Biodiversity of Nematode Communities Associated with Wheat (Triticum aestivum L.) in Southern Morocco and Their Contribution as Soil Health Bioindicators
Abstract
1. Introduction
2. Materials and Methods
2.1. Nematode Sampling Design
2.2. Nematode Processing
2.3. Soil Physico-Chemical Analyses
2.4. Diversity Assessment of Nematodes Taxa
2.5. Statistical Analyses
3. Results
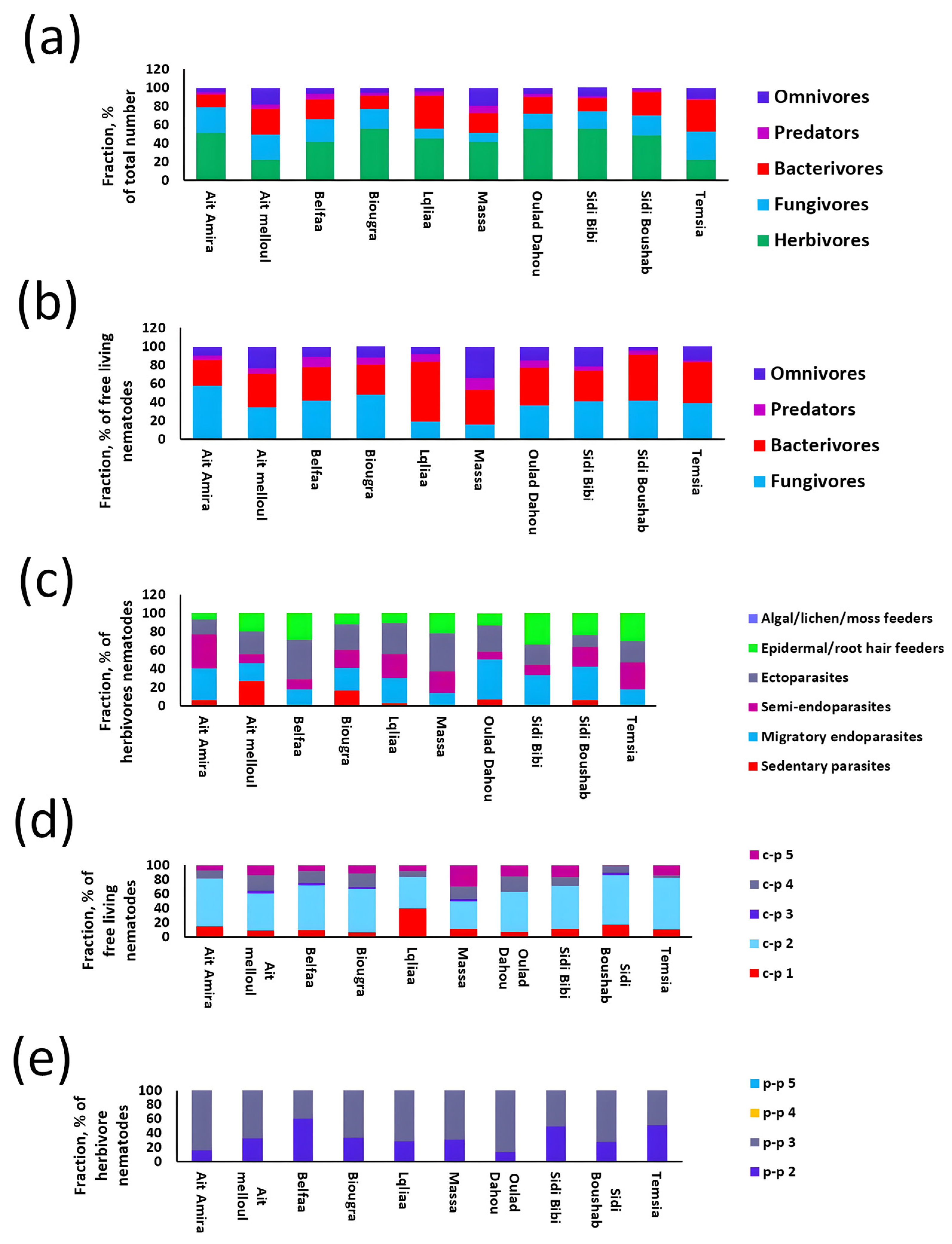
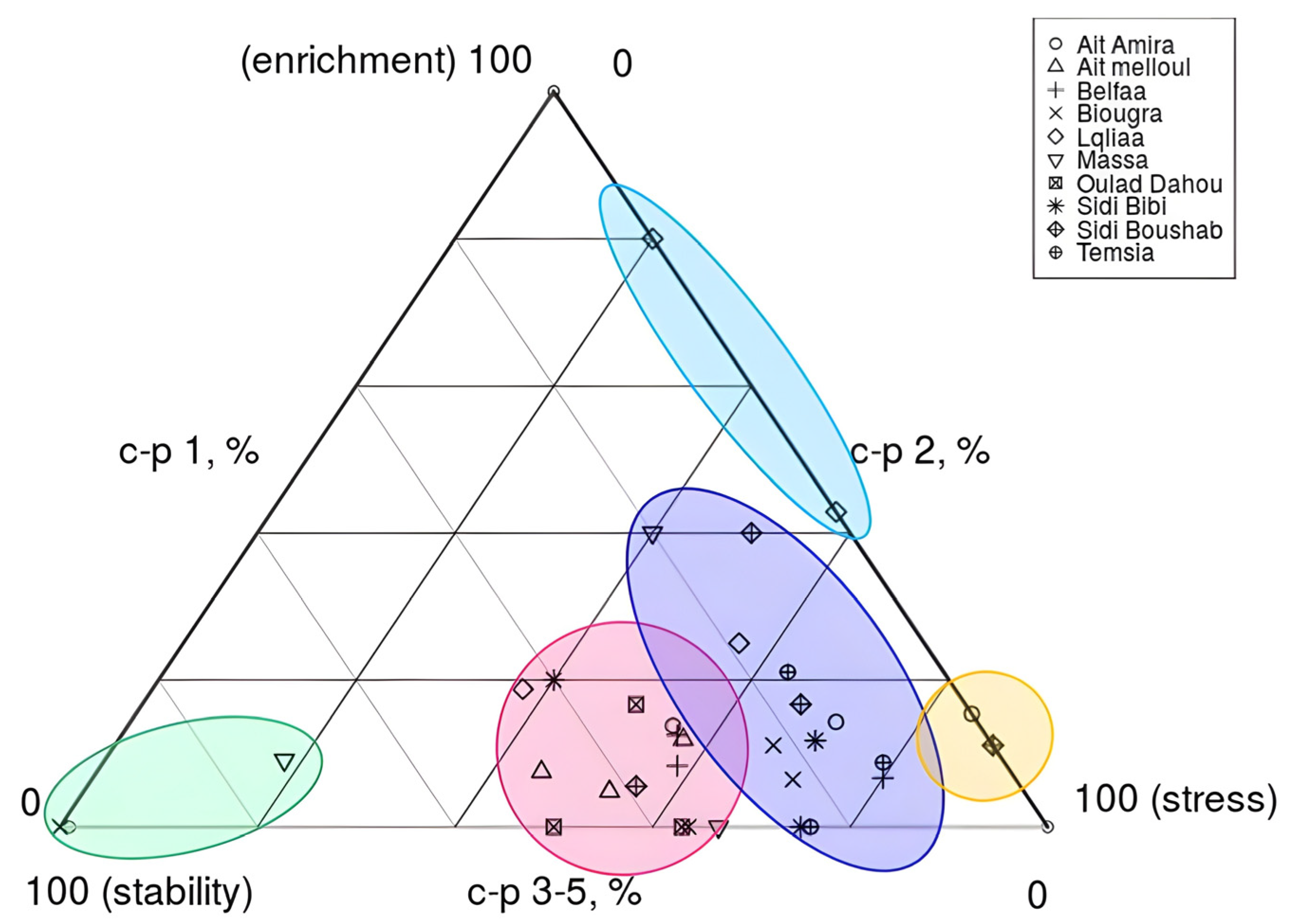
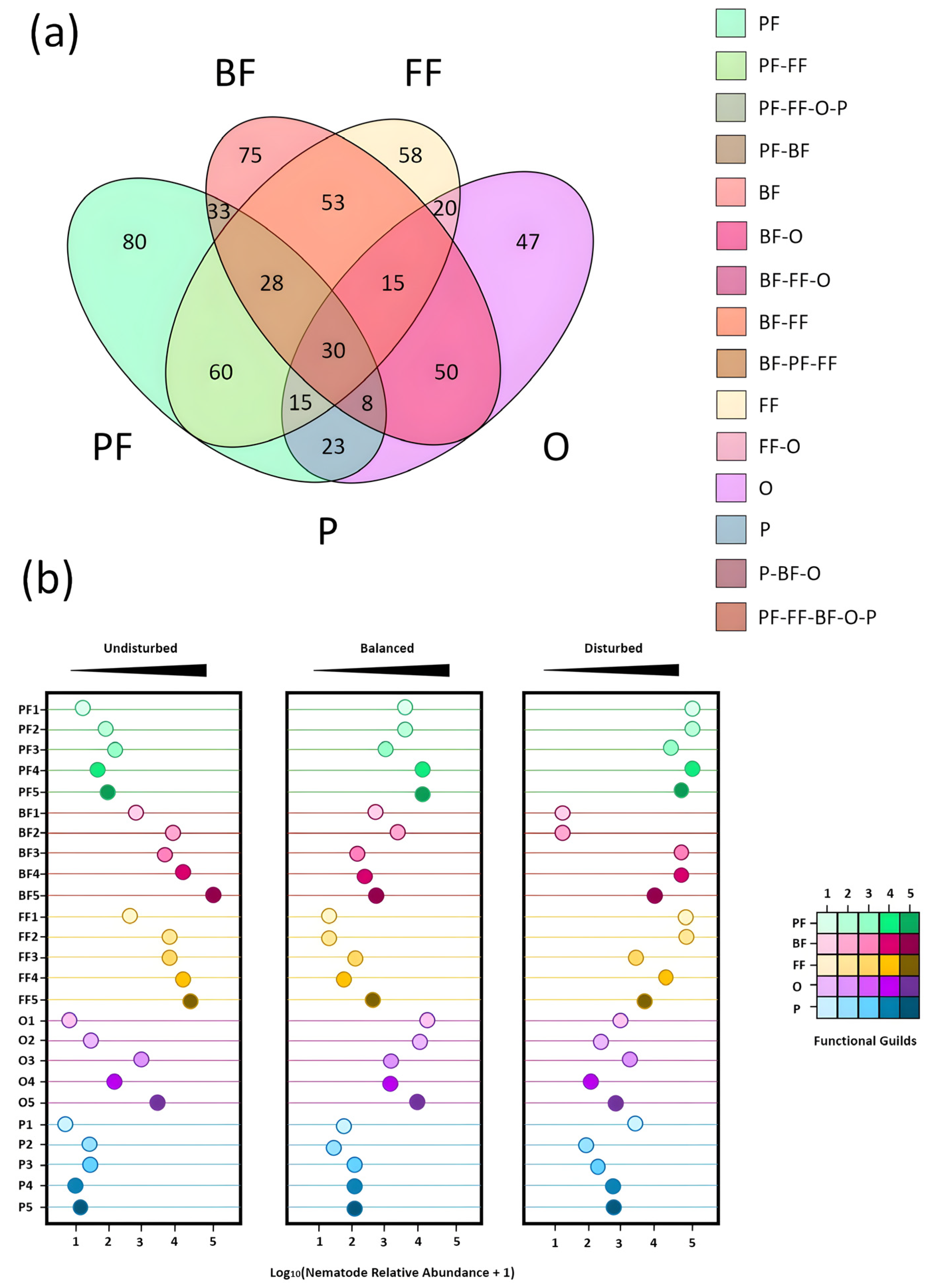
3.1. Nematode Community Patterns and Ecological Distribution in Wheat Fields
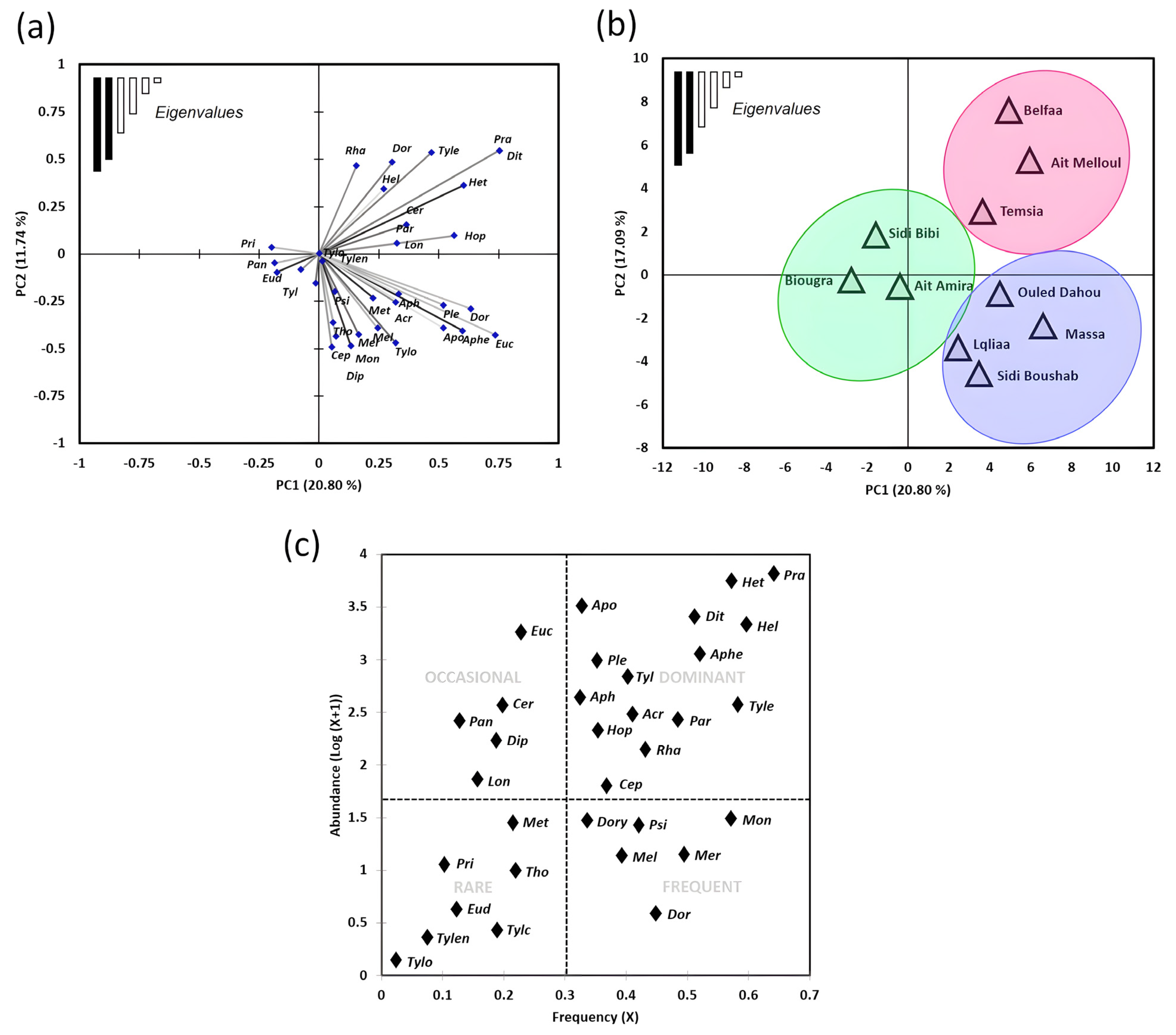
3.2. Diversity of Nematode Communities in Wheat
