A Strategy to Provide a Present and Future Scenario of Mexican Biodiversity of Tardigrada
Abstract
1. Introduction
2. Materials and Methods
2.1. List of the Mexican Tardigrades
2.2. Distribution Patterns of the Tardigrades of Mexico
2.3. Estimating Richness
2.3.1. Ratio of Known to Unknown Species
2.3.2. Accumulation Curves
2.4. Taxonomic Effort
3. Results
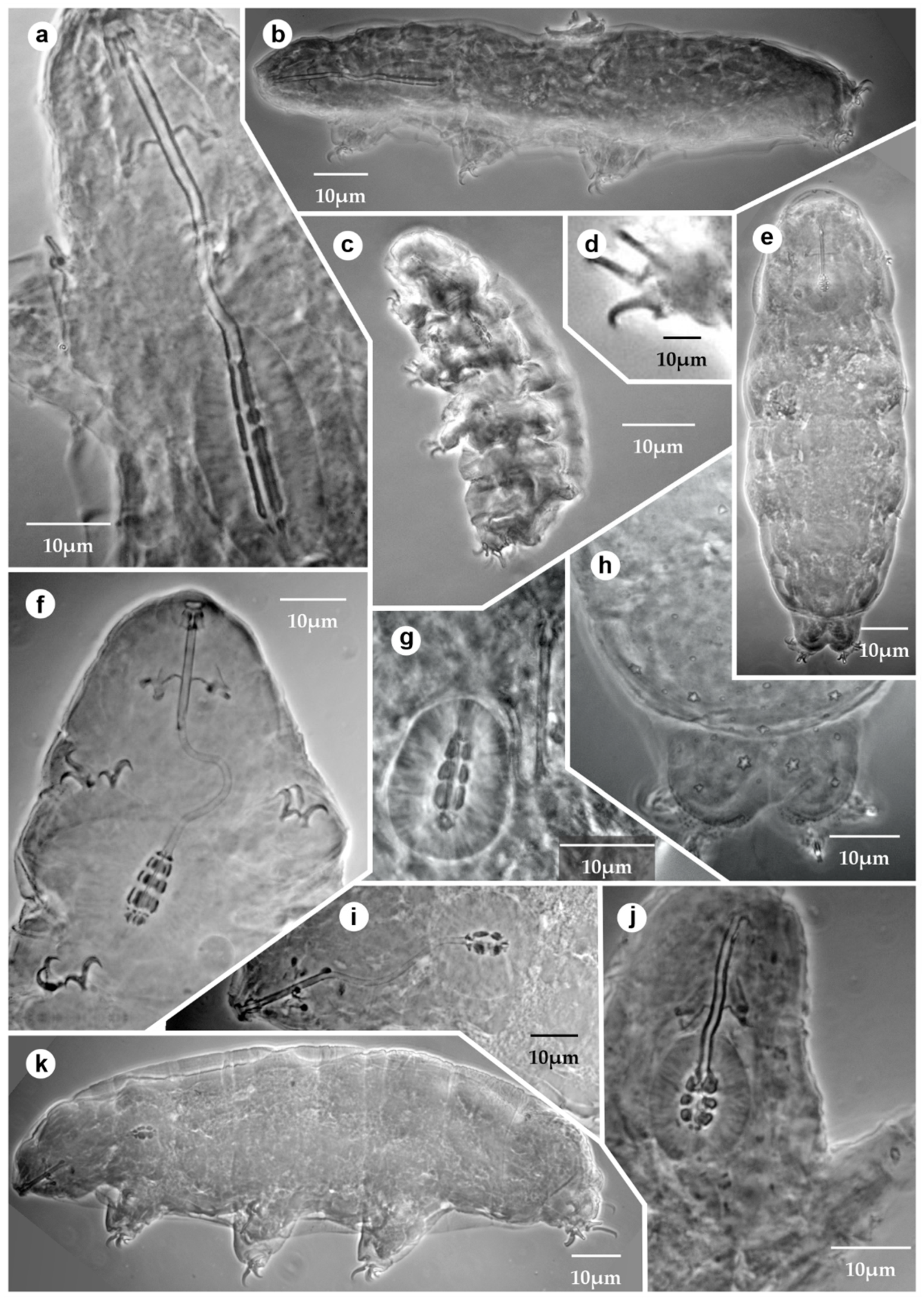
3.1. List of the Mexican Tardigrades
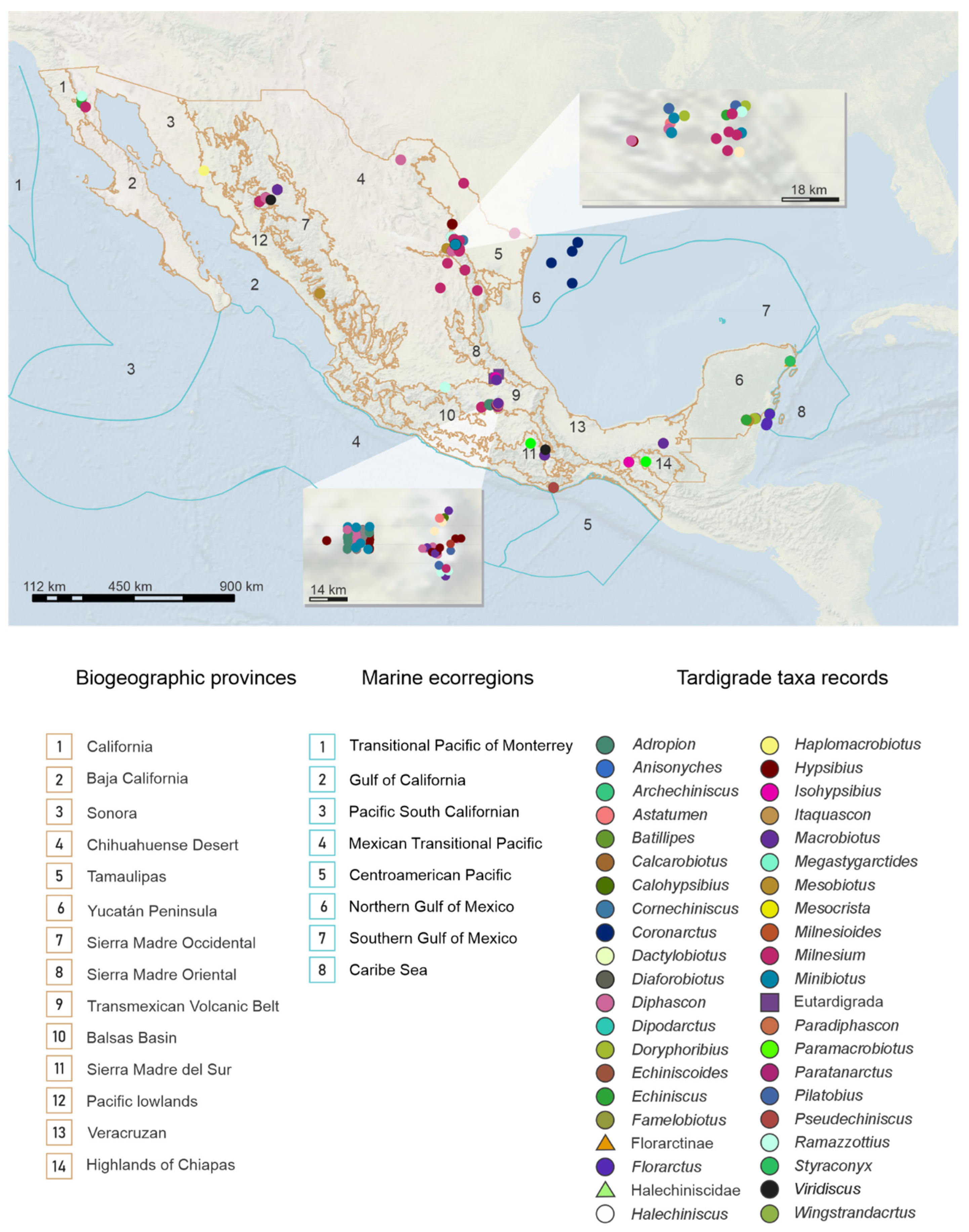
3.2. Distribution Patterns of the Tardigrades of Mexico
Geographic Distribution
3.3. Estimating Richness
3.3.1. Ratio of Known to Unknown Species
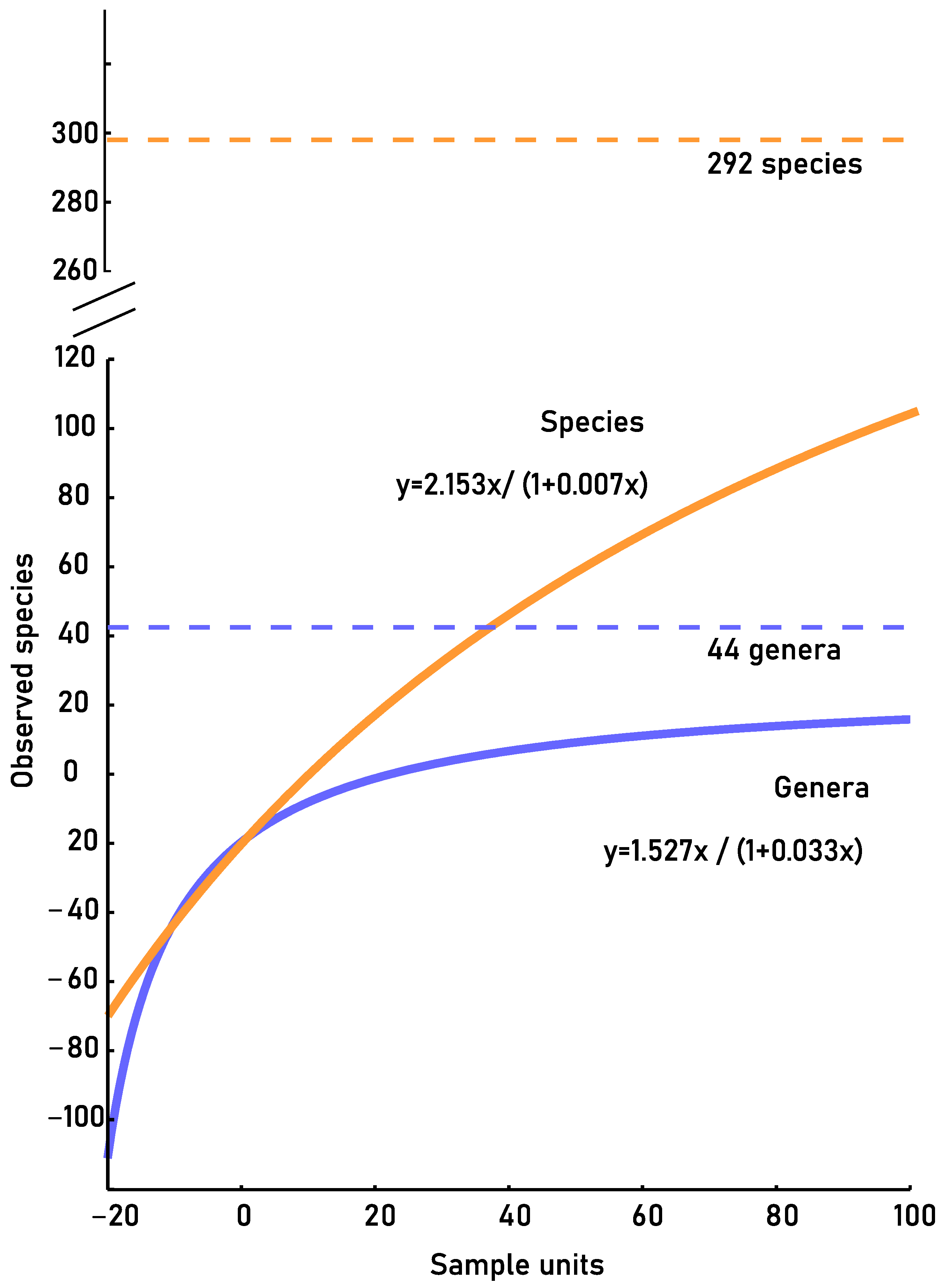
3.3.2. Accumulation Curves
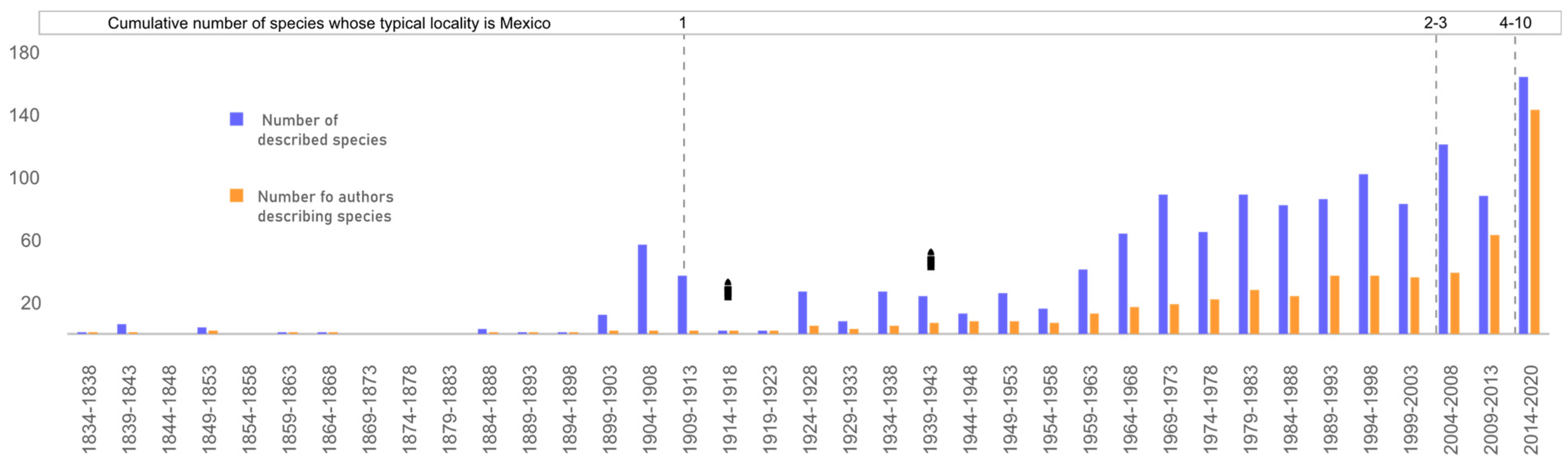
3.4. Taxonomic Effort
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guil, N. Molecular approach to micrometazoans. Are they here, there and everywhere? In Biogeography of Microscopic Organisms, 1st ed.; The Systematics Association/Cambridge University Press: Cambridge, UK, 2011; pp. 284–306. [Google Scholar]
- Garey, J.R.; McInnes, S.J.; Nichols, P.B. Developments in hydrobiology. In Freshwater Animal Diversity Assessment, 1st ed.; Balian, E.V., Lévêque, C., Segers, H., Martens, K., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 1, pp. 101–106. [Google Scholar]
- Nelson, D.R.; Guidetti, R.; Rebecchi, L. Phylum Tardigrada. In Ecology and General Biology: Thorp and Covich’s Freshwater Invertebrates, 4th ed.; Thorp, J.H., Rogers, D.C., Eds.; Academic Press: Cambridge, UK, 2015; Volume 1, pp. 347–380. [Google Scholar]
- Jönsson, K.I.; Harms-Ringdahl, M.; Torudd, J. Radiation tolerance in the tardigrade Richtersius coronifer. Int. J. Rad. Biol. 2005, 81, 649–656. [Google Scholar] [CrossRef] [PubMed]
- May, R.M.; Maria, M.; Guimard, J. Actions différentielles des rayons x et ultraviolets sur le tardigrade Macrobiotus areolatus, à l’état actif et desséché. Bull. Sci. Fr. Belg. 1964, 98, 349–367. [Google Scholar]
- Horikawa, D.D.; Sakashita, T.; Katagiri, C.; Watanabe, M.; Kikawada, T.; Nakahara, Y.; Hamada, N.; Wada, S.; Funayama, T.; Higashi, S.; et al. Radiation tolerance in the tardigrade Milnersium tardigradum. Int. J. Rad. Biol. 2006, 82, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Møbjerg, N.; Halberg, K.A.; Jørgensen, A.; Persson, D.; Bjørn, M.; Ramløv, H.; Kristensen, R.M. Survival in extreme environments on the current knowledge of adaptations in tardigrades. Acta Physiol. 2011, 202, 409–420. [Google Scholar] [CrossRef]
- Guidetti, R.; Altiero, T.; Rebecchi, L. On dormancy strategies in tardigrades. J. Insect Physiol. 2011, 57, 567–576. [Google Scholar] [CrossRef]
