Amphibian Biomass Export from Geographically Isolated Wetlands: Temporal Variability, Species Composition, and Potential Implications for Terrestrial Ecosystems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Data Collection
2.3. Data Analysis
| √TotalBiomassij | ~N (μij, s2) | (1) |
| E(√TotalBiomassij) | = μij | |
| μij | = β0 + β1Region + β2PondAreai + β3Rainfallj + β4Diversityij + β5PropAnuranij + β6jYearj + β7i Pondi | |
| β6j | ~N(0, σj2) | |
| β7i | ~ N(0, σi2) | |
| √BiomassPerAreaij | ~N (μij, s2) | (2) |
| E(√BiomassPerAreaij) | = μij | |
| μij | = β0 + β1Region + β2PondAreai + β3Rainfallj + β4Diversityij + β5PropAnuranij + β6jYearj + β7i Pondi | |
| β6j | ~ N(0, σj2) | |
| β7i | ~ N(0, σi2) | |
| Diversityij | ~ N (μij, s2) | (3) |
| E(Diversityij) | = μij | |
| μij | = β0 + β1Region + β2PondAreai + β3Rainfallj + β6jYearj + β7i Pondi | |
| β6j | ~ N(0, σj2) | |
| β7i | ~ N(0, σi2) | |
| sin−1(PropAnuranij) | ~ N (μij, s2) | (4) |
| E(sin−1(PropAnuranij)) | μij | |
| μij | = β0 + β1Region + β2PondAreai + β3Rainfallj + β6jYearj + β7i Pondi | |
| β6j | ~ N(0, σj2) | |
| β7i | ~ N(0, σi2) | |
3. Results
3.1. Amphibian Biomass Export
3.2. Species Composition and Diversity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polis, G.A.; Anderson, W.B.; Holt, R.D. Toward an integration of landscape and food web ecology: The dynamics of spatially subsidized food webs. Annu. Rev. Ecol. Syst. 1997, 28, 289–316. [Google Scholar] [CrossRef] [Green Version]
- Bump, J.K.; Tischler, K.B.; Schrank, A.J.; Peterson, R.O.; Vucetich, J.A. Large herbivores and aquatic-terrestrial links in southern boreal forests. J. Anim. Ecol. 2009, 78, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Helfield, J.M.; Naiman, R.J. Salmon and alder as nitrogen sources to riparian forests in a boreal Alaskan watershed. Oecologia 2002, 133, 573–582. [Google Scholar] [CrossRef]
- McCoy, M.W.; Barfield, M.; Holt, R.D. Predator shadows: Complex life histories as generators of spatially patterned indirect interactions across ecosystems. Oikos 2009, 118, 87–100. [Google Scholar] [CrossRef]
- Knight, T.M.; McCoy, M.W.; Chase, J.M.; McCoy, K.A.; Holt, R.D. Trophic cascades across ecosystems. Nature 2005, 437, 880–883. [Google Scholar] [CrossRef]
- Quinn, T.P.; Carlson, S.M.; Gende, S.M.; Rich, H.B., Jr. Transportation of Pacific salmon carcasses from streams to riparian forests by bears. Can. J. Zool. 2009, 87, 195–203. [Google Scholar] [CrossRef]
- Kremen, C.; Williams, N.M.; Aizen, M.A.; Gemmill-Herren, B.; LeBuhn, G.; Minckley, R.; Packer, L.; Potts, S.G.; Roulston, T.; Steffan-Dewenter, I.; et al. Pollination and other ecosystem services produced by mobile organisms: A conceptual framework for the effects of land-use change. Ecol. Lett. 2007, 10, 299–314. [Google Scholar] [CrossRef]
- Baker, M.R.; Schindler, D.E.; Holtgrieve, G.W.; St. Louis, V.L. Bioaccumulation and transport of contaminants: Migrating sockeye salmon as vectors of mercury. Environ. Sci. Technol. 2009, 43, 8840–8846. [Google Scholar] [CrossRef]
- Blais, J.M.; Kimpe, L.E.; McMahon, D.; Keatley, B.E.; Mallory, M.L.; Douglas, M.S.V.; Smol, J.P. Arctic seabirds transport marine-derived contaminants. Science 2005, 309, 445. [Google Scholar] [CrossRef] [Green Version]
- Walters, D.M.; Fritz, K.M.; Otter, R.R. The dark side of subsidies: Adult stream insects export organic contaminants to riparian predators. Ecol. Appl. 2008, 18, 1835–1841. [Google Scholar] [CrossRef]
- Marczak, L.B.; Thompson, R.M.; Richardson, J.S. Meta-analysis: Trophic level, habitat, and productivity shape the food web effects of resource subsidies. Ecology 2007, 88, 140–148. [Google Scholar] [CrossRef]
- Bartels, P.; Cucherousset, J.; Steger, K.; Eklöv, P.; Tranvik, L.J.; Hillebrand, H. Reciprocal subsidies between freshwater and terrestrial ecosystems structure consumer resource dynamics. Ecology 2012, 93, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.P.; Rover, J.A.; Stets, E.G.; Striegl, R.G. The regional abundance and size distribution of lakes and reservoirs in the United States and implications for estimates of global lake extent. Limnol. Oceanogr. 2012, 57, 597–606. [Google Scholar] [CrossRef]
