Spatial Variability in a Symbiont-Diverse Marine Host and the Use of Observational Data to Assess Ecological Interactions
Abstract
1. Introduction
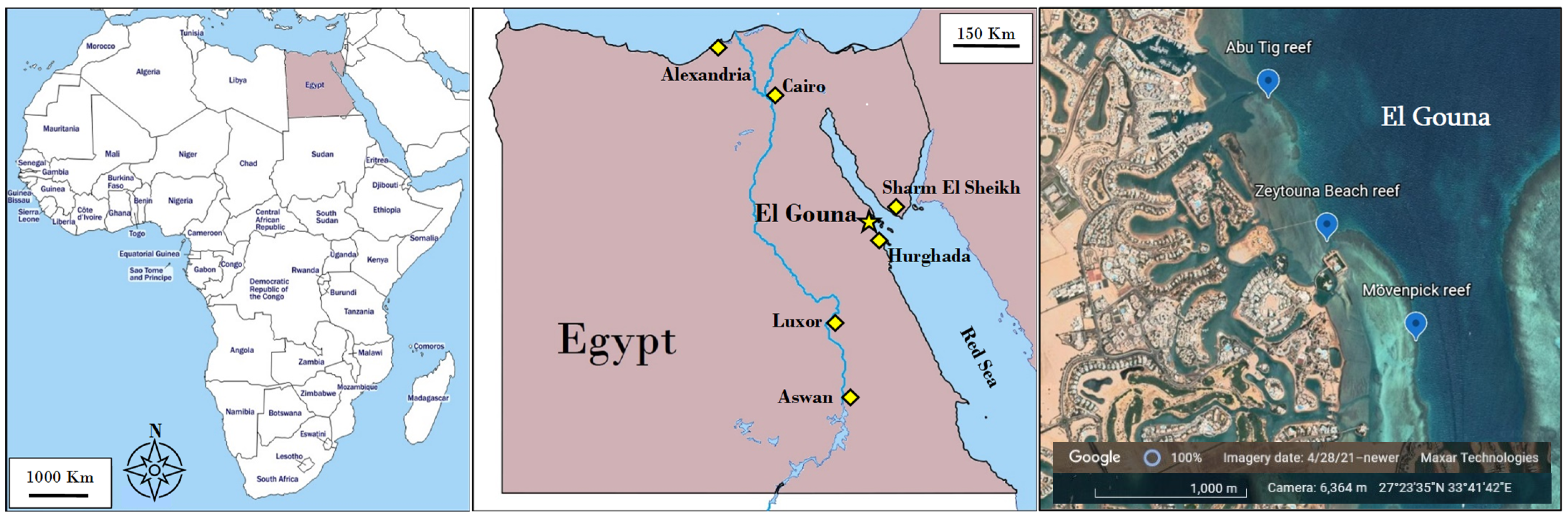
2. Materials and Methods
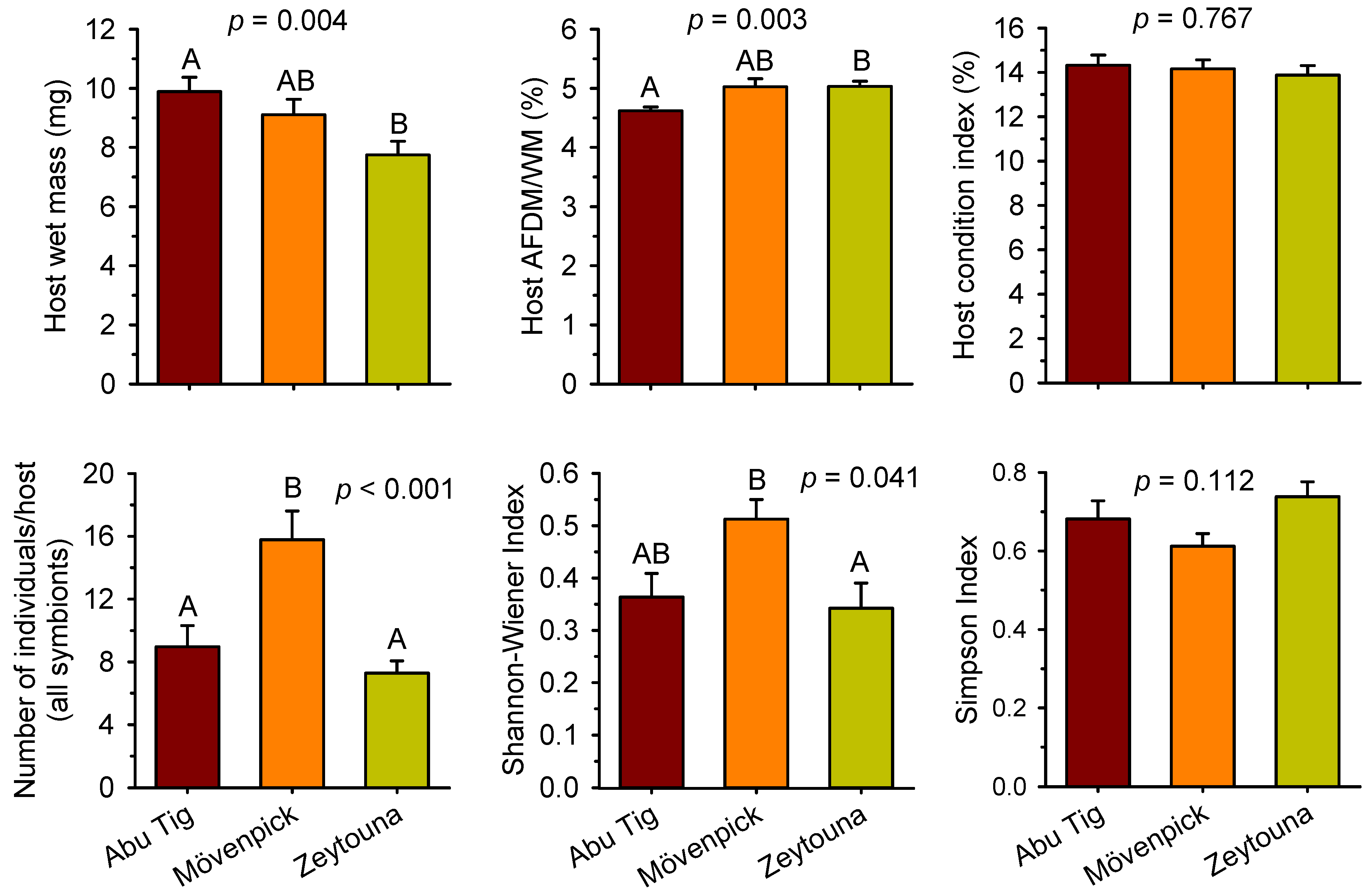
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| All Field Sites | Host Wet Mass (log x) | Host Total AFDM/WM | Host Tunic AFDM/WM | Host Body AFDM/WM | Host Condition Index | |
|---|---|---|---|---|---|---|
| Amphipods | 0.644 | 0.444 | 0.960 | 0.829 | 0.054 | |
| Copepods | 0.944 | 0.219 | 0.666 | 0.871 | 0.016 (0.039) | |
| All symbionts (log 1 + x) | 0.088 | 0.137 | 0.976 | 0.928 | 0.001 (0.068) | |
| Individual sites | ||||||
| Abu Tig | Amphipods Copepods All symbionts (log 1 + x) | 0.596 | 0.639 | 0.471 | 0.904 | 0.635 |
| 0.777 | 0.914 | 0.838 | 0.555 | 0.351 | ||
| 0.359 | 0.602 | 0.588 | 0.128 | 0.173 | ||
| Mövenpick | Amphipods Copepods All symbionts (log 1 + x) | 0.407 | 0.854 | 0.796 | 0.748 | 0.282 |
| 0.442 | 0.493 | 0.317 | 0.620 | 0.221 | ||
| 0.756 | 0.692 | 0.519 | 0.799 | 0.617 | ||
| Zeytouna Beach | Amphipods Copepods All symbionts (log 1 + x) | 0.113 | 0.260 | 0.976 | 0.989 | 0.020 (0.110) |
| 0.410 | 0.100 | 0.606 | 0.427 | <0.001 (0.212) | ||
| 0.027 (0.100) | 0.027 (0.100) | 0.653 | 0.780 | <0.001 (0.363) |
References
- Castro, P. Animal symbioses in coral reef communities: A review. Symbiosis 1988, 5, 161–184. [Google Scholar]
- Leung, T.L.F.; Poulin, R. Parasitism, commensalism, and mutualism: Exploring the many shades of symbioses. Vie Milieu 2008, 58, 107–115. [Google Scholar]
- Glynn, P.W.; Enochs, I.C. Invertebrates and their roles in coral reef ecosystems. In Coral Reefs: An Ecosystem in Transition; Dubinsky, Z., Stambler, N., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 273–325. ISBN 978-94-007-0113-7. [Google Scholar]
- Hirsch, A.M. Plant-microbe symbioses: A continuum from commensalism to parasitism. Symbiosis 2004, 37, 345–363. [Google Scholar]
- Cheney, K.L.; Côté, I.M. Mutualism or parasitism? The variable outcome of cleaning symbioses. Biol. Lett. 2005, 1, 162–165. [Google Scholar] [CrossRef]
- Brown, B.L.; Creed, R.P.; Skelton, J.; Rollins, M.A.; Farrell, K.J. The fine line between mutualism and parasitism: Complex effects in a cleaning symbiosis demonstrated by multiple field experiments. Oecologia 2012, 170, 199–207. [Google Scholar] [CrossRef]
- Hoeksema, J.D.; Bruna, E.M. Pursuing the big questions about interspecific mutualism: A review of theoretical approaches. Oecologia 2000, 125, 321–330. [Google Scholar] [CrossRef]
- Silknetter, S.; Kanno, Y.; Kanapeckas Métris, K.L.; Cushman, E.; Darden, T.L.; Peoples, B.K. Mutualism or parasitism: Partner abundance affects host fitness in a fish reproductive interaction. Freshw. Biol. 2019, 64, 175–182. [Google Scholar] [CrossRef]
- Saffo, M.B.; McCoy, A.M.; Rieken, C.; Slamovits, C.H. Nephromyces, a beneficial apicomplexan symbiont in marine animals. Proc. Natl. Acad. Sci. USA 2010, 107, 16190–16195. [Google Scholar] [CrossRef]