- Robertson, M.W.; Russo, N.J.; McInnes, S.J.; Goffinet, B.; Jiménez, J.E. Potential dispersal of tardigrades by birds through endozoochory: Evidence from Sub-Antarctic White-bellied Seedsnipe (Attagis malouinus). Polar Biol. 2020, 43, 899–902. [Google Scholar] [CrossRef]
- Guidetti, R.; Bertolani, R. The tardigrades of Emilia (Italy). III. Piane di Mocogno (Northern Apennines). Zool. Anz. 2001, 240, 377–383. [Google Scholar] [CrossRef]
- Guil, N. Diversity and distribution of tardigrades (Bilateria, Tardigrada) from the Iberian Peninsula, Balearic Islands and Chafarinas Islands. Graellsia 2002, 58, 75–94. [Google Scholar] [CrossRef]
- Bartels, P.J.; Nelson, D.R. A large-scale, multihabitat inventory of the Phylum Tardigrada in the Great Smoky Mountains National Park, USA: A preliminary report. Hydrobiologia 2006, 558, 111–118. [Google Scholar] [CrossRef]
- Johansson, C.; Miller, W.R.; Linder, E.T.; Adams, B.J.; Boreliz-Alvarado, E. Tardigrades of Alaska: Distribution patterns, diversity and species richness. Polar Res. 2013, 32, 18793. [Google Scholar] [CrossRef]
- Guidetti, R.; Bertolani, R.; Nelson, D.R. Ecological and faunistic studies on tardigrades in leaf litter of Beech Forests. Zool. Anz. 1999, 238, 215–223. [Google Scholar]
- Schuster, R.; Greven, H. A long-term study of population dynamics of tardigrades in the moss Rhytidiadelphus squarrosus (Hedw.) Warnst. J. Limnol. 2007, 66, 141–151. [Google Scholar] [CrossRef]
- Guil, N.; Sanchez-Moreno, S. Fine-scale patterns in micrometazoans: Tardigrade diversity, community composition and trophic dynamics in leaf litter. Syst. Biodivers. 2013, 11, 181–193. [Google Scholar] [CrossRef]
- Zawierucha, K.; Węgrzyn, M.; Ostrowska, M.; Wietrzyk, P. Tardigrada in Svalbard lichens: Diversity, densities and habitat heterogeneity. Polar Biol. 2017, 40, 1385–1392. [Google Scholar] [CrossRef]
- Stec, D.; Smolak, R.; Kaczmarek, Ł.; Michalczyk, Ł. An integrative description of Macrobiotus paulinae sp. nov. (Tardigrada: Eutardigrada: Macrobiotidae: Hufelandi group) from Kenya. Zootaxa 2015, 4052, 501–526. [Google Scholar] [CrossRef]
- Vecchi, M.; Cesari, M.; Bertolani, R.; Jönsson, R.I.; Rebecchi, L.; Guidetti, R. Integrative systematic studies on tardigrades from Antarctica identify new genera and new species within Macrobiotoidea and Echiniscoidea. Invertebr. Syst. 2016, 30, 303–322. [Google Scholar] [CrossRef]
- Tumanov, D.V.; Avdeeva, G.S. Integrative description of Hypsibius repentinus sp. nov. (Eutardigrada: Hypsibiidae) from Sweden. Zoosyst. Ross. 2021, 30, 101–115. [Google Scholar] [CrossRef]
- Degma, P.; Bertolani, R.; Guidetti, R. Actual Checklist of Tardigrada Species. Available online: https://iris.unimore.it/retrieve/handle/11380/1178608/358743/Actual%20checklist%20of%20Tardigrada%2040th%20Edition%2019-07-21.pdf (accessed on 3 February 2022).
