Population Genetic Structure and Diversity of Metaphire remanens (Oligochaeta: Megascolecidae) Based on Mitochondrial DNA Analysis, with a Note on a New Species of Metaphire remanens sp. nov.
Abstract
1. Introduction
2. Materials and Methods
2.1. Earthworm Sampling
2.2. DNA Extraction, Amplification, and Sequencing
2.3. Sequence Alignment, Population Genetic Structure and Diversity, Divergence Time, and Spread History
3. Results
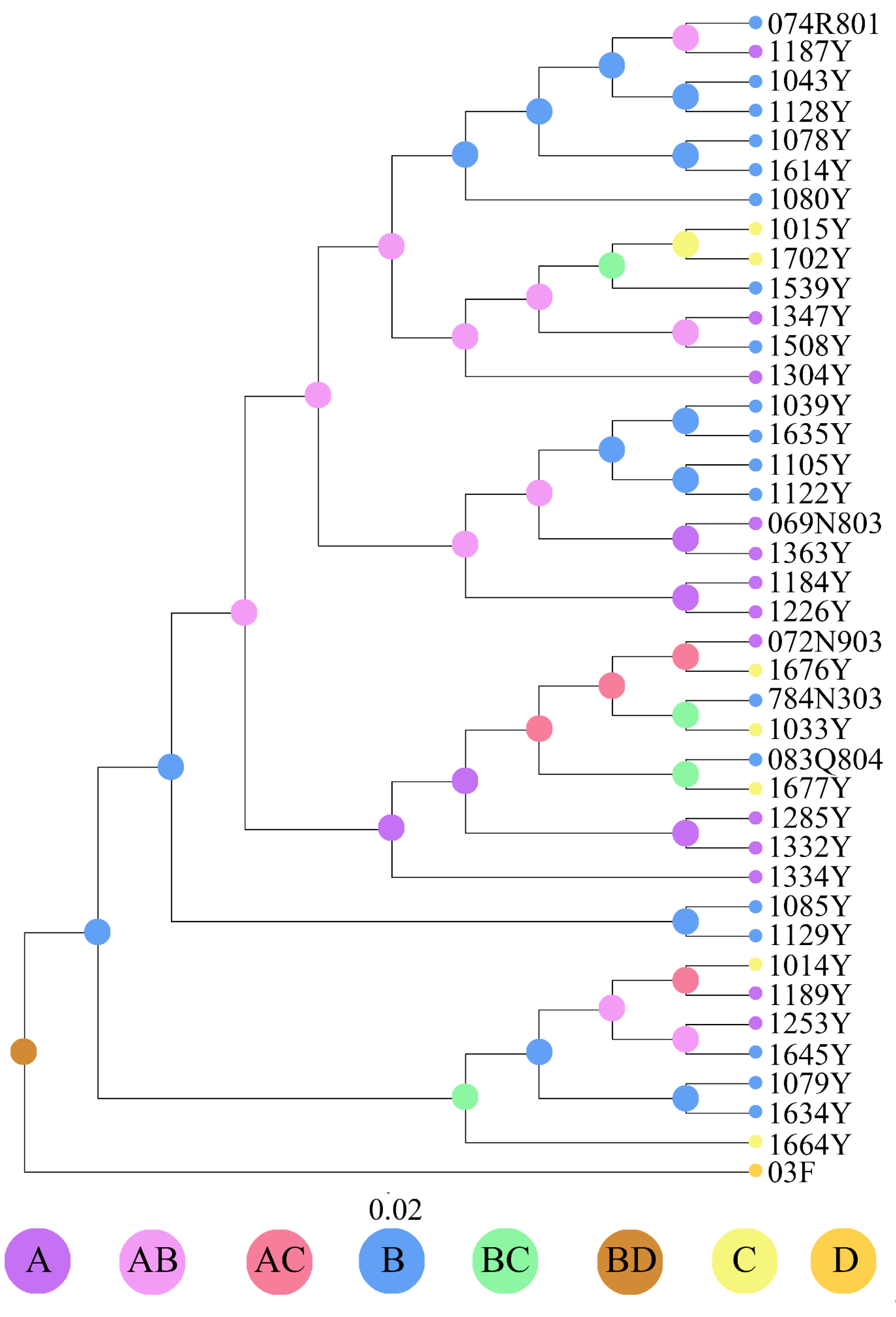
3.1. Population Genetic Structure and Diversity
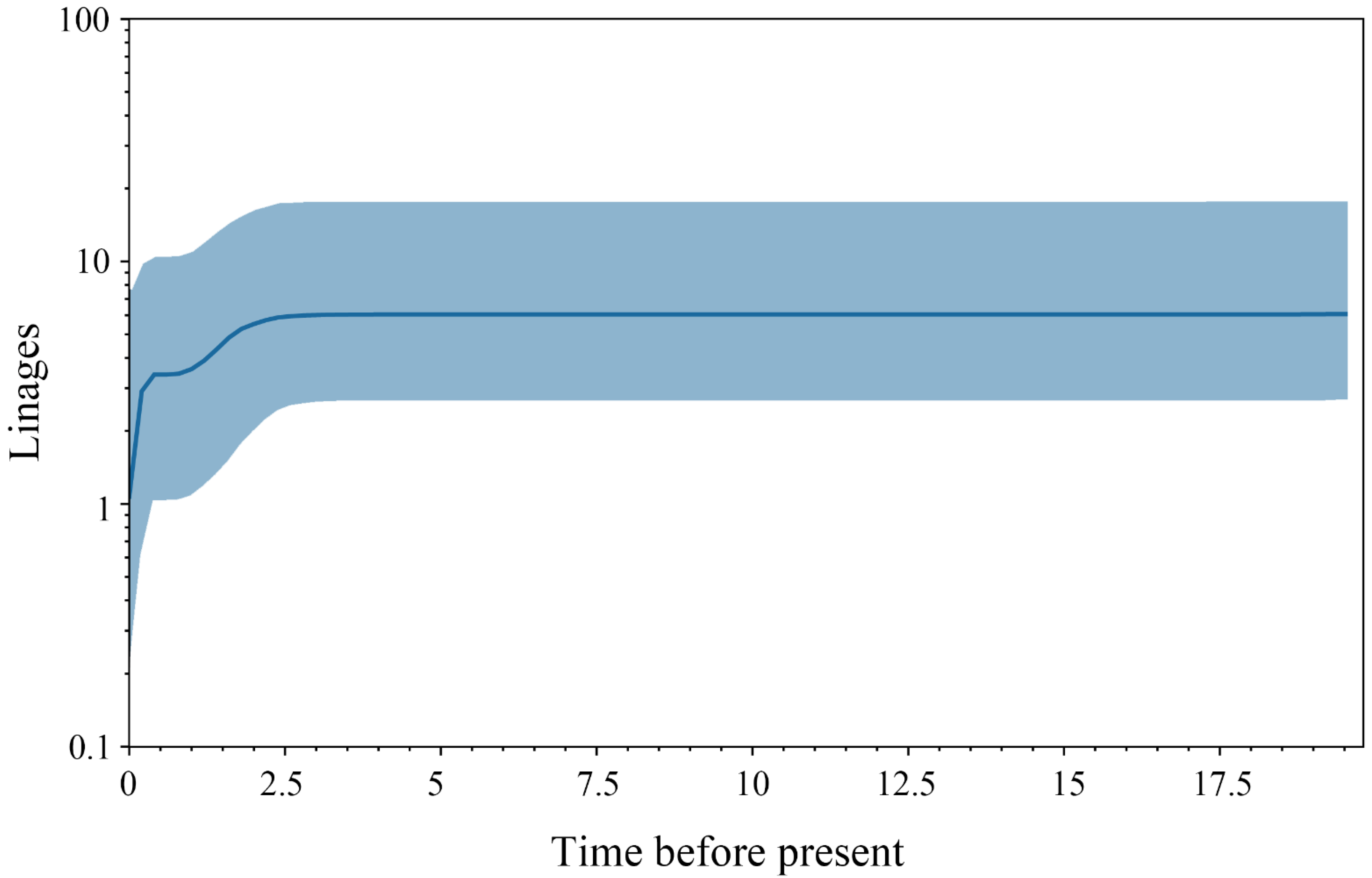
3.2. Divergence Time and Colonization History
4. Discussion
5. Conclusions
6. Description of Metaphire remanens sp. nov.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thakuria, D.; Schmidt, O.; Finan, D.; Egan, D.; Doohan, F.M. Gut Wall Bacteria of Earthworms: A natural Selection Process. ISME J. 2010, 4, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Groffman, P.M.; Fahey, T.J.; Fisk, M.C.; Yavitt, J.B.; Sherman, R.E.; Bohlen, P.J.; Maerz, J.C. Earthworms Increase Soil Microbial Biomass Carrying Capacity and Nitrogen Retention in Northern Hardwood Forests. Soil Biol. Biochem. 2015, 87, 51–58. [Google Scholar] [CrossRef]
- Sun, J.; James, S.W.; Jiang, J.; Yao, B.; Zhang, L.; Liu, M.; Qiu, J.; Hu, F. Phylogenetic Evaluation of Amynthas Earthworms from South China Reveals the Initial Ancestral State of Spermathecae. Mol. Phylogenet. Evol. 2017, 115, 106–114. [Google Scholar] [CrossRef] [PubMed]
- De Sosa, I.; Marchán, D.F.; Novo, M.; Díaz Cosín, D.J.; Giribet, G.; Fernández, R. Insights into the Origin of parthenogenesis in Oligochaetes: Strong Genetic Structure in a Cosmopolitan Earthworm is Not Related to Reproductive Mode. Eur. J. Soil Biol. 2017, 81, 31–38. [Google Scholar] [CrossRef]
- Shen, H.; Yu, H.; Chen, J. Parthenogenesis in Two Taiwanese Mountain Earthworms Amynthas catenus Tsai et al., 2001 and Amynthas hohuanmontis Tsai et al., 2002 (Oligochaeta, Megascolecidae) revealed by AFLP. Eur. J. Soil Biol. 2012, 51, 30–36. [Google Scholar] [CrossRef]
- Real, F.M.; Haas, S.A.; Franchini, P.; Xiong, P.; Simakov, O.; Kuhl, H.; Schöpflin, R.; Heller, D.; Moeinzadeh, M.H. The Mole Genome Reveals Regulatory Rearrangements Associated with Adaptive Intersexuality. Science 2020, 370, 208–214. [Google Scholar] [CrossRef]
- Lo, E.Y.Y.; Stefanović, S.; Dickinson, T.A. Geographical Parthenogenesis in Pacific Northwest Hawthorns (Crataegus; Rosaceae). Botany 2013, 91, 107–116. [Google Scholar] [CrossRef]
- Jared, C.; Alexandre, C.; Mailho-Fontana, P.L.; Pimenta, D.C.; Brodie, E.D.; Antoniazzi, M.M. Toads Prey upon Scorpions and are Resistant to Their Venom: A biological and Ecological Approach to Scorpionism. Toxicon 2020, 178, 4–7. [Google Scholar] [CrossRef]
- Kellner, K.; Seal, J.N.; Heinze, J. Sex at the Margins: Parthenogenesis vs. Facultative and Obligate Sex in a Neotropical Ant. J. Evol. Biol. 2013, 26, 108–117. [Google Scholar] [CrossRef]
- Jiang, J.B. Taxonomy and Phylogeny of the Family Megascolecidae Earthworm from China. Ph.D. Thesis, Shanghai Jiao Tong University, Shanghai, China, 2016. (In Chinese). [Google Scholar]
