Skeletal Transformations and the Origin of Baleen Whales (Mammalia, Cetacea, Mysticeti): A Study on Evolutionary Patterns
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources of Comparative Data
2.2. Anatomy
2.3. Phylogeny
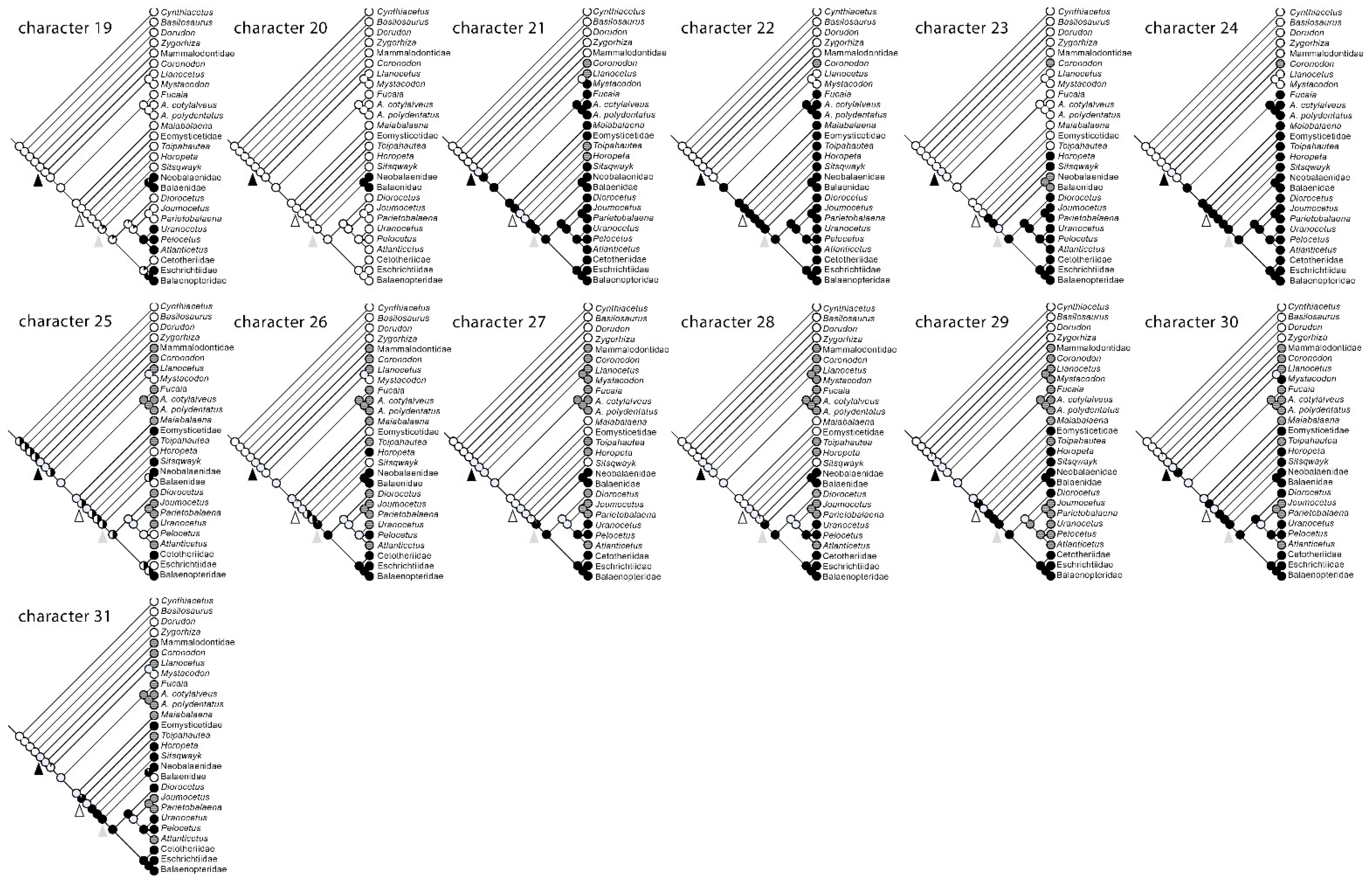
2.4. Character Mapping and Reconstruction of Characters at Ancestral Nodes
3. Results
3.1. Rostrum
3.2. Vertex
3.3. Temporal Fossa
3.4. Occipital Region
3.5. Earbones
3.6. Dentary
3.7. Appendicular Skeleton
3.8. Reconstruction of Character States at Ancestral Nodes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bannister, J.D. Baleen whales. In Encyclopedia of Marine Mammals; Perrin, W.F., Würsig, B., Thewissen, J.G.M., Eds.; Academic Press: New York, NY, USA, 2002; pp. 62–72. [Google Scholar]
- Savoca, M.S.; Czapanskiy, M.F.; Kahane-Rapport, S.R.; Gough, W.T.; Fahlbusch, J.A.; Bierlich, K.C.; Segre, P.S.; Di Clemente, J.; Penry, G.S.; Wiley, D.N.; et al. Baleen whale prey consumption based on high-resolution foraging measurements. Nature 2021, 599, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Pershing, A.J.; Christensen, L.B.; Record, N.R.; Sherwood, G.D.; Stetson, P.B. The impact of whaling on the oceancarbon cycle: Why bigger was better. PLoS ONE 2010, 5, e12444. [Google Scholar] [CrossRef] [PubMed]
- Pershing, A.J.; Stamieszkin, K. The North Atlantic ecosystem, from plankton to whales. Annu. Rev. Mar. Sci. 2020, 12, 339–359. [Google Scholar] [CrossRef]
- Sanderson, L.R.; Wassersug, R. Convergent and alternative designs for vertebrate suspension feeding. In The Skull. Functional and Evolutionary Mechanisms; Hanken, J., Hall, B.K., Eds.; University of Chicago Press: Chicago, IL, USA, 1993; Volume 3, pp. 37–112. [Google Scholar]
- Werth, A.J. Feeding in marine mammals. In Feeding: Form, Function and Evolution in Tetrapod Vertebrates; Schwenk, K., Ed.; Academic Press: New York, NY, USA, 2000; pp. 475–514. [Google Scholar]
- Deméré, T.A.; McGowen, M.R.; Berta, A.; Gatesy, J. Morphological and molecular evidence for a stepwise evolutionary transition from teeth to baleen in mysticete whales. Syst. Biol. 2008, 57, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Ekdale, E.G.; Deméré, T.A. Neurovascular evidence for a co-occurrence of teeth and baleen in an Oligocene mysticete and the transition to filter-feeding in baleen whales. Zool. J. Linn. Soc. 2021, 194, 395–415. [Google Scholar] [CrossRef]
- Marx, F.D.; Hocking, D.P.; Park, T.; Ziegler, T.; Evans, A.R.; Fitzgerald, E.M.G. Suction feeding preceded filtering in baleen whale evolution. Mem. Mus. Vic. 2016, 75, 71–82. [Google Scholar] [CrossRef][Green Version]
- Geisler, J.H.; Boessenecker, R.W.; Brown, M.; Beatty, B.L. The origin of filter feding in whales. Curr. Biol. 2017, 27, 2036–2042.e2. [Google Scholar] [CrossRef]
- Fitzgerald, E.M.G. The morphology and systematic of Mammalodon colliveri (Cetacea: Mysticeti), a toothed mysticete from the Oligocene of Australia. Zool. J. Linn. Soc. 2010, 158, 367–476. [Google Scholar] [CrossRef][Green Version]
- Boessenecker, R.W.; Churchill, M.; Buchholtz, E.A.; Beatty, B.L.; Geisler, J.H. Convergent Evolution of Swimming Adaptations in Modern Whales Revealed by a Large Macrophagous Dolphin from the Oligocene of South Carolina. Curr. Biol. 2020, 30, 3267–3273.e2. [Google Scholar] [CrossRef]