- Cumbo, V.R.; Baird, A.H.; Moore, R.B.; Negri, A.P.; Neilan, B.A.; Salih, A.; van Oppen, M.J.H.; Wang, Y.; Marquis, C.P. Chromera velia is endosymbiotic in larvae of the reef corals Acropora digitifera and A. tenuis. Protist 2013, 164, 237–244. [Google Scholar] [CrossRef]
- Kwong, W.K.; del Campo, J.; Mathur, V.; Vermeij, M.J.A.; Keeling, P.J. A widespread coral-infecting apicomplexan with chlorophyll biosynthesis genes. Nature 2019, 568, 103–107. [Google Scholar] [CrossRef]
- Halliday-Isaac, A.K.; Robinson, J.B.; Cruz-Rivera, E.; Campbell, A.G.; Sikkel, P.C. Environmental correlates of prevalence of an intraerythrocytic apicomplexan infecting Caribbean damselfish. Parasitologia 2021, 1, 69–82. [Google Scholar] [CrossRef]
- Carlton, J.T.; Blakeslee, A.M.H.; Fowler, A.E. Accidental associates are not symbionts: The absence of a non-parasitic endosymbiotic community inside the common periwinkle Littorina littorea (Mollusca: Gastropoda). Mar. Biol. 2020, 167, 97. [Google Scholar] [CrossRef]
- Hirose, E.; Oka, A.T.; Akahori, M. Sexual reproduction of the photosymbiotic ascidian Diplosoma virens in the Ryukyu Archipelago, Japan: Vertical transmission, seasonal change, and possible impact of parasitic copepods. Mar. Biol. 2005, 146, 677–682. [Google Scholar] [CrossRef]
- Parmentier, E.; Michel, L. Boundary lines in symbiosis forms. Symbiosis 2013, 60, 1–5. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Interspecific competition of symbiotic and fouling species of red king crab in the Barents Sea. Doklady Biol. Sci. 2011, 440, 300–302. [Google Scholar] [CrossRef]
- Devlaming, V.; Grossman, G.; Chapman, F. On the use of the gonosomatic index. Comp. Biochem. Physiol. A 1982, 73, 31–39. [Google Scholar] [CrossRef]
- Lucas, A.; Beninger, P.G. The use of physiological condition indices in marine bivalve aquaculture. Aquaculture 1985, 44, 187–200. [Google Scholar] [CrossRef]
- Crosby, M.P.; Gale, L.D. A review and evaluation of bivalve condition index methodologies with a suggested standard method. J. Shellfish Res. 1990, 9, 233–237. [Google Scholar]
- West, G. Methods of assessing ovarian development in fishes: A review. Mar. Freshw. Res. 1990, 41, 199–222. [Google Scholar] [CrossRef]
- Fernandez, C.; Boudouresque, C.-F. Phenotypic plasticity of Paracentrotus lividus (Echinodermata: Echinoidea) in a lagoonal environment. Mar. Ecol. Prog. Ser. 1997, 152, 145–154. [Google Scholar] [CrossRef]
- Fernandez, C.; Boudouresque, C.-F. Nutrition of the sea urchin Paracentrotus lividus (Echinodermata: Echinoidea) fed different artificial food. Mar. Ecol. Prog. Ser. 2000, 204, 131–141. [Google Scholar] [CrossRef]
- Lowerre-Barbieri, S.K.; Brown-Peterson, N.J.; Murua, H.; Tomkiewicz, J.; Wyanski, D.M.; Saborido-Rey, F. Emerging issues and methodological advances in fisheries reproductive biology. Mar. Coast. Fish. 2011, 3, 32–51. [Google Scholar] [CrossRef]
- Labocha, M.K.; Hayes, J.P. Morphometric indices of body condition in birds: A review. J. Ornithol. 2012, 153, 1–22. [Google Scholar] [CrossRef]
- Ouréns, R.; Freire, J.; Fernández, L. Definition of a new unbiased gonad index for aquatic invertebrates and fish: Its application to the sea urchin Paracentrotus lividus. Aquat. Biol. 2012, 17, 145–152. [Google Scholar] [CrossRef]