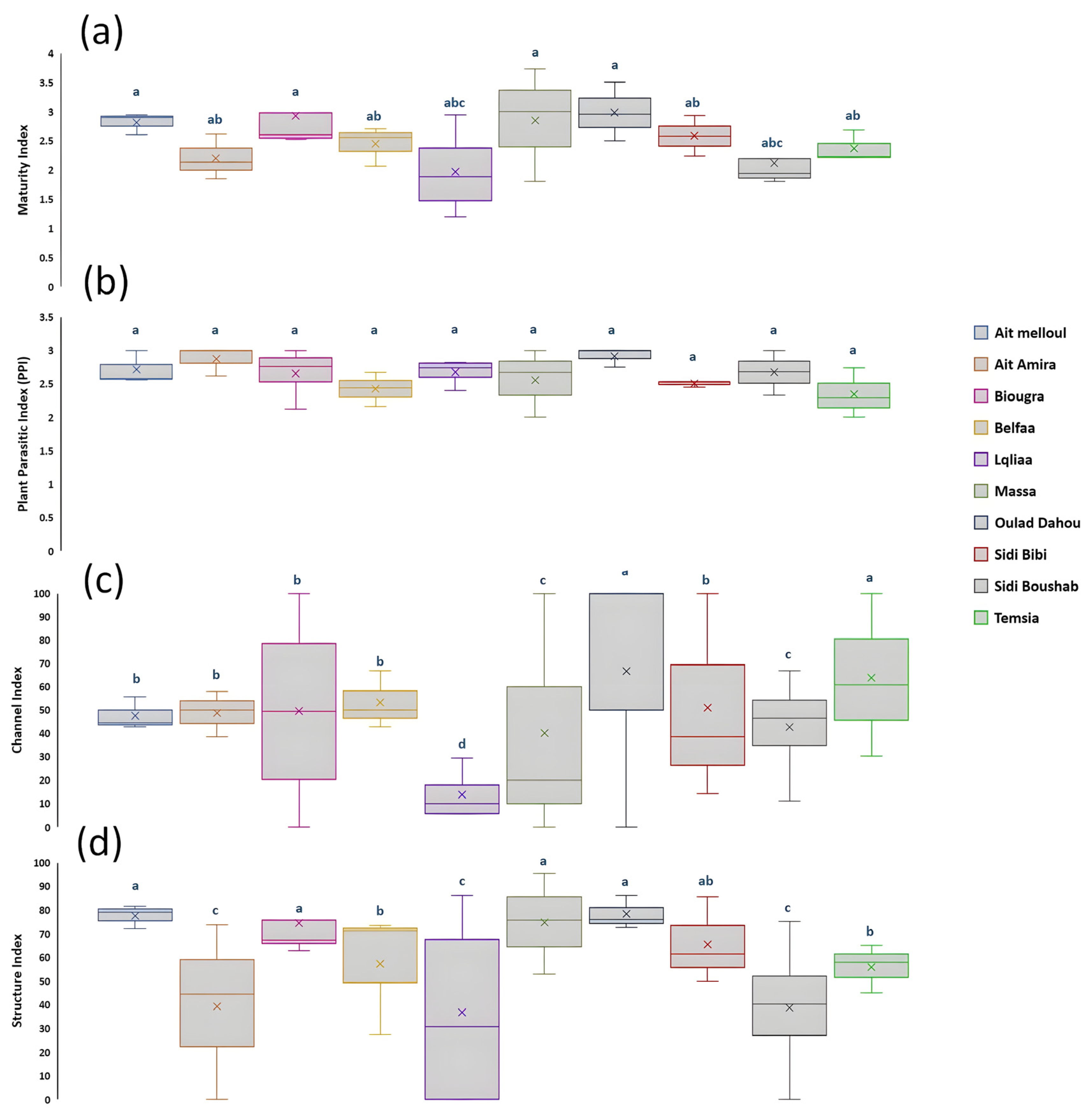
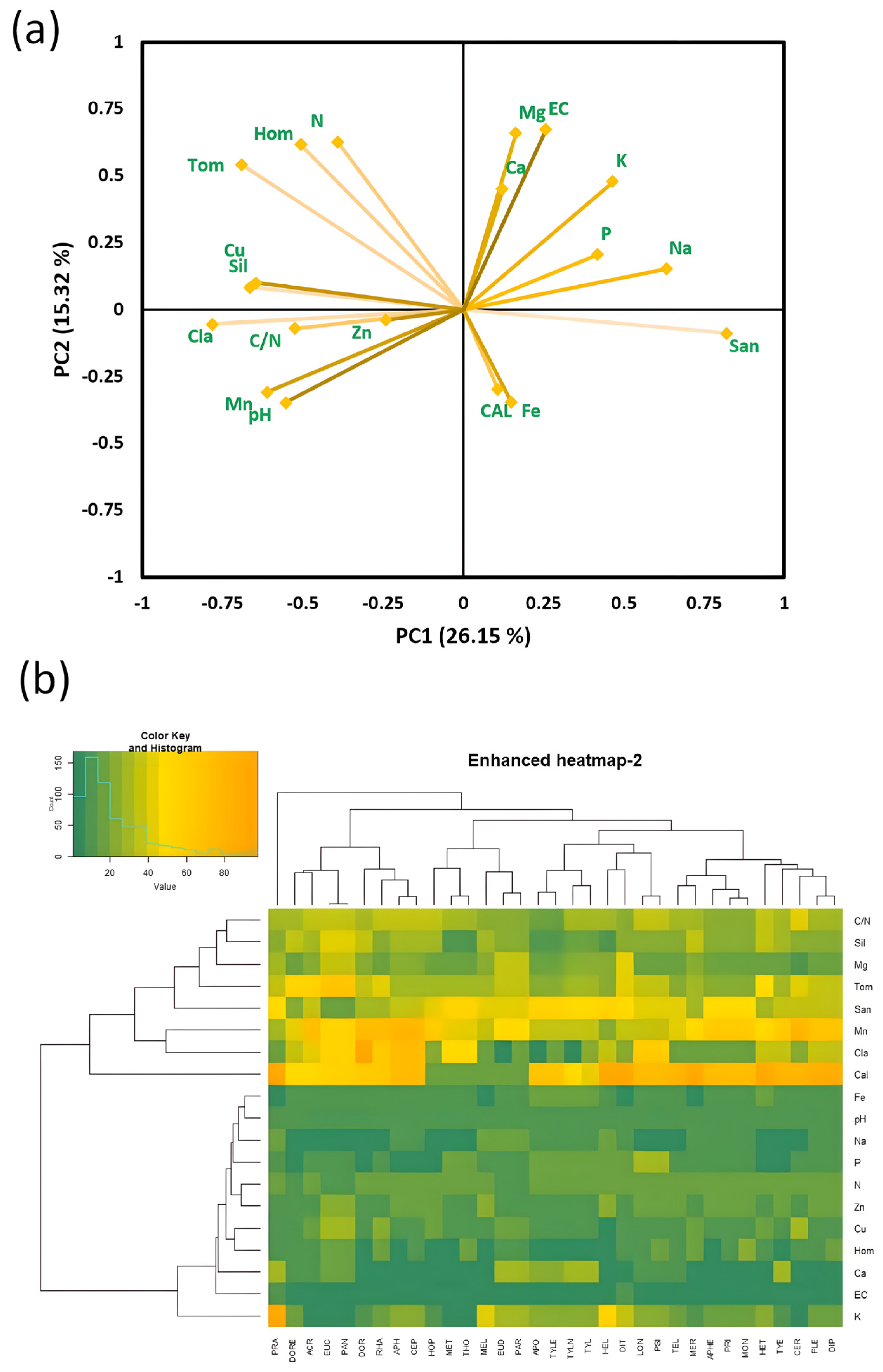
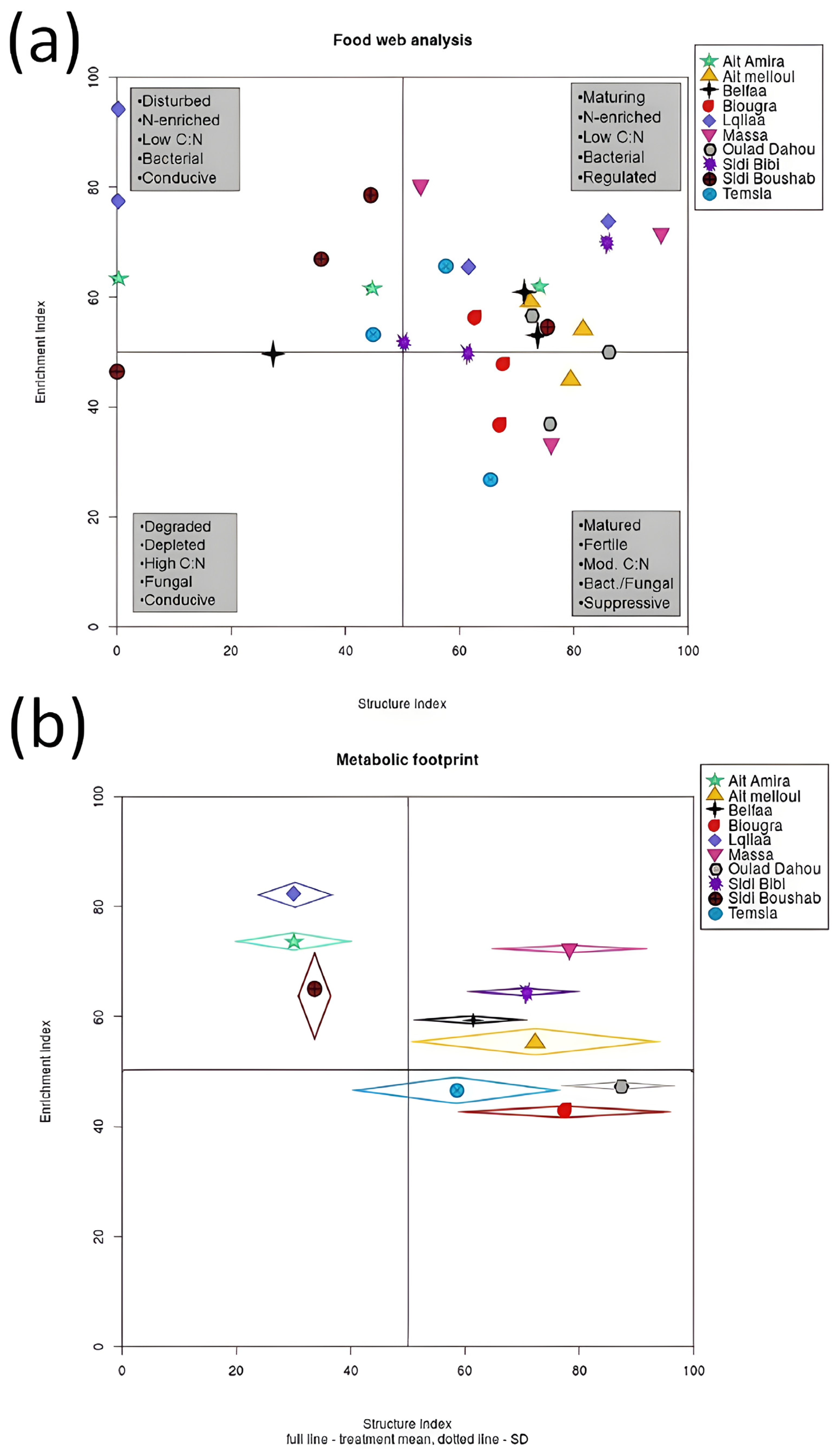
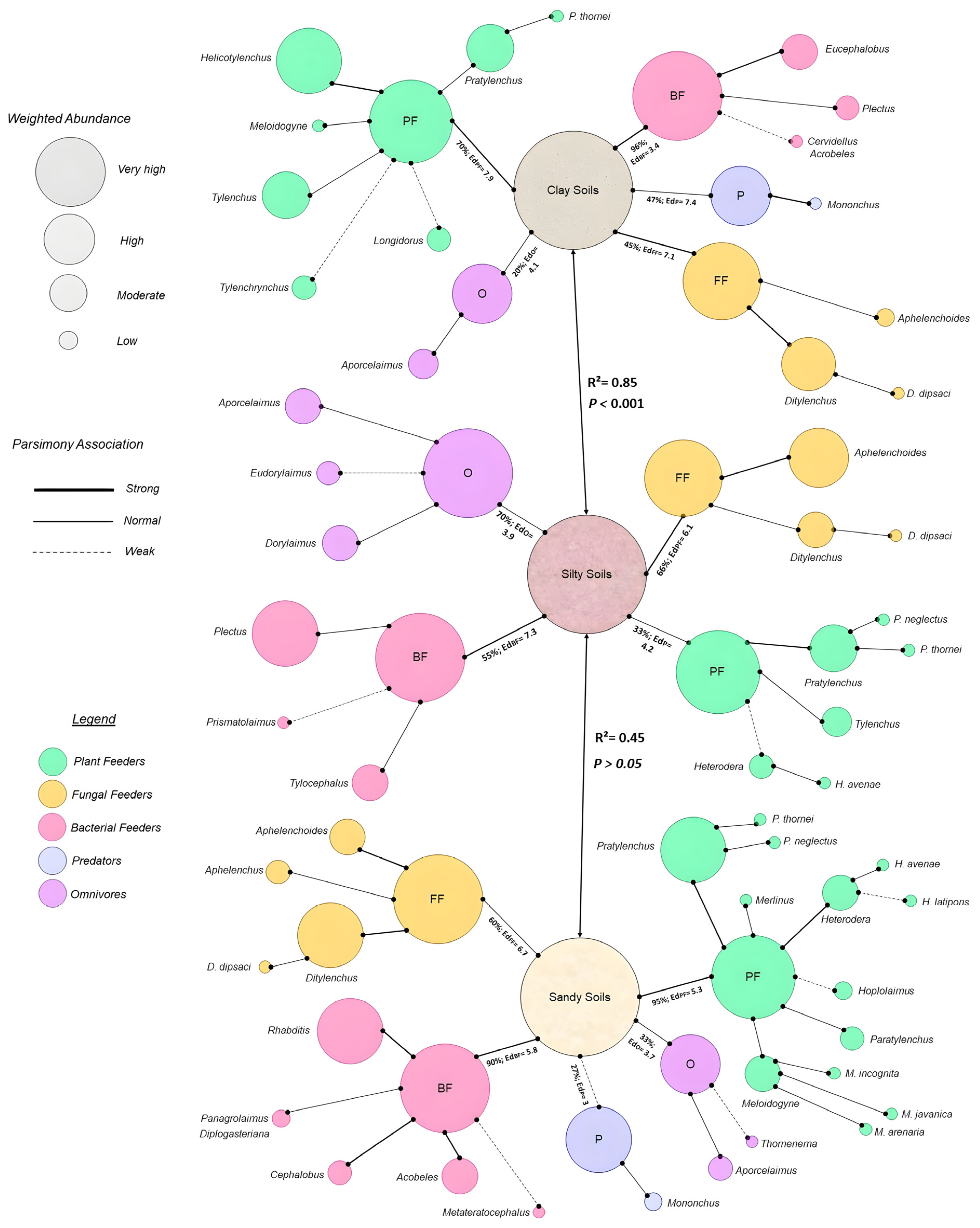
3.3. Nematodes as Potential Soil Bio-Indicators in Wheat Fields
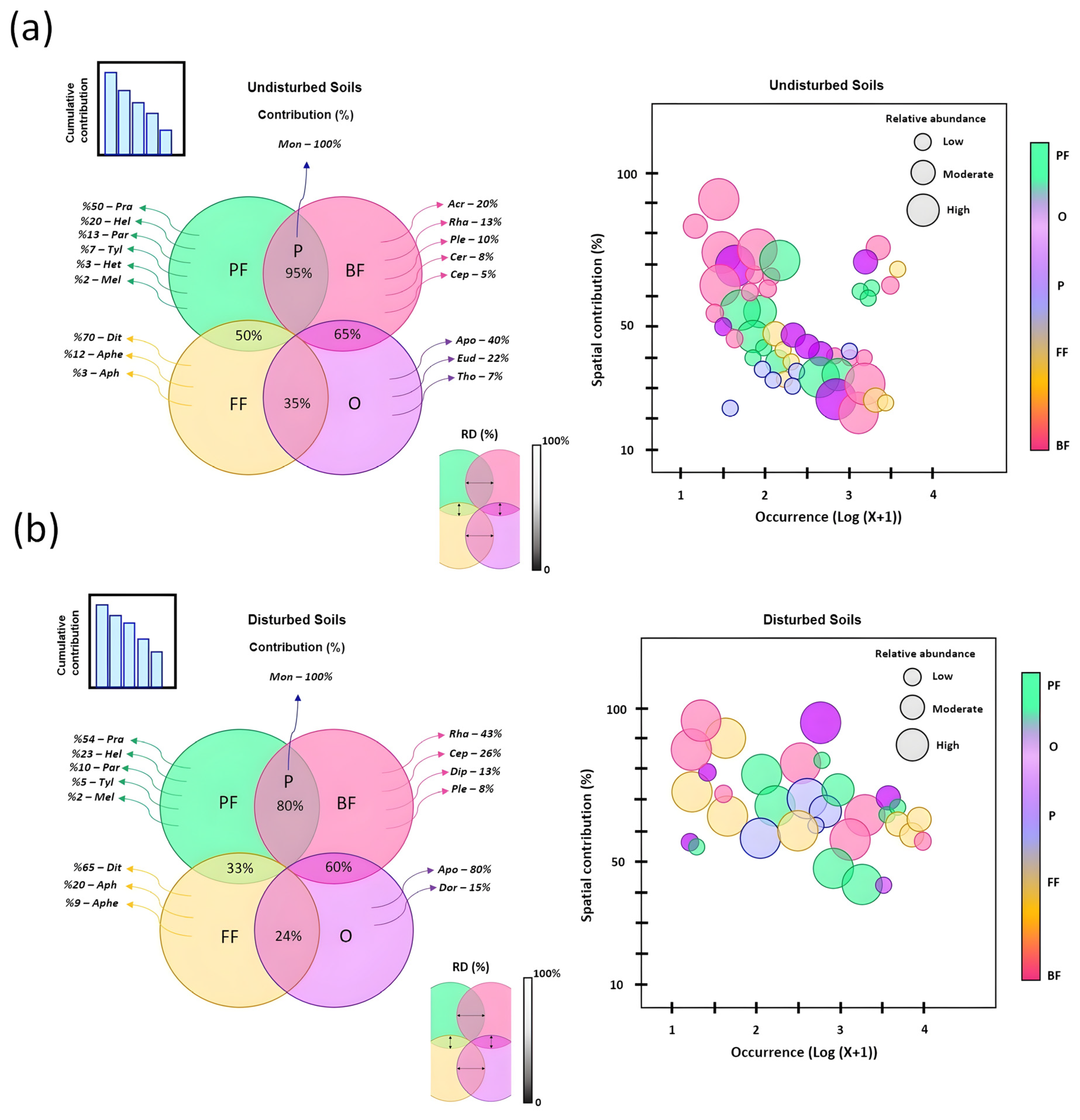
3.4. Nematode Guild Dynamics with Soil Health Attributes