- Cooper, L.N.; Berta, A.; Dawson, S.D.; Reidenberg, J.S. Evolution of hyperphalangy and digit reduction in the cetacean manus. Anat. Rec. 2007, 290, 654–672. [Google Scholar] [CrossRef]
- Benke, H. Investigations on the osteology and the functional morphology of the flipper of whales and dolphins (Cetacea). Investig. Cetacea 1993, 24, 9–252. [Google Scholar]
- Mitchell, E.D. A new cetacean from the late Eocene La Meseta Formation, Seymour Island, Antarctic Peninsula. Can. J. Fish. Aquat. Sci. 1989, 46, 2219–2235. [Google Scholar] [CrossRef]
- Geisler, J.H.; Sanders, A.E. Morphological evidence for the phylogeny of Cetacea. J. Mamm. Evol. 2003, 10, 23–129. [Google Scholar] [CrossRef]
- Bisconti, M.; Pellegrino, L.; Carnevale, G. Evolution of gigantism in right and bowhead whales (Cetacea: Mysticeti: Balaenidae). Biol. J. Linn. Soc. 2021, 134, 498–524. [Google Scholar] [CrossRef]
- Bisconti, M.; Damarco, P.; Mao, S.; Pavia, M.; Carnevale, G. The earliest baleen whale from the Mediterranean: Large-scale implications of an early Miocene thalassotherian mysticete from Piedmont, Italy. Pap. Palaeontol. 2021, 7, 1147–1166. [Google Scholar] [CrossRef]
- Bisconti, M.; Bosselaers, M. A new balaenopterid species from the southern North Sea Basin informs about phylogeny and taxonomy of Burtinopsis and Protororqualus (Cetacea, Mysticeti, Balaenopteridae). PeerJ 2020, 8, e9570. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Fordyce, R.E. A new archaic baleen whale Toipahautea waitaki (early Late Oligocene, New Zealand) and the origins of crown Mysticeti. R. Soc. Open Sci. 2018, 5, 172453. [Google Scholar] [CrossRef]
- Peredo, C.M.; Pyenson, N.D.; Marshall, C.D.; Uhen, M.D. Tooth loss precedes the origin of baleen in whales. Curr. Biol. 2018, 28, 3992–4000.e2. [Google Scholar] [CrossRef]
- de Muizon, C.; Bianucci, G.; Martínez-Cáceres, M.; Lambert, O. Mystacodon selenensis, the earliest known toothed mysticete (Cetacea, Mammalia) from the late Eocene of Peru: Anatomy, phylogeny, and feeding adaptations. Geodiversitas 2019, 41, 401–499. [Google Scholar] [CrossRef]
- Fordyce, R.E.; Marx, F.G. Gigantism precedes filter feeding in baleen whale evolution. Curr. Biol. 2018, 28, 1670–1676.e2. [Google Scholar] [CrossRef]
- Mead, J.G.; Fordyce, R.E. The therian skull: A lexicon with emphasis on the odontocetes. Smiths. Contrib. Zool. 2009, 627, 1–216. [Google Scholar] [CrossRef]
- Bisconti, M.; Varola, A. The oldest eschrichtiid mysticete and a new morphological diagnosis of Eschrichtiidae (gray whales). Riv. It. Paleontol. Strat. 2006, 112, 447–457. [Google Scholar]
- Ekdale, E.G.; Berta, A.; Deméré, T.A. The comparative osteology of the petrotympanic complex (ear region) of extant baleen whales (Cetacea: Mysticeti). PLoS ONE 2011, 6, e21311. [Google Scholar] [CrossRef] [PubMed]
- Uhen, M.D. Form, function, and anatomy of Dorudon atrox (Mammalia, Cetacea): An archaeocete from the middle to late Eocene of Egypt. Univ. Michigan P. Palaeont. 2004, 34, 1–238. [Google Scholar]
- True, F.W. The whalebone whales of the western North Atlantic. Smiths. Contrib. Knowl. 1904, 33, 1–322. [Google Scholar]
- Kellogg, R. Fossil marine mammals from the Miocene Calvert Formation of Maryland and Virginia. US Natl. Mus. Bull. 1968, 247, 103–197. [Google Scholar]