- Minamiya, Y.; Yokoyama, J.; Fukuda, T. A Phylogeographic Study of the Japanese Earthworm, Metaphire sieboldi (Horst, 1883) (Oligochaeta: Megascolecidae): Inferences from Mitochondrial DNA Sequences. Eur. J. Soil Biol. 2009, 45, 423–430. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Zhang, D.H.; Xu, Y.L.; Zhang, G.S.; Sun, Z.J. Effects of Fragmentation on Genetic Variation in Populations of the Terrestrial Earthworm Drawida Japonica Michaelsen, 1892 (Oligochaeta, Moniligastridae) in Shandong and Liaodong peninsulas, China. J. Nat. Hist. 2012, 46, 1387–1405. [Google Scholar] [CrossRef]
- Aspe, N.M.; James, S.W. Molecular Phylogeny and Biogeographic Distribution of Pheretimoid Earthworms (clitellata: Megascolecidae) of the Philippine Archipelago. Eur. J. Soil Biol. 2018, 85, 89–97. [Google Scholar] [CrossRef]
- Novo, M.; Almodovar, A.; Fernandez, R.; Giribet, G.; Cosin, D.J.D. Understanding the Biogeography of a Group of Earthworms in the Mediterranean Basin-The phylogenetic puzzle of Hormogastridae (Clitellata: Oligochaeta). Mol. Phylogenet. Evol. 2011, 61, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Dupont, L.; Gresille, Y.; Richard, B.; Decaens, T.; Mathieu, J. Dispersal Constraints and Fine-Scale Spatial Genetic Structure in Two Earthworm Species. Biol. J. Linn. Soc. 2015, 114, 335–347. [Google Scholar] [CrossRef]
- Arbogast, B.S. Phylogeography: The history and Formation of Species. Am. Zool. 2001, 41, 134–135. [Google Scholar] [CrossRef][Green Version]
- Pop, A.A.; Wink, M.; Pop, V.V. Use of 18S, 16S rDNA and Cytochrome C Oxidase Sequences in Earthworm Taxonomy (Oligochaeta, Lumbricidae). Pedobiologia 2003, 47, 428–433. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA Primers for Amplification of Mitochondrial Cytochrome C Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Bely, A.E.; Wray, G.A. Molecular Phylogeny of Naidid Worms (Annelida: Clitellata) Based on Cytochrome Oxidase I. Mol. Phylogenet. Evol. 2004, 30, 50–63. [Google Scholar] [CrossRef]
- Perez-Losada, M.; Ricoy, M.; Marshall, J.C.; Dominguez, J. Phylogenetic Assessment of the Earthworm Aporrectodea caliginosa species complex (Oligochaeta: Lumbricidae) Based on Mitochondrial and Nuclear DNA Sequences. Mol. Phylogenet. Evol. 2009, 52, 293–302. [Google Scholar] [CrossRef]
- Hillis, D.M.; Mable, B.K.; Larson, A.; Davis, S.K.; Zimmer, E.A. Nucleic acids, IV: Sequencing and Cloning. In Molecular Systematics, 2nd ed.; Hillis, D.M., Moritz, C., Mable, B.K., Eds.; Sinauer Associates, Inc.: Massachusetts, MA, USA, 1996; pp. 321–381. [Google Scholar]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More Models, New Heuristics and Parallel Computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choiceacross a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin ver. 3.0: An Integrated Software Package for Population Genetics Data Analysis. Evol. Bioinform. 2005, 1, 47–50. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. PopART: Full-Feature Software for Haplotype Network Construction. Methods. Ecol. Evol. 2015, 6, 9. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A Software for Comprehensive Analysis of DNA Poly-Morphism Data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Tajima, F. Statistical Method for Testing the Neutral Mutation Hypothesis by DNA Polymorphisms. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef]
