Abstract
This paper presents the results of research on the Ba, Li and Ti content in six species of herbs sampled from mid-field wet depressions and from the soil. These temporary flooded depressions were surrounded by arable crops, permanent grassland and shrubby vegetation. The research area was located in the eastern part of the Mazovian Voivodeship, east-central Poland. The following plants were used in the experiment: corn mint (Mentha arvensis L.), purple marshlocks (Comarum palustre L.), silverweed (Potentilla anserina L.), yarrow (Achillea millefolium L.), yellow loosestrife (Lysimachia vulgaris L.) and gypsy-wort (Lycopus europaeus L.). The Li, Ba and Ti content of plants, bottom sediment and soil was determined by the ICP-AES method after previous dry mineralization. Of the six herb species, Mentha arvensis L. was with the greatest accumulation potential of the chemical elements. However, no excessive Ba, Li and Ti content was found in herbs growing at different distances from arable fields, permanent grassland and shrubby vegetation. The highest Ba content was found in periodically flooded soil (zone II), while the highest amounts of Li and Ti were recorded in non-flooded soil (zone III).
1. Introduction
With the socio-economic development of rural areas and as a consequence of structural changes in agriculture, environmental degradation is observed, eliminating natural biocenoses and having a direct impact on the deterioration of water and air quality [1,2,3]. The share of such habitats in the landscape is difficult to determine so their ecological analysis and impact on the functioning of neighboring terrestrial ecosystems is important. Vegetation in small aquatic ecosystems and adjacent areas is usually exposed to excessive amounts of nutrients and heavy metals, resulting from improper farming methods. According to many authors [4,5], chemical composition of plants forming communities in such areas is affected by soil properties. environmental pollution and climatic conditions. Determination of the chemical composition of plants growing in small aquatic ecosystems is extremely important due to the possibility of obtaining herbal raw materials in their natural state [6,7,8,9]. The collection of medicinal plants in their natural state in agricultural, wasteland and other areas is supervised in Poland by the Regional Directorate for Environmental Protection. According to literature reports [10], herbal plants have a number of properties exerting a beneficial effect on human physical and mental health; they have antioxidant, antibacterial and anti-inflammatory properties, regulating digestion and preserving food. The aim of the paper is to assess the content of Ba, Li and Ti in bottom sediment, soil and in six herbal species growing in mid-field depressions of the Siedlce Plateau.
The choice of the chemical elements was determined by the fact that there were few scientific reports on Ba, Li and Ti in herbs from natural and agricultural ecosystems. The literature was mainly concerned with the content of heavy metals. The present research proves that even in unpolluted areas, the bioaccumulation of these elements is very diverse and requires constant monitoring.
2. Materials and Methods
The material was sampled between mid-June and the end of July, most often at the beginning of the flowering and full flowering stages. Only the aboveground parts of plants were used. Six species of herbs growing in the mid-field depressions of Siedlce Plateau were selected: silverweed (Potentilla anserina L.), corn mint (Mentha arvensis L.), yarrow (Achillea millefolium L.), purple marshlocks (Comarum palustre L.), yellow loosestrife (Lysimachia vulgaris L.) and gypsy-wort (Lycopus europaeus L.). Natural aquatic ecosystems were located at different places, with three mid-field ponds (A and B) in the commune of Jabłonna Lacka, in the village of Bujały Mikosze, and one area (C) in the commune of Sabnie, the village of Grodzisk (Figure 1). Each mid-field aquatic depression was surrounded by a different type of land cover: A—arable fields, B—permanent grassland and C—wild shrubs. Plants and soil were sampled from three different soil moisture zones: flooded (I), periodically flooded (II) and non-flooded (III). A mid-field location of a pond, diversity of growing vegetation and vicinity of agricultural crops were taken into account while selecting a study location. The pool area ranged from 15,000 m2 (Grodzisk—C) to 850 m2 (Bujały Mikosze—A). In administrative terms, the ponds were located in the eastern part of the Mazovian voivodeship, east-central Poland. The area constitutes an ecoregion called Green Lungs. According to the National Ecological Network ‘Econet-Polska’ [11], it is situated in the central part of the postglacial zone. Soils in that part of the country mainly developed from glacial tills during the central Polish glaciation [12]. They are dominated by luvisols, gleysols and brown soils, made from loamy sand and dusty loams. There are also rusty soils in the area [13].