- Bisconti, M.; Damarco, P.; Pavia, M.; Sorce, B.; Carnevale, G. Marzanoptera tersillae, a new balaenopterid genus and species from the Pliocene of Piedmont, north-west Italy. Zool. J. Linn. Soc. 2021, 192, 1253–1292. [Google Scholar] [CrossRef]
- Bisconti, M.; Munsterman, D.K.; Post, K. A new balaenopterid whale from the late Miocene of the Southern North Sea Basin and the evolution of balaenopterid diversity (Cetacea, Mysticeti). PeerJ 2019, 7, e6915. [Google Scholar] [CrossRef]
- Bisconti, M.; Munsterman, D.K.; Fraaije, R.H.B.; Bosselaers, M.E.J.; Post, K. A new species of rorqual whale (Cetacea, Mysticeti, Balaenopteridae) from the late Miocene of the Southern North Sea Basin and the role of the North Atlantic in the paleobiogeography of Archaebalaenoptera. PeerJ 2020, 8, e8315. [Google Scholar] [CrossRef] [PubMed]
- Bisconti, M.; Bosselaers, M. Fragilicetus velponi: A new mysticete genus and species and its implications for the origin of Balaenopteridae (Mammalia, Cetacea, Mysticeti). Zool. J. Linn. Soc. 2016, 177, 450–474. [Google Scholar] [CrossRef]
- Marx, F.G.; Post, K.; Bosselaers, M.; Munsterman, D. A large Late Miocene cetotheriid (Cetacea, Mysticeti) from the Netherlands clarifies the status of Tranatocetidae. PeerJ 2019, 7, e6426. [Google Scholar] [CrossRef] [PubMed]
- Peredo, C.M.; Uhen, M.D. A new basal Chaeomysticete (Mammalia: Cetacea) from the Oligocene Pysht Formation of Washington, USA. Pap. Palaeontol. 2016, 2016, 533–554. [Google Scholar] [CrossRef]
- El Adli, J.J.; Deméré, T.A.; Boessenecker, R.W. Herpetocetus morrowi (Cetacea: Mysticeti), a new species of diminutive baleen whale from the Upper Pliocene (Piacenzian) of California, USA, with observations on the evolution and relationships of the Cetotheriidae. Zool. J. Linn. Soc. 2014, 170, 400–466. [Google Scholar] [CrossRef]
- Geisler, J.H.; McGowen, M.R.; Yang, G.; Gatesy, J. A supermatrix analysis of genomic, morphological, and paleontological data from crown Cetacea. BMC Evol. Biol. 2011, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Marx, F.G. The more the merrier? A large cladistic analysis of mysticetes, and comments on the transition from teeth to baleen. J. Mamm. Evol. 2011, 18, 77–100. [Google Scholar] [CrossRef]
- Deméré, T.A.; Berta, A.; McGowen, M.R. The taxonomic and evolutionary history of fossil and modern balaenopteroid mysticetes. J. Mamm. Evol. 2005, 2, 99–143. [Google Scholar] [CrossRef]
- Boessenecker, R.W.; Fordyce, R.E. A new eomysticetid from the Oligocene Kokoamu Greensand of New Zealand and a review of the Eomysticetidae (Mammalia, Cetacea). J. Syst. Palaeontol. 2016, 15, 429–469. [Google Scholar] [CrossRef]
- Boessenecker, R.W.; Fordyce, R.E. A new genus and species of eomysticetid (Cetacea: Mysticeti) and a reinterpretation of “Mauicetus” lophocephalus Marples, 1956: Transitional baleen whales from the upper Oligocene of New Zealand. Zool. J. Linn. Soc. 2015, 175, 607–660. [Google Scholar] [CrossRef]
- Boessenecker, R.W.; Fordyce, R.E. Anatomy, feeding ecology, and ontogeny of a transitional baleen whale: A new genus and species of Eomysticetidae (Mammalia: Cetacea) from the Oligocene of New Zealand. PeerJ 2015, 3, e1129. [Google Scholar] [CrossRef]