- Fu, Y.X. Statistical Tests of Neutrality of Mutations Against Population Growth, Hitchhiking and Background Selection. Genetics 1997, 147, 915–925. [Google Scholar] [CrossRef]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian Phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Dong, X.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Pérez, L.M.; Breinholt, J.W.; Porto, P.G.; Aira, M.; Domínguez, J. An Earthworm Riddle: Systematics and Phylogeography of the Spanish Lumbricid Postandrilus. PLoS ONE 2011, 6, e28153. [Google Scholar] [CrossRef]
- Boore, J.L.; Brown, W.M. Complete DNA Sequence of the Mitochondrial Genome of the Annelid Worm Lumbricus Terrestris. Genet. Mol. Res. 1995, 141, 305–319. [Google Scholar]
- Welch, D.M.; Meselson, M. Evidence for the Evolution of Bdelloid Rotifers without Sexual Reproduction or Genetic Exchange. Science 2000, 288, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Graham, B. The Masterpiece of Nature: The Evolution and Genetics of Sexuality; University of Califormia Press: California, CA, USA, 1982; p. 635. [Google Scholar]
- Cosín, D.J.D.; Novo, M.; Fernández, R. Reproduction of earthworms: Sexual selection and parthenogenesis. In Biology of Earthworms; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Gates, G. Contributions to North American earthworms. No. 3. IV. The trapezoides species group. Bull. Tall Timbers Res. Stn. 1972, 12, 146. [Google Scholar]
- Qiu, J.; Bouché, M. Contribution to the taxonomy of Hormogastridae (Annelida: Oligochaeta) with Description of New Species from Spain. Doc. Pedozool. Integrol. 1998, 4, 164–177. [Google Scholar]
- Bonin, A.; Nicole, F.; Pompanon, F.; Miaud, C.; Taberlet, P. Population Adaptive Index: A New Method to Help Measure Intraspecific Genetic Diversity and Prioritize Populations for Conservation. Conserv. Biol. 2007, 21, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.A.S.; Bowen, B.W. Shallow Population Histories in Deep Evolutionary Lineages of Marine Fishes: Insights from Sardines and Anchovies and Lessons for Conservation. J. Hered. 1998, 89, 415–426. [Google Scholar] [CrossRef]
- Nei, M. Molecular Evolutionary Genetics; Columbia University Press: New York, NY, USA, 1987; pp. 92–145. [Google Scholar]
- Christensen, B. Asexual Propagation and Reproductive Strategies in Aquatic Oligochaeta. Hydrobiologia 1984, 115, 91–95. [Google Scholar] [CrossRef]
- Head, M.J.; Gibbard, P.L. Formal Subdivision of the Quaternary System/Period: Past, Present, and Future. Quat. Int. 2015, 383, 4–35. [Google Scholar] [CrossRef]
- Dong, Y.; Jiang, J.B.; Yuan, Z.; Zhao, Q.; Qiu, J.P. Population Genetic Structure Reveals Two Lineages of Amynthas triastriatus (Oligochaeta: Megascolecidae) in China, With Notes on a New Subspecies of Amynthas triastriatus. Int. J. Environ. Res. Public Health 2020, 17, 1538. [Google Scholar] [CrossRef]
- Hewitt, G.M. Genetic Consequences of Climatic Oscillations in the Quaternary. Philos. Tans. R. Soc. Lond. B Biol. Sci. 2004, 359, 183–195. [Google Scholar] [CrossRef]
- Qiu, Y.X.; Fu, C.X.; Comes, H.P. Plant Molecular Phylogeography in China and Adjacent Regions: Tracing the Genetic Imprints of Quaternary Climate and Environmental Change in the World’s Most Diverse Temperate Flora. Mol. Phylogenet. Evol. 2011, 59, 225–244. [Google Scholar] [CrossRef]