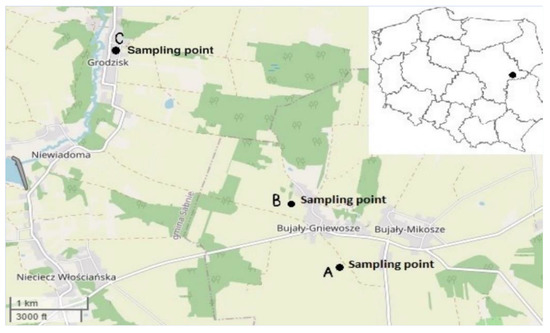
Figure 1.
Locations of the sampling points (https://www.openstreetmap.org/#map=12/52.1036/22.1357 (accessed on 4 March 2022)) [14].
From each moisture zone, 85 plant samples representing each species were collected. They were not rinsed with water before chemical analyses. Chemical composition of the above-ground part of the plant was determined. Samples of soil and bottom sediment from each mid-field depression were also collected. The outer layer of bottom sediment was sampled with a corer. After draining its water, the sediment was homogenized and dried at first at room temperature and then at 105 °C to constant weight. The plant material was dried in the same way. The plant material was ground to a diameter of 0.25 mm and 1 g was put into a porcelain crucible, after which the organic substance was dry-oxidized in a muffle furnace, at 450 °C for about 15 h. When oxidation was completed, what remained in the ash was minerals in the form of carbonates and oxides, and partly in the form of phosphates. Then, 10 mL of diluted HCl (1:1) was added to the crucible and the contents were evaporated on a sand bath to decompose carbonates and separate silica. After the addition of 5 mL of 10% HCl, crucible contents were passed through a hard filter to a 100 mL volume flask and supplemented with distilled water up to the mark. In the presence of hydrochloric acid, silicic acid was precipitated from silicates in the form of white gelatinous mass with high water content (H2SiO3·nH2O), which at a temperature of 100–200 °C converted into sparingly soluble SiO2 [15]. The content of selected chemical elements was determined using Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP–AES), while calibration was performed using standard Merck solutions. An internal quality control procedure was used to verify the accuracy of the methods. Two measurements were taken for each series of samples with the recovery being within the 85–115% range. The limit of detection for Ba, Li and Ti was 0.01 mg kg−1.
Soil samples were dried at 105 °C, then ground and sifted through a sieve with a mesh diameter of 1mm [15]. Samples of soil, bottom sediment and plant material were mineralized in the same way. In the soil and in the outer layer of sediment, pH in 1 mol KCl dm−3 was determined by the potentiometric method.
Accumulation coefficient (AC) of selected chemical elements in herbs were calculated on the basis of their content in plant biomass and soil. The following formula was used [16]:
AC = Cp/Cs
(Cp—content of the metal in the plant, Cs—content of the metal in the soil):
AC < 0.01—no accumulation;
AC < 0.1—slight accumulation;
AC = 1—medium accumulation;
AC > 1—high accumulation.
The results were statistically processed, with the significance of the results assessed using variance analysis. Tukey’s test at 5% significance level (p < 0.05) was used to determine significant differences between means. Statistica version 10.0 StatSoft was used for the calculations [17]. The values of linear correlation coefficients between the Ba, Li and Ti content of the soil from the individual moisture zones and their content in the aboveground parts of selected herbs were calculated. The standard deviation was calculated to determine the variability of Ba, Li and Ti content in plants and the soil.