- Unglaub, B.; Steinfartz, S.; Kühne, D.; Haas, A.; Schmidt, B.R. The relationships between habitat suitability, population size and body condition in a pond-breeding amphibian. Basic Appl. Res. 2018, 27, 20–29. [Google Scholar] [CrossRef]
- Dunphy, B.J.; Wells, R.M.G. Endobiont infestation, shell strength and condition index in wild populations of New Zealand abalone, Haliotis iris. Mar. Freshwater Res. 2001, 52, 781–786. [Google Scholar] [CrossRef]
- O’Connell-Milne, S.A.; Poulin, R.; Savage, C.; Rayment, W. Reduced growth, body condition and foot length of the bivalve Austrovenus stutchburyi in response to parasite infection. J. Exp. Mar. Biol. Ecol. 2016, 474, 23–28. [Google Scholar] [CrossRef]
- Bazterrica, M.C.; Bruschetti, C.M.; Alvarez, M.F.; Iribarne, O.; Botto, F. Effects of macroalgae on the recruitment, growth, and body condition of an invasive reef forming polychaete in a south-western Atlantic coastal lagoon. J. Sea Res. 2014, 88, 121–129. [Google Scholar] [CrossRef]
- Shenkar, N. Ascidian (Chordata, Ascidiacea) diversity in the Red Sea. Mar. Biodiv. 2012, 42, 459–469. [Google Scholar] [CrossRef]
- Vandepas, L.E.; Oliveira, L.M.; Lee, S.S.C.; Hirose, E.; Rocha, R.M.; Swalla, B.J. Biogeography of Phallusia nigra: Is it really black and white? Biol. Bull. 2015, 228, 52–64. [Google Scholar] [CrossRef]
- Rocha, R.M.D.; Lotufo, T.M.D.C.; de Almeida Rodrigues, S. The biology of Phallusia nigra Savigny, 1816 (Tunicata: Ascidiacea) in Southern Brazil: Spatial distribution and reproductive cycle. Bull. Mar. Sci. 1999, 64, 77–88. [Google Scholar]
- Kondilatos, G.; Corsini-Foka, M.; Pancucci-Papadopoulou, M.-A. Occurrence of the first non-indigenous ascidian Phallusia nigra Savigny, 1816 (Tunicata: Ascidiacea) in Greek waters. Aquat. Inv. 2010, 5, 181–184. [Google Scholar] [CrossRef]
- Naser, H.A. Variability of marine macrofouling assemblages in a marina and a mariculture centre in Bahrain, Arabian Gulf. Reg. Stud. Mar. Sci. 2017, 16, 162–170. [Google Scholar] [CrossRef]
- Goodbody, I. The biology of Ascidia nigra (Savigny). I. Survival and mortality in an adult population. Biol. Bull. 1962, 122, 40–51. [Google Scholar] [CrossRef]
- Goodbody, I. The biology of Ascidia nigra (Savigny). III. The annual pattern of colonization. Biol. Bull. 1965, 129, 128–133. [Google Scholar] [CrossRef]
- Goodbody, I.; Gibson, J. The biology of Ascidia nigra (Savigny) V. Survival in populations settled at different times of the year. Biol. Bull. 1974, 146, 217–237. [Google Scholar] [CrossRef]
- Al-Sofyani, A.M.A.; Satheesh, S. Recruitment patterns of the solitary ascidian Phallusia nigra Savigny, 1816 on artificial substrates submerged in the central Red Sea, Saudi Arabia. Oceanol. Hydrobiol. Stud. 2019, 48, 262–269. [Google Scholar] [CrossRef]
- Granot, I.; Shenkar, N.; Belmaker, J. Habitat niche breadth predicts invasiveness in solitary ascidians. Ecol. Evol. 2017, 7, 7838–7847. [Google Scholar] [CrossRef]