- National Center for Biotechnology Information (NCBI). Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 14 February 2022).
- Sarukhán, J.; Koleff, P.; Carabias, J.; Soberón, J.; Dirzo, R.; Llorente-Bousquets, J.; Halffter, G.; González, R.; March, I.; Mohar, A.; et al. Capital Natural de México. Síntesis: Conocimiento Actual, Evaluación y Perspectivas de Sustentabilidad, 1st ed.; Conabio: Mexico City, Mexico, 2009; pp. 21–43. [Google Scholar]
- Martínez-Meyer, E.; Sosa-Escalante, J.E.; Álvarez, F. El estudio de la biodiversidad en México: ¿Una ruta con dirección? Rev. Mex. Biodivers. 2014, 85, 1–9. [Google Scholar] [CrossRef]
- Moreno-Talamantes, A.; Roszkowska, M.; García-Aranda, M.A.; Flores-Maldonado, J.J.; Kaczmarek, Ł. Current knowledge on Mexican tardigrades with a description of Milnesium cassandrae sp. nov. (Eutardigrada: Milnesiidae) and discussion on the taxonomic value of dorsal pseudoplates in the genus Milnesium Doyère, 1840. Zootaxa 2019, 4691, 501–524. [Google Scholar] [CrossRef]
- Heinis, F. Beitrag zur kenntnis der zentralamerikanischen moosfauna. Rev. Suisse Zool. 1911, 19, 253–266. [Google Scholar] [CrossRef]
- May, R.M. Noveau genre et espece de Tardigrade du Mexique: Haplomacrobiotus hermosillensis. Bull. Soc. Zool. Fr. 1948, 84, 95–97. [Google Scholar]
- Schuster, R.O. Tardigrada from the Barranca del Cobre, Sinaloa and Chihuahua, Mexico. Proc. Biol. Soc. Wash. 1971, 84, 213–224. [Google Scholar] [CrossRef][Green Version]
- Beasley, C.W. Some tardigrades from Mexico. Southwest. Nat. 1972, 17, 21–29. [Google Scholar] [CrossRef]
- Ramazzotti, G.; Maucci, W. II Phylum Tardigrada. III Edizione riveduta e aggiornata. Mem. Ist. Ital. Idrobiol. 1983, 41, 1–102. [Google Scholar]
- Pilato, G.; Lisi, O. Notes on some tardigrades from southern Mexico with description of three new species. Zootaxa 2006, 68, 53–68. [Google Scholar] [CrossRef]
- Beasley, C.W.; Kaczmarek, Ł.; Michalczyk, Ł. Doryphoribius mexicanus, a new species of Tardigrada (Eutardigrada: Hypsibiidae) from Mexico (North America). Proc. Biol. Soc. Wash. 2008, 121, 34–40. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Diduszko, D.; Michalczyk, Ł. New records of Mexican Tardigrada. Rev. Mex. Biodivers. 2011, 82, 1324–1327. [Google Scholar] [CrossRef]
- Anguas-Escalante, A.; Pérez-Pech, W.A.; Guidetti, R.; Cutz-Pool, L.Q.; Ortiz-León, H. Tardígrados asociados a una plantación de cítricos de traspatio en la comunidad de El Palmar en Quintana Roo, México. Investig. y Cienc. de la Univ. Autónoma de Aguascalientes 2018, 26, 20–26. [Google Scholar] [CrossRef]
- Dueñas-Cedillo, A. Composición de la Comunidad de Tardígrados Asociados a Los Musgos de Milpa Alta (CICS), Ciudad de México. Bachelor’s Thesis, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, CDMX, Mexico, 12 August 2012. [Google Scholar]
- Pérez-Pech, W.A.; Cutz-Pool, L.; Guidetti, R.; Blanco-Piñón, A. Primer registro genérico de tardígrados, habitantes del área urbana de Chetumal Quintana Roo, México. Entomol. Mex. 2016, 3, 912–918. [Google Scholar]
- Pérez-Pech, W.A.; Anguas-Escalante, A.; de Jesús-Navarrete, A.; Hansen, J.G. Primer registro genérico de tardígrados marinos en costas de Quintana Roo, México. Acad. J. 2018, 4, 1909–1912. [Google Scholar]
- Pérez-Pech, W.A.; Hansen, J.G.; Demilio, E.; de Jesús-Navarrete, A.; Mendoza, I.M.; Olivares, A.R.; Vargas-Espositos, A. First records of marine tardigrades of the genus Coronarctus (Tardigrada, Heterotardigrada, Arthrotardigrada) from Mexico. Check List 2020, 16, 1–7. [Google Scholar] [CrossRef]
- Moreno-Talamantes, A.; Roszkowska, M.; Ríos-Guayasamín, P.; Flores-Maldonado, J.J.; Kaczmarek, Ł. First record of Dactylobiotus parthenogeneticus Bertolani, 1982 (Eutardigrada: Murrayidae) in Mexico. Check List 2015, 11, 1723. [Google Scholar] [CrossRef]
- Moreno-Talamantes, A.; León-Espinosa, G.A. Nuevo registro de Diaforobiotus islandicus (Richters, 1904) (Eutardigrada: Richtersiidae) para México. Árido-Ciencia 2019, 6, 5–12. [Google Scholar]
- León-Espinosa, G.A. Taxonomía de tardígrados (Tardigrada: Eutardigrada: Heterotardigrada) de Musgo en Localidades Selectas del Noreste de México. Bachelor’s Thesis, Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León, Nuevo León, Mexico, January 2018. [Google Scholar]
- Cutz-Pool, L.Q.; Crisanto, J.I.; Pérez-Pech, W.A.; Anguas-Escalante, A.; Guidetti, R. Caracterización de la fauna de tardígrados (Ecdysozoa: Tardigrada) de liquen y musgo en dos sitios con diferente uso de suelo, en Quintana Roo, México. In Agroecosistemas Tropicales: Conservación de Recursos Naturales y Seguridad Alimentaria, 1st ed.; Cetzal-Ix, W., Casanova-Lugo, F., Chay-Canul, A.J., Martínez-Puc, J.F., Eds.; Instituto Tecnológico de la Zona Maya: Quintana Roo, Mexico, 2018; Volume 1, pp. 193–200. [Google Scholar]
- Núñez, P.G.; León-Espinosa, G.A.; Vázquez, R.; Peña-Salinas, M.E.; Rodríguez-Almaraz, G.A.; Moreno-Talamantes, A. First tardigrade records from San Pedro Mártir, Baja California, Mexico. Check List 2021, 17, 1131–1136. [Google Scholar] [CrossRef]
- Pérez-Pech, W.A.; Guidetti, R.; Anguas-Escalante, A.; Cutz-Pool, L.Q.; Blanco-Piñón, A. Primer registro genérico de tardígrados para Pachuca Hidalgo, México y Áreas circundantes. Entomol. Mex 2017, 4, 688–694. [Google Scholar]
- Pérez-Pech, W.A.; Anguas-Escalante, A.; Cutz-Pool, L.Q.; Guidetti, R. Doryphoribius chetumalensis sp. nov. (Eutardigrada: Isohypsibiidae) a new tardigrade species discovered in an unusual habitat of urban areas of Mexico. Zootaxa 2017, 4344, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pech, W.A.; de Jesus-Navarrate, A.; Demilio, E.; Anguas-Escalante, A.; Hansen, J.G. Marine Tardigrada from the Mexican Caribbean with the description of Styraconyx robertoi sp. nov. (Arthrotardigrada: Styraconyxidae). Zootaxa 2020, 4731, 492–508. [Google Scholar] [CrossRef]
- Dueñas-Cedillo, A.; Martínez-Méndez, E.; García-Román, J.; Armendáriz-Toledano, F.; Ruiz, E.A. Tardigrades from Iztaccíhuatl Volcano (Trans-Mexican Volcanic Belt), with the description of Minibiotus citlalium sp. nov. (Eutardigrada: Macrobiotidae). Diversity 2020, 12, 271. [Google Scholar] [CrossRef]
- Anguas-Escalante, A.; de Jesús-Navarrete, A.; Demilio, E.; Pérez-Pech, W.A.; Hansen, G.J. A new species of Tardigrada from a Caribbean reef lagoon, Florarctus yucatanensis sp. nov. (Halechiniscidae: Florarctinae). Cah. Biol. Mar. 2020, 61, 377–385. [Google Scholar]
- Moreno-Talamantes, A.; León-Espinosa, G.A.; García-Aranda, M.A.; Flores-Maldonado, J.J.; Kaczmarek, Ł. The genus Milnesium Doyère, 1840 in Mexico with description of a new species. Ann. Zool. 2020, 70, 467–486. [Google Scholar] [CrossRef]
- Bartels, P.J.; Apodaca, J.J.; Mora, C.; Nelson, D.R. A global biodiversity estimate of a poorly known taxon: Phylum Tardigrada. Zool. J. Linn. Soc. 2016, 178, 730–736. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C. Nonparametric estimation and comparison of species richness. In eLS, 1st ed.; John Wiley & Sons, Ltd., Ed.: Chichester, UK, 2016; Volume 1, pp. 1–111. [Google Scholar] [CrossRef]
- Maciel-Mata, C.A.; Manríquez-Morán, N.; Octavio-Aguilar, P.; Sánchez-Rojas, G. El área de distribución de las especies: Revisión del concepto. Acta Univ. 2015, 25, 3–19. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Balmford, A.; Gaston, K.J.; Blyth, S.; James, A.; Kapos, V. Global variation in terrestrial conservation costs, conservation benefits, and unmet conservation needs. Proc. Natl. Acad. Sci. USA 2003, 100, 1046–1050. [Google Scholar] [CrossRef]
- Dirzo, R.; Raven, P.H. Global state of biodiversity and loss. Annu. Rev. Environ. Resour. 2003, 28, 137–167. [Google Scholar] [CrossRef]
- Mora, C.; Tittensor, D.P.; Adl, S.; Simpson, A.G.B.; Worm, B. How many species are there on earth and in the ocean? PLoS Biol. 2011, 9, e1001127. [Google Scholar] [CrossRef]
- Scheffers, B.R.; Joppa, L.N.; Pimm, S.L.; Laurance, W.F. What we know and don’t know about Earth’s missing biodiversity. Trends Ecol. Evol. 2012, 27, 501–510. [Google Scholar] [CrossRef]
- Morrone, J.J. Regionalización biogeográfica y evolución biótica de México: Encrucijada de la biodiversidad del Nuevo Mundo. Rev. Mex. Biodivers. 2019, 90, e902980. [Google Scholar] [CrossRef]
- Morrone, J.J.; Escalante, T.; Rodríguez-Tapia, G. Mexican biogeographic provinces: Map and shapefiles. Zootaxa 2017, 4277, 277–279. [Google Scholar] [CrossRef]
- Ecorregiones Marinas. Available online: https://www.biodiversidad.gob.mx/region/ecorregiones-marinas (accessed on 11 February 2022).