- Smith, L.L.; Subalusky, A.L.; Atkinson, C.L.; Earl, J.E.; Mushet, D.M.; Scott, D.E.; Lance, S.L.; Johnson, S.A. Biological connectivity of seasonally ponded wetlands across spatial and temporal scales. J. Am. Water Resour. Assoc. 2019, 55, 334–353. [Google Scholar] [CrossRef]
- Gibbons, J.W.; Winne, C.T.; Scott, D.E.; Willson, J.D.; Glaudas, X.; Andrews, K.M.; Todd, B.D.; Fedewa, L.A.; Wilkinson, L.; Tsaliagos, R.N.; et al. Remarkable amphibian biomass and abundance in an isolated wetland: Implications for wetland conservation. Conserv. Biol. 2006, 20, 1457–1465. [Google Scholar] [CrossRef]
- DeGregario, B.A.; Willson, J.D.; Dorcas, M.E.; Gibbons, J.W. Commercial value of amphibians produced from an isolated wetland. Am. Midl. Nat. 2014, 172, 200–204. [Google Scholar] [CrossRef]
- Fritz, K.A.; Whiles, M.R. Reciprocal subsidies between temporary ponds and riparian forests. Limnol. Oceanogr. 2021, 66, 3149–3161. [Google Scholar] [CrossRef]
- Schriever, T.A.; Cadotte, M.W.; Williams, D.D. How hydroperiod and species richness affect the balance of resource flows across aquatic-terrestrial boundaries. Aquat. Sci. 2013, 76, 131–143. [Google Scholar] [CrossRef]
- Regester, K.J.; Lips, K.R.; Whiles, M.R. Energy flow and subsidies associated with the complex life cycle of ambystomatid salamanders in ponds and adjacent forest in southern Illinois. Oecologia 2006, 147, 303–314. [Google Scholar] [CrossRef]
- Reinhardt, T.; Steinfartz, S.; Paetzold, A.; Weitere, M. Linking the evolution of habitat choice to ecosystem functioning: Direct and indirect effects of pond-reproducing fire salamanders on aquatic-terrestrial subsidies. Oecologia 2013, 173, 281–291. [Google Scholar] [CrossRef]
- Capps, K.A.; Berven, K.A.; Tiegs, S.D. Modelling nutrient transport and transformation by pool-breeding amphibians in forested landscapes using a 21-year dataset. Freshw. Biol. 2015, 60, 500–511. [Google Scholar] [CrossRef]
- Barzaghi, B.; Ficetola, G.F.; Pennati, R.; Manenti, R. Biphasic predators provide biomass subsidies in small freshwater habitats: A case study of spring and cave pools. Freshw. Biol. 2017, 62, 1637–1644. [Google Scholar] [CrossRef]
- Luhring, T.M.; DeLong, J.P.; Semlitsch, R.D. Stoichiometry and life-history interact to determine the magnitude of cross-ecosystem element and biomass fluxes. Front. Microbiol. 2017, 8, 814. [Google Scholar] [CrossRef]
- Fritz, K.A.; Whiles, M.R. Amphibian-mediated nutrient fluxes across aquatic-terrestrial boundaries of temporary wetlands. Freshw. Biol. 2018, 63, 1250–1259. [Google Scholar] [CrossRef]
- Fritz, K.A.; Whiles, M.R.; Trushenski, J.T. Subsidies of long-chain polyunsaturated fatty acids from aquatic to terrestrial environments via amphibian emergence. Freshw. Biol. 2019, 64, 832–842. [Google Scholar] [CrossRef]
- Pough, F.H. The advantages of ectothermy for tetrapods. Am. Nat. 1980, 115, 92–112. [Google Scholar] [CrossRef]
- Werner, E.E.; Wellborn, G.A.; McPeek, M.A. Diet composition in postmetamorphic bullfrogs and green frogs: Implications for interspecific predation and competition. J. Herpetol. 1995, 29, 600–607. [Google Scholar] [CrossRef] [Green Version]
- Crawford, J.A.; Shepard, D.B.; Conner, C.A. Diet composition and overlap between recently metamorphosed Rana areolata and Rana sphenocephala: Implications for a frog of conservation concern. Copeia 2009, 2009, 642–646. [Google Scholar] [CrossRef]
- Smith, L.M.; Gray, M.J.; Quarles, A. Diets of newly metamorphosed amphibians in west Texas playas. Southwest. Nat. 2004, 49, 257–263. [Google Scholar] [CrossRef]
- Bull, E.L.; Hayes, J.L. Selection of diet by metamorphic and juvenile western toads (Bufo boreas) in northeastern Oregon. Herpetol. Conserv. Biol. 2009, 4, 85–95. [Google Scholar]
- Hocking, D.J.; Babbitt, K.J. Amphibian contributions to ecosystem services. Herpetol. Conserv. Biol. 2014, 9, 1–17. [Google Scholar]
- Popescu, V.D.; Patrick, D.A.; Hunter, M.L., Jr.; Calhoun, A.J.K. The role of forest harvesting and subsequent vegetative regrowth in determining patterns of amphibian habitat use. For. Ecol. Manag. 2012, 170, 163–174. [Google Scholar] [CrossRef]
- Hocking, D.J.; Rittenhouse, T.A.G.; Rothermel, B.B.; Johnson, J.R.; Conner, C.A.; Harper, E.B.; Semlitsch, R.D. Breeding and recruitment phenology of amphibians in Missouri oak-hickory forests. Am. Midl. Nat. 2008, 160, 41–60. [Google Scholar] [CrossRef]
