Morphology and Molecular Phylogeny of Fuscheriides baugilensis sp. nov. (Protozoa, Ciliophora, Haptorida) from South Korea †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Identification
2.2. DNA Extraction, PCR Amplification and Sequencing
2.3. Phylogenetic Analysis
3. Results
3.1. Systematics
3.2. Species Diagnosis
3.3. Type Locality
3.4. Type Material
3.5. Etymology
3.6. Description
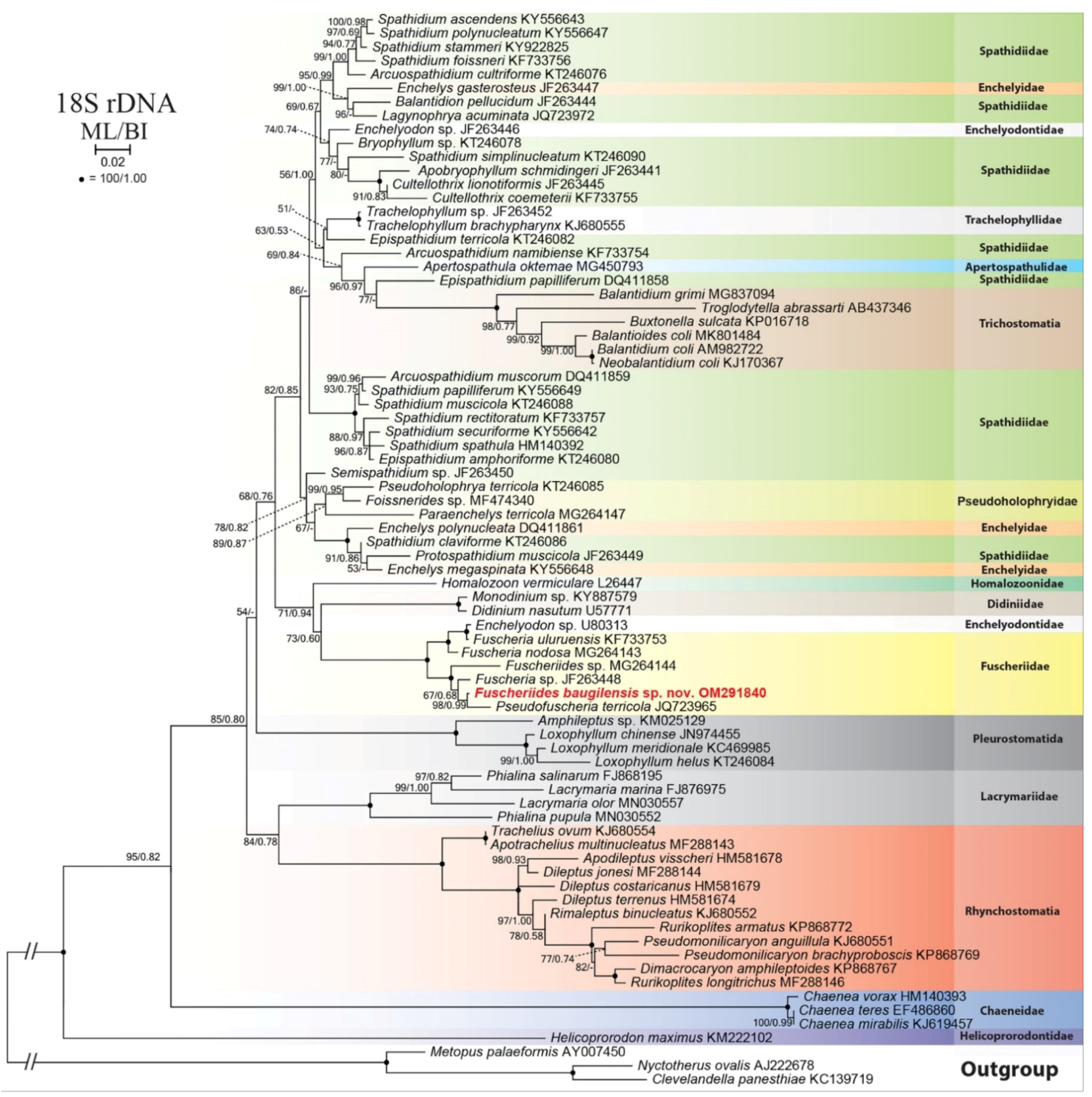
3.7. Phylogenetic Analysis of Fuscheriides baugilensis sp. nov.
4. Discussion
4.1. Morphological Comparison of Fuscheriides baugilensis sp. nov. with Similar Species
4.2. Phylogenetic Analyses
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foissner, W.; Agatha, S.; Berger, H. Part I: Text and line drawings. In Soil Ciliates (Protozoa, Ciliophora) from Namibia (Southwest Africa), with Emphasis on Two Contrasting Environments, the Etosha Region and the Namib Desert; Biologiezentrum der Oberösterreichischen Landesmuseums: Linz, Austria, 2002; Volume 5, pp. 1–1063. [Google Scholar]
- Gabilondo, R.; Foissner, W. Four new fuscheriid soil ciliates (Ciliophora: Haptorida) from four biogeographic regions. Acta Protozool. 2009, 48, 1–24. [Google Scholar]
- Foissner, W.; Foissner, I. The Fine Structure of Fuscheria terricola Berger et al., 1983 and a Proposed New Classification of the Subclass Haptoria Corliss, 1974 (Ciliophora, Litostomatea). Arch. Protistenkd. 1988, 135, 213–235. [Google Scholar] [CrossRef]
- Foissner, W. Faunistics, taxonomy and ecology of moss and soil ciliates (Protozoa, Ciliophora) from Antarctica, with description of new species, including Pleuroplitoides smithi gen. n., sp. n. Acta Protozool. 1996, 35, 95–123. [Google Scholar]
- Foissner, W. Gemeinsame Arten in der terricolen Ciliatenfauna (Protozoa: Ciliophora) von Australien und Afrika. Stapfia 1988, 17, 85–133. [Google Scholar]