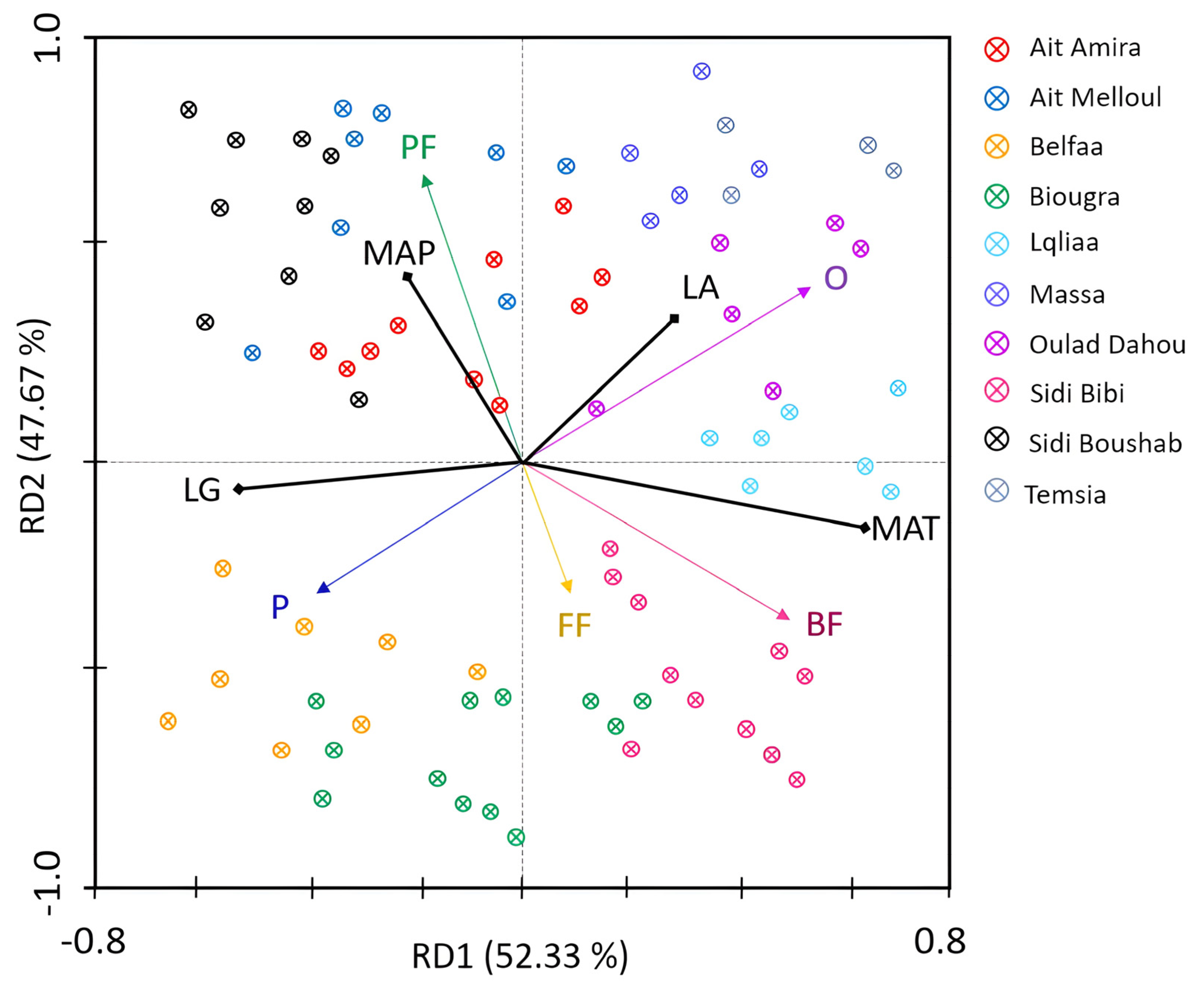
3.5. Effect of Climatic Factors on Nematode Trophic Patterns
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization Corporate Statistical Database (FAOSTAT). New Food Balances. 2020. Available online: http://www.fao.org/faostat/en/#data/FBS (accessed on 16 May 2020).