- Bisconti, M. Comparative osteology and phylogenetic relationships of Miocaperea pulchra, the first fossilpygmy right whale genus and species (Cetacea, Mysticeti, Neobalaenidae). Zool. J. Linn. Soc. 2012, 166, 876–911. [Google Scholar] [CrossRef]
- Bisconti, M. Skull morphology and phylogenetic relationships of a new diminutive balaenid from the lower Pliocene of Belgium. Palaeontology 2005, 48, 793–816. [Google Scholar] [CrossRef]
- Churchill, M.; Berta, A.; Deméré, T.D. The systematics of right whales (Mysticeti: Balaenidae). Mar. Mamm. Sci. 2012, 28, 497–521. [Google Scholar] [CrossRef]
- Kimura, T.; Hasegawa, Y. A new baleen whale (Mysticeti: Cetotheriidae) from the earliest late miocene of Japan and a reconsideration of the phylogeny of cetotheres. J. Vert. Paleontol. 2010, 30, 577–591. [Google Scholar] [CrossRef]
- Tanaka, Y.; Furusawa, H.; Kimura, M. A new member of fossil balaenid (Mysticeti, Cetacea) from the early Pliocene of Hokkaido, Japan. R. Soc. Open Sci. 2020, 7, 192182. [Google Scholar] [CrossRef]
- Maddison, W.; Maddison, W.; Mesquite: A Modular System for Evolutionary Analysis. Version: 3.61. 2019. Available online: http://www.mesquiteproject.org (accessed on 12 May 2020).
- Bisconti, M.; Damarco, P.; Tartarelli, G.; Pavia, M.; Carnevale, G. A natural endocast of an early Miocene odontocete and its implications in cetacean brain evolution. J. Comp. Neurol. 2021, 529, 1198–1227. [Google Scholar] [CrossRef]
- Bisconti, M.; Daniello, R.; Damarco, P.; Tartarelli, G.; Pavia, M.; Carnevale, G. High encephalization in a fossil rorqual illuminates baleen whale brain evolution. Br. Behav. Evol. 2021, 96, 78–90. [Google Scholar] [CrossRef]
- Bisconti, M. Evolutionary history of Balaenidae. Cranium 2003, 20, 9–50. [Google Scholar]
- McLeod, S.A.; Whitmore, F.C., Jr.; Barnes, L.G. Evolutionary relationships and classification. In The Bowhead Ehale; Burns, J.J., Montague, J.J., Cowles, C.J., Eds.; Society for Marine Mammalogy: Yarmouth Port, MA, USA, 1993; Volume 2, pp. 45–70. [Google Scholar]
- Bisconti, M. Anatomy of a new cetotheriid genus and species from the Miocene of Herentals, Belgium, and the phylogenetic and paleobiogeographic relationships of Cetotheriidae s.s. (Mammalia, Cetacea, Mysticeti). J. Syst. Palaeontol. 2015, 13, 377–395. [Google Scholar] [CrossRef]
- Bouetel, V.; de Muizon, C. The anatomy and relationships of Piscobalaena nana (Cetacea, Mysticeti), a Cetotheriidae s.s. from the early Pliocene of Peru. Geodiversitas 2006, 28, 319–395. [Google Scholar]
- Hanna, G.D.; McLellan, M. A new species of whale from the type locality of the Monterey Group. Proc. Calif. Acad. Sci. 1924, 13, 237–241. [Google Scholar]
- Kellogg, R. The history of whales—Their adaptation to life in the water. Q. Rev. Biol. 1928, 3, 29–76. [Google Scholar] [CrossRef]
- Portis, A. Catalogo descrittivo dei Talassoterii rinvenuti nei terreni terziari del Piemonte e della Liguria. Mem. R. Acc. Sci. Torino 1884, 37, 247–365. [Google Scholar]
- Bisconti, M. A new basal balaenopterid from the Early Pliocene of northern Italy. Palaeontology 2007, 50, 1103–1122. [Google Scholar] [CrossRef]
- Bisconti, M.; Ochoa, D.; Urbina, M.; Salas-Gismondi, R. Archaebalaenoptera eusebioi, a new rorqual from the late Miocene of Peru (Cetacea, Mysticeti, Balaenopteridae) and its impact in reconstructing body size evolution, ecomorphology and paleobiogeography of Balaenopteridae. J. Syst. Palaeontol. 2022, in press. [CrossRef]