- Ren, G.P.; Mateo, R.G.; Liu, J.Q.; Suchan, T.; Alvarez, N.; Guisan, A.; Conti, E.; Salamin, N. Genetic Consequences of Quaternary Climatic Oscillations in the Himalayas: Primula Tibetica as a Case Study Based on Restriction Site-Associated DNA Sequencing. New Phytol. 2017, 213, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.E. Late Pleistocene climate of Europe: A review. GSA Bull. 1961, 72, 933–984. [Google Scholar] [CrossRef]
- Jiang, J.B.; Qiu, J.P. Origin and Evolution of Earthworms Belonging to the Family Megascolecidae in China. Biodivers. Sci. 2018, 26, 1074–1082. [Google Scholar] [CrossRef]
- Sun, J. Taxonomy and Molecular Phylogeny of Amynthas Earthworms from China. Ph.D. Thesis, Shanghai Jiao Tong University, Shanghai, China, 2013. [Google Scholar]
- Sims, R.W.; Easton, E.G. A Numerical Revision of the Earthworm Genus Pheretima auct (Megascolecidae: Oligochaeta) with the Recognition of New Genera and an Appendix on the Earthworms Collected by the Royal Society North Borneo Expedition. Biol. J. Linn. Soc. 1972, 4, 169–268. [Google Scholar] [CrossRef]
- Zhang, W.X.; Li, J.X.; Fu, S.L.; Qiu, J.P. Four New Earthworm Species Belonging to Amynthas Kinberg and Metaphire Sims et Easton (Megascolecidae: Oligochaeta) from Guangdong, China. Ann. Zool. 2006, 56, 249–254. [Google Scholar]
- Bantaowong, U.; Chanabun, R.; James, S.W.; Panha, S. Seven New Species of the Earthworm Genus Metaphire Sims & Easton, 1972 from Thailand (Clitellata: Megascolecidae). Zootaxa 2016, 4117, 63. [Google Scholar] [PubMed]
- Quan, H.W. A New Species of Earthworm from Hainan Island. Acta Zootaxonom. Sin. 1985, 1, 18–20. (In Chinese) [Google Scholar]
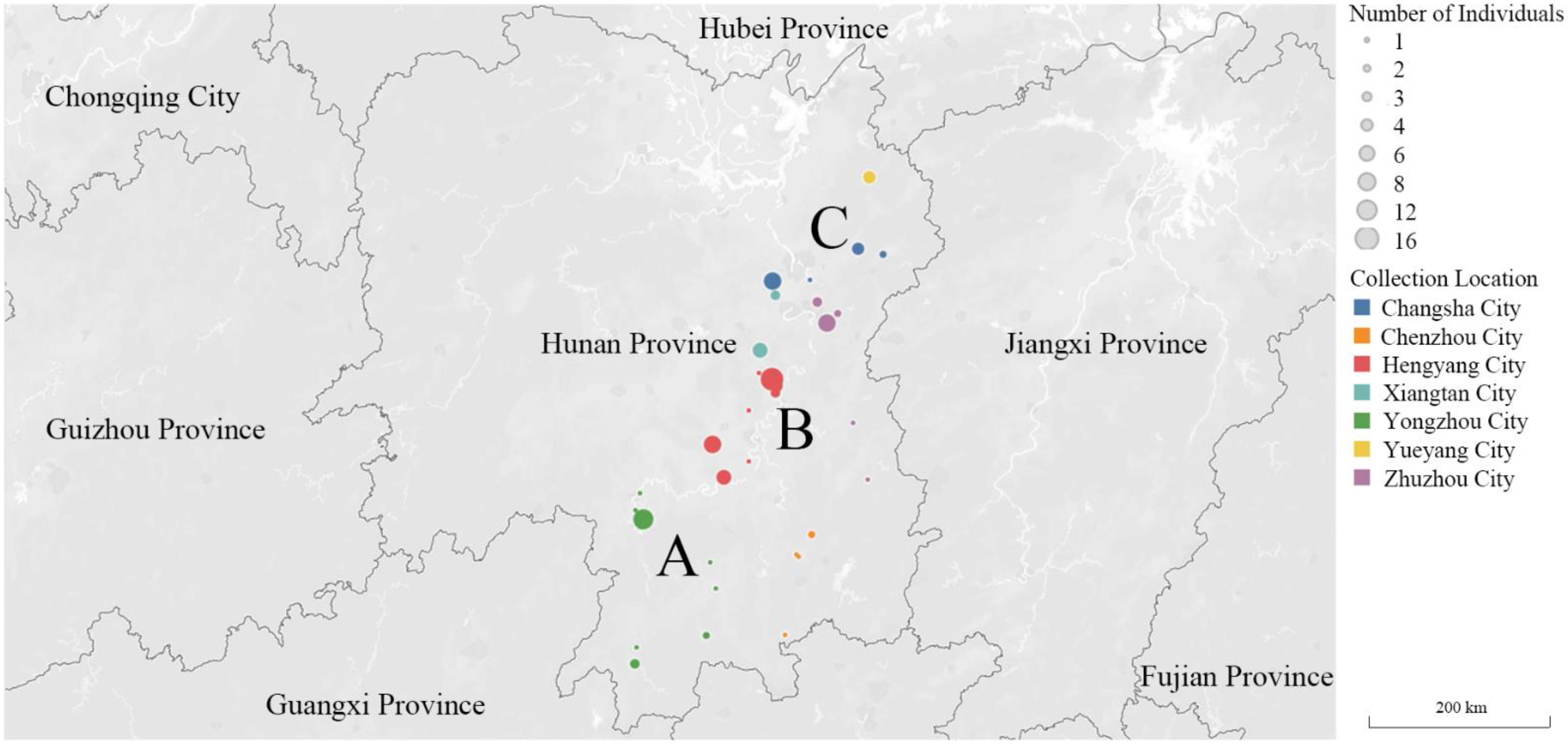

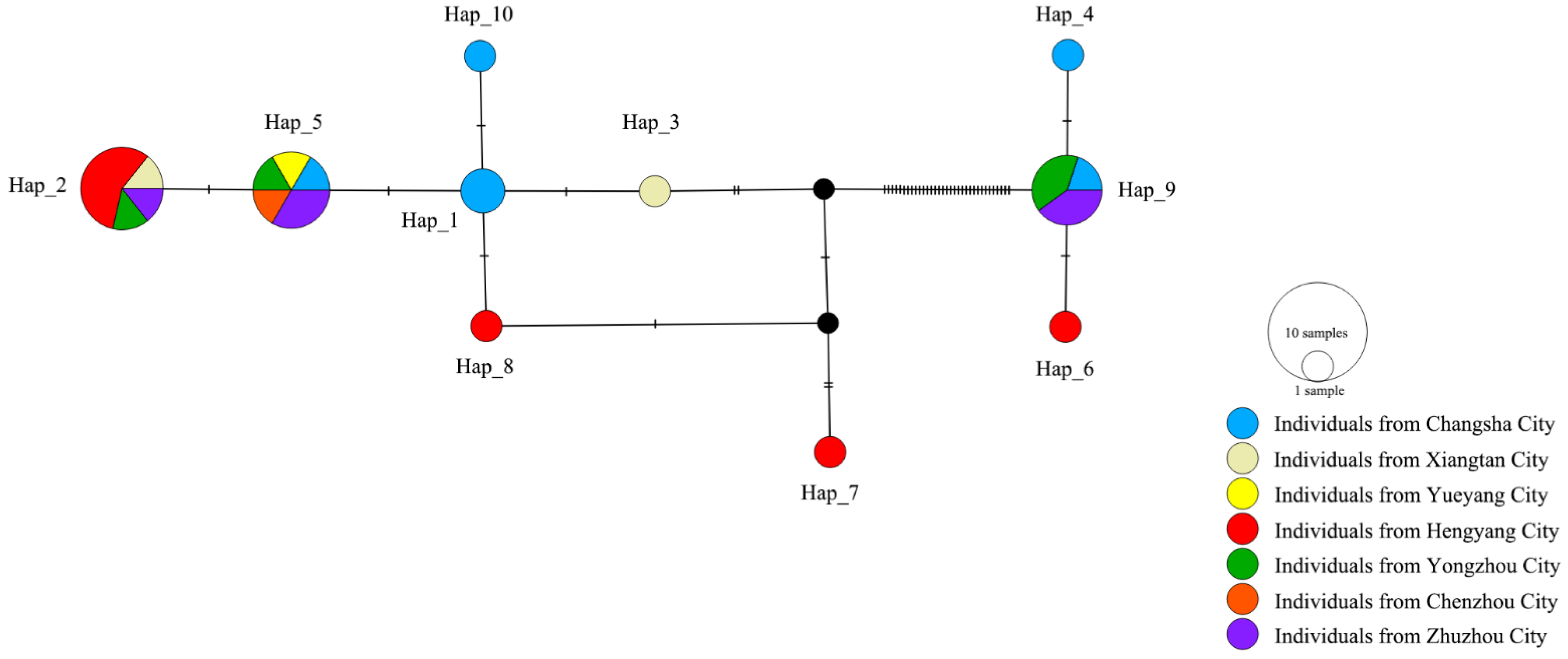

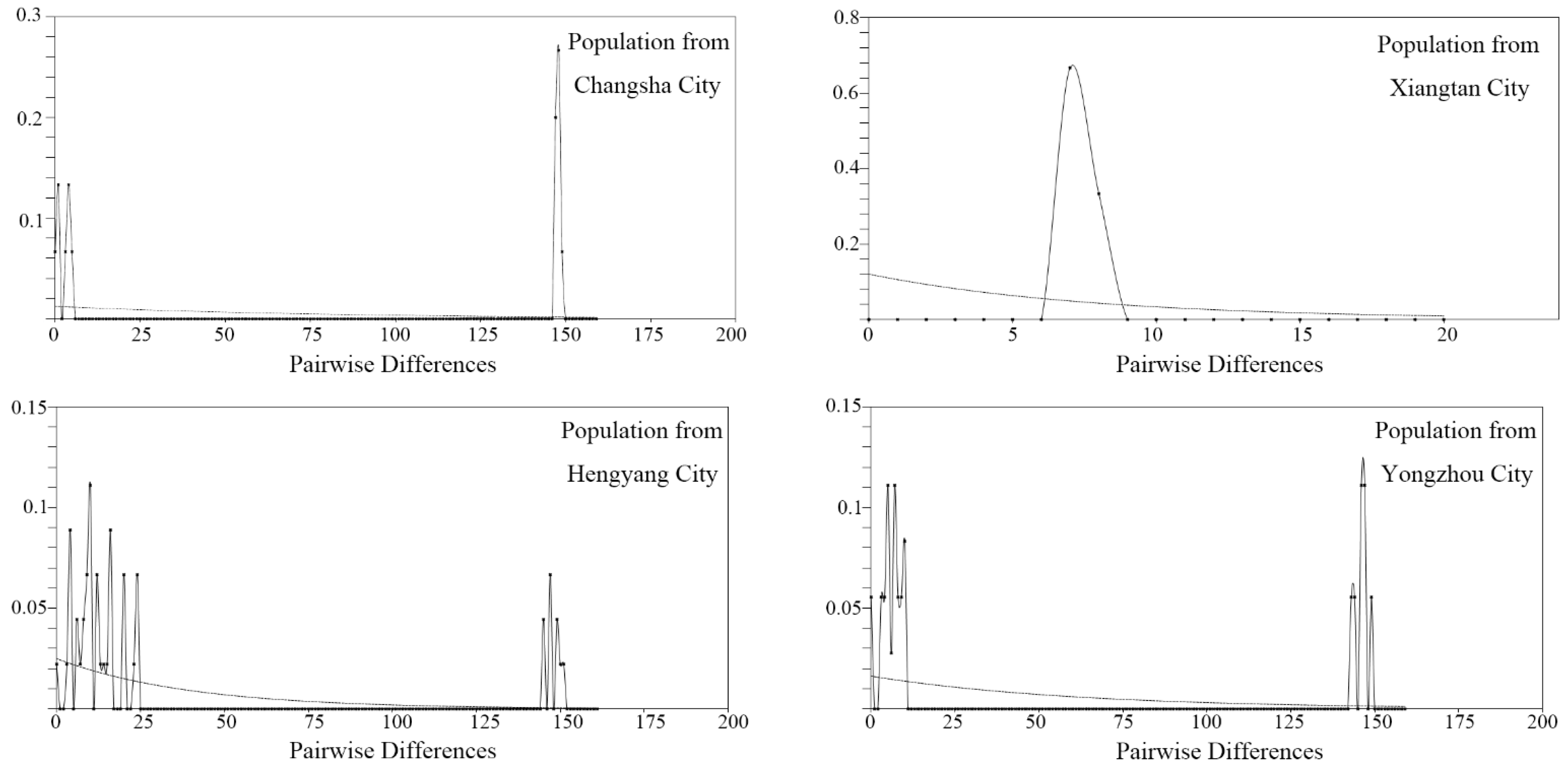
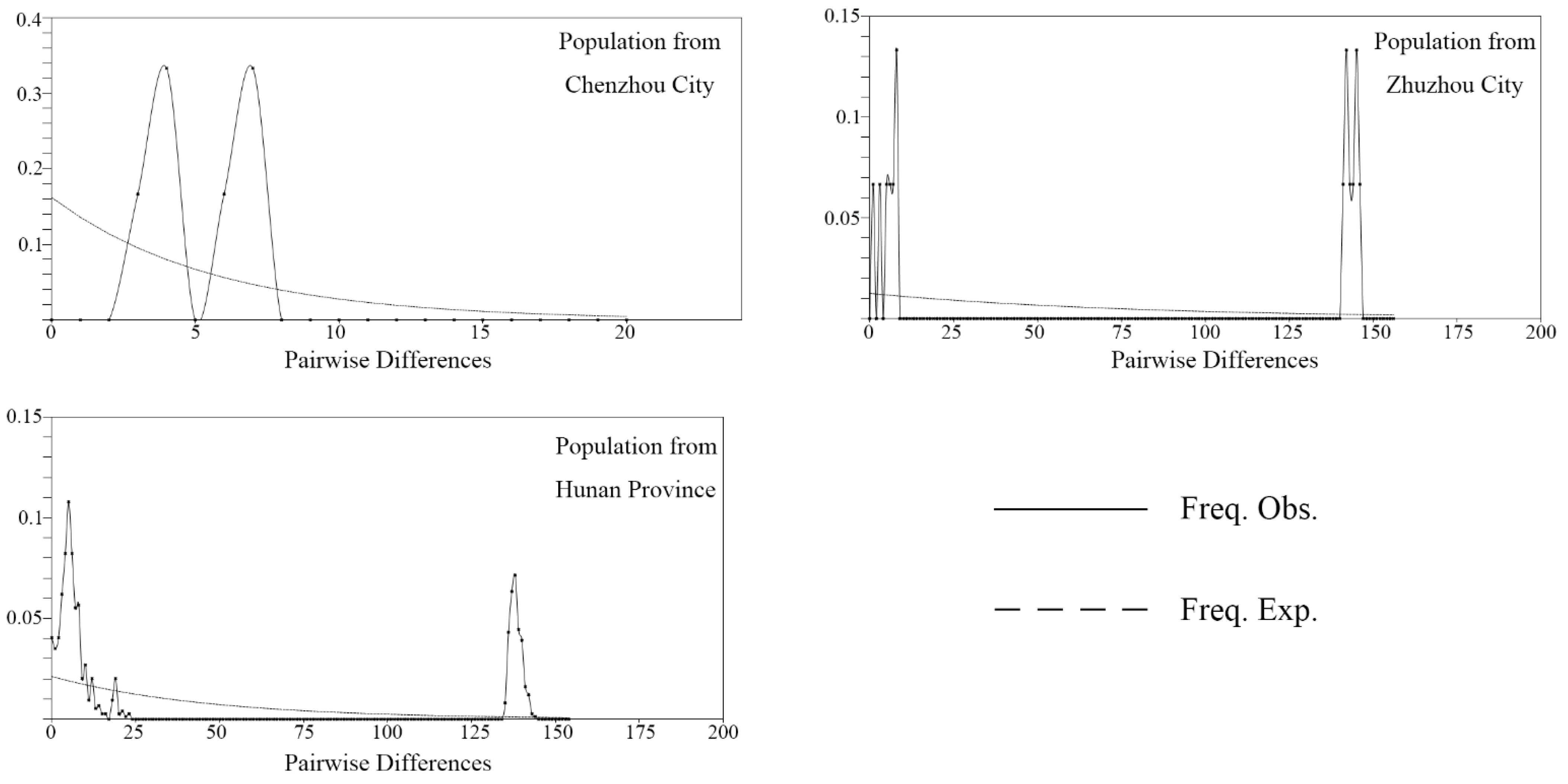


| Label | Collection Location | Longitude (°E) | Latitude (°N) | Collection Date | Elevation (m) | Gene Code | Number |
|---|---|---|---|---|---|---|---|
| P1CJHUSH190510089N5-01 | Changsha City | 112.784 | 28.086 | 10 May 2019 | 47 | Y1014 | 8 |
| P1CJHUSH190510089N5-02 | Changsha City | 112.784 | 28.086 | 10 May 2019 | 47 | Y1015 | 1 |
| P1CJHUSH190528089N9-04 | Changsha City | 113.102 | 28.094 | 28 May 2019 | 24 | Y1033 | 1 |
| P1CJHUSH190510779N4-04 | Changsha City | 113.726 | 28.2831 | 10 May 2019 | 70.65 | Y1664 | 2 |
| P1CJHUSH190511779N9-01 | Changsha City | 113.52 | 28.3279 | 11 May 2019 | 112.45 | Y1676 | 4 |
| P1CJHUSH190511779N9-02 | Changsha City | 113.52 | 28.3279 | 11 May 2019 | 112.45 | Y1677 | 1 |
| P1CJHUSH190510083N1-04 | Xiangtan City | 112.816 | 27.983 | 10 May 2019 | 47 | Y1039 | 1 |
| P1CJHUSH190510083N1-08 | Xiangtan City | 112.816 | 27.983 | 10 May 2019 | 47 | Y1043 | 3 |
| P1CJHUSH190511083Q8-04 | Xiangtan City | 112.681 | 27.568 | 11 May 2019 | 88 | 083Q804 | 6 |
| P1CJHUSH190512778Q9-03 | Yueyang City | 113.6092 | 28.8567 | 12 May 2019 | 178.15 | Y1702 | 4 |
| P1CJHUSH190512784N3-03 | Hengyang City | 112.815 | 27.246 | 12 May 2019 | 96 | 784N303 | 3 |
| P1CJHUSH190512784N4-04 | Hengyang City | 112.818 | 27.299 | 12 May 2019 | 67 | Y1078 | 1 |
| P1CJHUSH190512784N4-05 | Hengyang City | 112.818 | 27.299 | 12 May 2019 | 67 | Y1079 | 4 |
| P1CJHUSH190512784N5-01 | Hengyang City | 112.78 | 27.346 | 12 May 2019 | 114 | Y1080 | 16 |
| P1CJHUSH190512784R7-05 | Hengyang City | 112.669 | 27.395 | 12 May 2019 | 88 | Y1085 | 1 |
| P1CJHUSH190512079N3-01 | Hengyang City | 112.583 | 27.113 | 12 May 2019 | 110 | Y1105 | 1 |