- Foissner, W.; Berger, H. Terrestrial Ciliates (Protista, Ciliophora) from Australia and Some Other Parts of the World; Series Monographiae Ciliophorae; Consulting Engineering Office for Ecology: Salzburg, Austria, 2021; Volume 5, pp. i–xii, 1–380. [Google Scholar]
- Oertel, A.; Wolf, K.; Al-Rasheid, K.; Foissner, W. Revision of the Genus Coriplites Foissner, 1988 (Ciliophora: Haptorida), with Description of Apocoriplites nov. gen. and Three New Species. Acta Protozool. 2009, 47, 231–246. [Google Scholar] [PubMed]
- Dunthorn, M.; Stoeck, T.; Wolf, K.; Breiner, H.-W.; Foissner, W. Diversity and endemism of ciliates inhabiting Neotropical phytotelmata. Syst. Biodivers. 2012, 10, 195–205. [Google Scholar] [CrossRef]
- Rajter, Ľ.; Vďačný, P. Constraints on phylogenetic interrelationships among four Free-living litostomatean lineages inferred from 18S rRNA gene-ITS region sequences and secondary structure of the ITS2 molecule. Acta Protozool. 2017, 56, 255–281. [Google Scholar]
- VďAčný, P.; Bourland, W.A.; Orsi, W.; Epstein, S.S.; Foissner, W. Phylogeny and classification of the Litostomatea (Protista, Ciliophora), with emphasis on free-living taxa and the 18S rRNA gene. Mol. Phylogenet. Evol. 2011, 59, 510–522. [Google Scholar] [CrossRef]
- Vďačný, P.; Breiner, H.-W.; Yashchenko, V.; Dunthorn, M.; Stoeck, T.; Foissner, W. The Chaos Prevails: Molecular Phylogeny of the Haptoria (Ciliophora, Litostomatea). Protist 2014, 165, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jung, J.-H. Cytological staining of protozoa: A case study on the impregnation of hypotrichs (Ciliophora: Spirotrichea) using laboratory-synthesized protargol. Anim. Cells Syst. 2017, 21, 412–418. [Google Scholar] [CrossRef]