- Zeigler, C.V.; Chan, G.L.; Barnes, L.G. A new Late Miocene balaenopterid whale (Cetacea: Mysticeti), Parabalaenoptera baulinensis, (new genus and species) from the Santa Cruz Mudstone, Point Reyes Peninsula, California. Proc. Calif. Acad. Sci. USA 1997, 50, 115–138. [Google Scholar]
- Gol’Din, P.; Steeman, M.E. From problem taxa to problem solver: A new Miocene family, Tranatocetidae, brings perspective on baleen whale evolution. PLoS ONE 2015, 10, e0135500. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Oishi, M.; Yamada, T.K. A newly discovered species of living baleen whale. Nature 2003, 426, 278–281. [Google Scholar] [CrossRef]
- Miller, G.S. The telescoping of the cetacean skull. Smiths. Misc. Coll. 1923, 76, 1–70. [Google Scholar]
- Roston, R.A.; Roth, V.L. Cetacean Skull Telescoping Brings Evolution of Cranial Sutures into Focus. Anat. Rec. 2019, 302, 1055–1073. [Google Scholar] [CrossRef]
- Marx, F.G.; Fordyce, R.E. A link no longer missing: New evidence for the cetotheriid affinities of Caperea. PLoS ONE 2016, 11, e0164059. [Google Scholar] [CrossRef] [PubMed]
- Buono, M.R.; Fernández, M.S.; Cozzuol, M.A.; Cuitiño, J.I.; Fitzgerald, E.M.G. The early Miocene balaenid Morenocetus parvus from Patagonia (Argentina) and the evolution of right whales. PeerJ 2018, 5, e4148. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-H.; Fordyce, R.E. Disparate heterochronic processes in baleen whale evolution. Evol. Biol. 2014, 41, 299–307. [Google Scholar] [CrossRef]
- Kellogg, R. A review of the Archaeoceti. Carnegie Inst. Wash. Publ. 1936, 482, 1–455. [Google Scholar]
- Gol’Din, P.; Startsev, D. Brandtocetus, a new genus of baleen whales (Cetacea, Cetotheriidae) from the late Miocene of Crimea, Ukraine. J. Vert. Paleontol. 2014, 34, 419–433. [Google Scholar] [CrossRef]
- Kellogg, R. A new whalebone whale from the Miocene Calvert Formation. US Natl. Mus. Bull. 1965, 247, 1–45. [Google Scholar]
- Ritsche, I.S.; Fahlke, J.M.; Wieder, F.; Hilger, A.; Manke, I.; Hampe, O. Relationships of cochlear coiling shape and hearing frequencies in cetaceans, and the occurrence of infrasonic hearing in Miocene Mysticeti. Foss. Rec. 2018, 21, 33–45. [Google Scholar] [CrossRef]
- Park, T.; Evans, A.R.; Gallagher, S.J.; Fitzgerald, E.M.G. Low-frequency hearing preceded the evolution of giant body size and filter feeding in baleen whales. Proc. R. Soc. B 2017, 284, 20162528. [Google Scholar] [CrossRef]
- Ekdale, E.G. Morphological Variation Among the Inner Ears of Extinct and Extant Baleen Whales (Cetacea: Mysticeti). J. Morphol. 2016, 277, 1599–1615. [Google Scholar] [CrossRef]
- Shadwick, R.E.; Goldbogen, J.A.; Pyenson, N.D.; Whale, J.C.A. Structure and function in the lunge feeding apparatus: Mechanical properties of the fin whale mandible. Anat. Rec. 2017, 300, 1953–1962. [Google Scholar] [CrossRef]
- Pyenson, N.D.; Goldbogen, J.A.; Shadwick, R.E. Mandible allometry in extant and fossil Balaenopteridae (Cetacea: Mammalia): The largest vertebrate skeletal element and its role in rorqual lunge feeding. Biol. J. Linn. Soc. 2013, 108, 586–599. [Google Scholar] [CrossRef]