| P1CJHUSH190513074N1-05 | Hengyang City | 112.585 | 26.721 | 13 May 2019 | 60 | Y1122 | 1 |
| P1CJHUSH190513074Q5-03 | Hengyang City | 112.372 | 26.601 | 13 May 2019 | 88 | Y1128 | 6 |
| P1CJHUSH190513074Q5-05 | Hengyang City | 112.372 | 26.601 | 13 May 2019 | 88 | Y1129 | 1 |
| P1CJHUSH190513074R8-01 | Hengyang City | 112.272 | 26.852 | 13 May 2019 | 106 | 074R801 | 8 |
| P1CJHUSH190515072N4-04 | Yongzhou City | 111.661 | 26.483 | 16 May 2019 | 107 | Y1184 | 1 |
| P1CJHUSH190516072Q6-01 | Yongzhou City | 111.625 | 26.353 | 16 May 2019 | 116 | Y1187 | 1 |
| P1CJHUSH190516072Q8-01 | Yongzhou City | 111.691 | 26.283 | 16 May 2019 | 129 | Y1189 | 12 |
| P1CJHUSH190516072N9-03 | Yongzhou City | 111.674 | 26.252 | 16 May 2019 | 117 | 072N903 | 4 |
| P1CJHUSH190517069N4-01 | Yongzhou City | 111.617 | 25.173 | 17 May 2019 | 219 | Y1226 | 3 |
| P1CJHUSH190517069N8-03 | Yongzhou City | 111.634 | 25.301 | 17 May 2019 | 201 | 069N803 | 1 |
| P1CJHUSH190518807N4-02 | Yongzhou City | 112.227 | 25.398 | 18 May 2019 | 258 | Y1253 | 2 |
| P1CJHUSH190519808R2-02 | Yongzhou City | 112.31 | 25.753 | 19 May 2019 | 204 | Y1285 | 1 |
| P1CJHUSH190519808Q7-05 | Yongzhou City | 112.258 | 25.951 | 19 May 2019 | 230 | Y1304 | 1 |
| P1CJHUSH190520798N9-03 | Chenzhou City | 113.008 | 25.997 | 20 May 2019 | 90 | Y1332 | 1 |
| P1CJHUSH190520798Q10-01 | Chenzhou City | 112.988 | 26.015 | 20 May 2019 | 106 | Y1334 | 1 |
| P1CJHUSH190521799N1-03 | Chenzhou City | 112.897 | 25.4 | 21 May 2019 | 259 | Y1363 | 1 |
| P1CJHUSH190521800N5-04 | Chenzhou City | 113.125 | 26.167 | 21 May 2019 | 92 | Y1347 | 2 |
| P1CJHUSH190524073N1-03 | Zhuzhou City | 113.598 | 26.585 | 24 May 2019 | 174 | Y1508 | 1 |
| P1CJHUSH190525780Q2-01 | Zhuzhou City | 113.47 | 27.013 | 25 May 2019 | 151 | Y1539 | 1 |
| P1CJHUSH190527082Q1-01 | Zhuzhou City | 113.347 | 27.842 | 27 May 2019 | 82 | Y1614 | 2 |
| P1CJHUSH190527082N5-01 | Zhuzhou City | 113.251 | 27.771 | 27 May 2019 | 56 | Y1634 | 4 |
| P1CJHUSH190527082N5-02 | Zhuzhou City | 113.251 | 27.771 | 27 May 2019 | 56 | Y1635 | 8 |
| P1CJHUSH190528082N8-01 | Zhuzhou City | 113.164 | 27.93 | 28 May 2019 | 34 | Y1645 | 3 |
| 074R801 | 1187Y | 1043Y | 1128Y | 1078Y | 1614Y | 1080Y | 1015Y | 1702Y | 1539Y | 1347Y | 1508Y | 1304Y | 1039Y | 1635Y | 1105Y | 1122Y | 069N803 | 1363Y | 1184Y | 1226Y | 072N903 | 1676Y | 784N303 | 1033Y | 083Q804 | 1677Y | 1285Y | 1332Y | 1334Y | 1085Y | 1129Y | 1014Y | 1189Y | 1253Y | 1645Y | 1079Y | 1634Y | 1664Y | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 074R801 | |||||||||||||||||||||||||||||||||||||||
| 1187Y | 0.0 | ||||||||||||||||||||||||||||||||||||||
| 1043Y | 0.0 | 0.0 | |||||||||||||||||||||||||||||||||||||
| 1128Y | 0.0 | 0.0 | 0.0 | ||||||||||||||||||||||||||||||||||||
| 1078Y | 0.0 | 0.0 | 0.0 | 0.0 | |||||||||||||||||||||||||||||||||||
| 1614Y | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||||||||||||||||||||||||||||||||
| 1080Y | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||||||||||||||||||||||||||||||||
| 1015Y | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | ||||||||||||||||||||||||||||||||
| 1702Y | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.0 | |||||||||||||||||||||||||||||||
| 1539Y | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.0 | 0.0 | ||||||||||||||||||||||||||||||