- Ghazilou, A.; Koochaknejad, E.; Ershadifar, H.; Negarestan, H.; Kor, K.; Baskaleh, G. Infestation biology of Phallusia nigra (Tunicata, Phlebobranchia) on hard corals in a subtropical bay. Mar. Ecol. Prog. Ser. 2019, 626, 135–143. [Google Scholar] [CrossRef]
- Hirose, E.; Yamashiro, H.; Mori, Y. Properties of tunic acid in the ascidian Phallusia nigra (Ascidiidae, Phlebobranchia). Zool. Sci. 2001, 18, 309–314. [Google Scholar] [CrossRef]
- Odate, S.; Pawlik, J.R. The Role of vanadium in the chemical defense of the solitary tunicate, Phallusia nigra. J. Chem. Ecol. 2007, 33, 643–654. [Google Scholar] [CrossRef]
- Mayzel, B.; Haber, M.; Ilan, M. Chemical defense against fouling in the solitary ascidian Phallusia nigra. Biol. Bull. 2014, 227, 232–241. [Google Scholar] [CrossRef]
- Stock, J.H. Report on the Notodelphyidae (Copepoda, Cyclopoida) of the Israel South Red Sea Expedition. Bull. Sea Fish. Res. Stat. Israel 1967, 27, 1–126. [Google Scholar]
- White, K.N. A Taxonomic review of the Leucothoidae (Crustacea: Amphipoda). Zootaxa 2011, 3078, 1–113. [Google Scholar] [CrossRef]
- Kim, I.-H.; Cruz-Rivera, E.; Sherif, M.-E.-D.; El-Sahhar, S. Cyclopoid copepods (Ascidicolidae, Notodelphyidae) associated with Phallusia nigra Savigny, 1816 (Ascidiacea) in the Red Sea: A new ascidicolid and first descriptions of the males from two notodelphyids. J. Crust. Biol. 2016, 36, 553–566. [Google Scholar] [CrossRef]
- Martin, D.; Nygren, A.; Cruz-Rivera, E. Proceraea exoryxae sp. nov. (Annelida, Syllidae, Autolytinae), the first known polychaete miner tunneling into the tunic of an ascidian. PeerJ 2017, 5, e3374. [Google Scholar] [CrossRef]
- White, K.N.; Krapp-Schickel, T. Red Sea Leucothoidae (Crustacea: Amphipoda) including new and re-described species. Eur. J. Taxon. 2017, 324, 1–40. [Google Scholar] [CrossRef]
- White, K.N. Caribbean Leucothoidae (Crustacea: Amphipoda) of Panama. Gulf Caribb. Res. 2011, 23, 23–35. [Google Scholar] [CrossRef]
- Senna, A.R.; Andrade, L.F.; Ramos, B.S.; Skinner, L.F. A new ascidian-dwelling species of Leucothoe Leach, 1814 (Amphipoda: Leucothoidae) from Ilha Grande Bay, Rio de Janeiro State, Brazil. J. Nat. Hist. 2021, 55, 1441–1460. [Google Scholar] [CrossRef]
- Hernández, J.E.; Bolaños, J.A.; Palazón, J.L.; Hernández, G.; Lira, C.; Baeza, J.A. The enigmatic life history of the symbiotic crab Tunicotheres moseri (Crustacea, Brachyura, Pinnotheridae): Implications for its mating system and population structure. Biol. Bull. 2012, 223, 278–290. [Google Scholar] [CrossRef]
- Thomas, J.D.; Klebba, K.N. New species and host associations of commensal leucothoid amphipods from coral reefs in Florida and Belize (Crustacea:Amphipoda). Zootaxa 2007, 1494, 1–44. [Google Scholar] [CrossRef]
- White, K.; Reimer, J. Commensal Leucothoidae (Crustacea, Amphipoda) of the Ryukyu Archipelago, Japan. Part I: Ascidian-dwellers. ZooKeys 2012, 163, 13–55. [Google Scholar] [CrossRef]
- Ho, J.-S. Origin and evolution of the parasitic cyclopoid copepods. Int. J. Parasitol. 1994, 24, 1293–1300. [Google Scholar] [CrossRef]