3. Results and Discussion
The Ba content of the six herbs ranged between 6.7 and 82.1 mg kg−1 (Table 1). It significantly varied depending on the plant species and its habitat (moisture zone). The greatest variation of Ba content was recorded in Potentilla anserina L., Mentha arvensis L. and Comarum palustre L. in all sampling locations. The SD values for those plants were higher than for other herbs. As the average of all moisture zones, the highest content of Ba was recorded in the biomass of Potentilla anserina L. and Mentha arvensis L. The average content of Ba in Potentilla anserina L. was the largest in depression A (73.5 mg kg−1), surrounded by arable fields, while in the Mentha arvensis L., it was the largest in depression B (43.1 mg kg−1), surrounded by permanent grassland. The highest SD value for this chemical element was recorded for plants sampled from the depression surrounded by arable fields (A). Among the moisture zones, the highest standard deviation of Ba content, average from all locations, was recorded in herbs collected in the periodically flooded zone (II). The Ba content of Lycopus europaeus L., Lysimachia vulgaris L. and Achillea millefolium L. was several times lower than the average, not exceeding 14 mg kg−1. Kuziemska et al. [18] recorded higher Ba content in Dactylis glomerata L. growing on non-limed soil than in plants on limed soil, with 17.57 and 20.6 mg kg−1, respectively. According to Kabata-Pendias and Pendias [19], Ba concentration in plants is most often between 10 and 150 mg kg−1. In the present experiment, the average content of this element was within the same range.

Table 1.
Ba concentration (mg kg−1) in the biomass of some herbs.
The physiological role of Ba in plants has not yet been clarified, but the toxic effects of its high doses on the human body (gastrointestinal disorders, muscular hypoplasia and difficulty breathing) have been recorded [19,20]. For humans, the lethal dose of barium chloride (LD50) is approx. 14 mg kg−1. The mechanism of the toxicity of this metal consists in the displacement of potassium and the binding of sulphate anions [19]. When studying the effect of sampling location, it was found that the highest average concentration of Ba was in herbs from the depression surrounded by arable fields (A) and the lowest from that surrounded by permanent grassland (B). This might be due to the fact that the accumulation of this element, due to its chemical properties, was to some extent limited by the presence of Ca and Mg, acting antagonistically to Ba [21,22].
Varied Li content in the herb plants was recorded, depending on the species and moisture zone (Table 2). The greatest variation of Li content, based on the calculated SD values, was found for Achillea millefolium L. and Mentha arvensis L. in all sampling locations. In the case of moisture zones, the highest standard deviation of Li content was in herbs of the periodically flooded (II) and not-flooded (III) zones. As the average of all moisture zones, the highest content of Li was recorded in the biomass of Achillea millefolium L., Mentha arvensis L. and Lycopus europaeus L. This content varied depending on the surroundings of the mid-field ponds. In the area surrounded by arable fields (A), the most Li was found in the biomass of Achillea millefolium L., Mentha arvensis L. and Lycopus europaeus L., in the area surrounded by permanent grassland (B) in the biomass of Mentha arvensis L., Lycopus europaeus L. and Achillea millefolium L. and in the area surrounded by shrubs (C) in the biomass of Achillea millefolium L., Lycopus europaeus L. and Mentha arvensis L. Kabata-Pendias and Pendias [19] report that the accumulation of Li in plants varies between species, often proportional to its concentration in the soil. It is assumed that a content higher than 35 mg kg−1 Li in dry matter is toxic to plants [19]. In their research, Kabata-Pendias and Pendias [19] recorded the accumulation of this element in halophilic plants, reaching up to 1000 mg kg−1. This group included Solanacea and Rosacea families. In Datura inoxia (Solanacea) and Potentilla palustris (Rosacea), both of which are medicinal plants, Li concentrations did not exceed 2.4 mg kg−1 and 2.0 mg kg−1, respectively [23]. According to other data, the Li content of cereals and vegetables was 0.5–3.4 mg kg−1 [24]. The studies of Szilagyi et al. [25] confirm that Li is toxic. Kasperczyk and Wiśniowska-Kielian [26] reported that Li content in dicotyledonous plants was higher than in monocotyledonous ones. This chemical element regulates growth and the activity of certain enzymes, hormones, vitamins and translocation factors [27]. When analyzing moisture zones, it was found that herbs growing in the periodically flooded zone accumulated Li more intensively compared to the other zones of the aquatic depressions, especially in locations A (surrounded by arable fields) and B (grassland). At the location overgrown with shrubs (C), the highest average concentration of Li was recorded in the non-flooded zone.

