Biodiversity of Coleoptera (Insecta) in the Middle and Lower Volga Regions (Russia)
Abstract
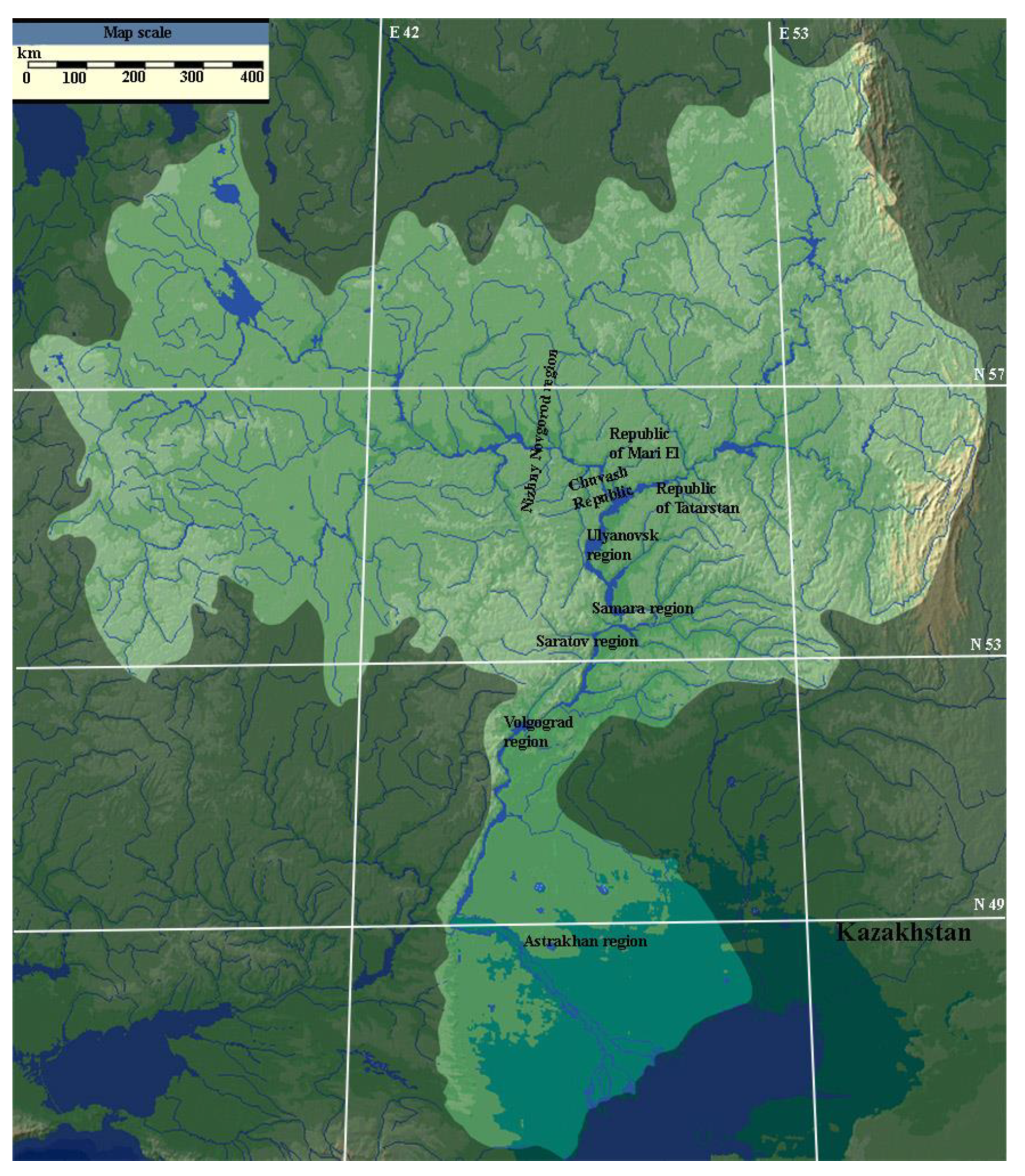
1. Summary
2. Data Description
2.1. Dataset Name
2.2. Figures, Tables and Schemes
3. Methods
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Didham, R.K.; Basset, Y.; Collins, C.M.; Leather, S.R.; Littlewood, N.A.; Menz, M.H.M.; Müller, J.; Packer, L.; Saunders, M.E.; Schönrogge, K.; et al. Interpreting insect declines: Seven challenges and a way forward. Insect Conserv. Divers. 2020, 13, 103–114. [Google Scholar] [CrossRef]
- Méndez, M.; Thomaes, A. Biology and conservation of the European stag beetle: Recent advances and lessons learned. Insect Conserv. Divers. 2021, 14, 271–284. [Google Scholar] [CrossRef]
- Wilson, M.C.; Chen, X.Y.; Corlett, R.T.; Didham, R.K.; Ding, P.; Holt, R.D.; Yu, M. Habitat fragmentation and biodiversity conservation: Key findings and future challenges. Landsc. Ecol. 2016, 31, 219–227. [Google Scholar] [CrossRef]
- Forister, M.L.; Pelton, E.M.; Black, S.H. Declines in insect abundance and diversity: We know enough to act now. Conserv. Sci. Pract. 2019, 1, e80. [Google Scholar] [CrossRef]
- Wagner, D.L. Insect declines in the anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef]
- Donkersley, P.; Ashton, L.; Lamarre, G.P.A.; Segar, S. Global insect decline is the result of wilful political failure: A battle plan for entomology. Ecol. Evolut. 2022, 12, e9417. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, C.A.; Zeegers, T.; van Klink, R.; Vermeulen, R.; van Wielink, P.; Spijkers, H.; van Deijk, J.; van Steenis, W.; Jongejans, E. Declining abundance of beetles, moths and caddisflies in the Netherlands. Insect Conser. Divers. 2020, 13, 127–139. [Google Scholar] [CrossRef]
- Schuch, S.; Meyer, S.; Bock, J.; van Klink, R.; Wesche, K. Drastic biomass loss in leafhopper and planthopper populations of various grasslands in Germany within six decades. Nat. Landschaft. 2019, 94, 141–145. [Google Scholar]
- Lindenmayer, D.; Burgman, M. Practical Conservation Biology; CSIRO: Collingwood, Australia, 2005. [Google Scholar]
- Cooke, S.J. Biotelemetry and biologging in endangered species research and animal conservation: Relevance to regional, national, and IUCN Red List threat assessments. Endang. Species Res. 2008, 4, 165–185. [Google Scholar] [CrossRef]