- Kim, I.-H.; Boxshall, G.A. Untold diversity: The astonishing species richness of the Notodelphyidae (Copepoda: Cyclopoida), a family of symbiotic copepods associated with ascidians (Tunicata). Megataxa 2020, 4, 1–660. [Google Scholar] [CrossRef]
- Kim, I.-H.; Boxshall, G.A. Copepods (Cyclopoida) associated with ascidian hosts: Ascidicolidae, Buproridae, Botryllophilidae, and Enteropsidae, with descriptions of 84 new species. Zootaxa 2021, 4978, 1–286. [Google Scholar] [CrossRef]
- Ortiz, M. Claves ilustradas para la clasificación de los anfípodos (Crustacea, Peracarida) de Cuba: Morfología y taxonomía. Rev. Investig. Mar. 2021, 41, 1–108. [Google Scholar]
- Thiel, M. Host-use and population demographics of the ascidian-dwelling amphipod Leucothoe spinicarpa: Indication for extended parental care and advanced social behaviour. J. Nat. Hist. 1999, 33, 193–206. [Google Scholar] [CrossRef]
- Boxshall, G.A.; Marchenkov, A. A new genus of notodelphyid copepod (Crustacea, Copepoda, Cyclopoida) from a compound ascidian host collected in the Suez Canal. Zoosystema 2005, 27, 483–497. [Google Scholar]
- Schellenberg, A. Neuo Notodelphyiden des Berliner und Hamburger Museums mit einer Übersicht der ascidien bowohnenden Gattungen und Arten. Mitt. Zool. Mus. 1922, 10, 219–274. [Google Scholar]
- Gurney, R. Report on the Crustacea:—Copepoda (littoral and semi-parasitic). Trans. Zool. Soc. 1927, 22, 451–577. [Google Scholar] [CrossRef]
- El Sherbiny Ahmed, H.; Sherif Ahmed, H.; Hassan Ali, N. Model for environmental risk assessment of tourism project construction on the Egyptian Red Sea Coast. J. Environ. Eng. 2006, 132, 1272–1281. [Google Scholar] [CrossRef]
- Vanderstraete, T.; Goossens, R.; Ghabour, T.K. The use of multi-temporal Landsat Images for the change detection of the coastal zone near Hurghada, Egypt. Int. J. Remote Sens. 2006, 27, 3645–3655. [Google Scholar] [CrossRef]
- Marshall, N.A.; Marshall, P.A.; Abdulla, A.; Rouphael, T.; Ali, A. preparing for climate change: Recognising its early impacts through the perceptions of dive tourists and dive operators in the Egyptian Red Sea. Curr. Issues Tour. 2011, 14, 507–518. [Google Scholar] [CrossRef]
- Nassar, K.; El-Adawy, A.; Zakaria, M.; Diab, R.; Masria, A. Quantitative appraisal of naturalistic/anthropic shoreline shifts for Hurghada: Egypt. Mar. Georesour. Geotechnol. 2021. [Google Scholar] [CrossRef]
- Moufaddal, W.M. Use of Satellite imagery as environmental impact assessment tool: A case study from the NW Egyptian Red Sea coastal zone. Environ. Monit. Assess. 2005, 107, 427–452. [Google Scholar] [CrossRef]
- Ballarin, L.; Burighel, P. Tunicata and Cephalochordata. In Biological Science Fundamentals and Systematics; Encyclopedia of Biological, Physiological and Health Sciences; Minelli, A., Contrafatto, G., Eds.; EOLSS Publishers, UNESCO: Oxford, UK, 2009; Volume IV, pp. 43–67. ISBN 978-84826-189-1. [Google Scholar]
- Cruz-Rivera, E.; Hay, M.E. Macroalgal traits and the feeding and fitness of an herbivorous amphipod: The roles of selectivity, mixing, and compensation. Mar. Ecol. Prog. Ser. 2001, 218, 249–266. [Google Scholar] [CrossRef]