- Jlibene, M.; Nsarellah, N. Wheat breeding in Morocco, a historical perspective. World Wheat Book 2011, 2, 425–442. [Google Scholar]
- Barakat, F.; Handoufe, A. Agroclimatic assessment of agricultural drought in Morocco. Sci. Chang. Planétaires Sécheresse 1998, 9, 201. [Google Scholar]
- Nicol, J.M.; Rivoal, R. Global knowledge and its application for the integrated control and management of nematodes on wheat. In Integrated Management ad Biocontrol of Vegetable and Grain Crops Nematodes; Ciancio, A., Mukerji, K.G., Eds.; Springer Academic Publishing: Dordrecht, The Netherlands, 2008; pp. 243–287. [Google Scholar]
- Dababat, A.A.; Imren, M.; Erginbas-Orakci, G.; Ashrafi, S.; Yavuzaslanoglu, E.; Toktay, H.; Pariyar, S.R.; Elekcioglu, H.I.; Morgounov, A.; Mekete, T. The importance and management strategies of cereal cyst nematodes, Heterodera spp., in Turkey. Euphytica 2015, 202, 173–188. [Google Scholar] [CrossRef]
- Dababat, A.A.; Fourie, H. Nematode parasites of cereals. Plant Parasitic Nematodes. In Subtropical and Tropical Agriculture; Sikora, R.A., Coyne, D., Hallmann, J., Timper, P., Eds.; CAB International: Wallingford, UK, 2018; pp. 163–221. [Google Scholar]
- Rivoal, R.; Cook, R. Nematode pests of cereals. In Plant Parasitic Nematodes in Temperate Agriculture; Evans, K., Trudgill, D.L., Webster, J.M., Eds.; CABI Publishing: Wallingford, UK, 1993; pp. 259–303. [Google Scholar]
- Sahin, E.; Nicol, J.M.; Elekcioglu, H.; Rivoal, R. Hatching of Heterodera filipjevi in controlled and natural temperature conditions in Turkey. Nematology 2009, 12, 277–287. [Google Scholar] [CrossRef]
- Yan, G.P.; Smiley, R.W. Discovery of Heterodera filipjevi on wheat in the USA. In Cereal Cyst Nematodes: Status, Research and Outlook; Riley, I.T., Nicol, J.M., Dababat, A.A., Eds.; CIMMYT: Ankara, Turkey, 2009; pp. 94–99. [Google Scholar]
- Seid, A.; İmren, M.; Ali, M.A.; Toumi, F.; Paulitz, T.; Dababat, A.A. Genetic Resistance of Wheat towards Plant-Parasitic Nematodes: Current Status and Future Prospects. Biotech Stud. 2021, 30, 43–62. [Google Scholar] [CrossRef]
- Mokrini, F.; Viaene, N.; Waeyenberge, L.; Dababat, A.A.; Moens, M. Characterization of cereal cyst nematodes (Heterodera spp.) in Morocco based on morphology, morphometrics and rDNA–ITS sequence analysis. J. Plant Prot. Res. 2017, 57, 219–227. [Google Scholar] [CrossRef][Green Version]
- Greco, N.; Di Vito, M. Nematodes of food legumes in the Mediterranean Basin 1. Eppo Bull. 1994, 24, 393–398. [Google Scholar] [CrossRef]
- Sabová, M.; Valocká, B.; Lišková, M.; Vargová, V.; Marek, J. Occurrence and distribution of Heterodera avenae Woll, 1924 in the Czech Republic. Ochr. Rostl. 1989, 25, 59–70. [Google Scholar]
- Subbotin, S.A.; Rumpenhorst, H.J.; Sturhan, D. Morphological and electrophoretic studies on populations of the Heterodera avenae complex from the former USSR. Russ. J. Nematol. 1996, 4, 29–38. [Google Scholar]
- Mokrini, F.; Waeyenberge, L.; Viaene, N.; Moens, M. First report of the cereal cyst nematode Heterodera latipons on wheat in Morocco. Plant Dis. 2012, 96, 774. [Google Scholar] [CrossRef]
- Castillo, P.; Vovlas, N. Pratylenchus (Nematoda: Pratylenchidae): Diagnosis, Biology, Pathogenicity and Management; Brill: Leiden, The Netherlands, 2007. [Google Scholar]
- Dababat, A.A.; Ferney, G.B.H.; Erginbas-Orakci, G.; Dreisigacker, S.; Imren, M.; Toktay, H.; Ogbonnaya, F. Association analysis of resistance to cereal cyst nematodes (Heterodera avenae) and root lesion nematodes (Pratylenchus neglectus and P. thornei) in CIMMYT advanced spring wheat lines for semi-arid conditions. Breed. Sci. 2016, 66, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Forge, T.A.; Larney, F.J.; Kawchuk, L.M.; Pearson, D.C.; Koch, C.; Blackshaw, R.E. Crop rotation effects on Pratylenchus neglectus populations in the root zone of irrigated potatoes in southern Alberta. Can. J. Plant Pathol. 2015, 37, 363–368. [Google Scholar] [CrossRef]
- Meskine, M.; Janati, A.; Abbad Andaloussi, F. Résultats préliminaires de l’étude sur les nématodes phytophages associés aux cultures de blé et orge. J. Natl. Phytiatr. 1984, 28–30. [Google Scholar]
- Mokrini, F.; Waeyenberge, L.; Viaene, N.; Andaloussi, F.A.; Moens, M. Diversity of root-lesion nematodes (Pratylenchus spp.) associated with wheat (Triticum aestivum and T. durum) in Morocco. Nematology 2016, 18, 781–801. [Google Scholar] [CrossRef]
- Doran, J.W. Soil health and global sustainability: Translating science into practice. Agric. Ecosyst. Environ. 2002, 88, 119–127. [Google Scholar] [CrossRef]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Villenave, C.; Ba, A.O.; Rabary, B. Analyse du fonctionnement biologique du sol par l’étude de la nématofaune: Semis direct versus labour sur les hautes terres près d’Antsirabé (Madagascar). Etude Gest. Sols 2009, 16, 369–378. [Google Scholar]
- Ferris, H.; Bongers, T.; de Goede, R.G. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Bongers, T.; van der Meulen, H.; Korthals, G. Inverse relationship between the nematode maturity index and plant parasite index under enriched nutrient conditions. Appl. Soil Ecol. 1997, 6, 195–199. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T. Nematode diversity in agroecosystems. In Invertebrate Biodiversity as Bioindicators of Sustainable Landscapes; Elsevier: Amsterdam, The Netherlands, 1999; Volume 1, pp. 113–135. [Google Scholar] [CrossRef]
- Boag, B.; Yeates, G.W. Soil nematode biodiversity in terrestrial ecosystems. Biodivers. Conserv. 1998, 7, 617–630. [Google Scholar] [CrossRef]
- Neher, D.A.; Wu, J.; Barbercheck, M.E.; Anas, O. Ecosystem type affects interpretation of soil nematode community measures. Appl. Soil Ecol. 2005, 30, 47–64. [Google Scholar] [CrossRef]
- Wasilewska, L. Soil invertebrates as bioindicators, with special reference to soil-inhabiting nematodes. Russ. J. Nematol. 1997, 5, 113–126. [Google Scholar]
- Pankhurst, C.; Doube, B.M.; Gupta, V. Biological Indicators of Soil Health; Oxon-New York; CAB International: Wallingford, UK, 2002. [Google Scholar]
- Barker, K.R.; Nusbaum, C.J.; Nelson, L. Effects of storage temperature and extraction procedure on recovery of plant-parasitic nematodes from field soils. J. Nematol. 1969, 1, 240. [Google Scholar] [PubMed]
- Hooper, D.J. Extraction of free-living nematode stages from soil. In Laboratory Methods for Work with Plant and Soil Nematodes; Southey, J.F., Ed.; Her Majesty’s Stationery Office: London, UK, 1986; pp. 5–22. [Google Scholar]
- De Grisse, A.T. Redescription ou modification de quelques techniques utilisés dans l’études dęs nématodes phytoparaires. Meded. Rijksfakulteit Landbouwwet. Gent 1969, 34, 351–356. [Google Scholar]
- Mai, W.F.; Lyon, H.H. Pictorial Key to the Genera of Plant-Parasitic Nematodes, 4th ed.; Cornell University Press: Ithaca, NY, USA, 1975. [Google Scholar]
- Mai, W.F.; Mullin, P.G. Plant-Parasitic Nematodes: A Pictorial Key to Genera; Cornell University Press: Ithaca, NY, USA, 1996. [Google Scholar]
- Brzeski, M.W. Review of the genus Ditylenchus filipjev, 1936 (Nematoda: Anguinidae). Rev. Nématol. 1991, 14, 9–59. [Google Scholar]
- Andrássy, I. Free-Living Nematodes of Hungary: Nematoda Errantia; Hungarian Natural History Museum: Budapest, Hungary, 2005; Volume 1. [Google Scholar]
- Geraert, E. The Tylenchidae of the World: Identification of the Family Tylenchidae (Nematoda); Academia Press: Ghent, Belgium, 2008. [Google Scholar]
- Geraert, E. The Dolichodoridae of the World: Identification of the Family Dolichodoridae (Nematoda); Academia Press: Ghent, Belgium, 2011. [Google Scholar]
- Taylor, D.P.; Netscher, C. An improved technique for preparing perineal patterns of Meloidogyne spp. Nematologica 1974, 20, 268–269. [Google Scholar]
- Handoo, Z.A. A key and compendium to species of the Heterodera avenae group (Nematoda: Heteroderidae). J. Nematol. 2002, 34, 250. [Google Scholar]
- Anderson, J.M.; Ingram, J.S.I. A Handbook of Methods; CAB International: Wallingford, UK, 1993. [Google Scholar]
- Richards, L.A. Diagnosis and Improvement Saline and Alkaline Soils; US Department of Agriculture: Washington, DC, USA, 1954.
- Sims, J.T.; Johnson, G.V. Micronutrient soil tests. Micronutr. Agric. 1991, 4, 427–476. [Google Scholar] [CrossRef]
- Allison, L.E. Wet-combustion apparatus and procedure for organic and inorganic carbon in soil. Soil Sci. Soc. Am. J. 1960, 24, 36–40. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. Methods of soil analysis: Part 3. Chem. Methods 1996, 5, 961–1010. [Google Scholar]
- Barbano, D.M.; Clark, J.L. Total nitrogen content of milk: A rapid microwave Kjeldahl digestion. J. Dairy Sci. 1990, 73, 83. [Google Scholar]
- Boag, B. Standardization of ecological terms in nematology. Fundam. Appl. Nematol. 1993, 16, 190–191. [Google Scholar]
- Krebs, C.J. Ecology: The Experimental Analysis of Distribution and Abundance; Harper and Row: New York, NY, USA, 1985. [Google Scholar]
- Ferris, H.; Matute, M.M. Structural and functional succession in the nematode fauna of a soil food web. Appl. Soil Ecol. 2003, 23, 93–110. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Austral Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Fortuner, R.; Merny, G. Les nématodes parasites des racines associés au riz en Basse-Casamance (Sénégal) et en Gambie. Cah. ORSTOM Série Biol. 1973, 21, 4–43. [Google Scholar]
- Sieriebriennikov, B.; Ferris, H.; de Goede, R.G. NINJA: An automated calculation system for nematode-based biological monitoring. Eur. J. Soil Biol. 2014, 61, 90–93. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Yellowbrick: Machine Learning Visualization. Available online: https://www.scikit-yb.org/en/latest/ (accessed on 28 January 2021).