| 1347Y | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | |||||||||||||||||||||||||||||
| 1508Y | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | ||||||||||||||||||||||||||||
| 1304Y | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||||||||||||||||||||||||||
| 1039Y | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | ||||||||||||||||||||||||||
| 1635Y | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.0 | |||||||||||||||||||||||||
| 1105Y | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.0 | 0.0 | ||||||||||||||||||||||||
| 1122Y | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | |||||||||||||||||||||||
| 069N803 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | ||||||||||||||||||||||
| 1363Y | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||||||||||||||||||||
| 1184Y | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | ||||||||||||||||||||
| 1226Y | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.0 | |||||||||||||||||||
| 072N903 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.2 | ||||||||||||||||||
| 1676Y | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 | 0.2 | |||||||||||||||||
| 784N303 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.2 | 0.0 | 0.2 | ||||||||||||||||
| 1033Y | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | |||||||||||||||
| 083Q804 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 | 0.2 | 0.3 | 0.2 | 0.2 | ||||||||||||||
| 1677Y | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.2 | 0.0 | 0.2 | 0.0 | 0.0 | 0.2 | |||||||||||||
| 1285Y | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.2 | 0.0 | 0.2 | 0.0 | 0.0 | 0.2 | 0.0 | ||||||||||||
| 1332Y | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.2 | 0.0 | 0.2 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | |||||||||||
| 1334Y | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.2 | 0.0 | 0.2 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | ||||||||||
| 1085Y | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.8 | 0.8 | 0.7 | 0.8 | 0.7 | 0.7 | 0.8 | 0.7 | 0.7 | 0.7 | 0.7 | |||||||||
| 1129Y | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 | 0.2 | 0.3 | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.5 | ||||||||
| 1014Y | 6.4 | 6.4 | 6.4 | 6.4 | 6.4 | 6.4 | 6.4 | 6.6 | 6.6 | 6.6 | 6.6 | 6.6 | 6.6 | 6.4 | 6.4 | 6.4 | 6.4 | 6.4 | 6.4 | 6.4 | 6.4 | 6.4 | 6.6 | 6.4 | 6.4 | 6.2 | 6.4 | 6.4 | 6.4 | 6.4 | 6.4 | 6.2 | |||||||
| 1189Y | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.3 | 6.3 | 6.3 | 6.3 | 6.3 | 6.3 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.3 | 6.3 | 6.1 | 6.3 | 6.1 | 6.2 | 6.0 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.0 | 0.2 | ||||||
| 1253Y | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.3 | 6.3 | 6.3 | 6.3 | 6.3 | 6.3 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.3 | 6.3 | 6.1 | 6.3 | 6.1 | 6.2 | 6.0 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.0 | 0.2 | 0.0 | |||||
| 1645Y | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.3 | 6.3 | 6.3 | 6.3 | 6.3 | 6.3 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.3 | 6.3 | 6.1 | 6.3 | 6.1 | 6.2 | 6.0 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.0 | 0.2 | 0.0 | 0.0 | ||||