- Prado, P.; Heck, K.L. Seagrass selection by omnivorous and herbivorous consumers: Determining factors. Mar. Ecol. Prog. Ser. 2011, 429, 45–55. [Google Scholar] [CrossRef]
- Cruz-Rivera, E.; Friedlander, M. Effects of algal phenotype on mesograzer feeding. Mar. Ecol. Prog. Ser. 2013, 490, 69–78. [Google Scholar] [CrossRef][Green Version]
- Okumuş, İ.; Stirling, H.P. Seasonal variations in the meat weight, condition index and biochemical composition of mussels (Mytilus edulis, L.) in suspended culture in two Scottish Sea lochs. Aquaculture 1998, 159, 249–261. [Google Scholar] [CrossRef]
- Adjei-Boateng, D.; Wilson, J.G. Body condition and gametogenic cycle of Galatea paradoxa (Mollusca: Bivalvia) in the Volta River Estuary, Ghana. Estuar. Coast. Shelf Sci. 2013, 132, 94–98. [Google Scholar] [CrossRef]
- Rainier, J.S.; Mann, R.L. A comparison of methods for calculating condition index in eastern oyster, Crassostrea virginica (Gmelin, 1791). J. Shellfish Res. 1992, 11, 55–58. [Google Scholar]
- Zeng, Y.; Yang, H. Review of molluscan bivalve condition index calculations and application in northern quahogs Mercenaria mercenaria. Aquac. Res. 2021, 52, 23–36. [Google Scholar] [CrossRef]
- Gage, J. Seasonal Cycles of Notodelphys and Ascidicola, copepod associates with Ascidiella (Ascidiacea). J. Zool. 1966, 150, 223–233. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; Tierno de Figueroa, J.M.; Navarro-Barranco, C.; Ros, M.; Sánchez-Moyano, J.E.; Moreira, J. Dietary analysis of the marine Amphipoda (Crustacea: Peracarida) from the Iberian Peninsula. J. Sea Res. 2014, 85, 508–517. [Google Scholar] [CrossRef]
- da Silva Ramos, E.K.; Batista Rosa, A.H.; Cobo, V.J. Influence of the endo-symbiont Leucothoe wuriti (Thomas & Klebba, 2007) (Crustacea, Leucothoidae) on the biomass of Phallusia nigra (Savigny, 1816) (Tunicata, Ascididae), in the northeastern coast of the São Paulo State, Brazil. Rev. Biociên. 2015, 21, 38–43. [Google Scholar]
- Egan, E.A. The seasonal occurrence of the copepod Pachypygus australis Gotto (Notodelphyidae) in its host Pyura pachydermatina (Herdman) Pyuridae: Ascidiacea. J. Exp. Mar. Biol. Ecol. 1984, 76, 247–262. [Google Scholar] [CrossRef]
- Svavarsson, J. Life Cycle and population dynamics of the symbiotic copepod Lichomolgus canui Sars associated with the ascidian Halocynthia pyriformis (Rathke). J. Exp. Mar. Biol. Ecol. 1990, 142, 1–12. [Google Scholar] [CrossRef]
- Saito, S. Density and adult ratio of the symbiotic harpacticoid copepod Idomene purpurocincta in the compound ascidian host Aplidium yamazii. Plankton Benthos Res. 2009, 4, 160–166. [Google Scholar] [CrossRef][Green Version]
- Jakob, E.M.; Marshall, S.D.; Uetz, G.W. Estimating fitness: A comparison of body condition indices. Oikos 1996, 77, 61–67. [Google Scholar] [CrossRef]
- Peig, J.; Green, A.J. The paradigm of body condition: A critical reappraisal of current methods based on mass and length. Funct. Ecol. 2010, 24, 1323–1332. [Google Scholar] [CrossRef]
- Labocha, M.K.; Schutz, H.; Hayes, J.P. Which body condition index is best? Oikos 2014, 123, 111–119. [Google Scholar] [CrossRef]