- Drivdal, L.; der Sluijs, J.P. Pollinator conservation requires a stronger and broader application of the precautionary principle. Curr. Opin. Insect Sci. 2021, 46, 95–105. [Google Scholar] [CrossRef]
- Harvey, D.J.; Gange, A.C.; Hawes, C.J.; Rink, M. Bionomics and distribution of the stag beetle, Lucanus cervus (L.) across Europe. Insect Conser. Divers. 2011, 4, 23–38. [Google Scholar] [CrossRef]
- Saunders, M.E. No simple answers for insect conservation. Am. Sci. 2019, 107, 148–151. [Google Scholar]
- Zamoroka, A.M. The longhorn beetles (Coleoptera, Cerambycidae) of Ukraine: Results of two centuries of research. Biosyst. Divers. 2022, 30, 46–74. [Google Scholar] [CrossRef] [PubMed]
- Polevoi, A.V. Fungus gnats (Diptera: Bolitophilidae, Diadocidiidae, Keroplatidae, Mycetophilidae) in the Kostomuksha State Nature Reserve, Russia. Nat. Conserv. Res. 2021, 6, 5–16. [Google Scholar] [CrossRef]
- Cardoso, P.; Erwin, T.L.; Borges, P.A.V.; New, T.R. The seven impediments in invertebrate conservation and how to overcome them. Biol. Conserv. 2011, 144, 2647–2655. [Google Scholar] [CrossRef]
- Sundukov, Y.N.; Makarov, K.V. The ground beetles of the tribus Trechini (Carabidae) on the Southern Kuril Islands. Nat. Conserv. Res. 2021, 6, 15–51. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuy, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Outhwaite, C.L.; Gregory, R.D.; Chandler, R.E.; Collen, B.; Isaac, N.J. Complex long-term biodiversity change among invertebrates, bryophytes and lichens. Nat. Ecol. Evolut. 2020, 4, 384–392. [Google Scholar] [CrossRef]
- Govorushko, S.M.; Nowicki, P. Lessons from insect conservation in Russia. J. Insect Conserv. 2019, 23, 1–14. [Google Scholar] [CrossRef]
- Dedyukhin, S.V. Phytophagous beetles (Coleoptera: Chrysomelidae and Curculionoidea), protected and recommended for protection in the regions of the Middle Volga and the Urals. Nat. Conserv. Res. 2020, 5, 1–27. [Google Scholar] [CrossRef]
- Shashkov, M.P.; Bobrovsky, M.V.; Shanin, V.N.; Khanina, L.G.; Grabarnik, P.Y.; Stamenov, M.N.; Ivanova, N.V. Data on 30-year stand dynamics in an old-growth broad-leaved forest in the Kaluzhskie Zaseki State Nature Reserve, Russia. Nat. Conserv. Res. 2022, 7, 24–37. [Google Scholar] [CrossRef]