- Morrone, J.J. Biogeographical regionalization of the world: A reappraisal. Aust. Syst. Bot. 2015, 28, 81–90. [Google Scholar] [CrossRef]
- Stork, N.E. How many species of insects and other terrestrial arthropods are there on Earth? Annu. Rev. Entomol. 2018, 63, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Villaseñor, J.L. Catálogo de las plantas vasculares nativas de México. Rev. Mex. Biodivers. 2016, 87, 559–902. [Google Scholar] [CrossRef]
- State of the World’s Plants 2016. Available online: https://stateoftheworldsplants.org/2016/#:~:text=The%20State%20of%20the%20World’s,the%20policies%20dealing%20with%20them (accessed on 3 February 2022).
- A Checklist of Mosses. Available online: http://www.mobot.org/MOBOT/tropicos/most/checklist.shtml (accessed on 3 February 2022).
- Delgadillo-Moya, C. Biodiversidad de Bryophyta (musgos) en México. Rev. Mex. Biodivers. 2014, 85, S100–S105. [Google Scholar] [CrossRef]
- Berlanga, H.; Gómez de Silva, H.; Vargas-Canales, V.M.; Rodríguez-Contreras, V.; Sánchez-González, L.A.; Ortega-Álvarez, R.; Calderón-Parra, R. Aves de México: Lista Actualizada de Especies y Nombres Comunes, 1st ed.; Conabio: Mexico City, Mexico, 2017; Volume 1, 18p. [Google Scholar]
- IOC World Bird List v 9.2. Available online: https://www.worldbirdnames.org/ (accessed on 3 February 2022).
- Flores-Villela, O.; García-Vázquez, U.O. Biodiversidad de reptiles en México. Rev. Mex. Biodivers. 2014, 85, 467–475. [Google Scholar] [CrossRef]
- The Reptile Database. Available online: http://www.reptile-database.org2019 (accessed on 4 February 2022).
- Sánchez-Cordero, V.; Botello, F.; Flores-Martínez, J.J.; Gómez-Rodríguez, R.A.; Guevara, L.; Gutiérrez-Granados, G.; Rodríguez-Moreno, Á. Biodiversity of Chordata (Mammalia) in Mexico. Rev. Mex. Biodivers. 2014, 85, 496–504. [Google Scholar] [CrossRef]
- Wilson, D.E.; Reeder, D.M. Mammal Species of the World: A Taxonomic and Geographic Reference. J. Mammal. 2005, 88, 824–830. [Google Scholar]
- Espinosa-Pérez, H. Biodiversidad de peces en México. Rev. Mex. Biodivers. 2014, 85, 450–459. [Google Scholar] [CrossRef]
- FishBase. Available online: https://www.fishbase.in/search.php (accessed on 4 February 2022).
- Llorente-Bousquets, J.; Ocegueda, S. Estado del conocimiento de la biota. In Capital Natural de México, Vol. I: Conocimiento Actual de la Biodiversidad, 1st ed.; Soberón, J., Halffter, G., Llorente-Bousquets, J., Eds.; Conabio: Mexico City, Mexico, 2008; Volume 1, pp. 283–322. [Google Scholar]
- Zhang, Z.Q. Animal biodiversity: An introduction to higher-level classification and taxonomic richness. Zootaxa 2011, 3148, 7–12. [Google Scholar] [CrossRef]
- Palacios-Vargas, J.G.; Figueroa, D. Biodiversity of Protura (Hexapoda: Entognatha) in Mexico. Rev. Mex. Biodivers. 2014, 85, 232–235. [Google Scholar] [CrossRef]
- Palacios-Vargas, J.G.; García-Gómez, A. Biodiversity of Diplura (Hexapoda: Entognatha) in Mexico. Rev. Mex. Biodivers. 2014, 85, 236–242. [Google Scholar] [CrossRef]
- Sendra, A.; Jiménez-Valverde, A.; Selfa, J.; Reboleira, A.S.P.S. Diversity, ecology, distribution and biogeography of Diplura. Insect. Conserv. Divers. 2021, 14, 415–425. [Google Scholar] [CrossRef]
- Gutiérrez, M.E.; Sarma, S.S.S. Zooplancton de Sistemas Acuáticos Epicontinentales Mexicanos en la Región Central de México; Conabio: Mexico City, Mexico, 1999; pp. 1–2. [Google Scholar]
- Humes, A.G. How many copepods? Hydrobiologia 1994, 292, 1–7. [Google Scholar] [CrossRef]
- Contreras-Félix, G.A.; Montiel-Parra, G.; Cupul-Magaña, F.G.; Pérez, T.M. Redescription of the velvet worm Oroperipatus eisenii (Onychophora: Peripatidae), through DNA sequencing, scanning electron microscopy and new collection records from Western Mexico. Rev. Mex. Biodivers. 2018, 89, 1033–1044. [Google Scholar] [CrossRef]
- Sena-Oliveira, I.; Read, V.M.S.J.; Mayer, G. A world checklist of Onychophora (velvet worms), with notes on nomenclature and status of names. ZooKeys 2012, 211, 1–70. [Google Scholar] [CrossRef] [PubMed]
- García-Prieto, L.; Osorio-Sarabia, D.; Lamothe-Argumedo, M.R. Biodiversidad de Nematoda parásitos de vertebrados en México Biodiversity. Rev. Mex. Biodivers. 2014, 85, 171–176. [Google Scholar] [CrossRef]
- Segers, H. Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature, taxonomy and distribution. Zootaxa 2007, 1564, 1–104. [Google Scholar] [CrossRef]
- Korovchinsky, N.M. How many species of Cladocera are there? Hydrobiologia 1996, 321, 191–204. [Google Scholar] [CrossRef]
- Tardigrados Asociados a Briofitas de la Zona Sur de la Ciudad de México. Available online: https://www.researchgate.net/publication/329797806_Tardigrados_asociados_a_briofitas_de_la_zona_sur_de_la_Ciudad_de_Mexico (accessed on 4 February 2022).
- Biodiversity of Tardigrades in Mexico. Available online: https://www.researchgate.net/publication/320597470_Biodiversity_of_Tardigrades_in_Mexico (accessed on 4 February 2022).
- Jiménez-Valverde, A.; Hortal, J. Las curvas de acumulación de especies y la necesidad de evaluar la calidad de los inventarios biológicos. Rev. Iber. Aracnol. 2003, 8, 151–161. [Google Scholar]
- EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples (Software and User’s Guide), Versión 6.0. Available online: http://viceroy.eeb.uconn.edu/estimates (accessed on 4 February 2022).
- Soberon, M.J.; Llorente-Bousquets, J. The use of species accumulation functions for the prediction of species richness. Conserv. Biol. 1993, 7, 480–488. [Google Scholar] [CrossRef]
- Gray, A.; Cavers, S. Island biogeography, the Effects of taxonomic effort and the importance of Island niche diversity to single-Island endemic species. Syst. Biol. 2014, 63, 55–65. [Google Scholar] [CrossRef]
- Costello, M.J.; Wilson, S.; Houlding, B. Predicting total global species richness using rates of species description and estimates of taxonomic effort. Syst. Biol. 2012, 61, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, Ł.; Bartels, P.J.; Roszkowska, M.; Nelson, D.R. The zoogeography of marine tardigrada. Zootaxa 2015, 4037, 1–189. [Google Scholar] [CrossRef] [PubMed]
- Global Biodiversity Information Facility. Available online: https://www.gbif.org/ (accessed on 3 February 2022).