- Addicott, J.F. A multispecies aphid–ant association: Density dependence and species-specific effects. Can. J. Zool. 1979, 57, 558–569. [Google Scholar] [CrossRef]
- Drew, G.; King, K. More or less? The effect of symbiont density in protective mutualisms. Am. Nat. 2022. [Google Scholar] [CrossRef]
- Illg, P.L. North American copepods of the family Notodelphyidae. Proc. US Natl. Mus. 1958, 107, 463–649. [Google Scholar] [CrossRef][Green Version]
- Dudley, P.L. A light and electron microscopic study of tissue interactions between a parasitic copepod, Scolecodes huntsmani (Henderson), and its host ascidian, Styela gibbsii (Stimpson). J. Morphol. 1968, 124, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Conradi, M.; López-González, P.J. A new family of cyclopoid copepods (Fratiidae) symbiotic in the ascidian (Clavelina dellavallei) from Cádiz, Spain. J. Zool. 1998, 246, 39–48. [Google Scholar]
- Ho, J.; Kim, I.-H. Two species of copepoda parasitic in the algal-bearing ascidian, Didemnum molle (Herdman), in Okinawa, Japan. Proc. Biol. Soc. Wash. 2009, 122, 414–425. [Google Scholar] [CrossRef]
- Marchenkov, A.; Boxshall, G.A. A new notodelphyid copepod, Paranotodelphys illgi n. sp. (Copepoda: Cyclopoida), parasitic in the ascidian Corynascidia herdmani Ritter in the North Pacific. Syst. Parasitol. 2003, 54, 43–52. [Google Scholar] [CrossRef]
- Stock, J.H. Parasite or commensal? Notodelphys weberi, a new South African ascidicole copepod. Amsterdam Nat. 1950, 1, 36–42. [Google Scholar]
- Illg, P.L. Occurrence in Sagami Bay, Japan, of Scolecodes, a remarkable copepod parasite of ascidians. Publ. Seto Mar. Biol. Lab. 1970, 18, 69–74. [Google Scholar] [CrossRef][Green Version]
- Thomas, J.D.; Taylor, G.W. Mouthpart morphology and feeding strategies of the commensal amphipod, Anamixis hanseni Stebbing. Bull. Mar. Sci. 1981, 31, 462–467. [Google Scholar]
- Fleming, T.H.; Holland, J.N. The evolution of obligate pollination mutualisms: Senita cactus and senita moth. Oecologia 1998, 114, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, G.E.D.; Downie, J.A. Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu. Rev. Plant Biol. 2008, 59, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Mortier, V.; Holsters, M.; Goormachtig, S. Never too many? How legumes control nodule numbers. Plant Cell Environ. 2012, 35, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.O.; Pitzschke, A. Plants make galls to accommodate foreigners: Some are friends, most are foes. New Phytol. 2020, 225, 1852–1872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yan, C.; Zhang, H. Mutualism between antagonists: Its ecological and evolutionary implications. Integr. Zool. 2021, 16, 84–96. [Google Scholar] [CrossRef]
- Alfaya, J.E.F.; Galván, D.E.; Machordom, A.; Penchaszadeh, P.E.; Bigatti, G. Malacobdella arrokeana: Parasite or commensal of the giant clam Panopea abbreviata? Zool. Sci. 2015, 32, 523–530. [Google Scholar] [CrossRef]
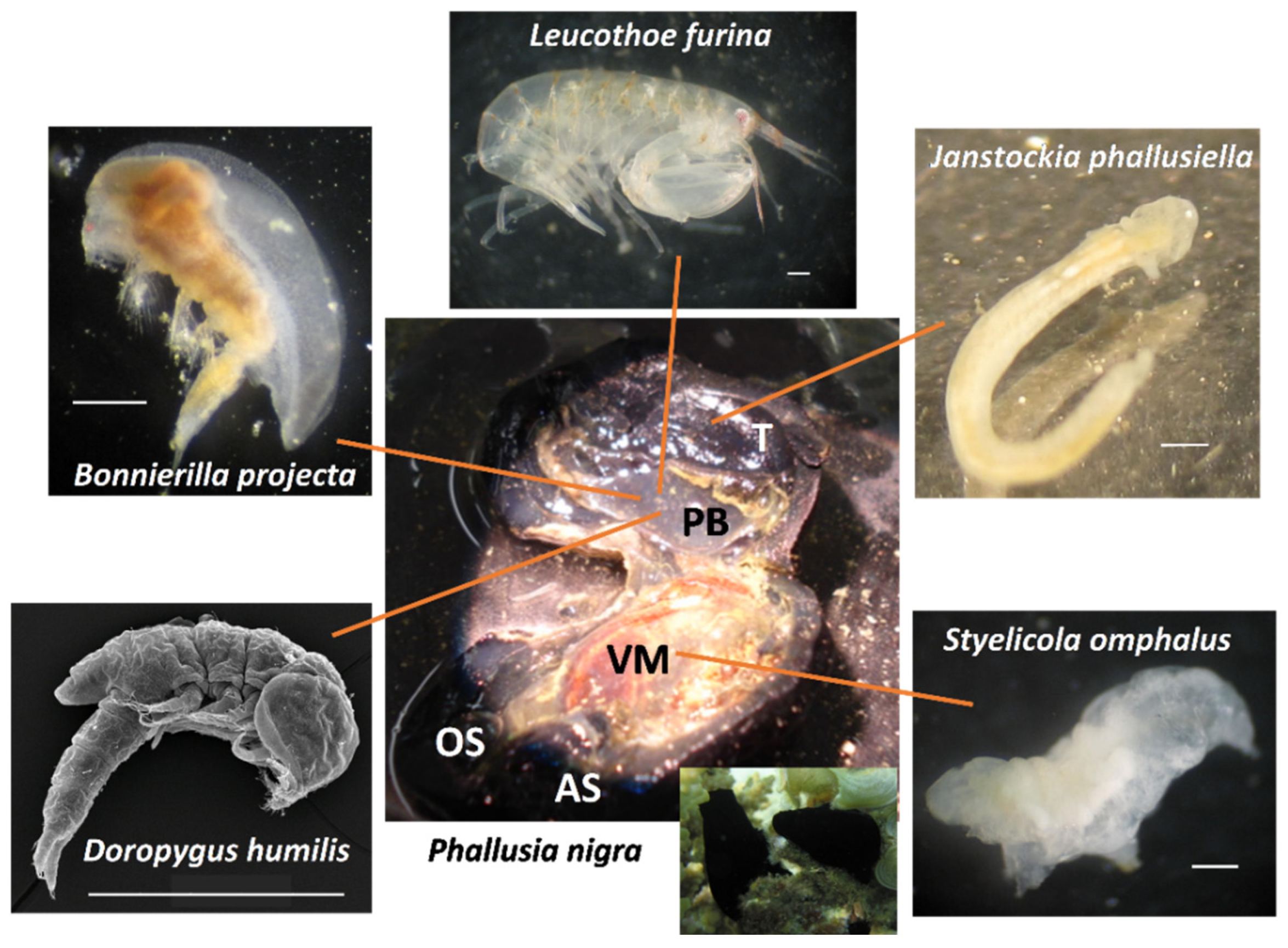


| Symbiont | Geographic Location | References |
|---|---|---|
| Crustacea Amphipoda | ||
| Amphilochus ascidicola Ortiz and Atienza, 2001 | Caribbean (Venezuela) | [57] |