Table 2.
Li concentration (mg kg−1) in the biomass of some herbs.
The accumulation of Ti in the plants from different habitats varied significantly under the influence of experimental factors (Table 3). On average, the most Ti was found in Mentha arvensis L., almost 4–5 times higher than in the other herbs. The highest SD of Ti was recorded for Mentha arvensis L. and Lycopus europaeus L. sampled from the depression surrounded by arable fields (A). In subsequent sampling locations (B) and (C), the greatest variation in Ti content was found for Mentha arvensis L., Achillea millefolium L. and Comarum palustre L. The highest standard deviation of Ti content in plants was recorded in the non-flooded zone (III). Depending on the plant species and their locations, the concentration of Ti ranged from 1.9 to 22.9 mg kg−1. Malinowska and Kalembasa [28] confirmed its varied accumulation depending on the plant species. According to the authors, Lolium multiflorum accumulated much more of this element than corn and sunflower. On average, the largest Ti accumulation was recorded in herbs sampled from the depression surrounded by permanent grassland (B) (7.3 mg kg−1) and the smallest from that covered with shrubs (C) (5.9 mg kg−1). Analyzing moisture zones around the natural aquatic ecosystems, it was found that the highest concentration of Ti was in plants of the periodically flooded zone in area A (arable fields) and B (permanent grassland), and in the plants of the flooded zone in area C (wild shrubs). The accumulation of Ti in various crops is significantly influenced by pH of the soil and its content of organic substance [28]. The authors reported that in forage plants from control, Ti content was higher than in plants treated with municipal sewage sludge. There are many factors that influence its uptake. With a long retention of water on the soil (more than 48 h), anaerobic processes begin to occur. Periodic flooding of the soil leads to many changes in its environment, which affects nutrient uptake by plants. According to the literature, Ti content of plants varies greatly between 0.2 and 80 mg kg−1 DM, while some species, such as horsetail, moss and nettle, may contain more than 300 mg Ti kg−1 DM [19,29].

Table 3.
Ti concentration (mg kg−1) in the biomass of some herbs.
The content of Ba, Li and Ti in the bottom sediment of the three mid-field ponds located in the Siedlce Plateau varied (Table 4). Most of the Li was recorded in the sediment from the pond surrounded by permanent grassland (B), with the greatest amounts of Ba and Ti in sediment of the depression surrounded by arable fields (A). The top layer of the bottom sediment from the three midfield ponds was characterized by similar pHKCl values, ranging from 6.4 to 6.5.

Table 4.
Ba, Li and Ti concentration in bottom sediment (mg kg−1 DM).
The Ba, Li and Ti content of the soil sampled from three mid-field depressions varied significantly (Table 5). Most Li and Ti was recorded in the soil of the non-flooded zone (III), on average 2.6 and 37.1 mg kg−1, respectively, and Ba in transitional zone soil (II), with 31.4 mg kg−1. Soil Ba content ranged from 10.1 to 51.1 mg kg−1, with its greatest accumulation in herbs collected in the non-flooded zone (Table 1). On average across sampling locations, Ba content was 28.2 mg kg−1, 2.3 mg kg−1 for Li and 29.2 mg kg−1 for Ti. According to Kabata–Pendias and Pendias [19], most Polish soils contain less than 50 mg Ba kg−1. The content of Ba ions in the soil is generally low as this element easily enters the soil sorption complex and is retained there [22]. Kabata–Pendias and Pendias [19] report that Ba is strongly bound by clay minerals, iron-manganese and phosphate concretions and sulfur compounds. Increased soil acidity and sulfur content can affect the uptake of this chemical element by plants. Barium easily migrates in soils together with circulating water, and is leached deep into the soil profile, or is subject to concentration in the top surface layer [19]. In non-contaminated soils, the amount of Ti usually ranges between 1.5 and 60 mg kg−1, and the amount of Li between 0.01 and 40 mg kg−1. However, some soils may contain even very significant amounts of Ti [19]. The lowest pH was recorded in the soil of flooded zones (I) of all depressions, due to the predominance of anaerobic conditions and biological sorption processes (Table 5). In the periodically flooded and non-flooded zones, soil pH was slightly higher, but it did not exceed 6.2. On average, the highest SD value for all chemical elements was recorded in soil from the depression surrounded by permanent grassland (B).