- Romano, F., III; Gallo, M.; D’Addabbo, R.; Accogli, J.; Baguley, J.; Montagna, P. Deep-sea tardigrades in the northern Gulf of Mexico with a description of a new species of Coronarctidae (Tardigrada: Arthrotardigrada), Coronarctus mexicus. J. Zool. Syst. Evol. Res. 2011, 49, 48–56. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Michalczyk, Ł.; Mcinnes, S.J. Annotated zoogeography of non-marine Tardigrada. Part III: North America and Greenland. Zootaxa 2016, 4203, 1–249. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Michalczyk, Ł.; Mcinnes, S.J. Annotated zoogeography of non-marine Tardigrada. Part II: South America. Zootaxa 2015, 3923, 1–107. [Google Scholar] [CrossRef]
- Mcinnes, S.J.; Michalczyk, Ł.; Kaczmarek, Ł. Annotated zoogeography of non-marine Tardigrada. Part IV: Africa. Zootaxa 2017, 4284, 1–74. [Google Scholar] [CrossRef]
- McInnes, S.J. Zoogeographic distribution of terrestrial/freshwater tardigrades from current literature. J. Nat. Hist. 1994, 28, 257–352. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Michalczyk, Ł.; McInnes, S.J. Annotated zoogeography of non-marine Tardigrada. Part I: Central America. Zootaxa 2014, 3763, 1–62. [Google Scholar] [CrossRef] [PubMed]
- Pilato, G. Remarks on the Macrobiotus polyopus group, with the description of two new species (Eutardigrada, Macrobiotidae). Zootaxa 2006, 1298, 37–47. [Google Scholar] [CrossRef]
- Fujimoto, S.; Suzuki, A.C.; Ito, M.; Tamura, T.; Tsujimoto, M. Marine tardigrades from Lützow-Holm Bay, East Antarctica with the description of a new species. Polar Biol. 2020, 43, 679–693. [Google Scholar] [CrossRef]
- Stec, D. Integrative descriptions of two new Mesobiotus species (Tardigrada, Eutardigrada, Macrobiotidae) from Vietnam. Diversity 2021, 13, 605. [Google Scholar] [CrossRef]
- Tumanov, D. Presence of Notahypsibius pallidoides (Tardigrada: Hypsibiidae) in the fauna of Russia confirmed with the methods of DNA barcoding. Commun. Biol. 2021, 66, 274–280. [Google Scholar] [CrossRef]
- Dueñas-Cedillo, A.; García-Román, J.; Ruiz, E.A.; Armendáriz-Toledano, F. Species-Specific cuticular phenotypes in Eutardigrada: A Morphometric Approach to Analyze the Variation of Star-Shaped Pores in Minibiotus Species. Diversity 2021, 13, 307. [Google Scholar] [CrossRef]
- Arakawa, K. Simultaneous metabarcoding of eukaryotes and prokaryotes to elucidate the community structures within tardigrade microhabitats. Diversity 2020, 12, 110. [Google Scholar] [CrossRef]
- Koleff, P.; Soberón, J.; Arita, H.J.; Dávila, P.; Flores-Villela, O.; Golubov, J.; Halffter, G.; Lira-Noriega, A.; Moreno, C.E.; Moreno, E.; et al. Patrones de diversidad espacial en grupos selectos de especies. In Capital Natural de México, Volumen I: Conocimiento Actual de la Biodiversidad.; Soberón, J., Halffter, G., Llorente-Bousquets, J., Eds.; Conabio: Mexico City, Mexico, 2008; Volume 1, pp. 323–364. [Google Scholar]
- El Medio Ambiente en México: Biodiversidad. Available online: https://apps1.semarnat.gob.mx:8443/dgeia/informe_resumen14/04_biodiversidad/4_1.html#:~:text=Los%20estados%20de%20Oaxaca%2C%20Veracruz,(Mapa%204.1.1) (accessed on 4 February 2022).
- Halffter, G.; Morrone, J.J. An analytical review of Halffter’s Mexican transition zone, and its relevance for evolutionary biogeography, ecology and biogeographical regionalization. Zootaxa 2017, 4226, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Morrone, J.J.; Escalante, T.; Rodríguez-Tapia, G.; Carmona, A.; Arana, M.; Mercado-Gómez, J.D. Biogeographic regionalization of the Neotropical region: New map and shapefile. An. Acad. Bras. Cienc. 2022, 94, e20211167. [Google Scholar] [CrossRef] [PubMed]
- Halffter, G. La zona de transición mexicana y la megadiversidad de México: Del marco histórico a la riqueza actual. Duguesiana 2017, 24, 77–89. [Google Scholar]
- Delgadillo-Moya, C. Moss distribution and the phytogeographical significance of the Neovolcanic Belt of Mexico. J. Biogeogr. 1987, 14, 69–78. [Google Scholar]
- Delgadillo-Moya, C. Patrones Biogeográficos de los musgos de México. In Una Perspectiva Latinoamericana de la Biogeografía, 1st ed.; Morrone, J.J., Llorente-Bousquets, J., Eds.; Conabio: Mexico City, Mexico, 2007; Volume 1, pp. 195–198. [Google Scholar]
- Thiel, H. Quantitative untersuchungen über die meiofauna des tiefseebodens. Veröff. Inst. Meeresf. 1966, 2, 131–148. [Google Scholar]
- Watling, L.; Guinotte, J.; Clark, M.R.; Smith, C.R. A proposed biogeography of the deep ocean floor. Prog. Oceanogr. 2013, 111, 91–112. [Google Scholar] [CrossRef]
- Cesari, M.; McInnes, S.J.; Bertolani, R.; Rebecchi, L.; Guidetti, L. Genetic diversity and biogeography of the south polar water bear Acutuncus antarcticus (Eutardigrada: Hypsibiidae)—Evidence that it is a truly pan-Antarctic species. Invertebr. Syst. 2016, 30, 635–649. [Google Scholar] [CrossRef]
- Gąsiorek, P.; Stec, D.; Morek, W.; Michalczyk, Ł. An integrative redescription of Hypsibius dujardini (Doyère, 1840), the nominal taxon for Hypsibioidea (Tardigrada: Eutardigrada). Zootaxa 2018, 4415, 45–75. [Google Scholar] [CrossRef] [PubMed]
- Gąsiorek, P.; Stec, D.; Morek, W.; Zawierucha, K.; Kaczmarek, Ł.; Lachowska-Cierlik, D.; Michalczyk, Ł. An integrative revision of Mesocrista Pilato, 1987 (Tardigrada: Eutardigrada: Hypsibiidae). J. Nat. Hist. 2016, 50, 45–46. [Google Scholar] [CrossRef]
- Blaxer, M.; Elsworth, B.; Daub, J. DNA taxonomy of a neglected animal phylum: An unexpected diversity of tardigrades. Proc. Royal Soc. B 2004, 271, S189–S192. [Google Scholar]
- Morek, W.; Surmacz, B.; López-López, A.; Michalczyk, Ł. “Everything is not everywhere”: Time-calibrated phylogeography of the genus Milnesium (Tardigrada). Mol. Ecol. 2021, 30, 3590–3609. [Google Scholar] [CrossRef]
- Guidetti, R.; Cesari, M.; Bertolani, R.; Altiero, T.; Rebecchi, L. High diversity in species, reproductive modes and distribution within the Paramacrobiotus richtersi complex (Eutardigrada, Macrobiotidae). Zool. Lett. 2019, 5, 1. [Google Scholar] [CrossRef]
- Gąsiorek, P.; Vončina, K.; Zając, K.; Michalczyk, Ł. Phylogeography and morphological evolution of Pseudechiniscus (Heterotardigrada: Echiniscidae). Sci Rep. 2021, 11, 7606. [Google Scholar] [CrossRef]
- Bonnet, L. Interet biogeographique et paleogeographique des thecamoebiens des sols. Ann. Stn. Biol. Besse 1984, 17, 298–334. [Google Scholar]
- Dragesco, J.; Dragesco-Kernéis, A. Ciliés libres de l’Afrique intertropicale. Faune Trop. 1986, 26, 1–559. [Google Scholar]
- Foissner, W. Protist diversity: Estimates of the near-imponderable. Protist 1999, 150, 363–368. [Google Scholar] [CrossRef]
- Foissner, W.; Agatha, S.; Berger, H. 2002. Soil ciliates (Protozoa, Ciliophora) from Namibia (Southwest Africa), with emphasis on two contrasting environments, the Etosha Region and the Namib Desert. Denisia 2002, 5, 1–1459. [Google Scholar]
- Tyler, P.A. Endemism in freshwater algae with special reference to the Australian region. Hydrobiologia 1996, 336, 1–9. [Google Scholar] [CrossRef]
- Vyverman, W. The Indo-Malaysian North-Australian phycogeographical region revised. Hydrobiologia 1996, 336, 107–120. [Google Scholar] [CrossRef]
- Palacios-Vargas, J.G. Biodiversidad de Collembola (Hexapoda: Entognatha) en México. Rev. Mex. Biodivers. 2014, 85, S220–S231. [Google Scholar] [CrossRef]
- Bebber, D.P.; Marriott, F.H.; Gaston, K.J.; Harris, S.A.; Scotland, R.W. Predicting unknown species numbers using discovery curves. Proc. Biol. Sci. 2007, 274, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Solow, A.R.; Smith, W.K. On estimating the number of species from the discovery record. Proc. R. Soc. B 2005, 272, 285–287. [Google Scholar] [CrossRef]
- Laforest, B.J.; Winegardner, A.K.; Zaheer, O.A.; Jeffery, N.W.; Boyle, E.E.; Adamowicz, S.J. Insights into biodiversity sampling strategies for freshwater microinvertebrate faunas through bioblitz campaigns and DNA barcoding. BMC Ecol. 2013, 13, 13. [Google Scholar] [CrossRef]
- Perbiche-Neves, G.; da Rocha, C.E.F.; Nogueira, M.G. Estimating cyclopoid copepod species richness and geographical distribution (Crustacea) across a large hydrographical basin: Comparing between samples from water column (plankton) and macrophyte stands. Zoologia 2014, 31, 239–244. [Google Scholar] [CrossRef]