- Rittenhouse, T.A.G.; Semlitsch, R.D. Grasslands as movement barriers for a forest-associated salamander: Migration behavior of adult and juvenile salamanders at a distinct habitat edge. Biol. Conserv. 2006, 131, 14–22. [Google Scholar] [CrossRef]
- Patrick, D.A.; Hunter, M.L., Jr.; Calhoun, A.J.K. Effects of experimental forestry treatments on a Maine amphibian community. For. Ecol. Manag. 2006, 234, 323–332. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Todd, B.D.; Blomquist, S.M.; Calhoun, A.J.K.; Gibbons, J.W.; Gibbs, J.P.; Graeter, G.J.; Harper, E.B.; Hocking, D.J.; Hunter, M.L., Jr.; et al. Effects of timber harvest on amphibian populations: Understanding mechanisms from forest experiments. Bioscience 2009, 59, 853–862. [Google Scholar] [CrossRef] [Green Version]
- Semlitsch, R.D.; Conner, C.A.; Hocking, D.J.; Rittenhouse, T.A.G.; Harper, E.B. Effects of timber harvesting on pond-breeding amphibian persistence: Testing the evacuation hypothesis. Ecol. Appl. 2008, 18, 283–289. [Google Scholar] [CrossRef]
- Osbourn, M.S. Initial Juvenile Movement fo Pond-Breeding Amphibians in Altered Forest Habitat. Ph.D. Thesis, University of Missouri, Columbia, MO, USA, 2012. [Google Scholar]
- Wilbur, H.M. Competition, predation, and the structure of the Ambystoma-Rana sylvatica community. Ecology 1972, 53, 3–21. [Google Scholar] [CrossRef]
- Takahashi, M.K.; Parris, M.J. Life cycle polyphenism as a factor affecting ecological divergence within Notophthalmus viridescens. Oecologia 2008, 158, 23–34. [Google Scholar] [CrossRef]
- McPherson, L.A.; Holásková, I.; Anderson, J.T. Functional equivalence of created wetland water quality: A comparison of amphibian metamorphic success. Open J. Ecol. 2020, 10, 101177. [Google Scholar] [CrossRef]
- Gordon, A.M.; Yougquist, M.B.; Boone, M.D. The effects of pond drying and predation on Blanchard’s cricket frogs (Acris blanchardi). Copeia 2016, 104, 482–486. [Google Scholar] [CrossRef]
- Rothermel, B.B.; Semlitsch, R.D. Consequences of forest fragmentation for juvenile survival in spotted (Ambystoma maculatum) and marbled (Ambystoma opacum) salamanders. Can. J. Zool. 2006, 84, 797–807. [Google Scholar] [CrossRef]
- Earl, J.E.; Semlitsch, R.D. Reciprocal subsidies in ponds: Does leaf input increase frog biomass export? Oecologia 2012, 170, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- O’Laughlin, B.E.; Harris, R.N. Models of metamorphic timing: An experimental evaluation with the pond-dwelling salamander Hemidactylium scutatum (Caudata: Plethodontidae). Oecologia 2000, 124, 343–350. [Google Scholar] [CrossRef]
- Hocking, D.J.; Semlitsch, R.D. Effects of experimental clearcut logging on gray treefrog (Hyla versicolor) tadpole performance. J. Herpetol. 2008, 42, 689–698. [Google Scholar] [CrossRef]
- Boone, M.D.; Little, E.E.; Semlitsch, R.D. Overwintered bullfrog tadpoles negatively affect salamanders and anurans in native amphibian communities. Copeia 2004, 2004, 683–690. [Google Scholar] [CrossRef]
- Boone, M.D.; Semlitsch, R.D.; Little, E.E.; Doyle, M.C. Multiple stressors in amphibian communities: Effects of chemical contamination, bullfrogs, and fish. Ecol. Appl. 2007, 17, 291–301. [Google Scholar] [CrossRef]
- Orlofske, S.A.; Hopkins, W.A. Energetics of metamorphic climax in the pickerel frog (Lithobates palustris). Comp. Biochem. Physiol. 2009, 154, 191–196. [Google Scholar] [CrossRef]
- Williams, B.K.; Rittenhouse, T.A.G.; Semlitsch, R.D. Leaf litter input mediates tadpole performance across forest canopy treatments. Oecologia 2008, 155, 377–384. [Google Scholar] [CrossRef]
- Earl, J.E.; Cohagen, K.E.; Semlitsch, R.D. Effects of leachate from tree leaves and grass litter on tadpoles. Environ. Toxicol. Chem. 2012, 31, 1511–1517. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Zuur, A.F.; Ieno, E.N. A protocol for conducting and presenting results of regression-type analyses. Methods Ecol. Evol. 2016, 7, 636–645. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, S.; Johnson, P.C.D.; Schielzeth, H. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 2017, 14, 20170213. [Google Scholar] [CrossRef] [Green Version]
- Bartoń, K. MuMIn: Multi-Model Inference. R Package Version 1.43.17. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 2 November 2021).