- Sviewgui 0.3.5. Available online: https://pypi.org/project/sviewgui/ (accessed on 28 January 2021).
- Bio.Phylo Package. Available online: https://biopython.org/docs/1.75/api/Bio.Phylo.html (accessed on 5 February 2021).
- NetworkX: Network Analysis in Python. Available online: https://networkx.org (accessed on 5 February 2021).
- Matplotlib-Venn: Venn Diagram Plotting Routines for Python/Matplotlib. Available online: https://github.com/konstantint/matplotlib-venn (accessed on 18 February 2021).
- Matplotlib-Lollipop: Lollipop Chart. Available online: https://www.data-to-viz.com/graph/lollipop (accessed on 18 February 2021).
- Ordination Methods (skbio.math.stats.ordination). Available online: http://scikit-bio.org/docs/0.1.3/math.stats.ordination.html (accessed on 27 February 2021).
- Neilsen, M.N.; Winding, A. Microorganisms as Indicators of Soil Health; Technical Report; National Environment Research Institute: Roskilde, Denmark, 2002. [Google Scholar]
- Bongers, T.; Ferris, H. Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 1999, 14, 224–228. [Google Scholar] [CrossRef]
- Yildiz, Ş.; Imren, M.; Duman, N. Nematode biodiversity in cereal growing areas of Bolu, Turkey. Turk. Entomol. Derg. 2017, 41, 159–168. [Google Scholar] [CrossRef]
- Liu, Y.B.; Li, X.Y.; Liu, Q.Z. Soil nematode communities in jujube (Ziziphus jujuba Mill.) rhizosphere soil under monoculture and jujube/wheat (Triticum aestivum Linn.) intercropping systems, a case study in Xinjiang arid region, northwest of China. Eur. J. Soil Biol. 2016, 74, 52–59. [Google Scholar] [CrossRef]
- Diakhaté, S. Influence de L’arbuste Piliostigma Reticulatum (D.C.) Hochst (Caesalpinioideae) sur les Communautés de Microorganismes et de Nématodes d’un sol Cultivé en mil au Sénégal (Nioro). Ph.D. Thesis, Université Cheikh Anta Diop, Dakar, Senegal, 2014. [Google Scholar]
- Yeates, G.W. Nematodes as soil indicators: Functional and biodiversity aspects. Biol. Fertil. Soils 2003, 37, 199–210. [Google Scholar] [CrossRef]
- Song, M.; Jing, S.; Zhou, Y.; Hui, Y.; Zhu, L.; Wang, F.; Wan, S. Dynamics of soil nematode communities in wheat fields under different nitrogen management in Northern China Plain. Eur. J. Soil Biol. 2015, 71, 13–20. [Google Scholar] [CrossRef]
- Didden, W.A.M.; Marinissen, J.C.Y.; Vreeken-Buijs, M.J.; Burgers, S.L.G.E.; De Fluiter, R.; Geurs, M.; Brussaard, L. Soil meso-and macrofauna in two agricultural systems: Factors affecting population dynamics and evaluation of their role in carbon and nitrogen dynamics. Agric. Ecosyst. Environ. 1994, 51, 171–186. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.; Freckman, D.W.; Georgieva, S. Feeding habits in soil nematode families and genera—An outline for soil ecologists. J Nematol. 1993, 25, 315. [Google Scholar] [PubMed]
- Ferris, H.; Bongers, T. Nematode indicators of organic enrichment. J. Nematol. 2006, 38, 3. [Google Scholar] [PubMed]
- Zhao, J.; Neher, D.A. Soil nematode genera that predict specific types of disturbance. Appl. Soil Ecol. 2013, 64, 135–141. [Google Scholar] [CrossRef]
- Ogolla, J.A. Diversity of Plant Parasitic Nematodes Associated with Maize (Zea mays L.) and Integrated Control of Lesion Nematodes (Pratylenchus spp.). Ph.D. Thesis, Kenyatta University, Nairobi, Kenya, 2005. [Google Scholar]
- Neher, D.A. Ecology of plant and free-living nematodes in natural and agricultural soil. Annu. Rev. Phytopathol. 2010, 48, 371–394. [Google Scholar] [CrossRef]
- Freckman, D.W.; Ettema, C.H. Assessing nematode communities in agroecosystems of varying human intervention. Agric. Ecosyst. Environ. 1993, 45, 239–261. [Google Scholar] [CrossRef]
- Ruess, L.; Ferris, H. Decomposition pathways and successional changes. Nematol. Monogr. Perspect. 2004, 2, 547–556. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 668. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Ayres, E.; Wall, D.H.; Li, G.; Bardgett, R.D.; Wu, T.; Garey, J.R. Global-scale patterns of assemblage structure of soil nematodes in relation to climate and ecosystem properties. Glob. Ecol. Biogeogr. 2014, 23, 968–978. [Google Scholar] [CrossRef]
- Godefroid, M.; Tixier, P.; Chabrier, C.; Djigal, D.; Quénéhervé, P. Associations of soil type and previous crop with plant-feeding nematode communities in plantain agrosystems. Appl. Soil Ecol. 2017, 113, 63–70. [Google Scholar] [CrossRef]
- Krif, G.; Mokrini, F.; Aissami, A.E.; Laasli, S.E.; Imren, M.; Özer, G.; Dababat, A.A. Diversity and Management Strategies of Plant Parasitic Nematodes in Moroccan Organic Farming and Their Relationship with Soil Physico-Chemical Properties. Agriculture 2020, 10, 447. [Google Scholar] [CrossRef]
- Van Diepeningen, A.D.; de Vos, O.J.; Korthals, G.W.; van Bruggen, A.H. Effects of organic versus conventional management on chemical and biological parameters in agricultural soils. Appl. Soil Ecol. 2006, 31, 120–135. [Google Scholar] [CrossRef]
- Cadet, P.; Thioulouse, J. Identification of soil factors that relate to plant parasitic nematode communities on tomato and yam in the French West Indies. Appl. Soil Ecol. 1998, 8, 35–49. [Google Scholar] [CrossRef][Green Version]
- Melakeberhan, H.; Maung, Z.; Lee, C.L.; Poindexter, S.; Stewart, J. Soil type-driven variable effects on cover-and rotation-crops, nematodes and soil food web in sugar beet fields reveal a roadmap for developing healthy soils. Eur. J. Soil Biol. 2018, 85, 53–63. [Google Scholar] [CrossRef]
- Prot, J.C.; Van Gundy, S.D. Effect of soil texture and the clay component on migration of Meloidogyne incognita second-stage juveniles. J. Nematol. 1981, 13, 213. [Google Scholar]
- Thompson, J.P.; Clewett, T.G.; Sheedy, J.G.; Reen, R.A.; O’Reilly, M.M.; Bell, K.L. Occurrence of root-lesion nematodes (Pratylenchus thornei and P. neglectus) and stunt nematode (Merlinius brevidens) in the northern grain region of Australia. Australas. Plant Pathol. 2010, 39, 254–264. [Google Scholar] [CrossRef]
- Mokrini, F.; Laasli, S.E.; Karra, Y.; El Aissami, A.; Dababat, A.A. Diversity and incidence of plant–parasitic nematodes associated with saffron (Crocus sativus L.) in Morocco and their relationship with soil physicochemical properties. Nematology 2019, 22, 87–102. [Google Scholar] [CrossRef]