| 1079Y | 6.3 | 6.3 | 6.3 | 6.3 | 6.3 | 6.3 | 6.3 | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 | 6.3 | 6.3 | 6.3 | 6.3 | 6.3 | 6.3 | 6.5 | 6.5 | 6.3 | 6.5 | 6.3 | 6.4 | 6.1 | 6.3 | 6.3 | 6.3 | 6.3 | 6.3 | 6.1 | 0.3 | 0.2 | 0.2 | 0.2 | |||
| 1634Y | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.3 | 6.3 | 6.3 | 6.3 | 6.3 | 6.3 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.3 | 6.3 | 6.1 | 6.3 | 6.1 | 6.2 | 6.0 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.2 | ||
| 1664Y | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.3 | 6.3 | 6.3 | 6.3 | 6.3 | 6.3 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.3 | 6.3 | 6.1 | 6.3 | 6.1 | 6.2 | 6.0 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 |
| Collection Location | Number of Sequences Used | Polymorphic Sites | Nucleotide Diversity | Number of Haplotypes | Haplotype Diversity | Fu’s FS | Tajima’s D |
|---|---|---|---|---|---|---|---|
| Changsha City | 6 | 152 | 0.02183 | 5 | 0.933 | 4.460 | 1.30792, p > 0.10 |
| Xiangtan City | 3 | 11 | 0.00203 | 3 | 1.000 | 0.807 | — |
| Yueyang City | 1 | — | — | — | — | — | — |
| Hengyang City | 10 | 176 | 0.01090 | 9 | 0.978 | 1.042 | −1.85453, p < 0.05 |
| Yongzhou City | 9 | 159 | 0.01714 | 7 | 0.944 | 4.360 | 0.17225, p > 0.10 |
| Chenzhou City | 4 | 10 | 0.00143 | 4 | 1.000 | −0.480 | −0.52807, p > 0.10 |
| Zhuzhou City | 6 | 150 | 0.02213 | 6 | 1.000 | 1.591 | 1.26797, p > 0.10 |
| Hunan Province | 39 | 176 | 0.01366 | 25 | 0.960 | 2.611 | 0.37830, p > 0.10 |
| Character | M. remanens sp. nov. | M. planata | M. jianfengensis | M. dadingmontis | M. saxicalcis |
|---|---|---|---|---|---|
| Body size (mm) | 68–110 × 3.9–5.7 | 125 × 4.8 | 160–250 × 6–10 | 80–85 × 2.9–3.4 | 254–377 × 9.6 |
| Segments | 88–98 | 138 | 131–173 | 82–90 | 147–157 |
| First dorsal pore | 10/11, 12/13 | 11/12 | 12/13 | 12/13 | 12/13 |
| Clitellum | XIV–XVI, no dorsal pores | XIV–XVI, visible setae | XIV–XVI | XIV–XVI, dorsal pores | XIV–XVI |
| Setae | 34–40/III, 38–44/V, 44–48/VIII, 58–62/XX, 56–66/XXV; 14–17 between male pores; 11–14 (VI), 12–15 (VII) between spermathecal pores; aa = 1.0–1.6ab, zz = 1.2–2.2zy. | 60–69/III, 75–87/VIII, 56–65/XX; 11 between male pores; aa = 1.0ab, zz = 1.0zy. | 52–56/III, 58–60/V, 65–66/VIII, 82–84/ XXV. | 32–40/III, 30–36/V, 38–42/VIII, 40–44/XX, 43–46/XXV; 7–9 between male pores; aa = 1.1–1.2ab, zz = 1.0–1.2zy. | 89–98/XX; 13–20 between male pores; aa = 1.0ab, zz = 1.0zy. |
| Spermathecal pores | Two pairs, 6/7–7/8, 0.33C; An obvious subsidence area; 0–5 papillae. | Two pairs, 6/7–7/8, 0.25C. | Two pairs, 6/7–7/8, 1/4C. | Two pairs, 6/7–7/8, 0.33C; 2 papillae. | Two pairs, 6/7–7/8. |
| Male pores | XVIII, 0.33C; Each on the bottom center of the longitudinally distributed copulatory chamber, slightly raised; 0–3 oval medium-sized flat-topped papillae on the cushion protrusion. Sometimes no male pores. | XVIII, 0.27C; transversely slit-like secondary apertures puckered radially around its margin. | XVIII, 0.25C; Each on the bottom center of the copulatory chamber; Two large-sized circular papillae and three small pads on the inner wall of the cavity are squeezed together to form a concave shape, surrounded by many longitudinal fold muscles; The mastoid is exposed when everted. | XVIII, 0.4C; each on the surface of a small protuberance situated in a large copulatory chamber in XVIII, surrounded by longitudinal epidermic folds. No papillae. | XVIII, 0.28C; secondary apertures transversely slit-like with puckered margin on segment XVIII, no genital markings. |