- Doğramaci, M.; DeBano, S.J.; Wooster, D.E.; Kimoto, C.A. A method for subsampling terrestrial invertebrate samples in the laboratory: Estimating abundance and taxa richness. J. Insect Sci. 2010, 10, 25. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Lobo, J.M. Determining a combined sampling procedure for a reliable estimation of Araneidae and Thomisidae assemblages (Arachnida, Araneae). J. Arachnol. 2005, 33, 33–42. [Google Scholar] [CrossRef]
- Grabowski, B. Ecological investigations on moss-dwelling water bears (Tardigrada) with a report on three species new to the German fauna. Acta Biol. 1995, 7, 77–98. [Google Scholar]
- McInnes, S.J. Is it real? Proceedings of the eighth international symposium on Tardigrada, Copenhagen. Zool. Anz. 2001, 240, 461–466. [Google Scholar] [CrossRef]
- Daza, A.; Caicedo, M.; Lisi, O.; Quiroga, S. New records of tardigrades from Colombia with the description of Paramacrobiotus sagani sp. nov. and Doryphoribius rosanae sp. nov. Zootaxa 2017, 4362, 29–50. [Google Scholar] [CrossRef] [PubMed]
- Lisi, O.; Londoño, R.; Quiroga, S. Tardigrada from a sub-Andean forest in the Sierra Nevada de Santa Marta (Colombia) with the description of Itaquascon pilatoi sp. nov. Zootaxa 2014, 3841, 551–562. [Google Scholar] [CrossRef][Green Version]
- Londoño, R.; Daza, A.; Caicedo, M.; Quiroga, S.; Kaczmarek, Ł. The genus Milnesium (Eutardigrada: Milnesiidae) in the Sierra Nevada de Santa Marta (Colombia), with the description of Milnesium kogui sp. nov. Zootaxa 2015, 3955, 561–568. [Google Scholar] [CrossRef][Green Version]
- Londoño, R.; Daza, A.; Lisi, O.; Quiroga, S. Nueva especie de osito de agua Minibiotus pentannulatus (Tardigrada: Macrobiotidae) de Colombia. Rev. Mex. Biodivers. 2017, 88, 807–814. [Google Scholar] [CrossRef]
- Mayo, S.J.; Allkin, R.; Baker, W.; Blagoderov, V.; Brake, I.; Clark, B.; Govaerts, R.; Godfray, C.; Haigh, A.; Hand, R.; et al. Alpha e-taxonomy: Responses from the systematics community to the biodiversity crisis. Kew Bull. 2008, 63, 1–16. [Google Scholar] [CrossRef]
- Rasmussen, C. Joseph Vachal (1838–1911): French entomologist and politician. Zootaxa 2012, 3442, 1–52. [Google Scholar] [CrossRef]
| Taxa | Terra Typica | Worldwide Records and Distribution | Records in Mexico | Mexican Biogeographic Provinces |
|---|---|---|---|---|
| Heterotardigrada Doyère, 1840 | ||||
| Order Arthrotardigrada Marcus, 1927 | ||||
| Family Anisonychidae Møbjerg, Jørgensen & Kristensen, 2019 | ||||
| Anisonyches sp. Pollock, 1975 | - | Persic Gulf, Indic Ocean, Atlantic, Caribbean [94,95]. Widely distributed | Yucatán [38] | YP (1) |
| Family Archechiniscidae Binda, 1978 | ||||
| Archechiniscus bahamensis Bartels, Fontoura & Nelson, 2018 | Bahamas | Caribbean [94,95]. Neotropical | Caribbean Sea [46] | CS (2) |
| Archechiniscus sp. Schulz, 1953 | - | Atlantic, Caribbean, Coral Sea, Indic Ocean, Ionian Sea, Pacific Ocean, Tasman Sea [94,95]. Widely distributed | Yucatán [37] | YP (1) |
| Family Batillipedidae Ramazzotti, 1962 | ||||
| Batillipes sp. Richters, 1909 | - | Europe, Asia [94], North America [95]. Holarctic | Yucatán [37,38] | YP (2) |
| Family Coronarctidae Renaud-Mornant, 1974 | ||||
| Coronarctus mexicus Romano III, Gallo, D’Addabbo, Accogli, Baguley & Montagna, 2011 | Gulf of Mexico | North America [96]. Only registered near of the type locality | Gulf of Mexico [38] | NGM (1) |
| Coronarctus sp. Renaud-Mornant, 1974 | - | Pacific Ocean, Atlantic Ocean, Gulf of México [94,95]. Widely distributed | Gulf of Mexico [38] | NGM (1) |
| Family Halechiniscidae Thulin, 1928 | ||||
| Dipodarctus cf. subterraneus (Renaud-Debyser, 1959) | Atlantic Ocean | Pacific Ocean, Atlantic Ocean, Mediterranean Sea, Caribbean. [94,95] Widely distributed | Caribbean Sea, Yucatán [46] | CS (1), YP (1) |
| Dipodarctus sp. Pollock, 1995 | - | Pacific Ocean, Caribbean, Atlantic Ocean, Mediterranean Sea [94,95]. Widely distributed | Caribbean Sea, Yucatán [37,46] | CS (1), YP (1) |
| Florarctinae sp. Renaud-Mornant, 1982 | - | Pacific Ocean, Atlantic Ocean, Caribbean [94,95]. Holarctic | Caribbean Sea [46] | CS (1) |
| Florarctus yucatanensis Anguas-Escalante, Navarrete, Demilio, Pérez-Pech & Hansen, 2020 | Mexico | North America [48]. Only registered in the type locality | The Caribbean Sea, Quintana Roo [48] | CS (1), YP (2) |
| Halechiniscidae sp. Thulin, 1928 | - | Pacific Ocean, Atlantic Ocecan, Indic Ocean [94,95]. Widely distributed | Yucatán [46] | YP (1) |
| Halechiniscus cf. perfectus Schulz, 1955 | Mediterranean Sea | Mediterranean Sea, Caribbean Sea [94]. Only registered in the type locality | Quintana Roo [37] | Unknown |
| Wingstrandarctus corallinus Kristensen, 1984 | Australia | North America, Australia, Caribbean [94,95]. Widely distributed | Caribbean Sea [33] | CS (1) |
| Wingstrandarctus sp. Kristensen, 1984 | - | Pacific Ocean, Atlantic Ocean, Caribbean [94,95]. Widely distributed | Yucatán [37] | YP (1) |
| Family Stygarctidae Schulz, 1951 | ||||
| Megastygarctides sp. McKirdy, Schmidt & McGinty-Bayly, 1976 * | - | India, Caribbean [94,95]. Tropical | Caribbean Sea [44] | CS (1) |
| Family Styraconyxidae Kristensen & Renaud-Mornant, 1983 | ||||
| Paratanarctus sp. D’Addabbo Gallo, Grimaldi de Zio, Morone De Lucia & Troccoli, 1992 * | - | The Mediterranean Sea, Caribbean [94,95]. Palearctic-Neotropical | Caribbean Sea [46] | CS (1) |
| Styraconyx robertoi Pérez-Pech, Navarrete, Demilio, Anguas-Escalante & Hansen, 2020 | Mexico | North America [46]. Only registered in the type locality | Caribbean Sea, Yucatán [46] | CS, YP |
| Order Echiniscoidea Richters, 1926 | ||||
| Family Echiniscidae Thulin, 1928 | ||||
| Cornechiniscus lobatus (Ramazzotti, 1943) | Italy | North America [97], South America [98], Africa [99]. Widely distributed | Tamaulipas, Coahuila [25,32,86] | TP (1), SMOrP (2) |
| Echiniscoides sp. Plate, 1888 * | - | North America [97], Africa [99], Europe, Asia, Oceania 100]. Widely distributed | Yucatán [37] | YP (1) |
| Echiniscus becki Schuster & Grigarick, 1966 | USA | North America [97]. Only registered in the type locality | Baja California [41] | BCP (1) |
| Echiniscus blumi Richters, 1903 | Spitsbergen | North America [97], Europe [100], Africa [99]. Widely distributed | Baja California [41] | BCP (1) |
| Echiniscus cf. tamus Mehlen, 1969 | USA | North America [97]. Nearctic | Chihuahua, Nuevo León [25,28] | SMOrP (1), SMOcP (1) |
| Echiniscus kerguelensis Richters, 1904 | Kerguelen Is. | Europe, Antarctic, India [100], North America [97], Africa [99]. Widely distributed | Mexico State, Morelos [29,86] | TVBP (2) |
| Echiniscus manuelae da Cunha & du Nascimento Ribeiro, 1962 | Portugal | Europe [100], North America [97], Central America [101], South America [98]. Widely distributed | Nuevo León [25] | SMOrP (1) |
| Echiniscus siergristi Heinis, 1911 | Mexico | North America [97]. Only registered in the type locality | Oaxaca [26,30] | LPP (1) |
| Echiniscus sp. C.A.S. Schultze, 1840 | - | Europe, India, Antarctic, Oceania [100], North America [97], Africa [99]. Widely distributed | Oaxaca, Hidalgo, Mexico City, Quintana Roo [26,35,36,42,44,45] | SMOrP (2), TVBP (10), YP (2) |
| Kristenseniscus kofordi (Schuster & Grigarick, 1966) | Santa Cruz Is. (Ecuador) | North America [97], Central America [101], South America [98]. American | Chiapas [31] | VP (1) |
| Pseudechiniscus cf. juanitae de Barros, 1939 | Brazil | North America [97], Central America [101], South America [98]. American | Chiapas, Nuevo León [25,31] | TP, (1), LPP (1), VP (1) |
| Pseudechiniscus facettalis Petersen 1951 | Switzerland | Europe, Asia, Australia [100], North America [97], Central America [101], South America [98], Africa [99]. Widely distributed | Chihuahua [28] | SMOcP (1) |
| Pseudechiniscus quadrilobatus Iharos, 1969 | Vietnam | North America [97]. Only registered in the type locality | Chiapas [31] | VP (1) |
| Pseudechiniscus sp. Thulin, 1911 | - | North America [97], South America [98], Africa [99], Europa, Asia [100]. Widely distributed | Chihuahua [28] | SMOcP (1) |
| Pseudechiniscus suillus (Ehrenberg, 1853) | Switzerland | North America [97], Europa [100], Africa [99]. Widely distributed | Oaxaca [26] | SMSP (1) |