- Coats, D.W.; Heinbokel, J.F. A study of reproduction and other life cycle phenomena in planktonic protists using an acridine orange fluorescence technique. Mar. Biol. 1982, 67, 71–79. [Google Scholar] [CrossRef]
- Foissner, W. An update of ‘basic light and scanning electron microscopic methods for taxonomic studies of ciliated protozoa’. Int. J. Syst. Evol. Microbiol. 2014, 64, 271–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medlin, L.; Elwood, H.J.; Stickel, S.; Sogin, M.L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 1988, 71, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.-H.; Baek, Y.-S.; Kim, S.; Choi, H.-G.; Min, G.-S. A new marine ciliate, Metaurostylopsis antarctica nov. spec. (Ciliophora, Urostylida) from the Antarctic Ocean. Acta Protozool. 2011, 50, 289–300. [Google Scholar]
- Sonnenberg, R.; Nolte, A.W.; Tautz, D. An evaluation of LSU rDNA D1-D2 sequences for their use in species identification. Front. Zool. 2007, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [Green Version]
- Hall, T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Guindon, S.; Gascuel, O. A Simple, Fast, and Accurate Algorithm to Estimate Large Phylogenies by Maximum Likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Foissner, W. Infraciliatur, Silberliniensystem und Biometrie Einiger neuer und Wenig Bekannter Terrestrischer, Limnischer und Mariner Ciliaten (Protozoa: Ciliophora) aus den Klassen Kinetofragminophora, Colpodea und Polyhymenophora; Botanische Arbeitsgemeinschaft am O.Ö. Landesmuseum: Linz, Austria, 1984; Volume 12, pp. 1–165. [Google Scholar]
- Foissner, W. Two new soil ciliates (Protozoa, Ciliophora) from Namibia: Diplites telmatobius nov. gen., nov. spec. and. Apobryophyllum etoschense nov. spec. Quekett J. Microsc. 1998, 38, 207–218. [Google Scholar]