Table 5.
Ba, Li and Ti concentration in soil (mg kg−1 DM).
The accumulation coefficient of Ba, Li and Ti was significantly dependent on the plant species and moisture zones (Table 6, Table 7 and Table 8). Thus, the accumulation coefficient of Ba and Li in herbs was estimated as average, while for Ti it was low [16]. The coefficient values for plants indicated varied accumulation of metals from the soil, which was also demonstrated in many other studies [19,30,31,32,33,34]. The highest Ba, Li and Ti accumulation coefficient was recorded in Mentha arvensis L., in addition to Ba in Potentilla anserina L. (Table 6) and Li in Achillea millefolium L. (Table 7). Coefficient values for some elements can be high, ranging from 1 to 10 [35]. However, for the elements studied in the present experiment those values were rather low. The highest accumulation coefficient of Ba and Li in the soil-plant system was recorded in plants from the area adjacent to the pond overgrown with shrubs (C), with 2.4 and 1.8, respectively (Table 6 and Table 7). For Ti, the highest value (0.39) was recorded in plants harvested from area B, with permanent grassland (Table 8). To date, little has been known about the content of Ba, Li and Ti in food and their amounts entering the human body, as the data available in the literature are incomplete.

Table 6.
Ba accumulation coefficient in the plants.

Table 7.
Li accumulation coefficient in the plants.

Table 8.
Ti accumulation coefficient in the plants.
The calculated values of the correlation coefficients between the Ba, Li and Ti content of the soil and in herbs showed no significant relationship between Ba and Ti (Table 9). A lack of significant correlations between the content of the elements in plants and in the soil may be related to the soil pH, ranging from 5.4 to 6.2 (Table 5), which could have affected the soluble forms of these elements. On the other hand, a positive significant correlation was found between the Li content of the soil and its amounts in the biomass of Mentha arvensis L. and Comarum palustre L. The values of the correlation coefficient (r) were, respectively, r = 0.711 and r = 0.815.

Table 9.
Linear correlation coefficient between the Ba, Li and Ti content of the soil and of herb biomass.
4. Conclusions
The content of those chemical elements varied significantly depending on the plant species and the place of growing. As the average of all moisture zone, the highest content of Ba was found in the biomass of Potentilla anserina L. and Mentha arvensis L. and Li in the biomass of Achillea millefolium L., Mentha arvensis L. and Lycopus europaeus L.
The content of Ti was several times higher in the biomass of Mentha arvensis L. than in other herbs. There were no excessive levels of Ba, Li and Ti in the biomass of the herbs growing at various distances from arable fields, permanent grassland and habitats overgrown with wild shrubs.
The content of Ba, Li and Ti in the soil of different moisture zones surrounding the three mid-field depressions of the Siedlce Plateau varied significantly. The highest Ba content was recorded in periodically flooded soil (zone—II), while the highest Li and Ti contents were recorded in the non-flooded soil (zone—III). In the soil of the flooded zone (I), the content of these elements was the lowest.
The results indicated that various systems of management of agricultural areas in the central-eastern region of Poland did not cause excessive bioaccumulation of the studied chemical elements in herbal plants and in the soil.
Author Contributions
E.M. and J.N. have equally contributed to this article. All authors have read and agreed to the published version of the manuscript.