- Atkinson, A.C.; Knapp, D.D.; Smith, L.L. Long-term patterns of amphibian diversity, abundance and nutrient export from small, isolated wetlands. Diversity 2021, 13, 598. [Google Scholar] [CrossRef]
- Pechmann, J.H.K.; Scott, D.E.; Semlitsch, R.D.; Caldwell, J.P.; Vitt, L.J.; Gibbons, J.W. Declining amphibian populations: The problem of separating human impacts from natural fluctuations. Science 1991, 253, 892–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berven, K.A. Factors affecting population fluctuations in larval and adult stages of the wood frog (Rana sylvatica). Ecology 1990, 71, 1599–1608. [Google Scholar] [CrossRef]
- Marsh, D.M. Fluctuations in amphibian populations: A meta-analysis. Biol. Conserv. 2001, 101, 327–335. [Google Scholar] [CrossRef]
- Altig, R.; Whiles, M.R.; Taylor, C.L. What do tadpoles really eat? Assessing the trophic status of an understudied and imperiled group of consumers in freshwater habitats. Freshw. Biol. 2007, 52, 386–395. [Google Scholar] [CrossRef]
- Earl, J.E.; Castello, P.O.; Cohagen, K.E.; Semlitsch, R.D. Effects of subsidy quality on reciprocal subsidies: How leaf litter species changes frog biomass export. Oecologia 2014, 175, 209–218. [Google Scholar] [CrossRef]
- Earl, J.E.; Luhring, T.M.; Williams, B.K.; Semlitsch, R.D. Biomass export of salamanders and anurans from ponds is affected differentially by changes in canopy cover. Freshw. Biol. 2011, 56, 2473–2482. [Google Scholar] [CrossRef]
- Roble, S.M. Dispersal movements and plant associations of juvenile gray treefrogs, Hyla versicolor Le Conte. Trans. Kans. Acad. Sci. 1979, 82, 235–245. [Google Scholar] [CrossRef]
- Laking, A.E.; Li, Z.; Goossens, E.; Miñarro, M.; Beukema, W.; Lens, L.; Bonte, D.; Verheyen, K.; Pasmans, F.; Martel, A. Salamander loss alters litter decomposition dynamics. Sci. Total Environ. 2021, 776, 145994. [Google Scholar] [CrossRef] [PubMed]
- Wyman, R.L. Experimental assessment of salamanders as predators of detrital food webs: Effects on invertebrates, decomposition and the carbon cycle. Biodivers. Conserv. 1998, 7, 641–650. [Google Scholar] [CrossRef]