- Pyenson, N.D.; Sponberg, S.N. Reconstructing body size in extinct crown Cetacea (Neoceti) using allometry, phylogenetic methods and tests from the fossil record. J. Mamm. Evol. 2011, 18, 269–288. [Google Scholar] [CrossRef]
- Kimura, T. Feeding strategy of an early Miocene cetothere from the Toyama and Akeyo Formations, central Japan. Paleontol. Res. 2002, 6, 179–189. [Google Scholar]
- Bisconti, M. New description, character analysis and preliminary phyletic assessment of two Balaenidae skulls from the Italian Pliocene. Palaeontogr. Ital. 2000, 87, 37–66. [Google Scholar]
- Lambertsen, R.H. Internal mechanism of rorqual feeding. J. Mamm. 1983, 64, 76–88. [Google Scholar] [CrossRef]
- Gol’Din, P.; Startsev, D.; Krakhmalnaya, T. The anatomy of the late Miocene baleen whale Cetotherium riabinini from Ukraine. Acta Palaeontol. Pol. 2014, 59, 795–814. [Google Scholar]
- Steeman, M.E. A new baleen whale from the late Miocene of Denmark and early mysticete hearing. Palaeontology 2009, 52, 1169–1190. [Google Scholar] [CrossRef]
- Lambertsen, R.H.; Ulrich, N.; Straley, J. Frontomandibular stay of Balaenopteridae: A mechanism for momentum recapture during feeding. J. Mamm. 1995, 76, 877–899. [Google Scholar] [CrossRef]
- Bisconti, M. Taxonomic revision and phylogenetic relationships of the rorqual-like mysticete from the Pliocene of Mount Pulgnasco, northern Italy (Mammalia, Cetacea, Mysticeti). Palaeontogr. Ital. 2007, 91, 85–108. [Google Scholar]
- Gillet, A.; Frédéric, B.; Parmentier, E. Divergent evolutionary morphology of the axial skeleton as a potential key innovation in modern cetaceans. Proc. R. Soc. B 2019, 286, 20191771. [Google Scholar] [CrossRef]
- Buchholtz, E.A. Vertebral and rib anatomy in Caperea marginata: Implications for evolutionary patterning of the mammalian vertebral column. Mar. Mamm. Sci. 2011, 27, 382–397. [Google Scholar] [CrossRef]
- Buchholtz, E.A. Modular evolution of the cetacean vertebral column. Evol. Dev. 2007, 9, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Buchholtz, E.A. Implications of vertebral morphology for locomotor evolution in early Cetacea. In The Emergence of Whales; Thewissen, J.G.M., Ed.; Plenum Press: New York, NY, USA, 1998; pp. 325–351. [Google Scholar]
- Duboys de Lavigerie, G.; Bosselaers, M.; Goolaerts, S.; Park, T.; Lambert, O.; Marx, F.G. New Pliocene right whale from Belgium informs balaenid phylogeny and function. J. Syst. Palaeontol. 2020, 18, 1141–1166. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Ando, T. Niche partitioning in Oligocene toothed mysticetes (Mysticeti: Aetiocetidae). J. Mamm. Evol. 2016, 23, 33–41. [Google Scholar] [CrossRef]
- Berta, A.; Lanzetti, A.; Ekdale, E.G.; Deméré, T.A. From teeth to baleen and raptorial to bulk filter feeding in mysticete cetaceans: The role of paleontological, genetic, and geochemical data in feeding evolution and ecology. Integr. Comp. Biol. 2016, 56, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Peredo, C.M.; Pyenson, N.D.; Uhen, M.D.; Marshall, C.D. Alveoli, teeth, and tooth loss: Understanding the homology of internal mandibular structures in mysticete cetaceans. PLoS ONE 2017, 12, e0178243. [Google Scholar] [CrossRef]