| Viridiscus viridis (Murray, 1910) | Oahu Is. (Hawaii) | North America [97] South America [98], Europe [100]. Widely distributed | Chihuahua [28] | SMOcP (1) |
| Viridiscus viridissimus (Péterfi, 1956) | Romania | Europe [100], North America [97], South America [98]. Widely distributed | Oaxaca [33] | BBP (1) |
| Class Eutardigrada Richters, 1926 | ||||
| Eutardigrada sp. Richters, 1926 | - | Europe, Asia, Oceania [100], North America [97], Central America [99], South America [98], Africa [99]. Widely distributed | Hidalgo [45] | TVBP (1) |
| Order Apochela Schuster, Nelson, Grigarick & Christenberry, 1980 | ||||
| Family Milnesiidae Ramazzotti, 1962 | ||||
| Milnesioides sp. Claxton, 1999 * | - | Australia [101], North America [97]. Australian-Nearctic | Mexico City [35] | TVBP (10) |
| Milnesium barbadosense Meyer & Hinton, 2012 * | Barbados | North America [97], South America [98]. American | Nuevo León, Tamaulipas [25,49] | SMOrP (7), TP (1) |
| Milnesium cassandrae Moreno-Talamantes, Roszkowska, García-Aranda, Flores-Maldonado & Kaczmarek, 2019 | Mexico | North America [97]. Only registered in the type locality | Nuevo León, Coahuila, Tamaulipas [25,49] | TP (9) |
| Milnesium fridae Moreno-Talamantes, León-Espinosa, García-Aranda, Flores-Maldonado & Kaczmarek, 2020 | Mexico | North America [97]. Only registered in the type locality | Nuevo León [49] | TP (2) |
| Milnesium sp. Doyère, 1840 | - | Europe, Asia, Oceania [100], North America [97], Central America [101], South America [98], Africa [99]. Widely distributed | Baja, California, Nuevo León, San Luis Potosí, Mexico City, Hidalgo, Quintana Roo [34,35,41,42,43,44,49,87] | BCP (1), ChDP (2), SMOrP (1), TVBP (1), YP (1) |
| Milnesium tardigradum Doyère, 1840 | France | Europe, Asia, Oceania [100], North America [97], Central America [101], South America [98], Africa [99]. Widely distributed | Chihuahua, Morelos, Chiapas, Mexico State [28,29,33] | SMOcP (6), TVBP (2), VP (1) |
| Order Parachela Schuster, Nelson, Grigarick & Christenberry, 1980 | ||||
| Family Calohypsibiidae Pilato, 1969 | ||||
| Calohypsibius cf. ornatus (Richters, 1900) | Ireland | North America [97], South America [98]. Widely distributed | Mexico State [35] | TVBP (1) |
| Family Doryphoribiidae Gąsiorek, Stec, Morek & Michalczyk, 2019 | ||||
| Doryphoribius chetumalensis Pérez-Pech, Anguas-Escalante, Cutz-Pool & Guidetti, 2017 | Mexico | North America [97]. Only registered in the type locality | Quintana Roo [45] | YP (4) |
| Doryphoribius dawkinsi Michalczyk & Kaczmarek, 2010 * | Costa Rica | North America [97], Central America. [101] American | Nuevo León [25] | SMOrP (2) |
| Doryphoribius evelinae (Marcus, 1928) | Germany | Europe [100], North America [97], South America [98]. Widely distributed | Chihuahua [28,30] | SMOcP (1) |
| Doryphoribius flavus (Iharos, 1966) | Hungary | Europe [100], North America [97], Central America [101]. Widely distributed | Chiapas [31] | VP (1) |
| Doryphoribius gibber Beasley & Pilato, 1987 | USA | North America [97], Asia [100]. Holarctic | Chiapas [31] | VP (1) |
| Doryphoribius mexicanus Beasley, Kaczmarek & Michalczyk, 2008 | Mexico | North America [97]. Only registered in the type locality | Oaxaca [29] | SMSP (1) |
| Doryphoribius quadrituberculatus Kaczmarek & Michalczyk, 2004 * | Costa Rica | North America [97], Central America. [101] American | Nuevo León [25] | TP (1) |
| Doryphoribius sp. Pilato, 1969 | - | North America [97], South America [98], Europe, Asia [100], Africa [99]. Widely distributed | Quintana Roo [36,45] | YP (5) |
| Paradiphascon sp. Dastych, 1992 * | - | Africa [99], North America [97]. Tropical-Nearctic | Mexico City [35] | TVBP (20) |
| Family Halobiotidae Gąsiorek, Stec, Morek & Michalczyk, 2019 | ||||
| Dianea sattleri (Richters, 1902) | Germany | Europe, Asia, Oceania [100], North America [97], Central America, [101] South America [98], Africa [99]. Widely distributed | Chiapas [31] | VP (1) |
| Haplomacrobiotus hermosillensis May 1948 | Mexico | North America [97]. Only registered in the type locality | Sonora [27,30] | SMOcP (1) |
| Family Hypsibiidae Pilato, 1969 | ||||
| Adropion scoticum (Murray, 1905) | Sweden | Europe [100], North America [97], South America [98], Africa [99]. Widely distributed | Mexico State [47] | TVBP (3) |
| Adropion sp. Pilato, 1987 | - | North America [97], South America [98], Africa [99], Europe, Asia [100]. Widely distributed | Mexico City [35] | TVBP (40) |
| Astatumen trinacriae Arcidiacono, 1962 | Italy | Europe [100], North America [97], South America [98], Africa [99]. Widely distributed | Nuevo León [25] | SMOrP (1), DChP (1) |
| Diphascon chilenense Plate, 1888 | Chile | North America [97], South America [98]. American | Chihuahua [28] | SMOcP (1) |
| Diphascon mitrense Piato, Binda, & Qualtieri, 1999 | Argentina | North America [97], South America [98]. American | Mexico State [47] | TVBP (2) |
| Diphascon pingue (Marcus, 1936) | Germany | North America [97], Central America [101], South America [98], Africa [99], Europe, Asia, Antarctic [100]. Widely distributed | Nuevo León, Coahuila, Mexico State [25,47] | TVBP (4), SMOrP (3), ChDP (1) |
| Diphascon sp. Plate, 1888 | - | North America [97], South America [98], Antarctica, Europe, Asia [100], Africa [99]. Widely distributed | Hidalgo, Mexico City [35,44] | TVBP (31) |
| Guidettion carolae (Binda & Pilato, 1969) | Italy | Europe [100] and North America [95]. Holarctic | Nuevo León [25] | SMOrP (1) |
| Hypsibius cf. convergens (Urbanowicz, 1925) | Lithuania | Europe, Asia, Antarctic, Oceania [100], Africa [99], North America [97], South America [98]. Widely distributed | Chihuahua [28] | ChDP (1), SMOcP (4) |
| Hypsibius cf. microps Thulin, 1928 | Sweden | Europe, Asia [100], North America [97], South America [98], Africa [99]. Widely distributed | Mexico State [47] | TVBP (4) |
| Hypsibius cf. pallidus Thulin, 1911 | Sweden | Europe, Asia [100], North America [97], South America [98], Africa [99]. Widely distributed | Mexico State [30,47] | TVBP (3) |
| Hypsibius sp. Ehrenberg, 1848 | - | North America [97], South America [98], Antarctica, Europe [100], Africa [98]. Widely distributed | Mexico City, Mexico State [35] | TVBP (40) |
| Isohypsibius sculptus (Ramazzotti, 1962) | Chile | Europe [100], South America [98], North America [97]. Widely distributed | Morelos [29] | TVBP (1) |
| Isohypsibius sp. Thulin, 1928 | - | North America [97], Europa, Asia, Antarctica [100], Africa [99]. Widely distributed | Hidalgo [44] | SMOrP (1) |
| (?) Isohypsibius sp. Thulin, 1928 | - | North America [97], Europa, Asia, Antarctica [100], Africa [99]. Widely distributed | Chihuahua [28] | SMOcP (1) |
| Itaquascon umbellinae de Barros, 1939 | Brazil | North America [97], South America [98], Africa [99]. Widely distributed | Chihuahua [28] | SMOcP (1) |
| Pilatobius nodulosus Ramazzotti, 1957 | USA | North America [97]. Nearctic | Mexico State [29,47] | SMOrP (2), TVBP (3) |
| Family Macrobiotidae Thulin, 1928 | ||||
| Calcarobiotus cf. polygonatus (Binda & Guglielmino, 1991) | Tanzania | Africa [99], North America [97]. Tropical-Nearctic | Nuevo León [39] | SMOrP (1) |
| Famelobiotus sp. Pilato, Binda & Lisi, 2004 * | - | Asia [100], North America [97]. Oriental-Nearctica | Mexico City [35] | TVBP (10) |
| Macrobiotus alvaroi Pilato & Kaczmarek, 2007 | Costa Rica | Central America [101], North America [97]. American | Chiapas [33] | VP (1) |
| Macrobiotus anemone Meyer, Domingue & Hinton, 2014 * | USA | North America [97]. Nearctic | Tamaulipas [25] | TP (1) |
| Macrobiotus ascensionis Richters, 1908 | Ascension Is. | Atlantic Ocean [94,95]. Only registered in the type locality | Unknown [30] | |
| Macrobiotus cf. acadianus (Meyer & Domingue, 2011) * | USA | North America [97]. Nearctic | Nuevo León [25] | TP (1) |
| Macrobiotus echinogenitus Richters, 1903 | Spitsbergen (Norway) | Europe, Asia [100], Africa [99], North America [97], South America. [98] Widely distributed | Mexico State [29] | TVBP (1) |