- Georgieva, S.S.; McGrath, S.P.; Hooper, D.J.; Chambers, B.S. Nematode communities under stress: The long–term effects of heavy metals in soil treated with sewage sludge. Appl. Soil Ecol. 2002, 20, 27–42. [Google Scholar] [CrossRef]
- Oteifa, B.A. Nitrogen source of the host nutrition in relation to infection by a root-knot nematode, Meloidogyne incognita. Plant Dis. Report. 1955, 39, 902–903. [Google Scholar]
- Widmer, T.L.; Mitkowski, N.A.; Abawi, G.S. Soil organic matter and management of plant-parasitic nematodes. J. Nematol. 2002, 34, 289. [Google Scholar] [PubMed]
- Hominick, W.M.; Hunt, D.J.; Reid, A.; Briscoe, B.R.; Bohan, D.A. Biosystematics, phylogeny, and population genetics of entomopathogenic nematodes. In Taxonomy, Phylogeny and Gnotobiological Studies of Entomopathogenic Nematode Bacterium Complexes; Boemare, N., Richardson, P., Coudert, F., Eds.; European Commission Publication: Brussels, Belgium, 1999; pp. 45–53. [Google Scholar]
- Hu, C.; Qi, Y. Effective microorganisms and compost favor nematodes in wheat crops. Agron. Sustain. Dev. 2013, 33, 573–579. [Google Scholar] [CrossRef]
- Barros, P.Â.; Pedrosa, E.M.R.; de Oliveira Cardoso, M.S.; Rolim, M.M. Relationship between soil organic matter and nematodes in sugarcane fields. Semin. Cienc. Agrar. 2017, 38, 551–559. [Google Scholar] [CrossRef]
- Papatheodorou, E.M.; Argyropoulou, M.D.; Stamou, G.P. The effects of large-and small-scale differences in soil temperature and moisture on bacterial functional diversity and the community of bacterivorous nematodes. Appl. Soil Ecol. 2004, 25, 37–49. [Google Scholar] [CrossRef]
- Berry, S.; Rhodes, R. Green manure crops: Agronomic characteristics and effect on nematodes. In Proceedings of the 80th Annual Congress of the South African Sugar Technologists’ Association, Durban, South Africa, 18–20 July 2006; South African Sugar Technologists’ Association: Durban, South Africa, 2006; pp. 269–273. [Google Scholar]
- Ferris, H. Form and function: Metabolic footprints of nematodes in the soil food web. Eur. J. Soil Biol. 2010, 46, 97–104. [Google Scholar] [CrossRef]
- Imren, M.; Ciftci, V.; Yildiz, Ş.; Kutuk, H.; Dababat, A.A. Occurrence and population dynamics of the root lesion nematode Pratylenchus thornei (Sher and Allen) on wheat in Bolu, Turkey. Turk. J. Agric. For. 2017, 41, 35–41. [Google Scholar] [CrossRef]
- Bird, A.F. Plant Response to Root-Knot Nematode. Annu. Rev. Phytopathol. 1974, 12, 69–85. [Google Scholar] [CrossRef]
- Boag, B. Effects of temperature on rate of feeding of the plant parasitic nematodes Rotylenchus robustus, Xiphinema diversicaudatum, and Hemicycliophora conida. J. Nematol. 1980, 12, 193. [Google Scholar]
- Duyck, P.F.; Dortel, E.; Tixier, P.; Vinatier, F.; Loubana, P.M.; Chabrier, C.; Quénéhervé, P. Niche partitioning based on soil type and climate at the landscape scale in a community of plant-feeding nematodes. Soil Biol. Biochem. 2012, 44, 49–55. [Google Scholar] [CrossRef]
- Pritchard, S.G. Soil organisms and global climate change. Plant Pathol. 2011, 60, 82–99. [Google Scholar] [CrossRef]
- Riley, I.T.; Kelly, S.J. Endoparasitic nematodes in cropping soils of Western Australia. Aust. J. Exp. Agric. 2002, 42, 49–56. [Google Scholar] [CrossRef]
- Kandel, S.L.; Smiley, R.W.; Garland-Campbell, K.; Elling, A.A.; Huggins, D.; Paulitz, T.C. Spatial distribution of root lesion nematodes (Pratylenchus spp.) in a long-term no-till cropping system and their relationship with soil and landscape properties. Eur. J. Soil Biol. 2018, 150, 1011–1021. [Google Scholar] [CrossRef]
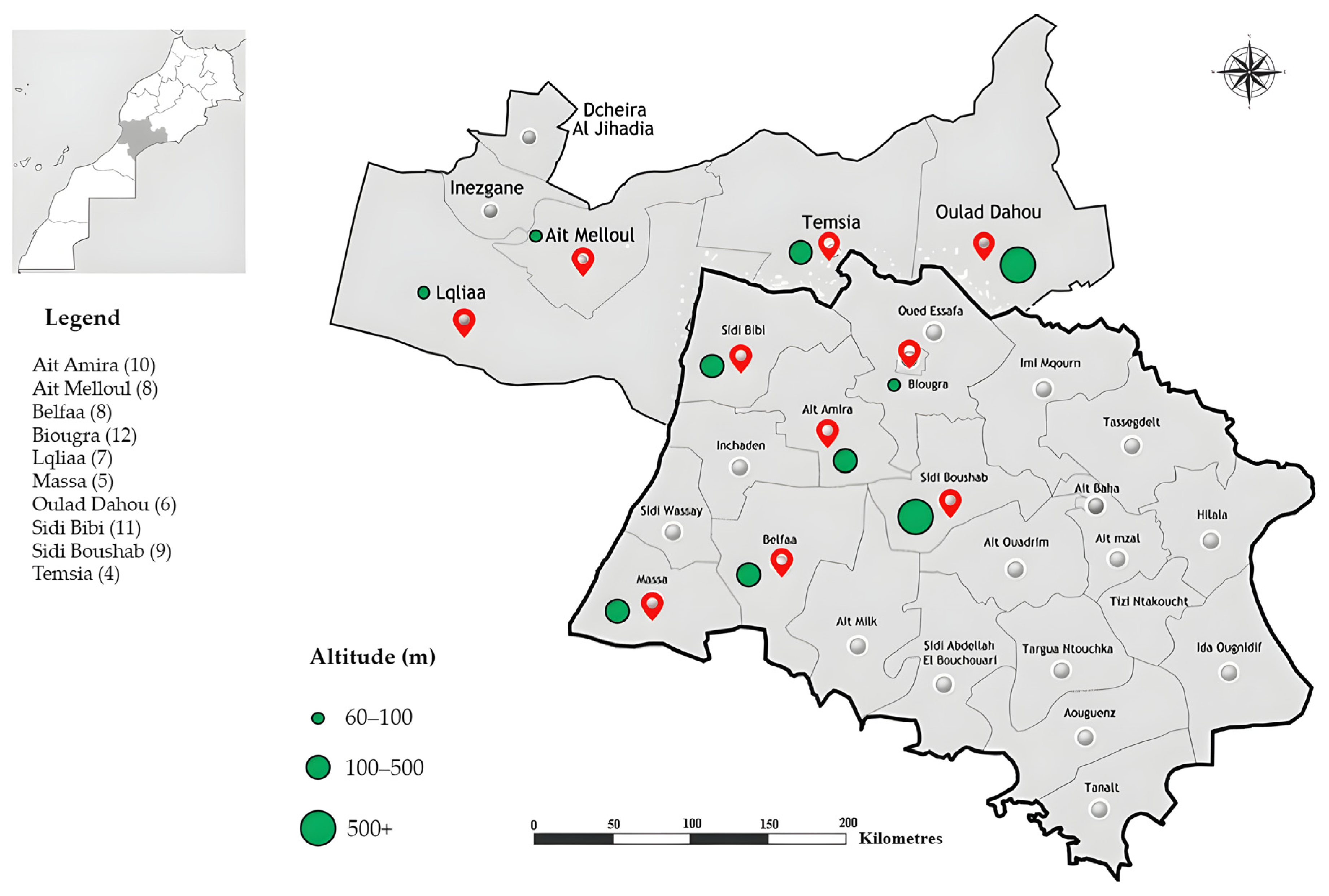
| Locality (Province) | GPS (Latitude, Longitude) | Soil Texture | Organic Matter | pH | Samples per Site |
|---|---|---|---|---|---|
| Ait Amira (AA) | +30° 10′ 35.4″ N, 9° 29′ 18.96″ | Sandy clay loam | 1.7% | 8.5 | 10 |
| Ait Melloul (AM) | +30° 20′ 3″ N, 9° 29′ 50″ | Medium loam | 2.7% | 9.3 | 8 |
| Belfaa (BE) | +30° 1′ 53″ N, 9° 33′ 15″ | Sandy loam | 2.0% | 8.1 | 8 |
| Biougra (Bi) | +30° 12′ 52″ N, 9° 22′ 15″ | Clay loam | 2.5% | 8.5 | 12 |
| Lqliaa (Lq) | +30° 17′ 27″ N, 9° 27′ 58″ | Sandy loam | 1.9% | 7.4 | 7 |
| Massa (Ma) | +30° 02′ 00″ N, 9° 38′ 00″ | Clay loam | 2.3% | 8.3 | 5 |
| Oulad Dahou (OD) | +30° 21′ 42.6″, 8° 35′ 51.50″ | Sandy clay loam | 1.9% | 8.6 | 6 |
| Sidi Bibi (SBi) | +30° 14′ 0″ N, 9° 32′ 0″ | Medium loam | 2.6% | 9.4 | 11 |
| Sidi Boushab (Sbo) | +30° 4′ 26.4″ N, 9° 16′ 44.4″ | Sandy loam | 1.7% | 7.6 | 9 |
| Temsia (TE) | +30° 21′ 36″ N, 9° 24′ 50.4″ | Clay loam | 2.3% | 7.7 | 4 |
| Family | Genus | Trophic Group | c-p Class | p-p Class | Functional Guild | Code |
|---|---|---|---|---|---|---|
| Rhabditidae | Rhabditis | Bacterivores | 1 | 0 | Ba1 | Rha |
| Cephalobidae | Acrobeles | Bacterivores | 2 | 0 | Ba2 | Acr |
| Cervidellus | Bacterivores | 2 | 0 | Ba2 | Cer | |
| Eucephalobus | Bacterivores | 2 | 0 | Ba2 | Euc | |
| Cephalobus | Bacterivores | 3 | 0 | Ba3 | Cep | |
| Metateratocephalidae | Metateratocephalus | Bacterivores | 2 | 0 | Ba2 | Met |
| Diplogasteridae | Diplogasteriana | Bacterivores | 1 | 0 | Ba1 | Dip |
| Panagrolaimidae | Panagrolaimus | Bacterivores | 1 | 0 | Ba1 | Pan |
| Plectidae | Plectus | Bacterivores | 2 | 0 | Ba2 | Ple |
| Prismatolaimidae | Prismatolaimus | Bacterivores | 3 | 0 | Ba3 | Pri |
| Tylocephalus | Bacterivores | 2 | 0 | Ba2 | Tylo | |
| Aphelenchoididae | Aphelenchoides | Fungivores | 2 | 0 | Fu2 | Aph |
| Aphelenchidae | Aphelenchus | Fungivores | 2 | 0 | Fu2 | Aphe |
| Mydonomidae | Dorylaimoides | Fungivores | 4 | 0 | Fu4 | Dory |
| Anguinidae | Ditylenchus | Fungivores | 2 | 0 | Fu2 | Dit |
| Tylencholaimidae | Tylencholaimus | Fungivores | 4 | 0 | Fu4 | Tylc |
| Tylencholaimellus | Fungivores | 4 | 0 | Fu4 | Tylen | |
| Dorylaimidae | Dorylaimus | Omnivores | 4 | 0 | Om4 | Dor |
| Qudsianematidae | Eudorylaimus | Omnivores | 4 | 0 | Om4 | Eud |
| Aporcelaimidae | Aporcelaimus | Omnivores | 5 | 0 | Om5 | Apo |
| Thornematidae | Thornenema | Omnivores | 5 | 0 | Om5 | Tho |
| Mononchidae | Mononchus | Predators | 4 | 0 | Pr4 | Mon |
| Meloidogynidae | Meloidogyne | Herbivores | 0 | 3 | He3 | Mel |
