
- Bond, M.O.; Gaoue, O.G. Prestige and homophily predict network structure for social learning of medicinal plant knowledge. PLoS ONE 2020, 15, e0239345. [Google Scholar] [CrossRef]
- Coe, M.A.; Gaoue, O.G. Most cultural importance indices do not predict species’ cultural keystone status. Hum. Ecol. 2020, 48, 721–732. [Google Scholar] [CrossRef]
- Muleba, I.; Yessoufou, K.; Rampedi, I.T. Testing the non-random hypothesis of medicinal plant selection using the woody flora of the Mpumalanga Province, South Africa. Environ. Dev. Sustain. 2021, 23, 4162–4173. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).