Funding
The results of the research carried out under the research theme No. 134/21/B were financed from the science grant, granted by the Ministry of Science and Higher Education.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The datasets supporting the conclusions of the article are included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nicolet, P.; Ruggiero, A.; Biggs, J. Second European pond workshop: Conservation of pond biodiversity in a changing European landscape. Ann. Limnol. Int. J. Limnol. 2007, 43, 77–80. [Google Scholar] [CrossRef]
- Boix, D.; Biggsb, J.; Cereghino, R.; Hull, A.; Kalettka, T.; Oertli, B. Pond research and management in Europe: Small is beautiful. Hydrobiologia 2012, 689, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gruchelski, M.; Niemczyk, J. Requirements and activities in the area of rationalization of polish agricultural policy (in a socio-economic and environmental protection aspects). Post. Techn. Przetw. Spożyw. 2018, 2, 103–107. (In Polish) [Google Scholar]
- Chan, K. Some aspects of toxic contaminants in herbal medicines. Chemosphere 2003, 52, 1361–1371. [Google Scholar] [CrossRef] [Green Version]
- Zheljazkova, V.D.; Jeliazkova, E.A.; Kovacheva, N.; Dzuhurmanski, A. Metal uptake by medicinal plant species grown in soils contaminated by a smelter. Environ. Exp. Bot. 2008, 64, 207–216. [Google Scholar] [CrossRef]
- Regulations of the Ministry of the Environment (Dz. U. z 2004 r. Nr 168, poz. 1764). 2004. Available online: http://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20041681764 (accessed on 1 April 2021).
- Deng, H.; Ye, Z.H.; Wong, M.H. Accumulation of lead, zinc, copper and cadmium by 12 wetland plants species thriving in metal-contaminated sites in China. Environ. Pollut. 2004, 132, 29–40. [Google Scholar] [CrossRef]
- Garcia-Rico, L.; Leyva-Perez, J.; Jara-Marini, M.E. Content and daily intake of copper, zinc, lead, cadmium and mercury from dietary supplements in Mexico. Food Chem. Toxicol. 2007, 45, 1599–1605. [Google Scholar] [CrossRef]
- Arslan, H.; Güleryüz, G.; Leblebici, Z.; Kırmızı, S.; Aksoy, A. Verbascum bombyciferum Boiss. (Scrophulariaceae) as a possible bio-indicator for the assessment of heavy metals in the environment of Bursa, Turkey. Environ. Monit. Assess. 2010, 1631, 105–113. [Google Scholar] [CrossRef]
- Bielicka-Giełdoń, A.; Ryłko, E. Estimation of metallic elements in herbs and species available on the Polish market. Pol. J. Environ. Stud. 2013, 22, 1251–1256. [Google Scholar]
- Liro, A. The Concept of the National Ecological Network ECONET—Poland; IUCN Poland Foundation: Warsaw, Poland, 1995. [Google Scholar]
- Kuźnicki, F.; Białousz, S.; Składowski, P.; Szafranek, A.; Kamińska, K.; Ziemińska, A. Physicochemical properties of soils of southeastern part of the Masovian Lowland as a criterion of their typology. Soil Sci. Annu. Wars. 1979, 30, 3–20. (In Polish) [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Location of the Analysed Samples. Available online: http://www.openstreetmap.org/#map=12/52.1036/22.1357 (accessed on 16 September 2021).
- Kalembasa, S.; Kalembasa, D.; Żądełek, J. Soil Science and Agricultural Chemistry; Practice Script; WSRP: Siedlce, Poland, 1989; p. 434. (In Polish) [Google Scholar]
- Wesołowski, M.; Radecka, I. Medicinal plants. Elemental composition, source of minerals for plants, indicators of environmental contamination by heavy metals. Pol. Pharm. 2003, 59, 911–919. (In Polish) [Google Scholar]
- StatSoft, Inc. 2011 STATISTICA (Data Analysis Software System), Version 10. Available online: www.statsoft.com (accessed on 2 April 2021).