| Macrobiotus hufelandi C.A.S. Schultze, 1834 | Germany | Europe, Asia [100], North America [97], Central America [101], South America [98], Africa [99]. Widely distributed | Mexico State, Oaxaca, Chihuahua [26,28,29,41] | TVBP (1), LPP (1), SMOcP (1) |
| Macrobiotus kazmierskii Kaczmarek & Michalczyk, 2009 * | Argentina | North America [97] South America. [98] American | Nuevo León [25] | TP (1) |
| Macrobiotus ocotensis Pilato, 2006 | Mexico | North America [97]. Only registered in the type locality | Chiapas [102] | VP (1) |
| Macrobiotus persimilis Binda & Pilato 1972 * | Italy | Europe [100], North America [97]. Holarctic | Chiapas [33] | ChHP (1) |
| Macrobiotus rubens Murray, 190 | Himalayas (India) | India, Australia [100], North America [97], South America [98], Africa [99]. Widely distributed | Oaxaca [26,30] | LPP (1) |
| Macrobiotus sp. C.A.S. Schultze, 1834 | - | Europe, India, Antarctic, Oceania [100], North America [97], Africa [99]. Widely distributed | Oaxaca, Hidalgo, Mexico City, Mexico State, Quintana Roo [26,30,42,44,47] | SMOrP (4), TVBP (45), LPP (1) YP (2) |
| Macrobiotus terminalis (Bertolani & Rebecchi, 1993) | Italy | Europe [100] North America [97]. Holarctic | Oaxaca [33,36] | BBP (1) |
| Mesobiotus contii Pilato & Lisi, 2006 | Mexico | North America [97]. Only registered in the type locality | Chiapas [31] | VP |
| Mesobiotus coronatus (de Barros 1942) | Brazil | South America [98], Central America [101], North America [97], Europa [100], Africa [99]. Widely distributed | Oaxaca, Chihuahua [28,33] | BBP (1), SMOcP (2) |
| Mesobiotus diffusus (Binda & Pilato, 1987) * | Tunisia (Africa) | Africa [99], North America [97]. Tropical-Nearctic | Mexico State, Coahuila [39,88] | TVBP (1), SMOrP (1) |
| Mesobiotus harmsworthii (Murray, 1907) | Franz Joseph Land (Russia) | Europe [100], North America [97], Central America [101], South America [98], Africa [99]. Widely distributed | Sinaloa, Oaxaca [26,29] | LPP (1), SMOcP (2) |
| Mesobiotus sp. Vecchi, Cesari, Bertolani, Jönsson, Rebecchi & Guidetti, 2016a | - | North America [97], South America [98], Europe, Asia [100], Africa [99]. Widely distributed | Hidalgo, Mexico City, Quintana Roo [34,42,44,87] | SMOrP (1), TVBP (2), YP (1) |
| Mesocrista sp. Pilato, 1987 | - | Europe, Asia [100], North America [97]. Holarctic | Mexico City [35] | TVBP (10) |
| Minibiotus cf. intermedius (Plate, 1888) | Chile | Europe, Asia [100], South America [98], North America [97]. Widely distributed | Chihuahua, Nuevo León [28] | SMOcP (1) |
| Minibiotus citlalium Dueñas-Cedillo & García-Román, 2020 in Dueñas-Cedillo et al. 2020 | Mexico | North America [97]. Only registered in the type locality | Mexico State [47] | TVBP (1) |
| Minibiotus continuus Pilato & Lisi, 2006 | Mexico | North America [97]. Only registered in the type locality | Chiapas [31] | TP (1), VP (1), SMOrP (2) |
| Minibiotus furcatus (Ehrenberg, 1859) * | Switzerland | Europe [100], North America [97]. Holarctic | Mexico State, Morelos [29] | TVBP (1) |
| Minibiotus sidereus Pilato, Binda & Lisi, 2003 | Ecuador | South America [98], North America [97]. American | Mexico State [47] | TVBP (6) |
| Minibiotus sp. R.O. Schuster, 1980 | - | North America [97], South America [98], Antarctica, Europe, Asia, Oceania [100], Africa [99]. Widely distributed | Mexico City, Nuevo León [35,39] | TVBP (40), SMOrP (1) |
| Paramacrobiotus cf. klymenki Pilato, Kiosya, Lisi & Sabella, 2012 * | Ukraine | Europe [100], North America [97]. Holarctic | Nuevo León [39] | SMOrP (1) |
| Paramacrobiotus areolatus (Murray, 1907) | Svalbard (Norway) | Europe [100], North America [97], Central America [101], South America [98], Africa [99]. Widely distributed | Chihuahua [28,45] | SMOcP (1) |
| Paramacrobiotus richtersi (Murray, 1911) | Ireland | Europe [100], North America [97], Central America [101], South America [98], Africa [99]. Widely distributed | Nuevo León [28] | SMOcP |
| Paramacrobiotus sp. Guidetti, Schill, Bertolani, Dandekar & Wolf, 2009 | - | North America [97], South America [98], Europe [100], Africa [99]. Widely distributed | Hidalgo, Mexico, City, Nuevo León [35,41,87] | TVBP (10), SMOrP (3) |
| Family Microhypsibiidae Pilato, 1998 | ||||
| Ramazzottius baumanni (Ramazzotti, 1962) | Chile | North America [97], Central America [101], South America [98], Australia [100]. Widely distributed | Mexico State, Michoacán, Morelos [29] | TVBP (3) |
| Ramazzottius cf. oberhaeuseri (Doyère, 1840) | Germany | Europe, Asia, Oceania [100], North America [97], South America [98], Africa [99]. Widely distributed | Mexico State, Michoacán, Nuevo León [25,29,34] | TP (1), SMOrP (1), TVBP (2) |
| Ramazzottius sp. Binda & Pilato, 1986 | - | North America [97], Antarctica, Europe [100], Africa [99]. Widely distributed | Baja California, Mexico City, Quintana Roo [35,43] | BCP (1), TVBP (30), YP (1) |
| Family Murrayidae Guidetti, Rebecchi & Bertolani, 2000 | ||||
| Dactylobiotus parthenogeneticus Bertolani, 1982 * | Italy | Europe [100], North America [97], South America [98]. Widely distributed | Nuevo León [39] | SMOrP (1) |
| Family Richtersiusidae Guidetti, Schill, Giovannini, Massa, Goldoni, Ebel, Förschler, Rebecchi & Cesari, 2021 | ||||
| Diaforobiotus islandicus (Richters, 1904) | Iceland | North America [97], Central America [101], Europa, Asia [100], Africa [99]. Widely distributed | Nuevo León [40] | SMOrP |
| Number of Expected Tardigrades Species | |||||
|---|---|---|---|---|---|
| Taxa | Known Species in the World | Known Species in Mexico | Percentage of Known Species in Mexico (%) | Actual Richness * | Estimated Richness ++ |
| Plants | 390,900 | 23,314 | 5.96 | 82 | 158 |
| Mosses | 12,754 | 984 | 7.71 | 106 | 205 |
| Birds | 20,034 | 1115 | 5.56 | 77 | 148 |
| Reptiles | 10,970 | 864 | 7.87 | 109 | 209 |
| Mammals | 5629 | 564 | 10.01 | 138 | 266 |
| Fishes | 34,200 | 2763 | 8.07 | 111 | 214 |
| Insects | 955,025 | 69,163 | 7.24 | 100 | 192 |
| Arthropods | 1,013,825 | 80,000 | 7.89 | 109 | 209 |
| Protura | 804 | 17 | 2.11 | 29 | 56 |
| Diplura | 1008 | 48 | 4.76 | 66 | 126 |
| Copepods | 11,500 | 62 | 0.53 | 7 | 14 |
| Onychophorans | 186 | 3 | 1.61 | 22 | 43 |
| Nematodes | 8375 | 402 | 4.8 | 66 | 127 |
| Rotifers | 3000 | 184 | 6.13 | 85 | 163 |
| Cladocerans | 568 | 108 | 19.01 | 262 | 505 |
| Martínez-Meyer et al. [24] ° | 8.5 | 117 | 226 | ||
| Mean | 7.27 | 91 | 176 | ||
| The Ratio of Known to Unknown Species | |||
|---|---|---|---|
| Global richness | Range of species estimated | Mean of species estimated | |
| Known richness | 1380 a | 77–262 | 91 |
| Estimated richness | 2654 b | 126–505 | 176 |
| Accumulation curve | |||
| Effectively record | Samples needed c | Estimated | |
| Genus | 78% | 572 | 44 |
| Species | 39% | 2714 | 292 |
| GENUS | SPECIES | |
|---|---|---|
| R2 | 0.99 | 0.99 |
| a | 1.527 | 2.153 |
| b | 0.033 | 0.007 |
| m | 0.089 | 0.78 |
| Recorded proportion | 78% | 39% |
| Missing samples | 572 | 2714 |
| Estimated richness | 44 | 292 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Román, J.; Dueñas-Cedillo, A.; Cervantes-Espinoza, M.; Flores-Martínez, J.J.; Vargas-Mendoza, C.F.; Ruiz, E.A.; Armendáriz-Toledano, F. A Strategy to Provide a Present and Future Scenario of Mexican Biodiversity of Tardigrada. Diversity 2022, 14, 280. https://doi.org/10.3390/d14040280
García-Román J, Dueñas-Cedillo A, Cervantes-Espinoza M, Flores-Martínez JJ, Vargas-Mendoza CF, Ruiz EA, Armendáriz-Toledano F. A Strategy to Provide a Present and Future Scenario of Mexican Biodiversity of Tardigrada. Diversity. 2022; 14(4):280. https://doi.org/10.3390/d14040280
Chicago/Turabian StyleGarcía-Román, Jazmín, Alba Dueñas-Cedillo, Montserrat Cervantes-Espinoza, José Juan Flores-Martínez, Carlos Fabián Vargas-Mendoza, Enrico Alejandro Ruiz, and Francisco Armendáriz-Toledano. 2022. "A Strategy to Provide a Present and Future Scenario of Mexican Biodiversity of Tardigrada" Diversity 14, no. 4: 280. https://doi.org/10.3390/d14040280
APA StyleGarcía-Román, J., Dueñas-Cedillo, A., Cervantes-Espinoza, M., Flores-Martínez, J. J., Vargas-Mendoza, C. F., Ruiz, E. A., & Armendáriz-Toledano, F. (2022). A Strategy to Provide a Present and Future Scenario of Mexican Biodiversity of Tardigrada. Diversity, 14(4), 280. https://doi.org/10.3390/d14040280