- Huang, C.; Wang, C.; Hou, P.L. Toads (Bufo bankorensis) influence litter chemistry but not litter invertebrates and litter decomposition rates in a subtropical forest of Taiwan. J. Trop. Ecol. 2007, 23, 161–168. [Google Scholar] [CrossRef]
- Beard, K.H.; Vogt, K.A.; Kulmatiski, A. Top-down effects of a terrestrial frog on forest nutrient dynamics. Oecologia 2002, 133, 583–593. [Google Scholar] [CrossRef]
- Beard, K.H.; Eschtruth, A.K.; Vogt, K.A.; Vogt, D.J.; Scatena, F.N. The effects of the frog Eleutherodactylus coqui on invertebrates and ecosystem processes at two scales in the Luquillo Experimental Forest, Puerto Rico. J. Trop. Ecol. 2003, 19, 607–617. [Google Scholar] [CrossRef] [Green Version]
- Shuman-Goodier, M.E.; Diaz, M.I.; Almazan, M.L.; Singleton, G.R.; Hadi, B.A.R.; Propper, C.R. Ecosystem hero and villain: Native frog consumes rice pests, while the invasive cane toad feasts on beneficial arthropods. Agric. Ecosyst. Environ. 2019, 279, 100–108. [Google Scholar] [CrossRef]
- Pittman, S.E.; Osbourn, M.S.; Semlitsch, R.D. Movement ecology of amphibians: A missing component for understanding population declines. Biol. Conserv. 2014, 169, 44–53. [Google Scholar] [CrossRef]
- Earl, J.E.; Zollner, P.A. Advancing research on animal-transported subsidies by integrating animal movement and ecosystem modelling. J. Anim. Ecol. 2017, 86, 987–997. [Google Scholar] [CrossRef] [Green Version]
- Earl, J.E.; Zollner, P.A. Effects of animal movement strategies and costs on the distribution of active subsidies across simple landscapes. Ecol. Model. 2014, 283, 45–52. [Google Scholar] [CrossRef]
- Patrick, D.A.; Harper, E.B.; Hunter, M.L., Jr.; Calhoun, A.J.K. Terrestrial habitat selection and strong density-dependent mortality in recently metamorphosed amphibians. Ecology 2008, 89, 2563–2574. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.E.; Komoroski, M.J.; Croshaw, D.A.; Dixon, P.M. Terrestrial distribution of pond-breeding salamanders around an isolated wetland. Ecology 2013, 94, 2537–2546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rittenhouse, T.A.G.; Semlitsch, R.D. Distribution of amphibians in terrestrial habitat surrounding wetlands. Wetlands 2007, 27, 153–161. [Google Scholar] [CrossRef]