| Leucothoe angraensis Senna, Andrade, Ramos & Skinner, 2021 | South Atlantic (Brazil) | [50] |
| L. flammosa Thomas and Klebba 2007 | Caribbean (Cuba) | [57] |
| L. furina (Savigny, 1816) | Red Sea (Egypt) | [46] |
| L. spinicarpa (Abildgaard, 1789) | North Atlantic (USA) | [58] |
| L. wuriti Thomas and Klebba 2007 | North Atlantic (USA), Caribbean (Belize, Panama) | [49,52] |
| Brachiura | ||
| Tunicotheres moseri (Rathbun, 1918) | Caribbean (Jamaica, Venezuela) | [35,51] |
| Copepoda | ||
| Bonnierilla projecta Stock, 1967 | Red Sea (Egypt, Erithrea) | [44,46] |
| Doropygus humilis1 Stock, 1967 | Red Sea (Egypt, Erithrea) | [44,46] |
| Janhius brevis2 (Stock, 1967) | Red Sea (Erithrea) | [44] |
| Janstockia phallusiella Boxshall & Marchenkov, 2005 | Red Sea (Egypt) | [46,59] |
| Lonchidiopsis tripes Stock, 1967 | Red Sea (Erithrea) | [44] |
| Notodelphys ciliata Schellenberg, 1922 | Red Sea (Egypt) | [60] |
| Notodelphys steinitzi Stock, 1967 | Red Sea (Erithrea) | [44] |
| Paranotodelphys phallusiae (Gurney, 1927) | Red Sea (Egypt) | [61] |
| Styelicola omphalus Kim I.H., Cruz-Rivera, Sherif & El-Sahhar, 2016 | Red Sea (Egypt) | [46] |
| Annelida Polychaeta | ||
| Proceraea exoryxae Martin, Nygren & Cruz-Rivera, 2017 | Red Sea (Egypt) | [47] |
| All Field Sites | Bonnierilla | Doropygus | Janstockia | Styelicola | |
|---|---|---|---|---|---|
| Leucothoe | 0.037 | 0.251 | 0.502 | 0.773 | |
| Bonnierilla | 0.092 | 0.474 | 0.817 | ||
| Doropygus | 0.579 | 0.078 | |||
| Janstockia | 0.761 | ||||
| Individual sites | |||||
| Abu Tig | Leucothoe | 0.593 | 0.526 | 0.863 | 0.360 |
| Bonnierilla | 0.861 | 0.418 | 0.548 | ||
| Doropygus | 0.641 | 0.774 | |||
| Janstockia | 0.553 | ||||
| Mövenpick | Leucothoe | 0.048 | - | 0.553 | 0.832 |
| Bonnierilla | - | 0.985 | 0.847 | ||
| Doropygus | - | - | |||
| Janstockia | 0.731 | ||||
| Zeytouna Beach | Leucothoe | 0.645 | 0.421 | 0.81 | 0.657 |
| Bonnierilla | <0.001 | 0.950 | 0.741 | ||
| Doropygus | 0.656 | 0.839 | |||
| Janstockia | 0.755 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Rivera, E.; Sherif, M.-E.-D.; El-Sahhar, S.; Lombardi, T. Spatial Variability in a Symbiont-Diverse Marine Host and the Use of Observational Data to Assess Ecological Interactions. Diversity 2022, 14, 197. https://doi.org/10.3390/d14030197
Cruz-Rivera E, Sherif M-E-D, El-Sahhar S, Lombardi T. Spatial Variability in a Symbiont-Diverse Marine Host and the Use of Observational Data to Assess Ecological Interactions. Diversity. 2022; 14(3):197. https://doi.org/10.3390/d14030197
Chicago/Turabian StyleCruz-Rivera, Edwin, Mohy-El-Din Sherif, Salma El-Sahhar, and Thomas Lombardi. 2022. "Spatial Variability in a Symbiont-Diverse Marine Host and the Use of Observational Data to Assess Ecological Interactions" Diversity 14, no. 3: 197. https://doi.org/10.3390/d14030197
APA StyleCruz-Rivera, E., Sherif, M.-E.-D., El-Sahhar, S., & Lombardi, T. (2022). Spatial Variability in a Symbiont-Diverse Marine Host and the Use of Observational Data to Assess Ecological Interactions. Diversity, 14(3), 197. https://doi.org/10.3390/d14030197