| Pratylenchidae | Pratylenchus | Herbivores | 0 | 3 | He3 | Pra |
| Paratylenchidae | Paratylenchus | Herbivores | 0 | 2 | He2 | Par |
| Tylenchidae | Tylenchus | Herbivores | 0 | 2 | He2 | Tyl |
| Psilenchus | Herbivores | 0 | 2 | He2 | Psi | |
| Dolichodoridae | Tylenchorhynchus | Herbivores | 0 | 3 | He3 | Tyle |
| Merlinius | Herbivores | 0 | 3 | He3 | Mer | |
| Longidoridae | Longidorus | Herbivores | 0 | 5 | He5 | Lon |
| Heteroderidae | Heterodera | Herbivores | 0 | 3 | He3 | Het |
| Hoplolaimidae | Hoplolaimus | Herbivores | 0 | 3 | He3 | Hop |
| Helicotylenchus | Herbivores | 0 | 3 | He3 | Hel |
| TG | Nematode Taxa | Localities * | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | AM | BE | BI | LQ | MA | OD | SBi | SBo | TE | ||
| Bacterivores | Rhabditis | 25.9 c | 45.6 ab | 15.3 d | 7.5 e | 57.8 a | 15 d | 35.4 b | 22 c | 15 d | 55.5 a |
| Acrobeles | 10 d | 48.96 a | 35 b | 15 d | 15.2 d | 0 f | 22.3 c | 20 c | 7.5 e | 50.3 a | |
| Cervidellus | 0 d | 26.1 a | 0 d | 3.75 cd | 5.7 c | 0 d | 0 d | 2.25 cd | 7.75 c | 15 b | |
| Eucephalobus | 0 d | 35 a | 5.6 c | 0 d | 0 d | 0 d | 0 d | 5.1 c | 2.25 cd | 13.9 b | |
| Cephalobus | 20.6 bc | 15.9 c | 14.4 c | 9 d | 17 c | 33 b | 30.2 b | 10.6 c | 22.5 bc | 80 a | |
| Metateratocephalus | 0 c | 20.3a | 5.2 b | 0 c | 0 c | 8 b | 0 c | 0 c | 3.75 b | 0 c | |
| Diplogasteriana | 5.75 b | 5.5 b | 5 b | 4 b | 7.5 a | 0 c | 4 b | 0 c | 7.3 a | 10.4 a | |
| Panagrolaimus | 5.3 b | 12.8 a | 5.2 b | 5 b | 5 b | 7 b | 4 b | 5.3 b | 4 b | 0 c | |
| Plectus | 8.65 c | 66.2 a | 10.4 c | 28.6 bc | 11.3 c | 25.6 bc | 13.4 c | 39.3 b | 0 d | 33.4 b | |
| Prismatolaimus | 0 c | 10 a | 5 b | 3.75 b | 0 c | 1 bc | 0 c | 0 c | 0 c | 0 c | |
| Tylocephalus | 0 b | 0 b | 0 b | 0 b | 0 b | 0 b | 0 b | 5.6 a | 0 b | 0 b | |
| Fungivores | Aphelenchoides | 20.4 c | 130.3 a | 25.1 c | 11.3 d | 15.1 d | 10 d | 10.2 d | 25 c | 15 d | 70 b |
| Aphelenchus | 11.6 c | 50.66 a | 30.2 b | 26.5 b | 7.6 d | 0 e | 20 bc | 20.3 bc | 3.75 d | 25 b | |
| Dorylaimoides | 0 d | 32.1 a | 5.6 c | 7.5 c | 0 d | 0 d | 11.4 b | 0 d | 3.2 c | 5.5 c | |
| Ditylenchus D. dipsaci | 98.3 a + | 22.8 c + | 55.7 b + | 63.7 b + | 15.9 cd + | 0 d – | 25.8 c + | 35.1 bc + | 45.7 bc + | 65.6 b + | |
| Tylencholaimus | 0 c | 10.6 a | 0 c | 0 c | 0 c | 0 c | 0 c | 5.25 b | 0 c | 0 c | |
| Tylencholaimellus | 0 b | 5.3 a | 0 b | 0 b | 0 b | 0 b | 0 b | 0 b | 0 b | 0 b | |
| Omnivores | Dorylaimus | 12.4 b | 22.9 a | 10.9 c | 0 d | 0 d | 4.4 cd | 0 d | 10 c | 7.5 c | 15 b |
| Eudorylaimus | 0 b | 0 b | 0 b | 3.75 a | 0 b | 0 b | 0 b | 5 a | 0 b | 5 a | |
| Aporcelaimus | 23.6 cd | 80.2 a | 15.7 d | 30.5 c | 15.3 d | 26.7 c | 35.06 c | 25.6 c | 3.75 e | 65.2 b | |
| Thornenema | 0 c | 20.5 a | 5.3 b | 0 c | 3.8 b | 0 c | 0 c | 5.3 b | 0 c | 0 c | |
| Predators | Mononchus | 15 c | 45.4 a | 18.7 c | 14.6 c | 30.2 b | 11 c | 20.8 b | 9.7 c | 13 c | 5 d |
| Herbivores | Meloidogyne M. incognita M. javanica M. arenaria | 15.6 a + + – | 20.25 a + – – | 5.8 c – + – | 7.5 c + – + | 3.75 c + – – | 0 d – – – | 11.2 b + – – | 0 d – – – | 11.3 b – + + | 0 d – – – |
| Pratylenchus P. thornei P. neglectus | 70.8 b + + | 40.3 c + – | 35.4 c + + | 71.3 b + – | 48.6 c + – | 14.3 d + + | 70.4 b + – | 95.3 a + + | 67.5 b + – | 45.05 c + + | |
| Paratylenchus | 14.9 c | 22.3 b | 65.2 a | 33.8 b | 26.4 b | 10.4 c | 0 d | 55.5 ab | 3.75 d | 50 ab | |
| Tylenchus | 2.55 d | 25.6 b | 30.7 b | 26.3 b | 0 e | 8.5 c | 15.77 c | 90.5 a | 38.5 b | 15 c | |
| Psilenchus | 10 b | 15 b | 25.3 a | 11 b | 1.5 c | 10 b | 0 d | 0 d | 0 d | 2.5 c | |
| Tylenchorhynchus | 82.3 a | 33.8 cd | 15.5 d | 8.6 d | 24.3 cd | 31.5 cd | 45.5 c | 26.3 cd | 60 b | 43.9 bc | |
| Merlinius | 9.77 b | 23.1 a | 21.1 a | 3.75 c | 4.25 c | 10 b | 4.9 c | 0 d | 11.3 b | 0 d | |
| Longidorus | 0 c | 15 a | 0 c | 7.5 b | 12.3 a | 15.1 a | 0 c | 0 c | 0 c | 0 c | |
| Heterodera H. avenae H. latipons | 97.5 a + + | 30.6 d – + | 105 a + + | 17.2 e + – | 42 c + + | 13.2 e – + | 36 d + – | 75.88 b + + | 46.3 c + + | 24.3 de + – | |
| Hoplolaimus H. indicus | 120 a + | 5.3 c + | 60 b + | 0 d – | 0 d – | 6.2 c + | 9.75 c + | 0 d – | 0 d – | 0 d – | |
| Helicotylenchus | 105 a | 19.6 d | 27.1 c | 31.09 c | 29.3 c | 25.6 c | 15.9 d | 35 c | 41.3 b | 19.6 d | |
| Localities (Province) | Diversity Parameters | ||
|---|---|---|---|
| Mean Number of Taxa | Shannon Diversity Index (H’) | Evenness (J) | |
| Ait Amira | 17.4 c | 1.2 bc | 0.68 a |
| Ait Melloul | 28.56 a | 2.7 a | 0.8 a |
| Belfaa | 23.4 ab | 2.54 a | 0.73 a |
| Biougra | 6.27 d | 1.05 bc | 0.71 a |
| Lqliaa | 21.83 b | 2.2 ab | 0.83 a |
| Massa | 19.48 b | 2.17 ab | 0.78 a |
| Oulad Dahou | 30.7 a | 2.91 a | 0.89 a |
| Sidi Bibi | 15.4 c | 1.83 ab | 0.87 a |
| Sidi Boushab | 25 ab | 2.49 a | 0.77 a |
| Temsia | 20.55 b | 1.63 b | 0.74 a |
| p | < 0.001 | 0.0365 | 0.795 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laasli, S.-E.; Mokrini, F.; Lahlali, R.; Wuletaw, T.; Paulitz, T.; Dababat, A.A. Biodiversity of Nematode Communities Associated with Wheat (Triticum aestivum L.) in Southern Morocco and Their Contribution as Soil Health Bioindicators. Diversity 2022, 14, 194. https://doi.org/10.3390/d14030194
Laasli S-E, Mokrini F, Lahlali R, Wuletaw T, Paulitz T, Dababat AA. Biodiversity of Nematode Communities Associated with Wheat (Triticum aestivum L.) in Southern Morocco and Their Contribution as Soil Health Bioindicators. Diversity. 2022; 14(3):194. https://doi.org/10.3390/d14030194
Chicago/Turabian StyleLaasli, Salah-Eddine, Fouad Mokrini, Rachid Lahlali, Tadesse Wuletaw, Timothy Paulitz, and Abdelfattah A. Dababat. 2022. "Biodiversity of Nematode Communities Associated with Wheat (Triticum aestivum L.) in Southern Morocco and Their Contribution as Soil Health Bioindicators" Diversity 14, no. 3: 194. https://doi.org/10.3390/d14030194
APA StyleLaasli, S.-E., Mokrini, F., Lahlali, R., Wuletaw, T., Paulitz, T., & Dababat, A. A. (2022). Biodiversity of Nematode Communities Associated with Wheat (Triticum aestivum L.) in Southern Morocco and Their Contribution as Soil Health Bioindicators. Diversity, 14(3), 194. https://doi.org/10.3390/d14030194