- Lang, F.; Bauhus, J.; Frossard, E.; George, E.; Kaiser, K.; Kaupenjohann, M.; Kruger, J.; Matzner, E.; Polle, A.; Prietzel, J.; et al. Phosphorus in forest ecosystems: New insights from an ecosystem nutrition perspective. J. Plant Nutr. Soil Sci. 2016, 179, 129–135. [Google Scholar] [CrossRef]
- Schaberg, P.G.; DeHayes, D.H.; Hawley, G.J. Anthropogenic calcium depletion: A unique threat to forest ecosystem health? Ecosyst. Health 2001, 7, 214–228. [Google Scholar] [CrossRef]
- Knapp, D.D.; Smith, L.L.; Atkinson, C.L. Larval anurans follow predictions of stoichiometric theory: Implications for nutrient storage in wetlands. Ecosphere 2021, 12, e03466. [Google Scholar] [CrossRef]
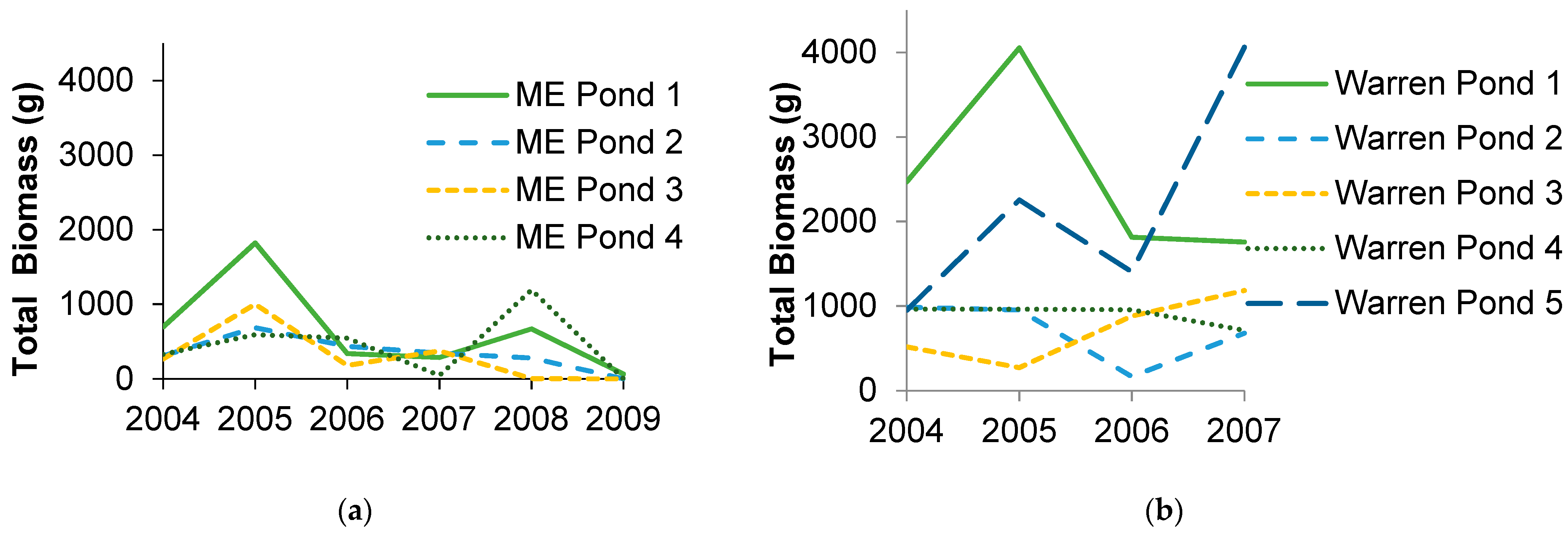
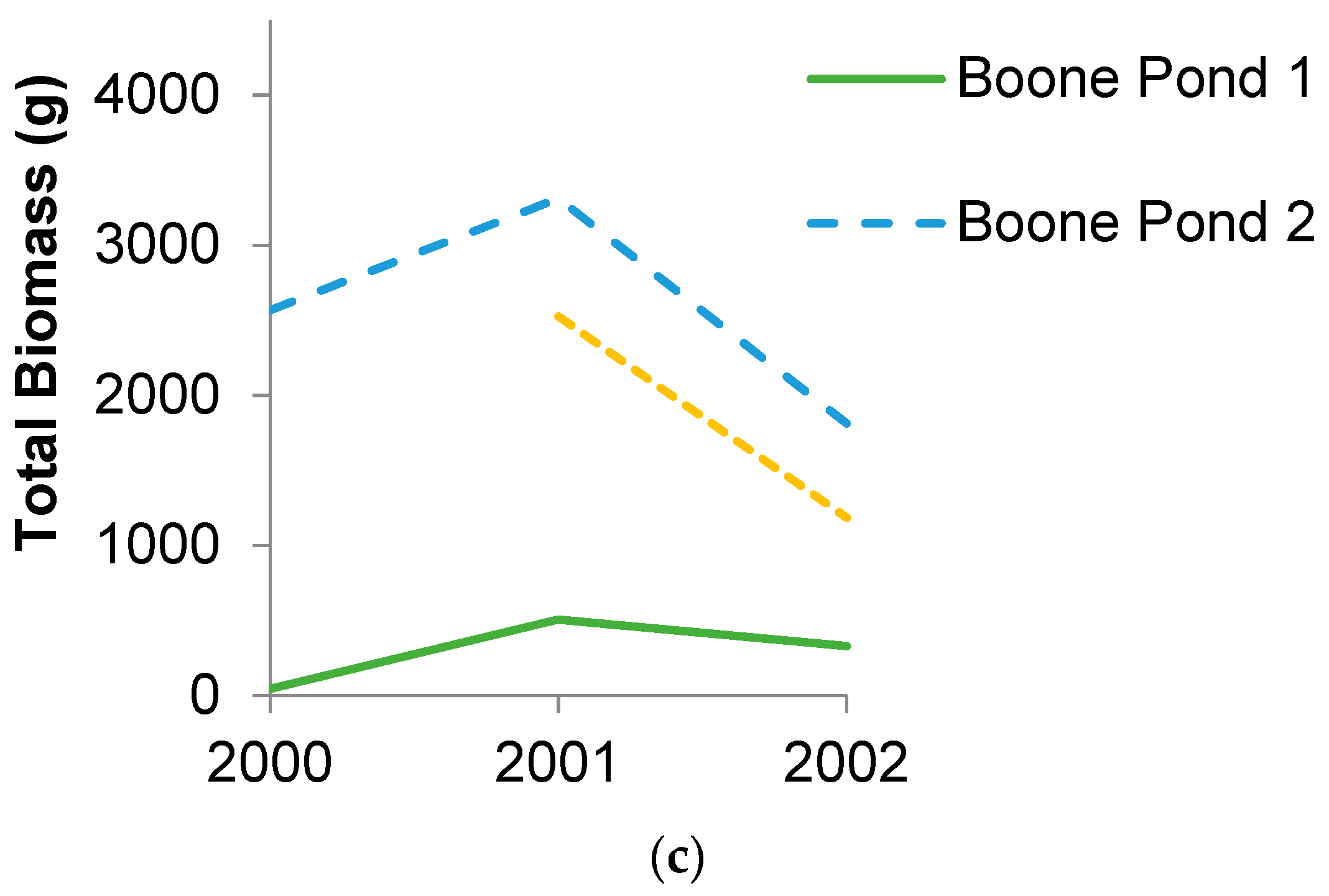
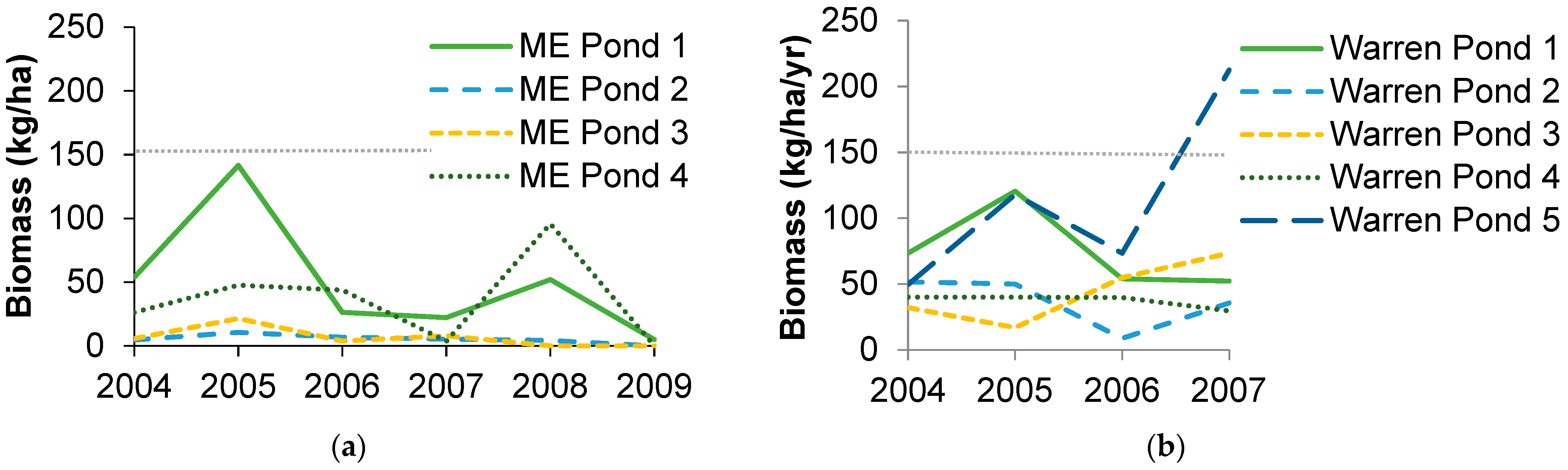
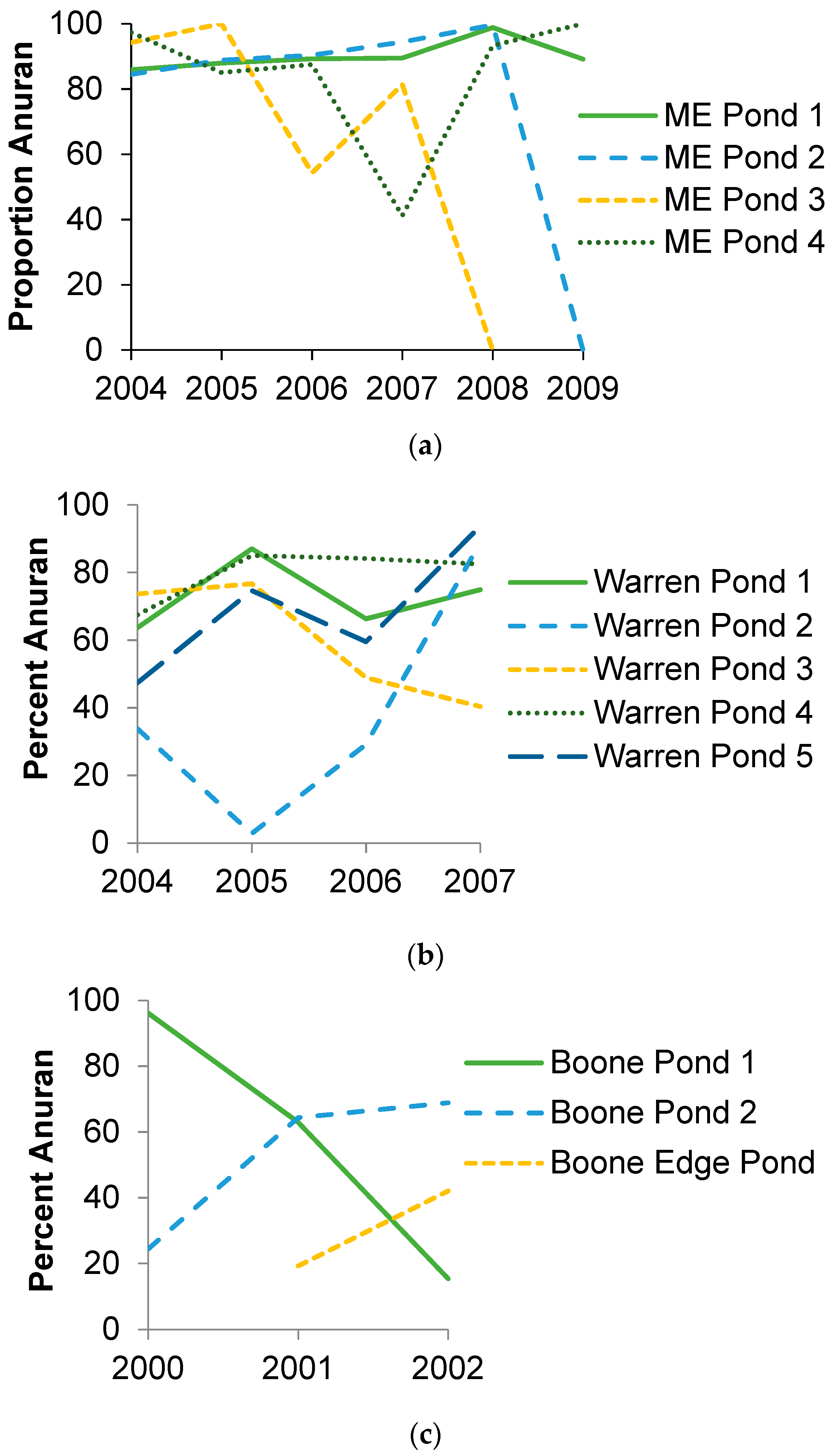
| Species | Abundance (per Pond per Year) | Mean Wet Mass at Metamorphosis (g) | Source |
|---|---|---|---|
| Blue-Spotted Salamander (Ambystoma laterale) | 0–31 | 0.94 | Michigan [39] |
| Spotted Salamander (Ambystoma maculatum) | 0–356 | 0.62 | Maine LEAP [35] |
| Green Frog (Lithobates clamitans) | 2–119 | 2.72 | DBCA pitfall traps [38] |
| Wood Frog (Lithobates sylvaticus) | 0–2626 | 0.61 | Maine LEAP [35] |
| Eastern Newt (Notophthalmus viridescens) | 0–7 | 0.37 | West Virginia [40] |
| Spring Peeper (Pseudacris crucifer) | 0–1 1 | 0.18 | West Virginia [41] |
| Species | Abundance (per Pond per Year) | Mean Wet Mass at Metamorphosis (g) | Source |
|---|---|---|---|
| Blanchard’s Cricket Frog (Acris blanchardi) | 0–19 1 | 0.22 | Ohio [42] |
| Ringed Salamander (Ambystoma annulatum) | 7–635 2 | 1.40 | DBCA pitfall traps [38] |
| Spotted Salamander (Ambystoma maculatum) | 0–1720 | 1.02 | DBCA pitfall traps [38] |
| Marbled Salamander (Ambystoma opacum) | 0–15 2 | 0.77 | Mesocosms using larvae from DBCA [43] |
| American Toad (Anaxyrus americanus) | 0–126 | 0.09 | DBCA mesocosms [44] |
| Four-Toed Salamander (Hemidactylium scutatum) | 0–2 2 | 0.07 | Virginia [45] |
| Eastern Gray Treefrog (Hyla versicolor) | 0–41 1 | 0.45 | DBCA mesocosms [46] |
| Bullfrog (Lithobates catesbieanus) | 0–23 | 7.55 | Mesocosms using tadpoles from Baskett [47,48] |
| Green Frog (Lithobates clamitans) | 3–1383 | 2.72 | DBCA pitfall traps [38] |