- Foissner, W. Taxonomische Studien über die Ciliaten des Großglocknergebietes (Hohe Tauern, Österreich) I. Familien Holophryidae, Prorodontidae, Plagiocampidae, Colepidae, Enchelyidae und Lacrymariidae nov. fam. Annln naturh. Mus. Wien 1983, 84/B, 49–85. [Google Scholar]
- Foissner, W. Terrestrial and Semiterrestrial Ciliates (Protozoa, Ciliophora) from Venezuela and Galápagos; Biologiezentrum: Linz, Austria, 2016; Volume 35, pp. 1–912. [Google Scholar]
- Berger, H.; Foissner, W.; Adam, H. Morphology and morphogenesis of Fuscheria terricola n. sp. and Spathidium muscorum (Ciliophora: Kinetofragminophora). J. Protozool. 1983, 30, 529–535. [Google Scholar] [CrossRef]
- Foissner, W.; O’Donoghue, P.J. Morphology and infraciliature of some freshwater ciliates (Protozoa: Ciliophora) from Western and South Australia. Invertebr. Taxon. 1990, 3, 661–696. [Google Scholar] [CrossRef]
- Huang, J.B.; Zhang, T.; Zhang, Q.; Li, Y.; Warren, A.; Pan, H.; Yan, Y. Further insights into the highly derived haptorids (Ciliophora, Litostomatea): Phylogeny based on multigene data. Zoȯl. Scr. 2018, 47, 231–242. [Google Scholar] [CrossRef]
- Jang, S.W.; Vďačný, P.; Shazib, S.U.A.; Shin, M.K. Morphology, ciliary pattern and molecular phylogeny of Trachelophyllum brachypharynx Levander, 1894 (Litostomatea, Haptoria, Spathidiida). Acta Protozool. 2015, 54, 123–135. [Google Scholar]
- Jiang, L.; Wang, C.; Zhuang, W.; Li, S.; Hu, X. Taxonomy, phylogeny, and geographical distribution of the little-known Helicoprorodon multinucleatum Dragesco, 1960 (Ciliophora, Haptorida) and key to species within the genus. Eur. J. Protistol. 2021, 78, 125769. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, T.; Wang, Y.; Katz Laura, A.; Gao, F.; Song, W. Disentangling sources of variation in SSU rDNA sequences from single cell analyses of ciliates: Impact of copy number variation and experimental error. Proc. R. Soc. B Boil. Sci. 2017, 284, 20170425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foissner, W.; Xu, K. Monograph of the Spathidiida (Ciliophora, Haptoria). Vol I: Protospathidiidae, Arcuospathidiidae, Apertospathulidae; Monographiae Biologicae Series; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; Volume 81, pp. 1–485. [Google Scholar] [CrossRef]
- Foissner, W.; Wolf, K.W.; Kumar, S.; Xu, K.; Quintela-Alonso, P. Five new spathidiids (Ciliophora: Haptoria) from Caribbean tank bromeliads. Acta Protozool. 2014, 53, 159–194. [Google Scholar]
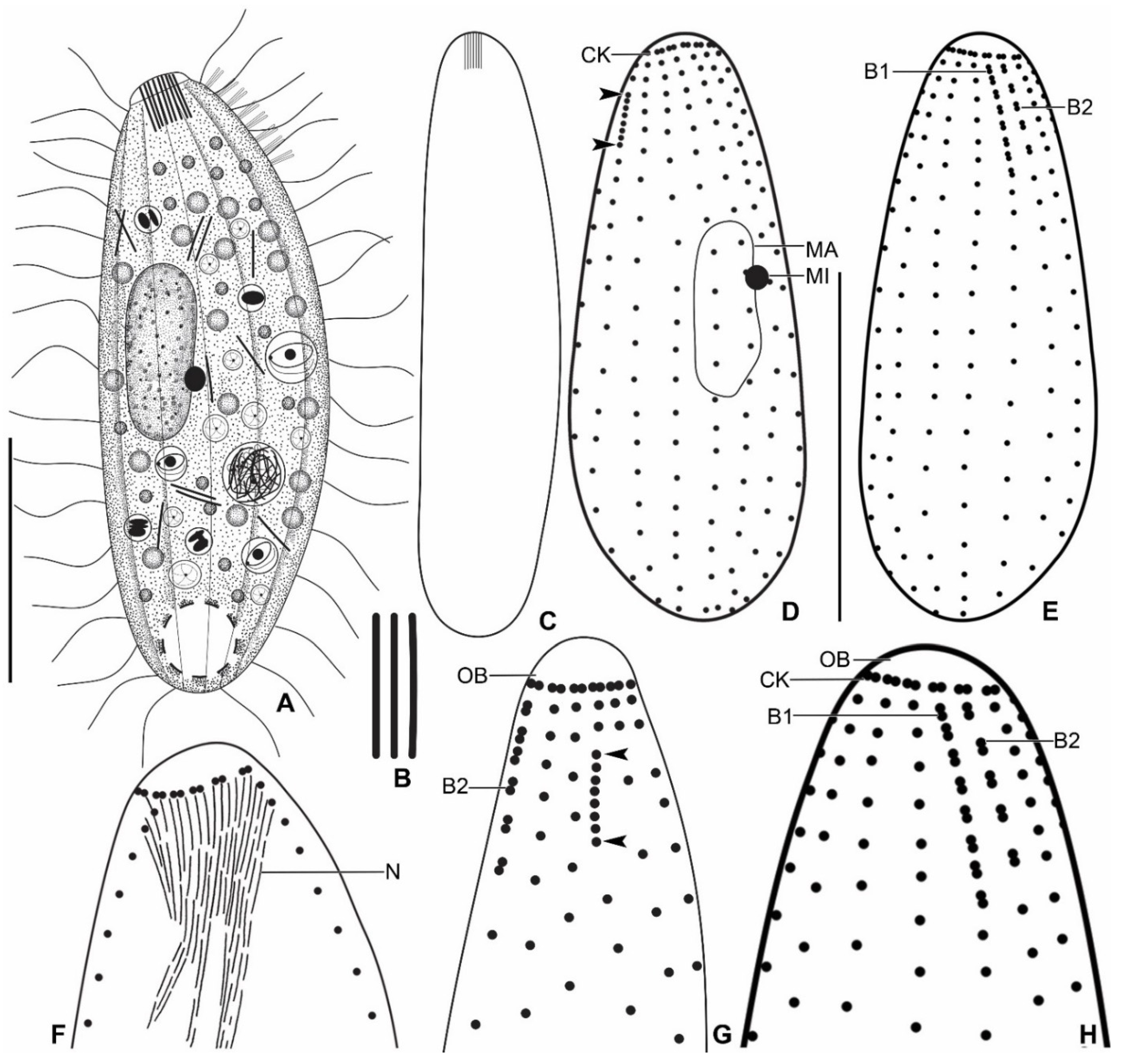
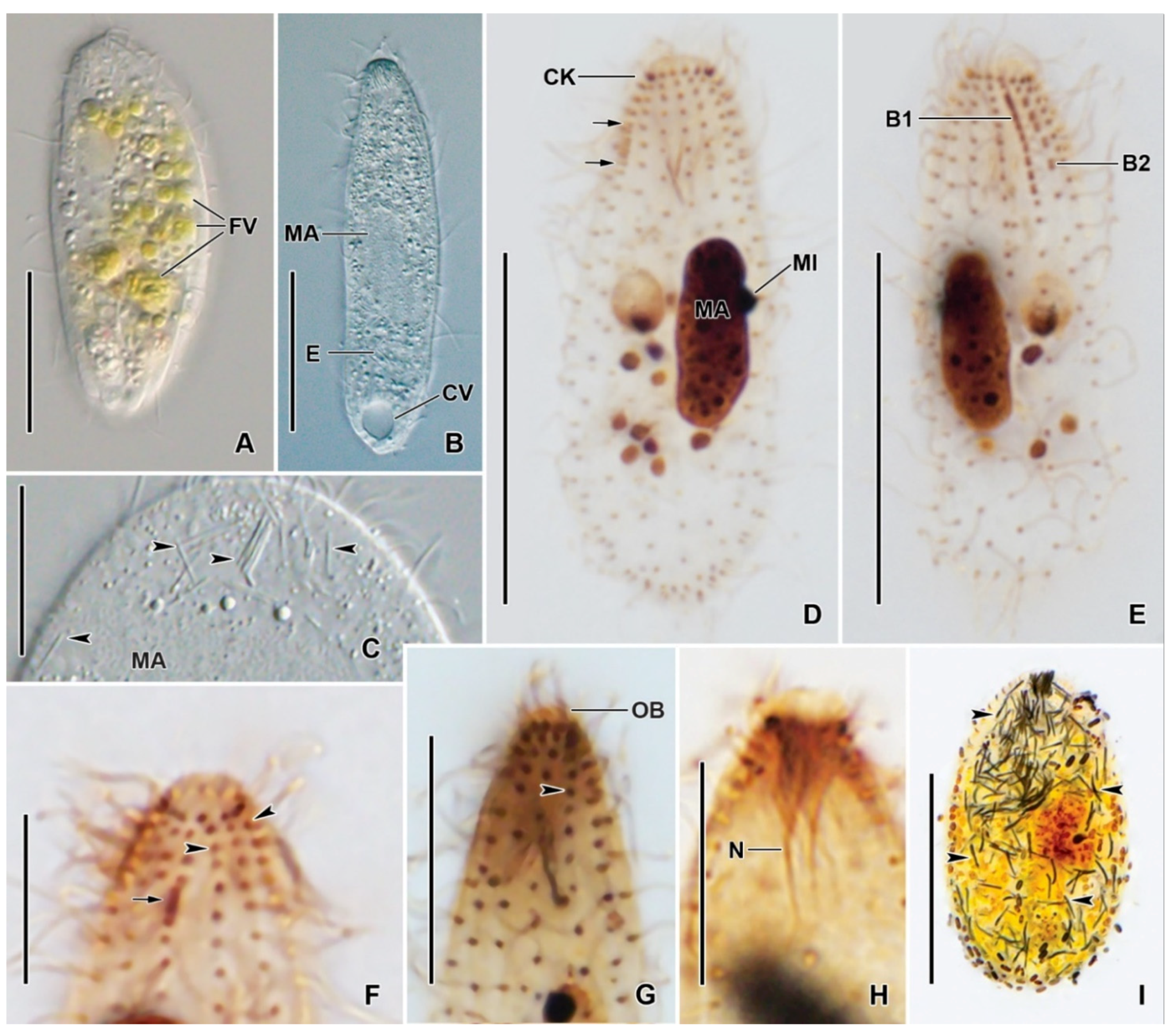
| Characteristics a | Mean | M | SD | SE | CV | Min | Max | n |
|---|---|---|---|---|---|---|---|---|
| Body, length | 29.0 | 28.9 | 3.6 | 0.8 | 12.4 | 21.3 | 36.2 | 22 |
| Body, width | 13.1 | 13.1 | 1.5 | 0.3 | 11.6 | 10.5 | 16.8 | 22 |
| Body length:width, ratio | 2.2 | 2.2 | 0.3 | 0.1 | 13.1 | 1.8 | 2.8 | 22 |
| Oral bulge, height | 1.2 | 1.2 | 0.2 | 0.0 | 13.9 | 0.9 | 1.5 | 14 |
| Oral bulge, width | 3.5 | 3.4 | 0.5 | 0.1 | 13.1 | 2.5 | 4.5 | 23 |
| Body width:oral bulge width, ratio | 3.6 | 3.6 | 0.3 | 0.1 | 8.8 | 2.9 | 4.2 | 14 |
| Circumoral kinety to macronucleus, distance | 8.5 | 7.9 | 2.9 | 0.6 | 34.7 | 4.9 | 17.0 | 21 |
| Macronucleus, length | 8.3 | 8.0 | 1.5 | 0.3 | 18.5 | 6.0 | 11.6 | 22 |
| Macronucleus, width | 5.4 | 5.3 | 0.7 | 0.2 | 13.2 | 4.3 | 6. 9 | 22 |
| Macronucleus length:width, ratio | 1. 6 | 1.5 | 0.4 | 0.1 | 23.7 | 1.1 | 2.7 | 22 |
| Macronucleus, number | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 23 |
| Micronucleus, length | 2.0 | 1.9 | 0.4 | 0.1 | 21.6 | 1.1 | 2.8 | 18 |
| Micromucleus, width | 1.6 | 1.5 | 0.4 | 0.1 | 22.0 | 1.1 | 2.3 | 18 |
| Micronucleus, number | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 18 |