- Thewissen, J.G.M.; Cohn, M.J.; Stevens, L.S.; Bajpai, S.; Heyning, J.; Horton, W.E., Jr. Developmental basis for hind-limb loss in dolphins and origin of the cetacean bodyplan. Proc. Natl. Acad. Sci. USA 2006, 103, 8414–8418. [Google Scholar] [CrossRef] [PubMed]
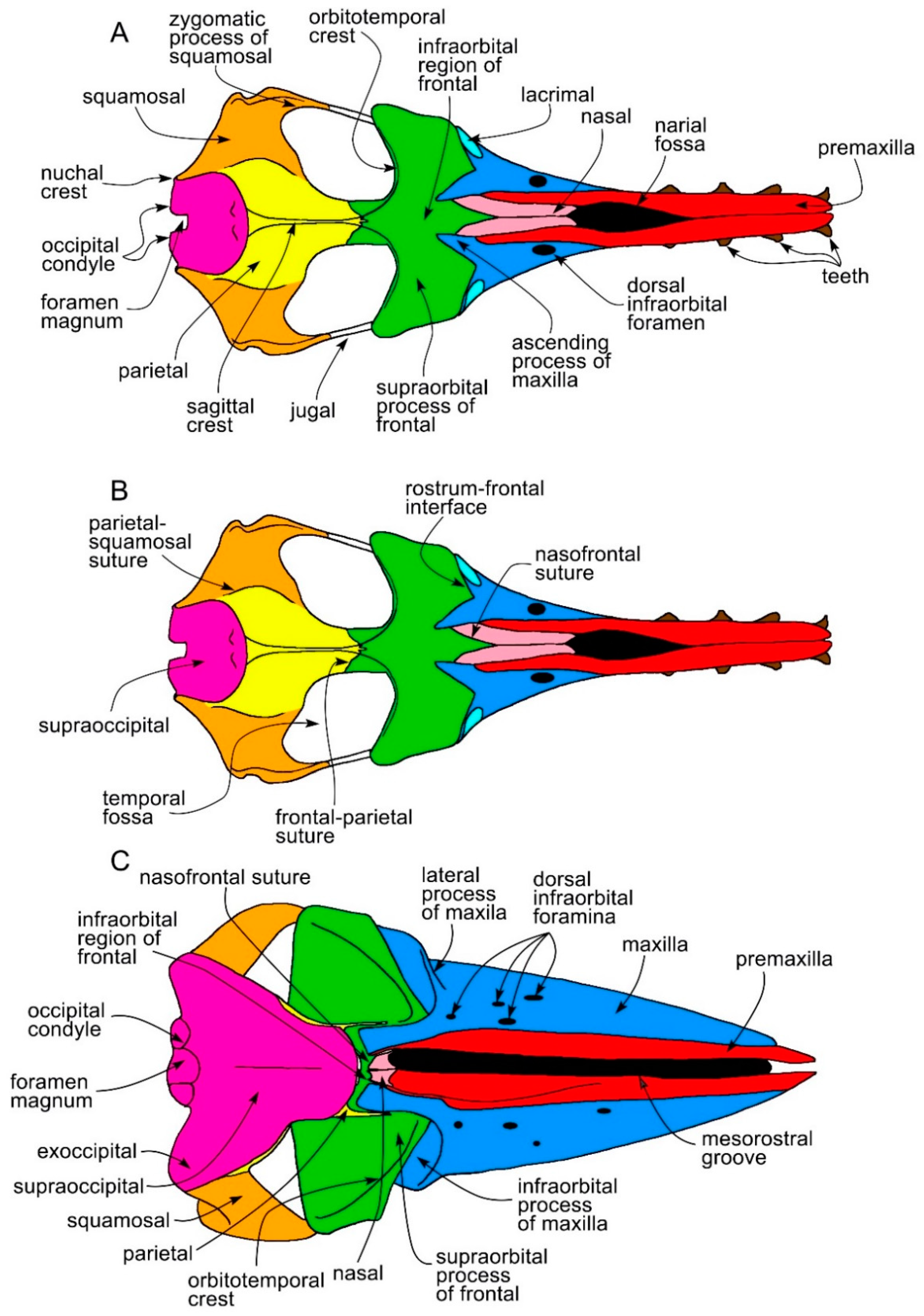
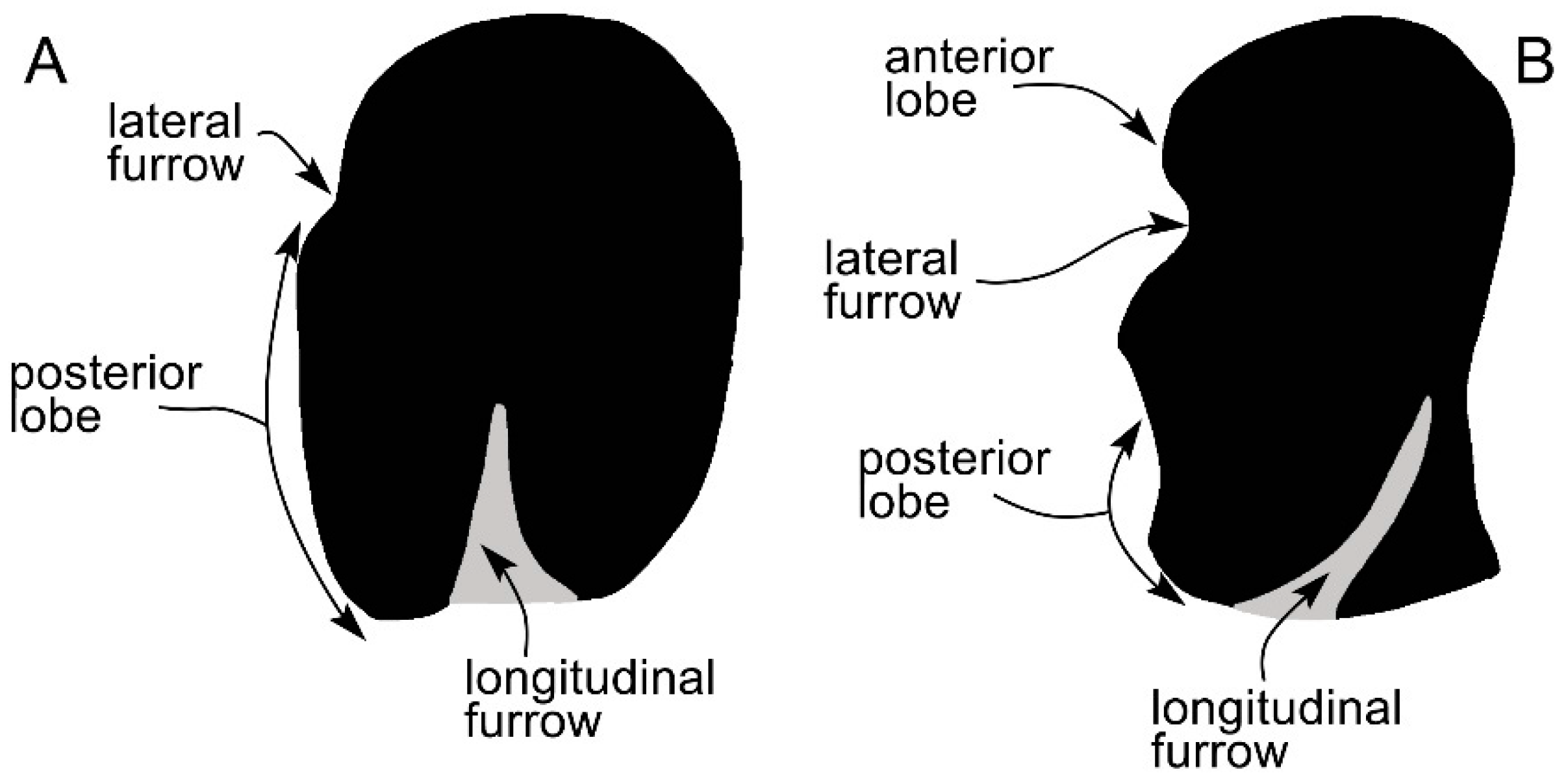
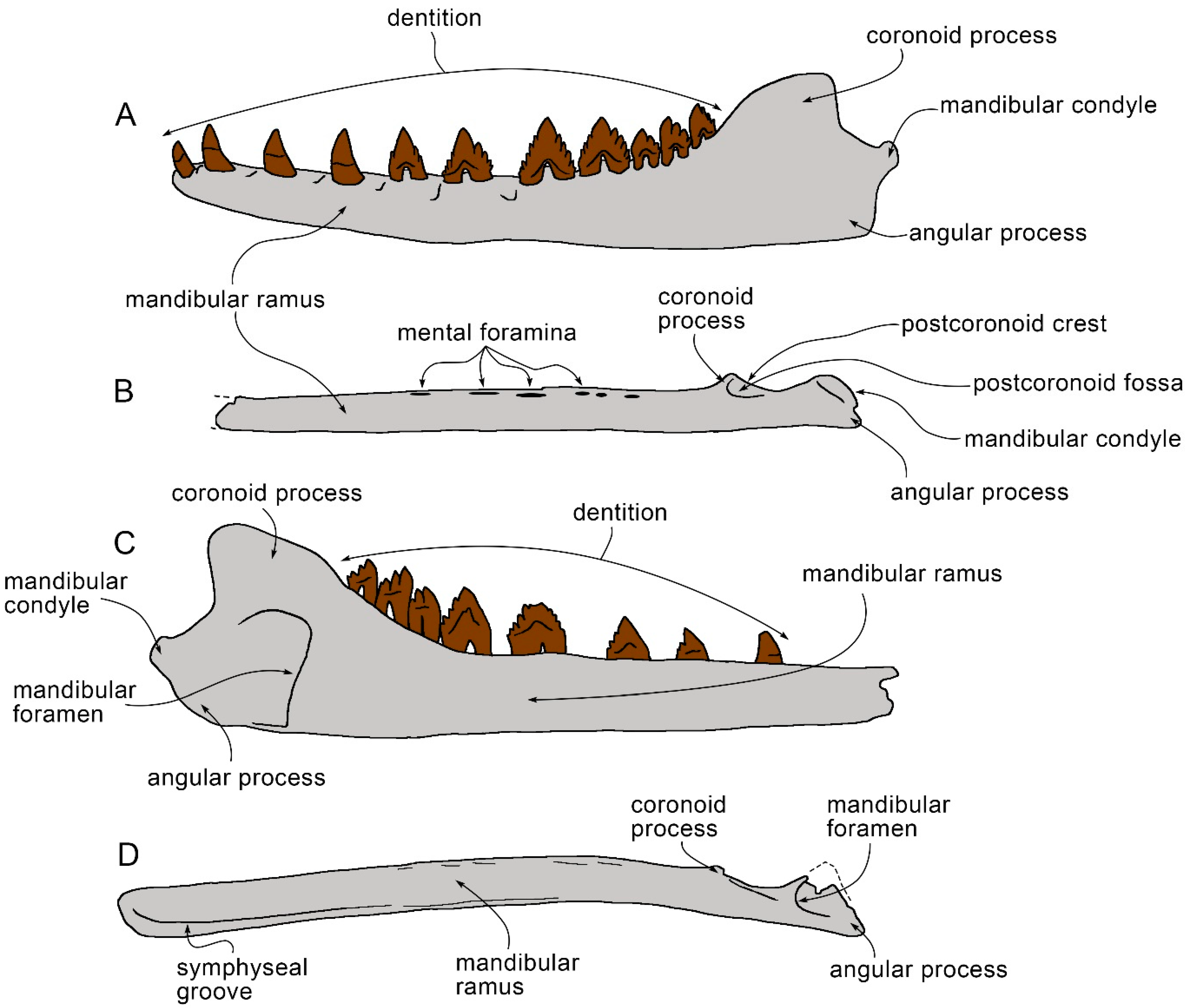


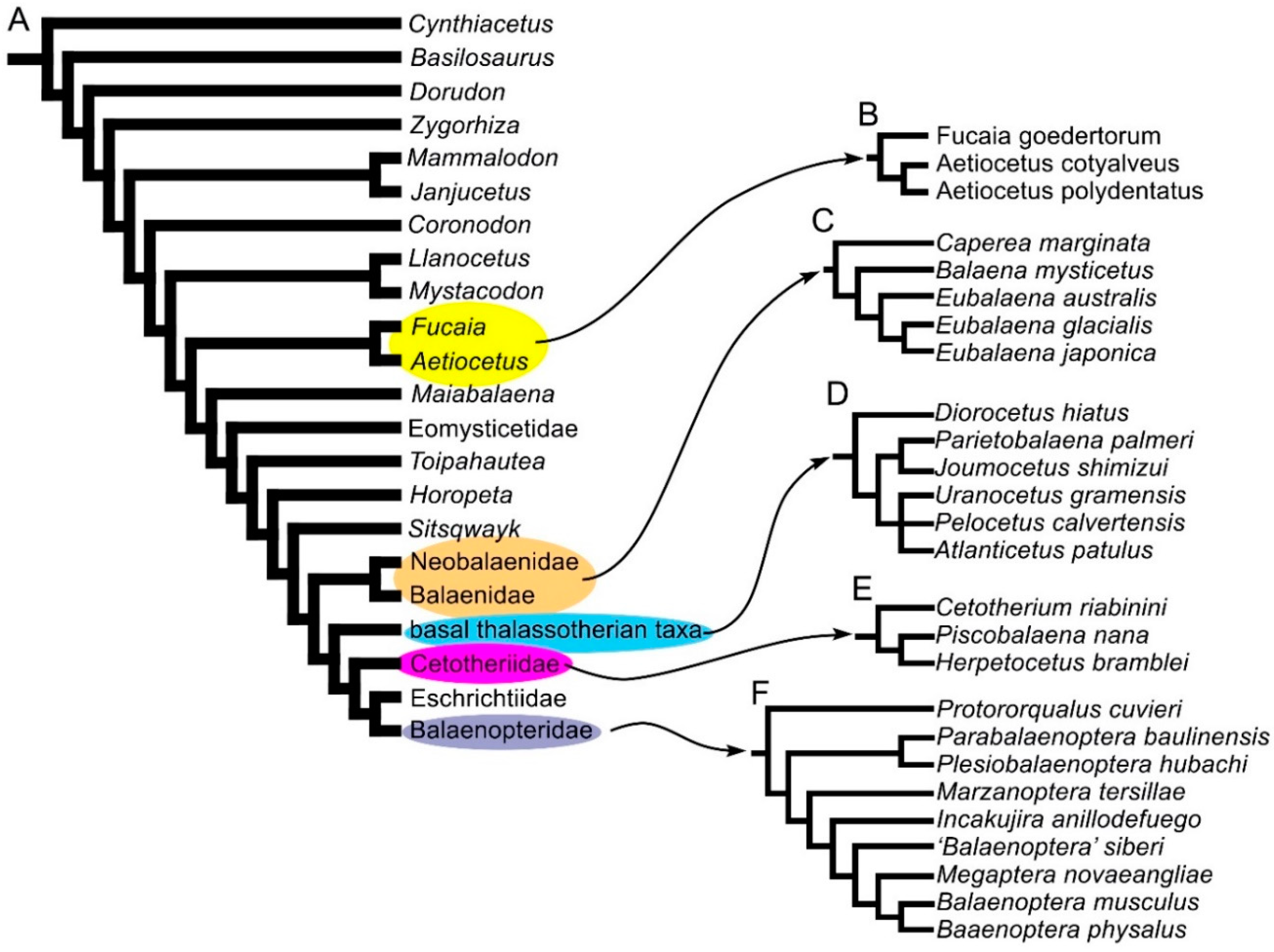

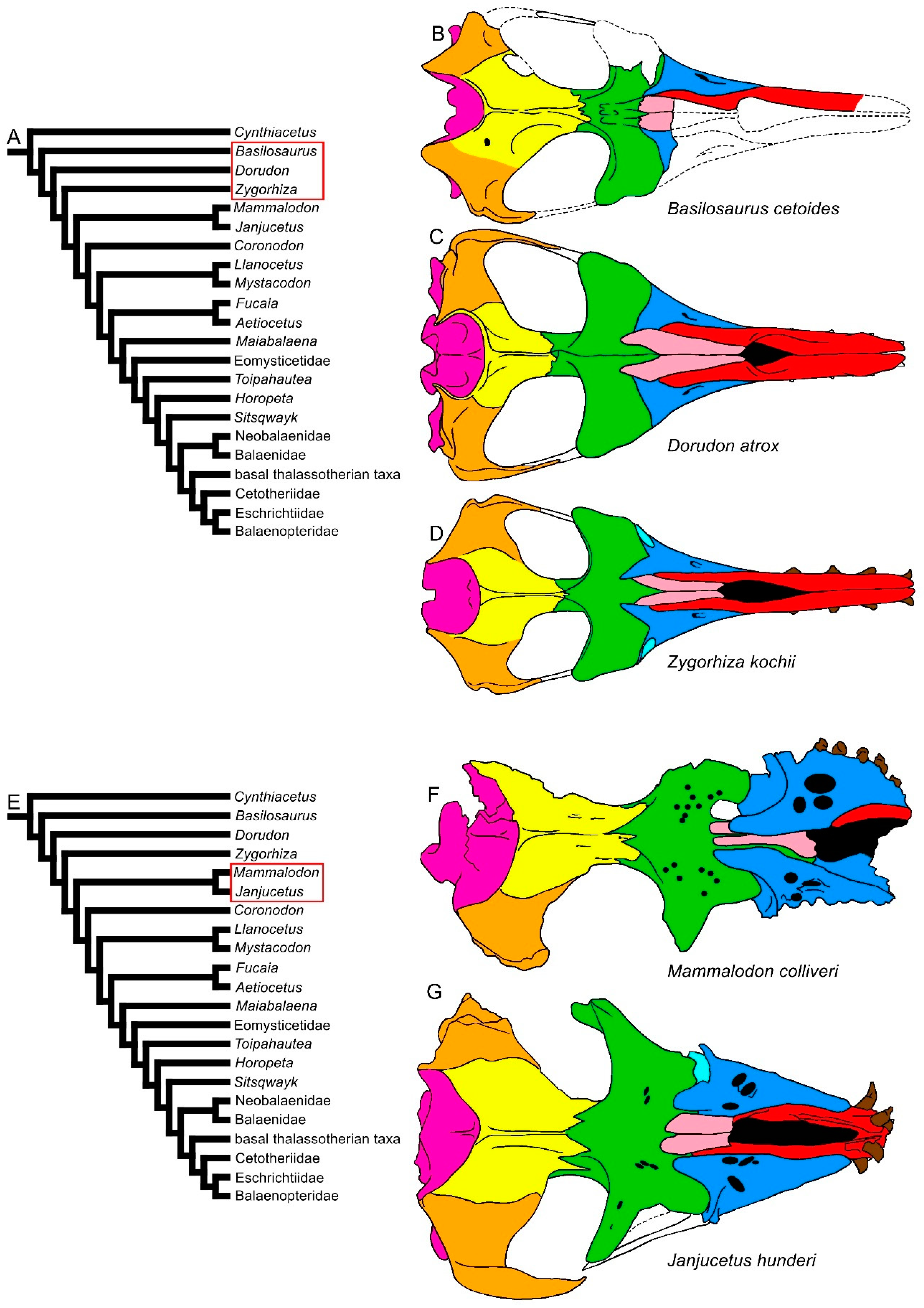
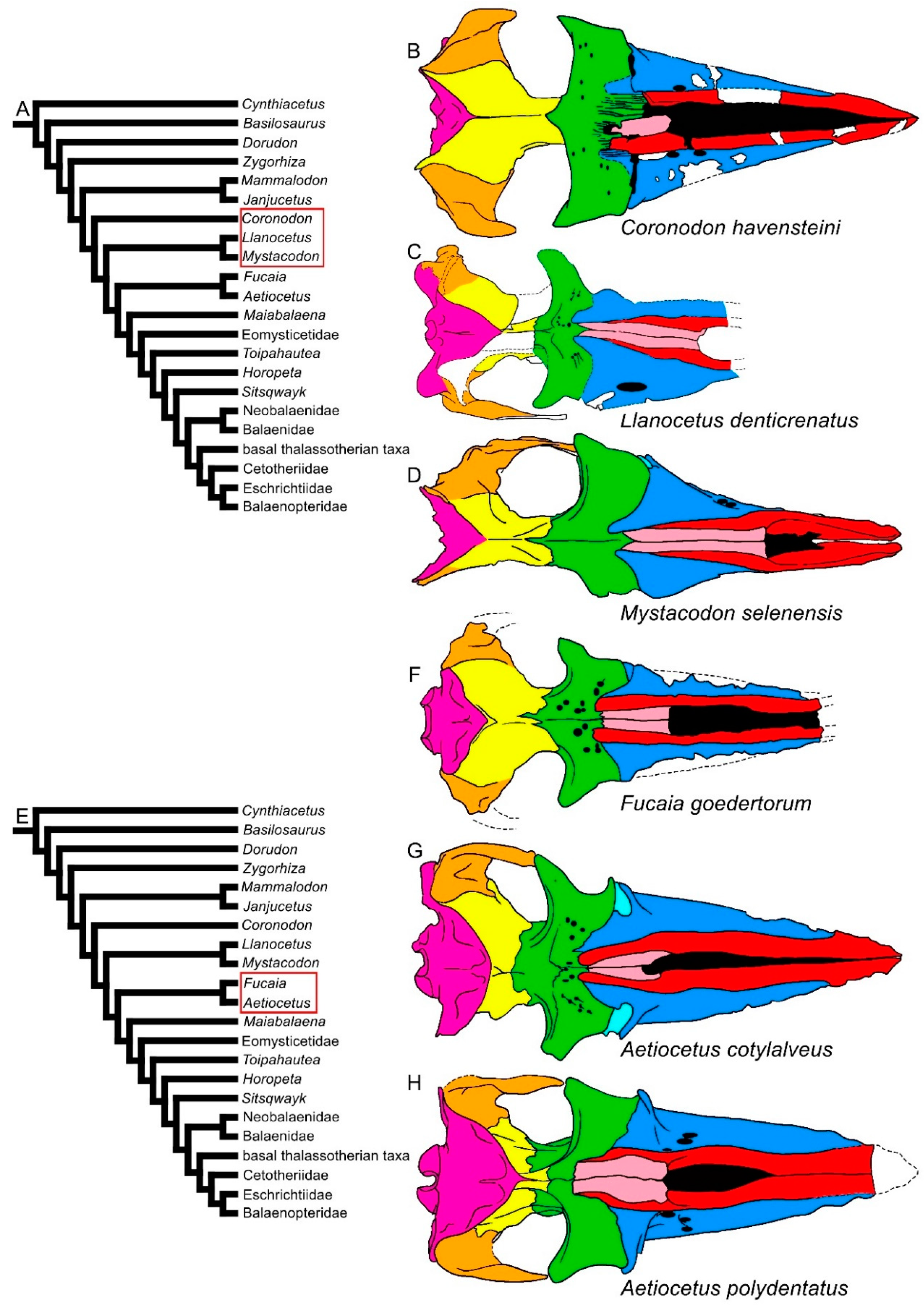

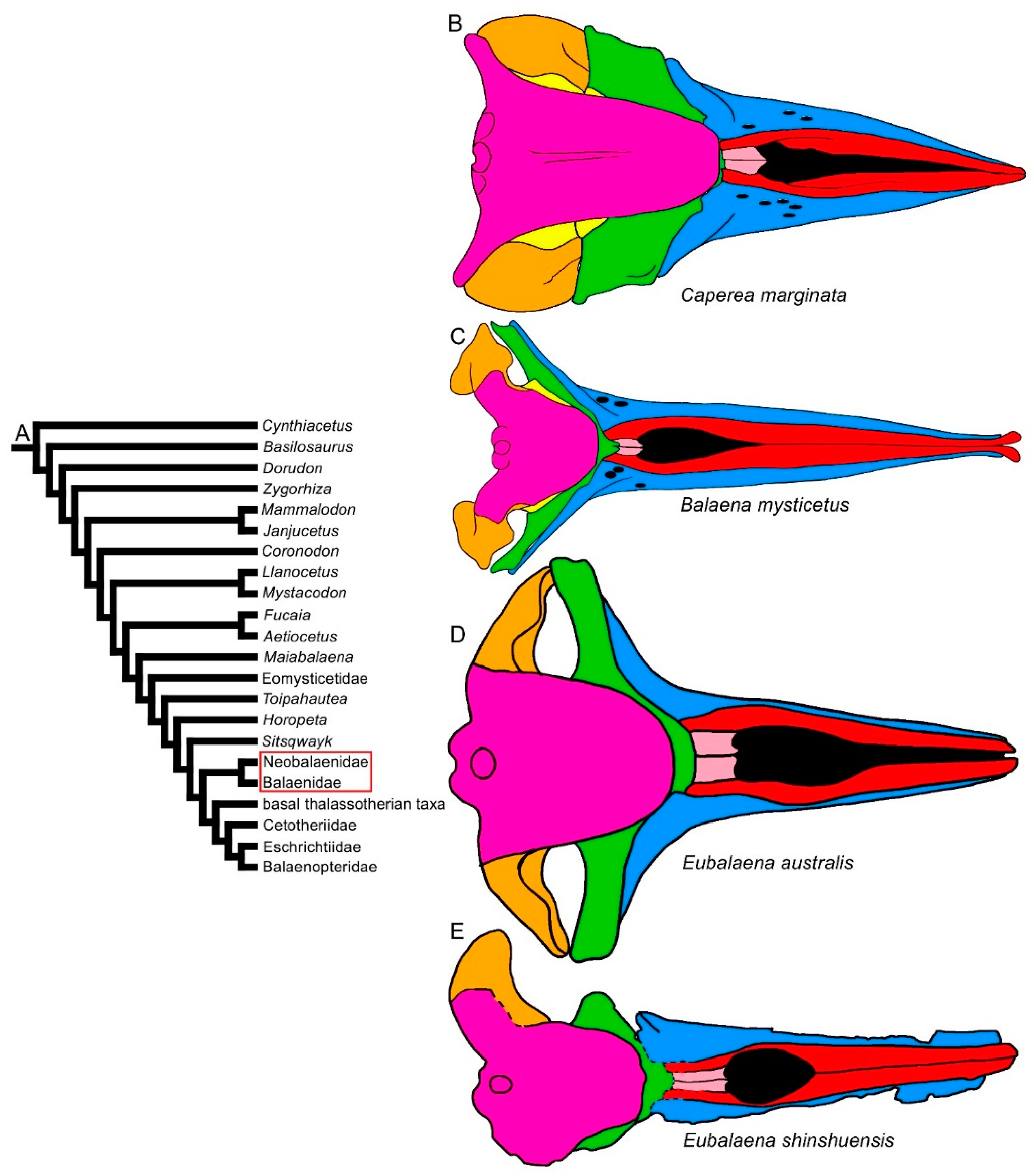
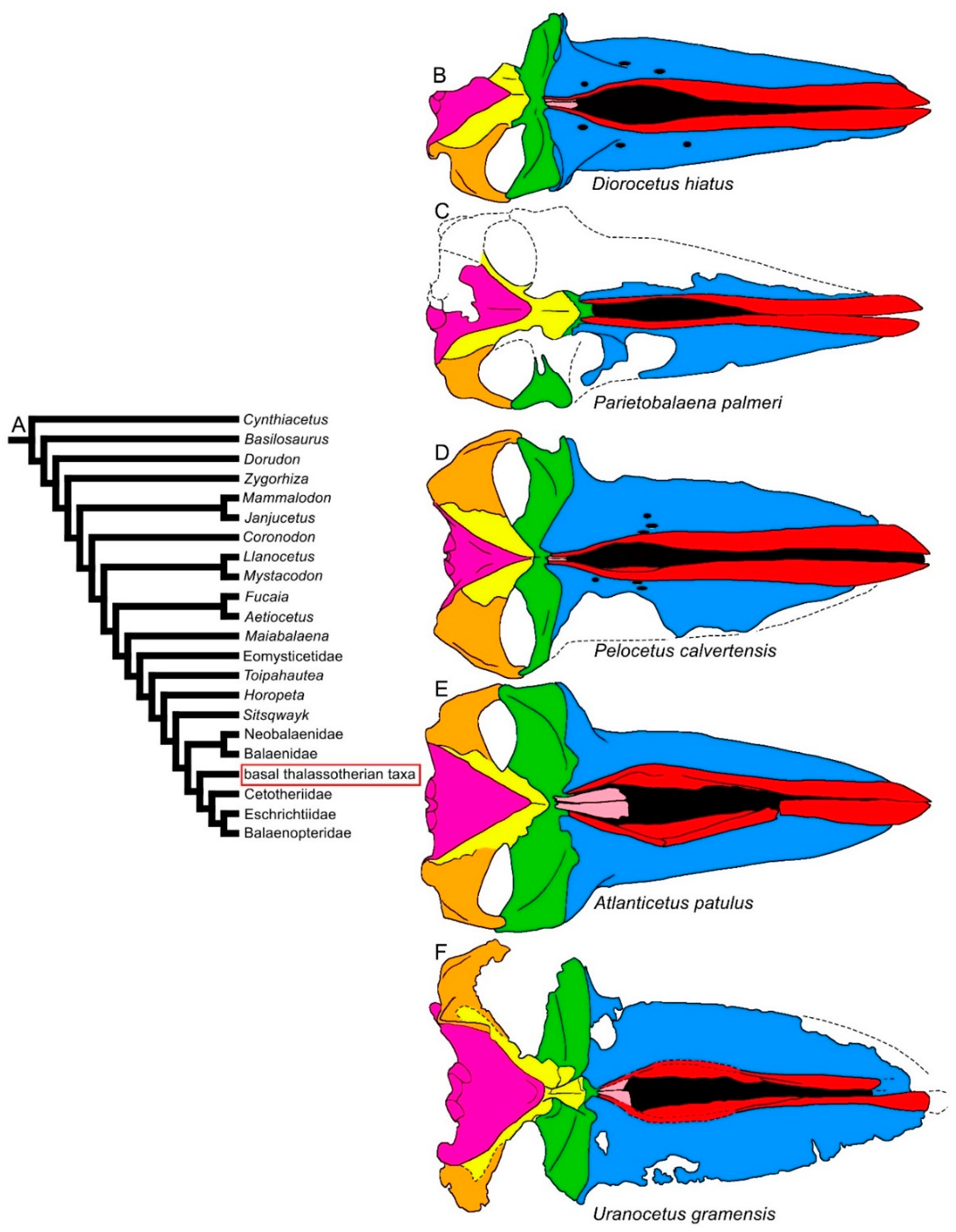
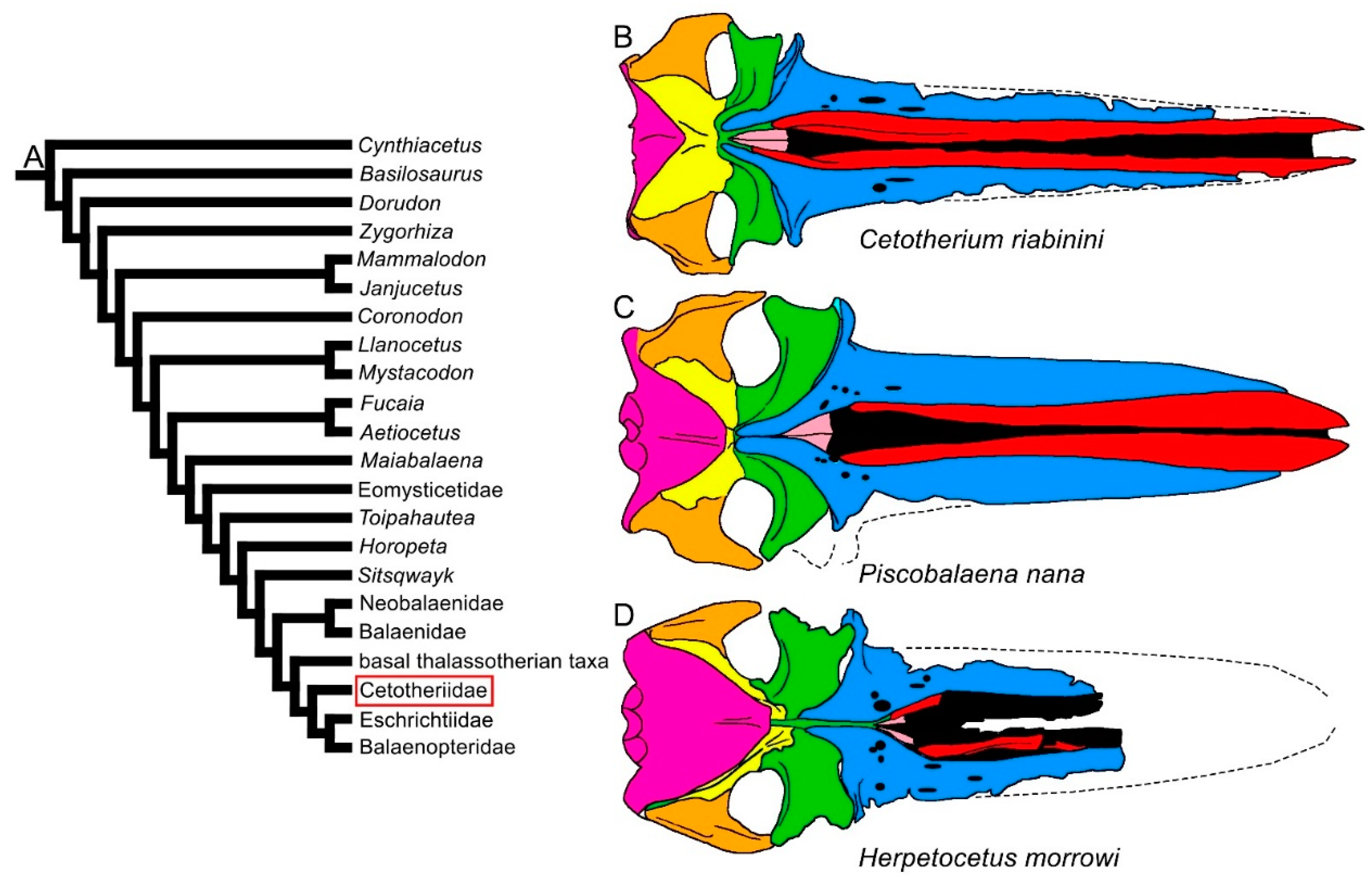
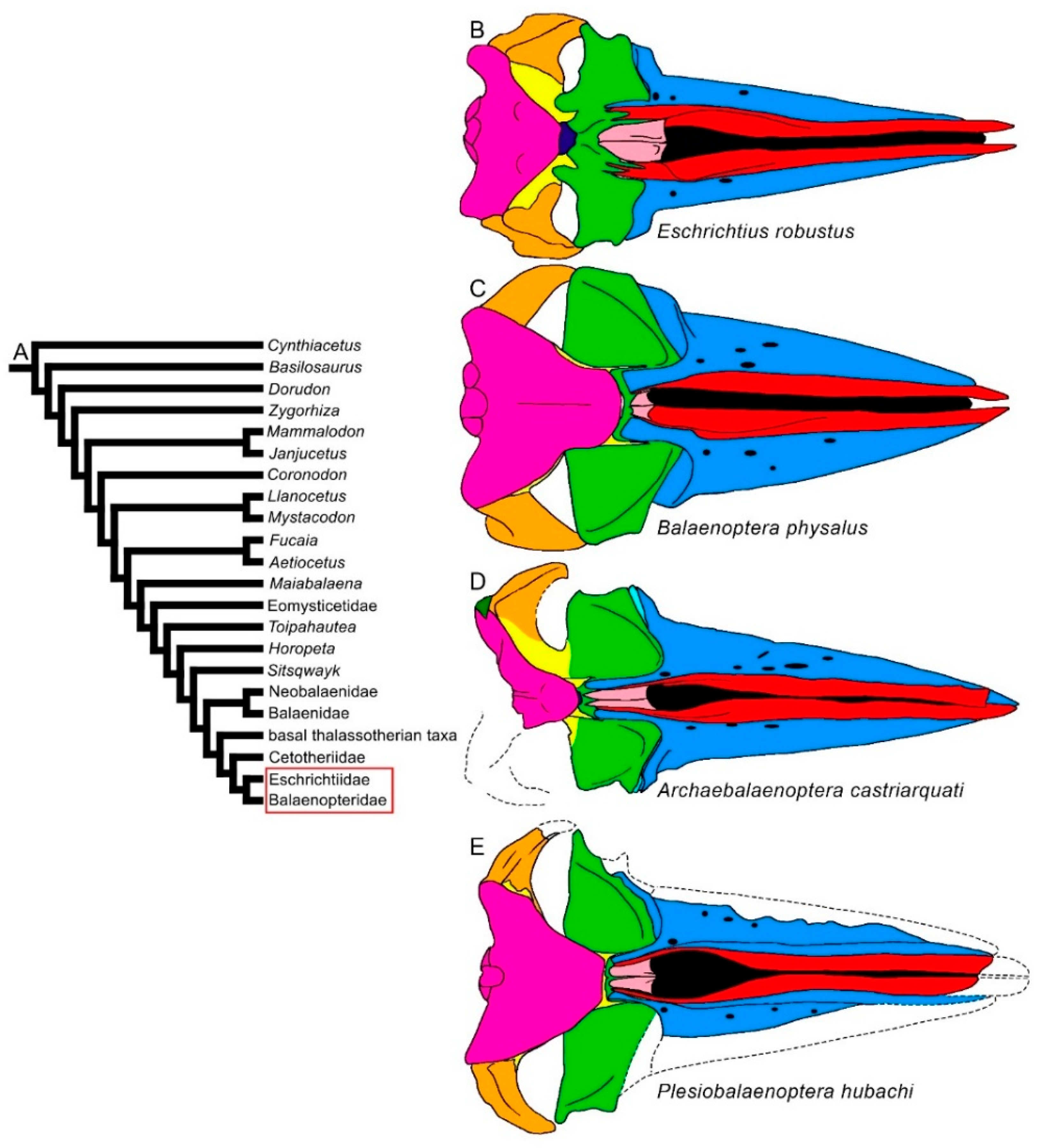

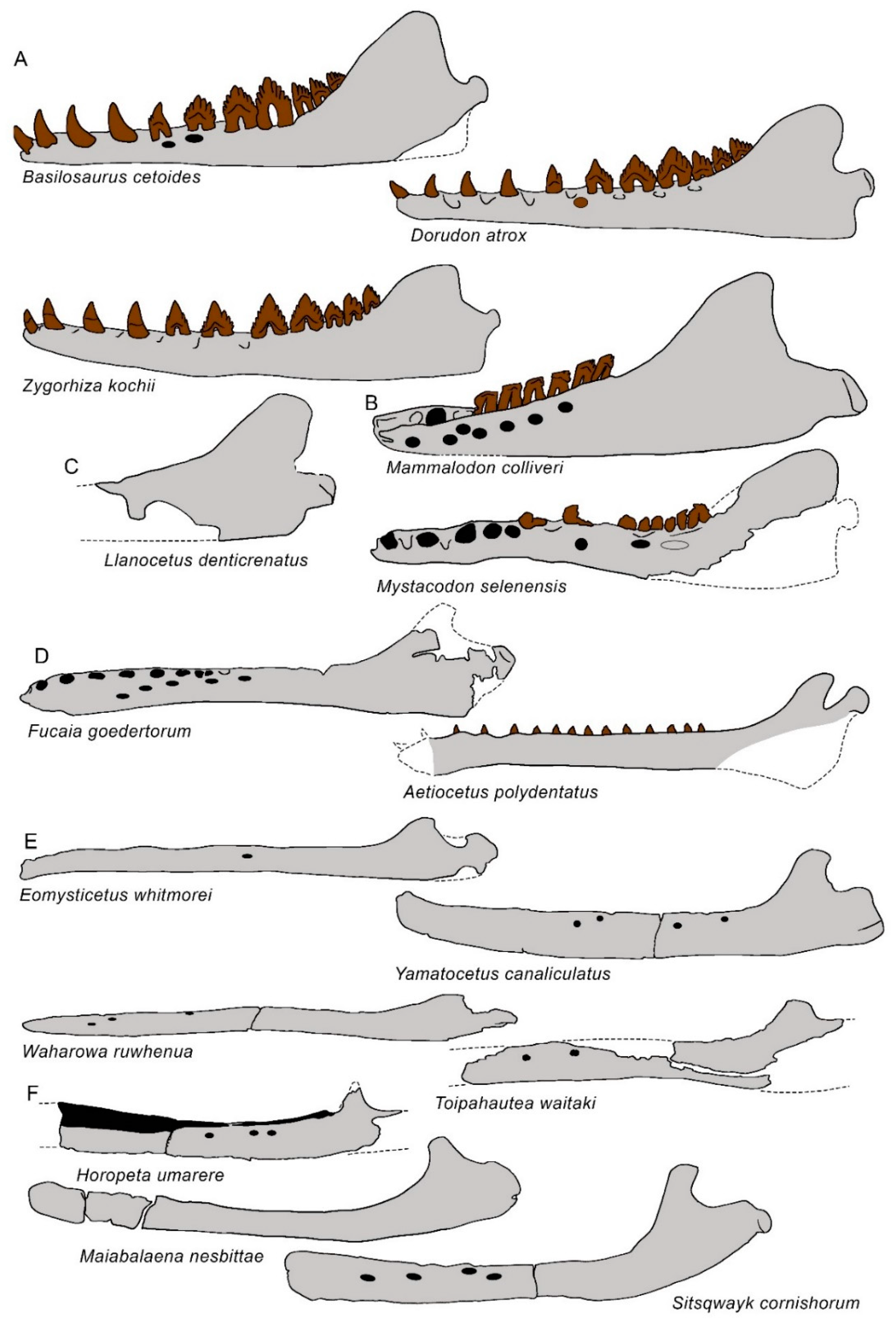
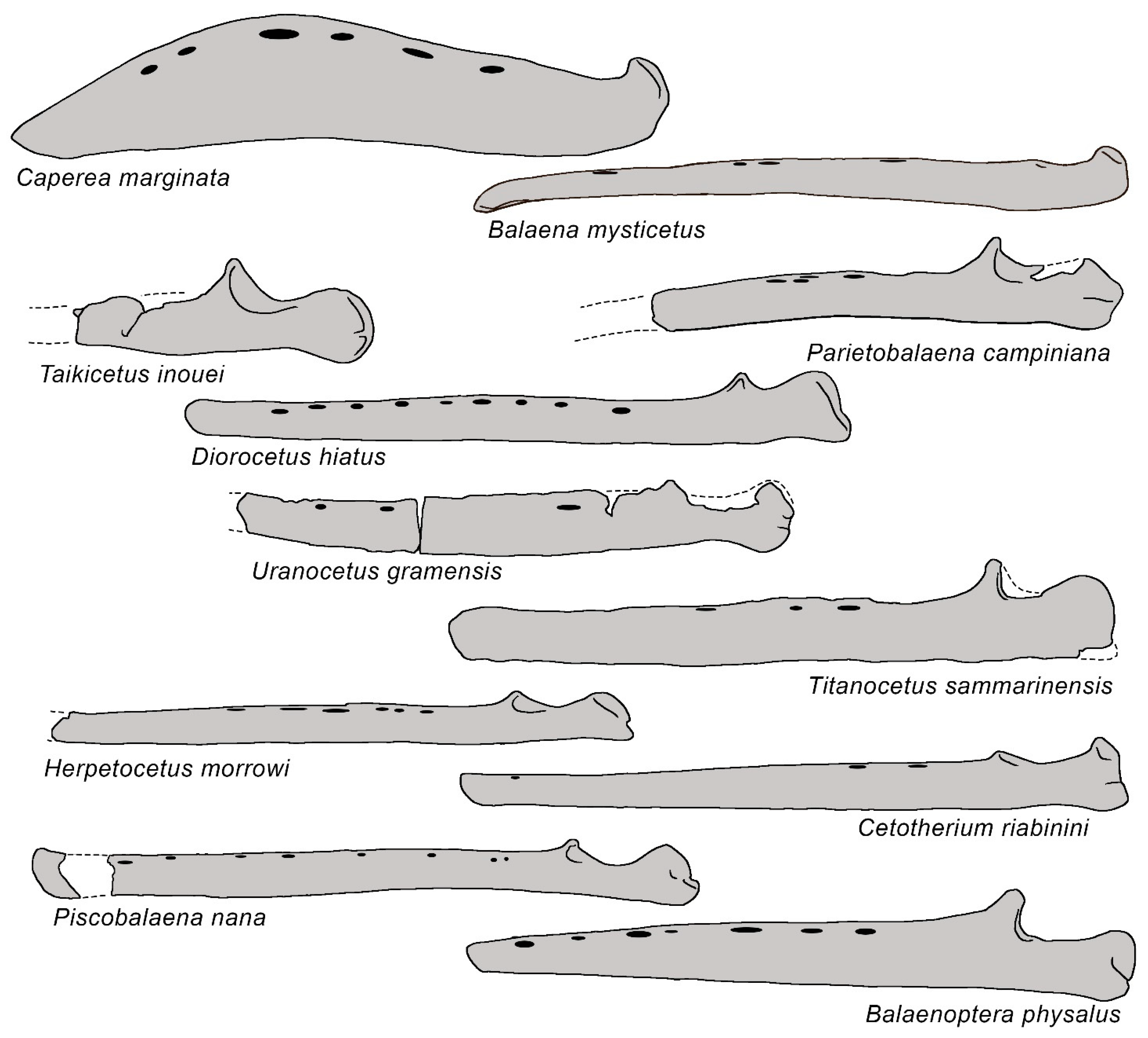

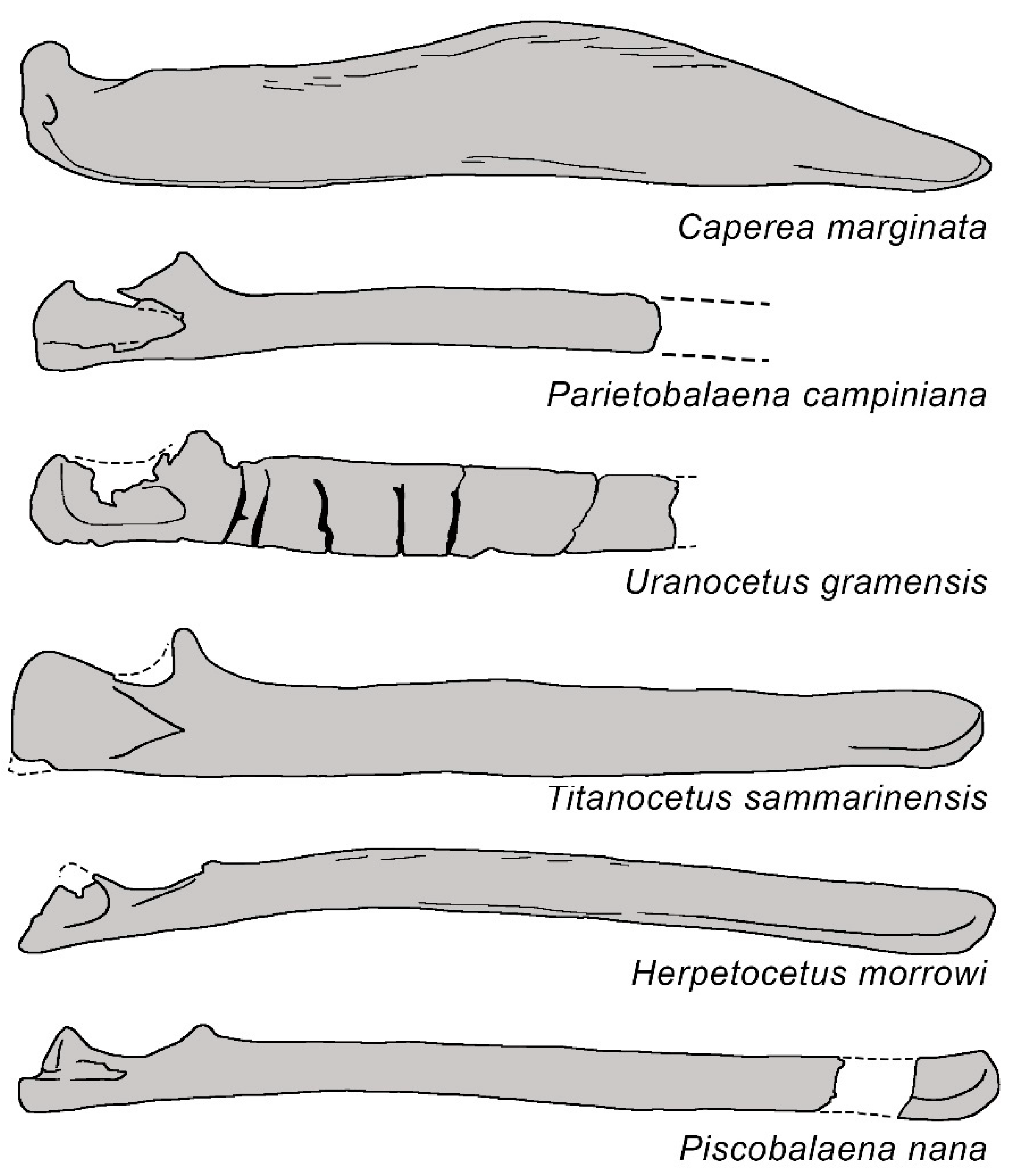




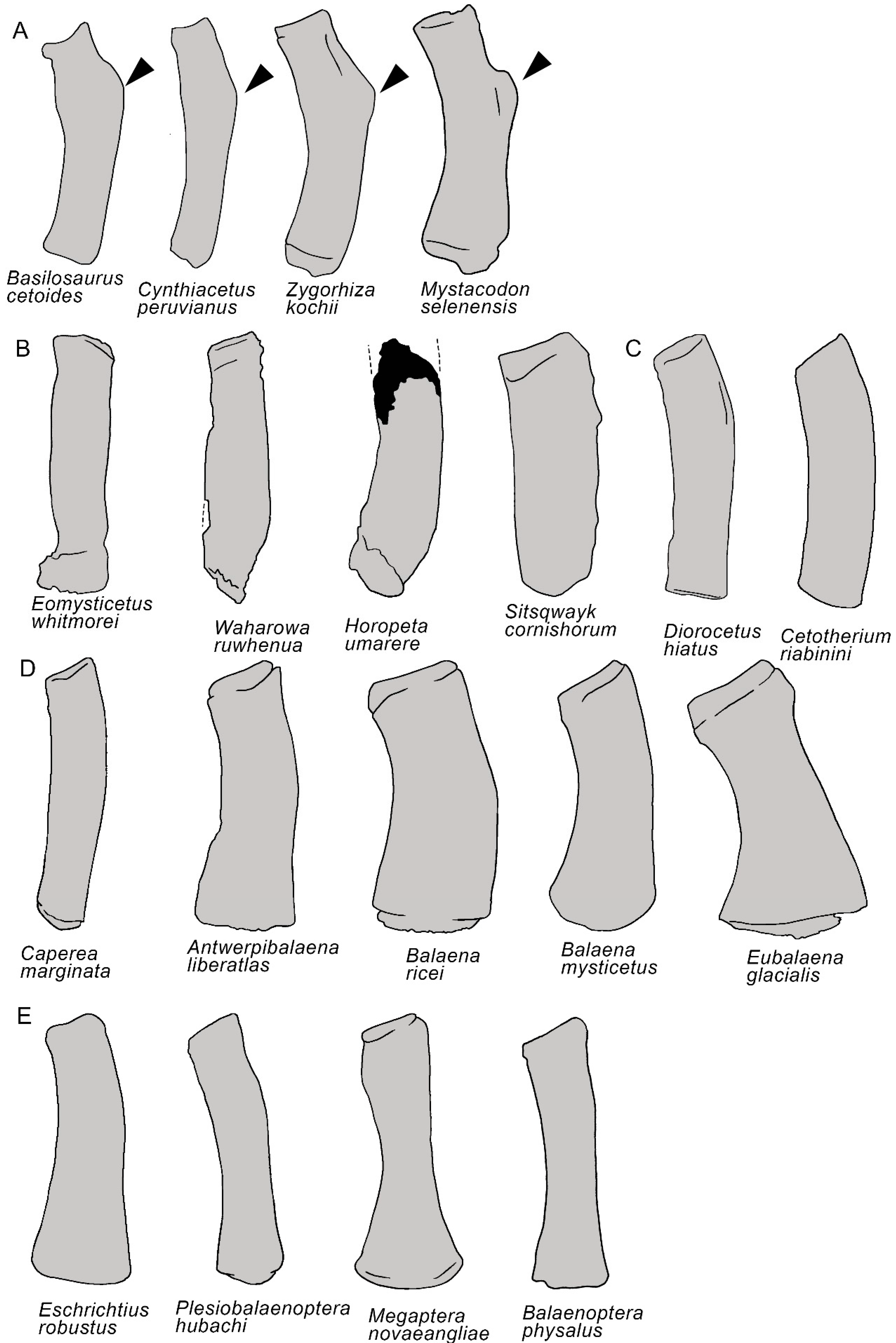
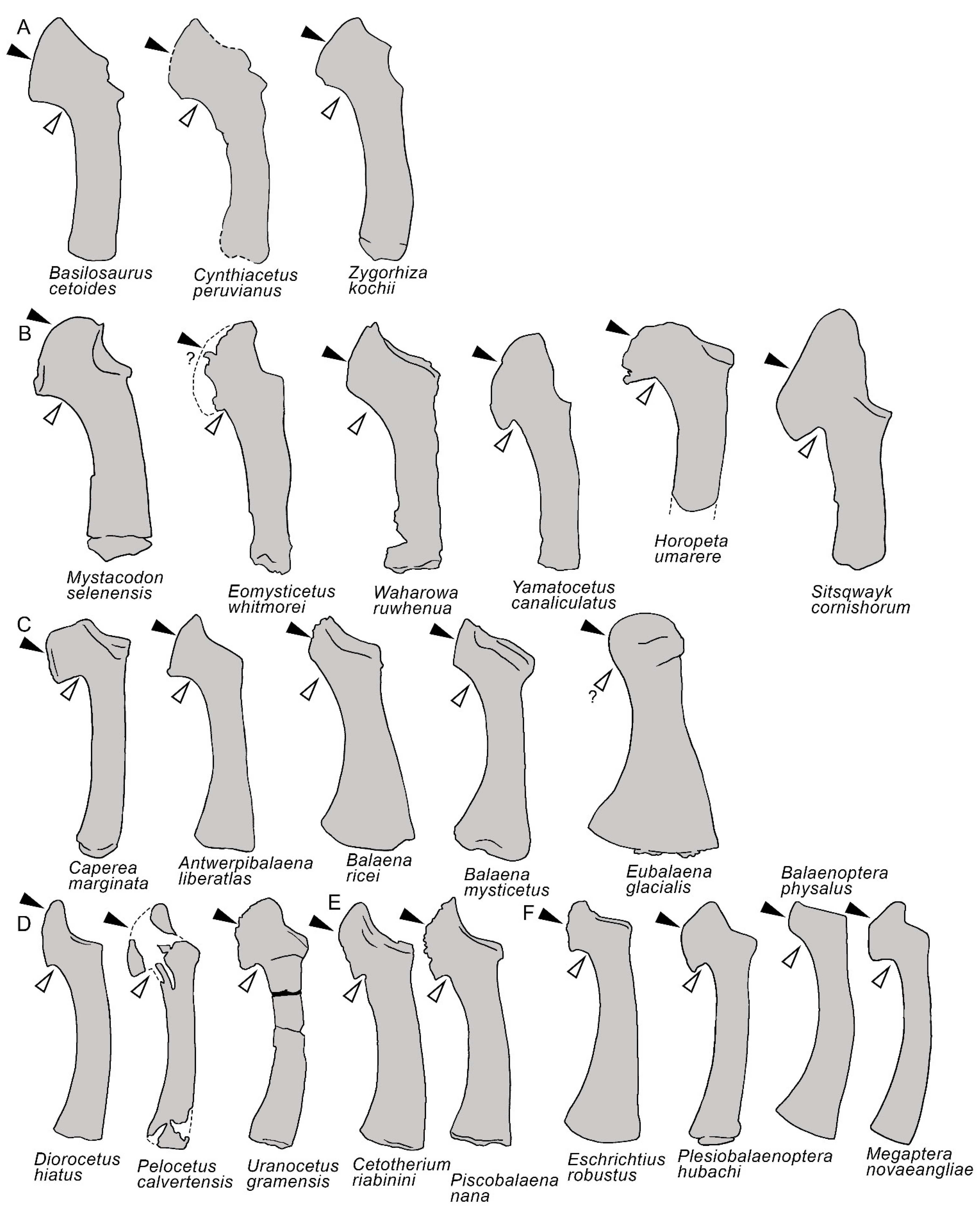

| Taxon | Age (Ma) |
|---|---|
| Cynthiacetus peruvianus | 38–33.9 |
| Dorudon atrox | 38–33.9 |
| Zygorhiza kochii | 38–33.9 |
| Mammalodontidae | 27.3–23.03 |
| Aetiocetidae | 33.9–25.2 |
| Eomysticetidae | 28.4–20.44 |
| Neobalaenidae | 11.6–0.0 |
| Balaenidae | 23.03–0.0 |
| Cetotheriidae | 28.1–2.0 |
| Balaenopteridae | 23.03–0.0 |
| Eschrichtiidae | 11.6–0.0 |
| Maiabalaena nesbittae | 33.9–28.1 |
| Sitsqwayk cornishorum | 28.1–23.03 |
| Toipahautea waitaki | 34.3–27.3 |
| Llanocetus denticrenatus | 38–33.9 |
| Coronodon havensteini | 33.9–28.1 |
| Horopeta umarere | 27.3–25.2 |
| Pelocetus calvertensis | 15.97–13.82 |
| Isanacetus laticephalus | 23.03–15.97 |
| Atlanticetus | 15.97–13.82 |
| Diorocetus hiatus | 15.97–13.82 |
| Parietobalaena palmeri | 20.44–13.82 |
| Uranocetus gramensis | 11.62–7.246 |
| Character | State 0 | State 1 | |
|---|---|---|---|
| Rostrum | |||
| 1 | Lateral process of maxilla | Absent | Present |
| 2 | Infraorbital plate | Absent | Present |
| 3 | Dorsal infraorbital foramina | Single | Multiple |
| 4 | Mesorostral groove | Absent | Present |
| 5 | Border of maxilla | Thick | Thin |
| 6 | Teeth on maxilla, premaxilla, dentary | Present | Absent |
| 7 | Position of anterior border of nasal with respect to total maxillary length | In the anterior half | In the posterior half |
| Vertex | |||
| 8 | Position of nasofrontal suture | Anterior border of interorbital region of frontal | Within interorbital region of frontal |
| 9 | Ascending process of maxilla | Short (length < 5 times width) | Long (length > 5 times width) |
| 10 | Supraoccipital superimposed on interorbital region of frontal | no | Yes |
| 11 | Parietal exposure at vertex | Long (posterior border in the posterior half of temporal fossa) | Short (posterior border in the anterior half of temporal fossa) |
| 12 | Sagittal crest | Acute | Double and flat |
| Temporal fossa | |||
| 13 | Orbitotemporal crest location | Posterodorsal edge of supraorbital process of frontal | Diagonal on supraorbital process of frontal (with variations) |
| 14 | Nuchal crest posterior to occipital condyles | Yes | No |
| 15 | Intertemporal constriction | Narrow | Wide |
| 16 | Intertemporal constriction | Long | Short |
| Occipital region | |||
| 17 | Supraoccipital orientation | More vertical | More horizontal |
| 18 | Posterior transverse constriction of occipital region at level of nuchal crest | Present | Absent |
| Tympanic bulla | |||
| 19 | Anterior lobe | Absent | Present |