| Pickerel Frog (Lithobates palustris) | 0–30 | 1.57 | Virginia [49] |
| Southern Leopard Frog (Lithobates sphenocephalus) | 0–174 | 1.86 | DBCA mesocosms [44] |
| Wood Frog (Lithobates sylvaticus) | 0–400 1,2 | 0.75 | DBCA mesocosms [44] |
| Eastern Newt (Notophthalmus viridescens) | 0–1086 | 0.50 | Arkansas [40] |
| Spring Peeper (Pseudacris crucifer) | 0–434 1 | 0.24 | Mesocosms using eggs from Baskett [50] |
| Boreal Chorus Frog (Pseudacris maculata) | 0–14 1,3 | 0.15 | Lab using eggs from DBCA [51] |
| Dependent Variable | Independent Variable | Estimate ± SE | t | p-Value | Effect |
|---|---|---|---|---|---|
| Total Biomass Export r2 = 0.51 | Intercept | 7.19 ± 2.82 | 2.55 | 0.02 | - |
| Region:MO | 25.88 ± 7.88 | 3.29 | 0.01 | MO > ME | |
| Pond Area | 0.001 ± 0.019 | 0.07 | 0.95 | - | |
| Rainfall | −0.31 ± 0.23 | −1.36 | 0.22 | - | |
| Diversity | −20.75 ± 10.31 | −2.01 | 0.05 | Negative | |
| Proportion from Anurans | 13.01 ± 5.41 | 2.41 | 0.02 | Positive | |
| Biomass Export/Pond Area r2 = 0.44 | Intercept | 8.09 ± 2.69 | 3.01 | 0.01 | - |
| Region:MO | 4.71 ± 1.62 | 2.90 | 0.02 | MO > ME | |
| Pond Area | −0.007 ± 0.004 | −1.92 | 0.12 | - | |
| Rainfall | −0.08 ± 0.05 | −1.59 | 0.16 | - | |
| Diversity | −5.20 ± 2.49 | −2.09 | 0.04 | Negative | |
| Proportion from Anurans | 1.93 ± 1.32 | 1.46 | 0.16 | - | |
| Species Diversity r2 = 0.58 | Intercept | 0.35 ± 0.13 | 2.82 | 0.02 | - |
| Region:MO | 0.33 ± 0.07 | 4.84 | 0.002 | MO > ME | |
| Pond Area | −0.00009 ± 0.00012 | −0.80 | 0.43 | - | |
| Rainfall | −0.005 ± 0.004 | −1.40 | 0.21 | - | |
| Proportion of Biomass from Anurans r2 = 0.17 | Intercept | 1.34 ± 0.23 | 5.72 | <0.0001 | - |
| Region:MO | −0.33 ± 0.12 | −2.77 | 0.008 | ME > MO | |
| Pond Area | −0.0002 ± 0.0003 | −0.75 | 0.46 | - | |
| Rainfall | −0.008 ± 0.006 | −1.19 | 0.24 | - |
| Citation | Study Site | Species Included | Number of Wetlands | Number of Years | Amphibian Biomass Export (g AFDM/m2/year) | |
|---|---|---|---|---|---|---|
| Maximum | Mean | |||||
| This Study 1 | Maine | All Amphibians | 4 | 6 | 2.22 | 0.39 |
| This Study 1 | Missouri (DBCA) | All Amphibians | 5 | 4 | 3.34 | 0.96 |