| Septa | 5/6–7/8 thick and muscular, 10/11–13/14 slightly thickened, 8/9 and 9/10 absent. | 6/7–7/8 muscular, 10/11–12/13 slightly muscular, 8/9–9/10 absent. | 4/5–7/8 thick and muscular, 10/11–13/14 slightly thickened, 8/9 and 9/10 absent. | Thin, 8/9 and 9/10 absent. | 5/6–7/8 thick, 10/11–11/12 thin, 8/9–9/10 absent. |
| Gizzard | IX–X, spherical | IX–X, spherical | IX–X | VIII–X, small and drum-like | Behind 7/8 |
| Intestine | Enlarged from XV | Enlarged from XV | Enlarged from XV | Enlarged from XVI | Enlarged from XV |
| Intestinal caeca | XXVII–XXII | XXVII–XXI | XXVII–XXV | XXVII–XXIV | XXVII–XXII |
| Testis sacs | No. | Two pairs, X and XI. | Two pairs, X and XI, not developed. | Two pairs, X and XI. | Two pairs, X and XI. |
| Prostate glands | Left degeneration, right not developed/degeneration; 0–7 glands. | 1/2 XVII–XIX, not developed. | XVII–XIX, developed; no glands. | XVIII, moderately large, prostatic duct simple curved. two glands. | XVI–XXI, developed. |
| Spermathecae | Two pairs, VII and VIII; Ampulla bag-shaped, ampulla duct as long as or 1.5 times of ampulla; Diverticulum as long as main pouch, slender, terminal 1/3 dilated into bag-shaped seminal chamber. Sometimes no spermathecae. A total of 0–14 glands. | Two pairs, VII and VIII; Ampulla heart-shaped, ampulla duct 1/3 of ampulla. Diverticulum as long as main pouch, slender, terminal 1/3 dilated into club-shaped seminal chamber. Four glands. | Two pairs, VII and VIII; Ampulla oval, ampulla duct as long as ampulla; Diverticulum shorter than main pouch, curved, terminal 1/4 dilated into seminal chamber. | Two pairs, VII–VIII; ampulla small oval-shaped, ampulla duct 1/4 of ampulla. Diverticulum as long as the whole spermatheca, slender, terminal 1/5 dilated into oval seminal chamber. Has glands. | Two pairs, VII and VIII; ampulla paddle-shaped, ampulla duct 1/3 of ampulla. Diverticulum longer than main pouch, slender, terminal 1/4 dilated into seminal chamber. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Q.; Jiang, J.; Li, J.; Qiu, J. Population Genetic Structure and Diversity of Metaphire remanens (Oligochaeta: Megascolecidae) Based on Mitochondrial DNA Analysis, with a Note on a New Species of Metaphire remanens sp. nov. Diversity 2022, 14, 275. https://doi.org/10.3390/d14040275
Jin Q, Jiang J, Li J, Qiu J. Population Genetic Structure and Diversity of Metaphire remanens (Oligochaeta: Megascolecidae) Based on Mitochondrial DNA Analysis, with a Note on a New Species of Metaphire remanens sp. nov. Diversity. 2022; 14(4):275. https://doi.org/10.3390/d14040275
Chicago/Turabian StyleJin, Qing, Jibao Jiang, Jiali Li, and Jiangping Qiu. 2022. "Population Genetic Structure and Diversity of Metaphire remanens (Oligochaeta: Megascolecidae) Based on Mitochondrial DNA Analysis, with a Note on a New Species of Metaphire remanens sp. nov." Diversity 14, no. 4: 275. https://doi.org/10.3390/d14040275
APA StyleJin, Q., Jiang, J., Li, J., & Qiu, J. (2022). Population Genetic Structure and Diversity of Metaphire remanens (Oligochaeta: Megascolecidae) Based on Mitochondrial DNA Analysis, with a Note on a New Species of Metaphire remanens sp. nov. Diversity, 14(4), 275. https://doi.org/10.3390/d14040275