| 20 | Ventral furrow | Short | Long |
| Dentary | |||
| 21 | Comparative length of ramus | Short | Long |
| 22 | Height of coronoid process | High | Low |
| 23 | Orientation of coronoid process | In line with ramus | Deflected |
| 24 | Symphyseal groove | Absent | Present |
| Appendicular skeleton | |||
| 25 | Orientation of margo caudalis of scapula | About 50° | About 30° |
| 26 | Supraspinous fossa of scapula | Wide | Narrow |
| 27 | Greater tubercle of humerus | Well-developed | Reduced |
| 28 | Deltopectoral crest of humerus | Well-developed and long | Reduced-to-absent |
| 29 | Radial crest of radius | Present | Absent |
| 30 | Articulation between humerus, radius and ulna | Rotational | Nonrotational |
| 31 | Angle below olecranon | Wide and curve | Acute |
| Cynthiacetus peruvianus | 0000000000000000000000000000000 |
| Basilosaurus cetoides | 0000000000000000000000000000000 |
| Dorudon atrox | 0000000000000000000000000000000 |
| Zygorhiza kochii | 0000000000000000000000000000000 |
| Mammalodontidae | 111100000000010011000000??????? |
| Coronodon havensteini | 11111000000001001100??????????? |
| Llanocetus denticrenatus | 11111000000001001100?000??????? |
| Mystacodon selenensis | 11111000000001001100100000???10 |
| Fucaia goedertorum | 111110000000010011001101??????? |
| Aetiocetus cotylalveus | 111110000000010011001101??????? |
| Aetiocetus polydentatus | 111110110000010011001101??????? |
| Maiabalaena nesbittae | 111110000000010011001101??00??? |
| Eomysticetidae | 1111110000000100110011011000111 |
| Toipahautea waitaki | 111111??????0?001100?101??????? |
| Horopeta umarere | 111111??????0?001100?11101??111 |
| Sitsqwayk cornishorum | 1111110000000100110011111000111 |
| Neobalaenidae | 1111111000—1111111111-11111111 |
| Balaenidae | 1111111000—1111111111-10111110 |
| Diorocetus hiatus | 111111100110111111001111????111 |
| Parietobalaena palmeri | 111111100010111111001111??????? |
| Joumocetus shimizui | 111111100010111111001111??????? |
| Pelocetus calvertensis | 1111111000101011111011110111?11 |
| Atlanticetus patulus | 111111100010101111101111??????? |
| Uranocetus gramensis | 111111100010101111101111??11?11 |
| Cetotheriidae | 1111111110110011110011111111111 |
| Eschrichtiidae | 1111111110111011111011110111111 |
| Balaenopteridae | 1111111110-11111111011111111111 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bisconti, M.; Carnevale, G. Skeletal Transformations and the Origin of Baleen Whales (Mammalia, Cetacea, Mysticeti): A Study on Evolutionary Patterns. Diversity 2022, 14, 221. https://doi.org/10.3390/d14030221
Bisconti M, Carnevale G. Skeletal Transformations and the Origin of Baleen Whales (Mammalia, Cetacea, Mysticeti): A Study on Evolutionary Patterns. Diversity. 2022; 14(3):221. https://doi.org/10.3390/d14030221
Chicago/Turabian StyleBisconti, Michelangelo, and Giorgio Carnevale. 2022. "Skeletal Transformations and the Origin of Baleen Whales (Mammalia, Cetacea, Mysticeti): A Study on Evolutionary Patterns" Diversity 14, no. 3: 221. https://doi.org/10.3390/d14030221
APA StyleBisconti, M., & Carnevale, G. (2022). Skeletal Transformations and the Origin of Baleen Whales (Mammalia, Cetacea, Mysticeti): A Study on Evolutionary Patterns. Diversity, 14(3), 221. https://doi.org/10.3390/d14030221