| Atkinson, et al. [56] 2 | Georgia | All Amphibians | 2 | 3 & 9 | 26.11 | 2.29 |
| Gibbons, et al. [15] 1 | South Carolina | All Amphibians | 1 | 1 | 2.50 | - |
| Schriever, et al. [18] | Ontario | All Amphibians | 6 | 1 | 41.91 | 18.03 |
| Fritz and Whiles [17] | Illinois | All Amphibians | 8 | 1 | 6.10 | 3.00 |
| Regester, et al. [19] | Illinois | Ambystoma spp. | 5 | 1 | 0.57 | 0.23 |
| Reinhardt, et al. [20] | Germany | Fire salamanders | 6 | 1 | 0.35 | 0.52 |
| Capps, et al. [21] | Michigan | Wood frogs | 1 | 21 | 2.40 3 | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Earl, J.E.; Blomquist, S.M.; Harper, E.B.; Hocking, D.J.; Hunter, M.L., Jr.; Johnson, J.R.; Osbourn, M.S.; Patrick, D.A.; Popescu, V.D.; Rittenhouse, T.A.G.; et al. Amphibian Biomass Export from Geographically Isolated Wetlands: Temporal Variability, Species Composition, and Potential Implications for Terrestrial Ecosystems. Diversity 2022, 14, 163. https://doi.org/10.3390/d14030163
Earl JE, Blomquist SM, Harper EB, Hocking DJ, Hunter ML Jr., Johnson JR, Osbourn MS, Patrick DA, Popescu VD, Rittenhouse TAG, et al. Amphibian Biomass Export from Geographically Isolated Wetlands: Temporal Variability, Species Composition, and Potential Implications for Terrestrial Ecosystems. Diversity. 2022; 14(3):163. https://doi.org/10.3390/d14030163
Chicago/Turabian StyleEarl, Julia E., Sean M. Blomquist, Elizabeth B. Harper, Daniel J. Hocking, Malcolm L. Hunter, Jr., Jarrett R. Johnson, Michael S. Osbourn, David A. Patrick, Viorel D. Popescu, Tracy A. G. Rittenhouse, and et al. 2022. "Amphibian Biomass Export from Geographically Isolated Wetlands: Temporal Variability, Species Composition, and Potential Implications for Terrestrial Ecosystems" Diversity 14, no. 3: 163. https://doi.org/10.3390/d14030163
APA StyleEarl, J. E., Blomquist, S. M., Harper, E. B., Hocking, D. J., Hunter, M. L., Jr., Johnson, J. R., Osbourn, M. S., Patrick, D. A., Popescu, V. D., Rittenhouse, T. A. G., & Rothermel, B. B. (2022). Amphibian Biomass Export from Geographically Isolated Wetlands: Temporal Variability, Species Composition, and Potential Implications for Terrestrial Ecosystems. Diversity, 14(3), 163. https://doi.org/10.3390/d14030163