| Somatic kineties, number | 15.2 | 16.0 | 1.0 | 0.2 | 6.6 | 14.0 | 16.0 | 23 |
| Circumoral dikinetids, number | 15.2 | 16.0 | 1.0 | 0.2 | 6.6 | 14.0 | 16.0 | 23 |
| Kinetids in mid-ventral kinety, number | 18.2 | 18.0 | 2.2 | 0.5 | 11.9 | 16.0 | 23.0 | 17 |
| Dorsal brush row 1, length | 5.9 | 6.0 | 0.7 | 0.2 | 11.7 | 4.4 | 6.6 | 11 |
| Dorsal brush row 1 dikinetid, number | 8.3 | 8.0 | 0.9 | 0.3 | 10.9 | 7.0 | 10.0 | 11 |
| Dorsal brush row 2, length | 4.9 | 4.9 | 0.8 | 0.2 | 15.8 | 4.0 | 6.2 | 11 |
| Dorsal brush row 2 dikinetid, number | 5.5 | 5.0 | 0.5 | 0.2 | 9.6 | 5.0 | 6.0 | 11 |
| Dorsal brush rows, number | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 11 |
| Subapical condensation, length | 2.8 | 2.6 | 0.6 | 0.1 | 19.9 | 1.8 | 3.9 | 21 |
| Kinetids in subapical condensation, number | 6.4 | 7.0 | 0.9 | 0.2 | 13.5 | 5.0 | 8.0 | 21 |
| Kinetids anterior to subapical condensation, number | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 21 |
| Nematodesmata, length | 9.2 | 9.0 | 1.2 | 0.3 | 13.3 | 7.7 | 11.5 | 14 |
| Characteristics | Fuscheriides baugilensis sp. nov. | Fuscheriides tibetensis | Pseudofuscheria terricola | P. magna | Fuscheria nodosa nodosa | Fuscheria nodosa salisburgensis | Fuscheria uluruensis |
|---|---|---|---|---|---|---|---|
| Body, length (μm) | 21–43 | 33–62 | 44–78 | 85–145 | 35–46 | 82–137 | 65–87 |
| Body, width (μm) | 10–18 | 5–17 | 12–28 | 33–95 | 18–26 | 45–97 | 45–62 |
| Macronucleus, numbers | 1 | 1 | 1 | 1 | 1 | 1 | 8–28 |
| Macronucleus, shape | Ellipsoidal | Reniform | Horseshoe-shaped | Oblong, curved oblong, or horseshoe | Horseshoe-shaped | Strand-shaped | Elongate ellipsoidal |
| Somatic kineties, number | 14–16 | 7 | 12–19 | 25–34 | 24–28 | 42–45 | 42–50 |
| Kinetids in ventral kinety, number | 16–23 | 11–27 | 12–45 | 40–80 | 20–35 | 31–66 | 41–88 |