- Kuziemska, B.; Jaremko, D.; Bik, B.; Jakubicka, M. Content of lead and barium in biomass of cocksfoot (Dactylis glomerata L.) cultivated on soil contaminated with nickel under different liming and organic fertilization. Zesz. Nauk. UP Wrocław Roln. CV 2013, 594, 27–36. (In Polish) [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Biogeochemistry of Trace Elements; PWN: Warsaw, Poland, 1999; p. 398. (In Polish) [Google Scholar]
- Migaszewski, Z.M.; Gałuszka, A. Fundamentals of Environmental Geochemistry; WNT: Warsaw, Poland, 2007. (In Polish) [Google Scholar]
- Kalembasa, D.; Jaremko, D. Total content of lithium, barium and strontium in soils of South Podlasie Lowland. Pol. J. Environ. Stud. 2006, 15, 320–325. [Google Scholar]
- Jaremko, D.; Kalembasa, D. A comparison of methods for the determination of cation exchange capacity of soils. Ecol. Chem. Eng. S 2014, 21, 487–498. [Google Scholar]
- Lovkova, M.Y.; Sokolova, S.M.; Buzuk, G.N. Lithium-concentrating plant species and their pharmaceutical usage. Dokl. Biol. Sci. 2007, 412, 64–66. [Google Scholar] [CrossRef]
- Armendáriz, C.R.; Gonzalez-Weller, D.; Gutierrez Fernandez, A.J.; Hardisson De La Torre, A. Levels of lithium in the six most taken groups of food among the canarian population. Abstr. Toxicol. Lett. 2010, 196S, 298. [Google Scholar] [CrossRef]
- Szilagyi, M.; Balogh, I.; Anke, A.; Szentmihalyi, S. Biochemical aspects of lithium toxicity. Acta Med. Leg. Soc. 1985, 35, 167–180. [Google Scholar]
- Kasperczyk, M.; Wiśniowska-Kielian, B. The influence of fertilization and undersowing on cadmium, nickel and lithium contents in meadow sward. Zesz. Probl. Post. Nauk Rol. 1997, 448, 207–212. (In Polish) [Google Scholar]
- Rogóż, A.; Wiśniowska-Kielian, B. Lithium Content and Circulation in the Natural Environment; AGH: Kraków, Poland, 2019; p. 216. (In Polish) [Google Scholar]
- Malinowska, E.; Kalembasa, S. The influence of sewage sludge doses and liming on the content of Li, Ti, Ba, Sr and As in the test plants biomass. J. Ecol. Eng. 2011, 27, 110–119. (In Polish) [Google Scholar]
- Kabata-Pendias, A.; Szteke, B. Trace Elements in the Geo- and Biosphere; Wyd. IUNG-PIB: Puławy, Poland, 2012; pp. 229–233. (In Polish) [Google Scholar]
- Królak, E. Heavy Metal Content in Filling Dusts, Soil and Dandelion (Taraxacum officinale Webb.) in Suthern Podlasie Lowland. EJPAU. 2001. Environ Dev 4,1. Available online: www.ejpau.pl/series/volume4/environmental/art-01.html (accessed on 10 April 2021).
- Lock, K.; Jansen, C.R. Influence of Aging on Metal availability in Soil. Rev. Environ. Contam. Toxicol. 2003, 178, 1–21. [Google Scholar]
- Rosselli, W.; Keller, C.; Bosch, K. Phytoextraction capacity of trees growing a metal contaminated soil. Plant Soil 2003, 256, 265–272. [Google Scholar] [CrossRef]
- Malinowska, E.; Jankowski, K. Impact of agricultural chemicals on selected heavy metals accumulation in herb plants. Appl. Ecol. Environ. Res. 2016, 14, 479–487. [Google Scholar] [CrossRef]
- Malinowska, E.; Jankowski, K. Copper and zinc concentrations of medicinal herbs and soil surrounding ponds on agricultural land. Landsc. Ecol. Eng. 2017, 13, 83–188. [Google Scholar] [CrossRef] [Green Version]
- Kloke, A.; Sauerbeck, D.R.; Vetter, H. The contamination of plants and soils with heavy metals and the transport of metals in the terrestrial food chains. In Changing Metal Cycles and Human Health; Nriagu, J.O., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Tokyo, Japan, 1984; pp. 113–141. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).