| Brush rows, numbers | 2 | 2 | 2 | 2 | 2 | 2 (rarely 3) | 2 |
| Subapical condensation | Present | Present | Present | Present | Absent | Absent | Absent |
| Subapical condensation rows, number | 1 | 1 | 1 or 2 | 2 | - | - | - |
| Kinetids in front of condensation, number | 2 | 3 | 4 | 5–6 | - | - | - |
| Extrusomes, shape | Oblong to rod-shaped | Oblong | Nail-shaped | Nail-shaped | Nail-shaped | Nail-shaped | Nail-shaped |
| Extrusomes, size (μm) | ~4 × 0.3 | ~2 × 0.3 | ~4–7 | ~10 | ~10 | ~9–15 | |
| Habitat, country | Temporary pond after rainfall, Korea | Salty vegetation soil (10‰), South Tibet | Soil, Austria | Floodplain soil, Australia | Pond, Australia | Soil, Austria | Soil, Austria |
| Reference | Present study | [2] | [30] | [6] | [31] | [2] | [2] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.W.; Omar, A.; Nam, S.W.; Jung, J.-H. Morphology and Molecular Phylogeny of Fuscheriides baugilensis sp. nov. (Protozoa, Ciliophora, Haptorida) from South Korea. Diversity 2022, 14, 70. https://doi.org/10.3390/d14020070
Jang SW, Omar A, Nam SW, Jung J-H. Morphology and Molecular Phylogeny of Fuscheriides baugilensis sp. nov. (Protozoa, Ciliophora, Haptorida) from South Korea. Diversity. 2022; 14(2):70. https://doi.org/10.3390/d14020070
Chicago/Turabian StyleJang, Seok Won, Atef Omar, Seung Won Nam, and Jae-Ho Jung. 2022. "Morphology and Molecular Phylogeny of Fuscheriides baugilensis sp. nov. (Protozoa, Ciliophora, Haptorida) from South Korea" Diversity 14, no. 2: 70. https://doi.org/10.3390/d14020070
APA StyleJang, S. W., Omar, A., Nam, S. W., & Jung, J.-H. (2022). Morphology and Molecular Phylogeny of Fuscheriides baugilensis sp. nov. (Protozoa, Ciliophora, Haptorida) from South Korea. Diversity, 14(2), 70. https://doi.org/10.3390/d14020070