- Ruchin, A.; Alekseev, S.; Egorov, L.; Artaev, O.; Semishin, G.; Esin, M. Ground beetle fauna (Coleoptera, Carabidae) of Mordovia State Nature Reserve (Russia). Biodivers. Data J. 2021, 9, e69807. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, F.; Callois, J.M. On the time lag between human activity and biodiversity in Europe at the national scale. Anthropocene 2021, 35, 100303. [Google Scholar] [CrossRef]
- Essl, F.; Dullinger, S.; Rabitsch, W.; Hulme, P.E.; Pyšek, P.; Wilson, J.R.U.; Richardson, D.M. Historical legacies accumulate to shape future biodiversity in an era of rapid global change. Divers. Distrib. 2015, 21, 534–547. [Google Scholar] [CrossRef]
- Grames, E.M.; Montgomery, G.A.; Boyes, D.H.; Dicks, L.V.; Forister, M.L.; Matson, T.A.; Nakagawa, S.; Prendergast, K.S.; Taylor, N.G.; Tingley, M.W.; et al. A framework and case study to systematically identify long-term insect abundance and diversity datasets. Conserv. Sci. Pract. 2022, 4, e12687. [Google Scholar] [CrossRef]
- Van Klink, R.; Bowler, D.E.; Driessen, M.M.; Ernest, S.K.M.; Gentile, A.; Gilbert, F.; Gongalsky, K.B.; Owen, J.; Peer, G.; Peer, I.; et al. InsectChange: A global database of temporal changes in insect and arachnid assemblages. Ecology 2021, 102, e03354. [Google Scholar] [CrossRef]
- Vasenkova, N.V.; Kuznetsova, N.A. A multiscale approach to evaluate the structure of diversity of Collembola in boreo-nemoral forests of the Russian Plain. Nat. Conserv. Res. 2022, 7, 38–51. [Google Scholar] [CrossRef]
- Salguero-Gómez, R.; Jackson, J.; Gascoigne, S.J.L. Four key challenges in the open-data revolution. J. Anim. Ecol. 2021, 90, 2000–2004. [Google Scholar] [CrossRef]
- Hughes, A.C.; Orr, M.C.; Ma, K.; Costello, M.J.; Waller, J.; Provoost, P.; Yang, Q.; Zhu, C.; Qiao, H. Sampling biases shape our view of the natural world. Ecography 2021, 44, 1259–1269. [Google Scholar] [CrossRef]
- Seebens, H.; Kaplan, E. DASCO: A workflow to downscale alien species checklists using occurrence records and to re-allocate species distributions across realms. NeoBiota 2022, 74, 75–91. [Google Scholar] [CrossRef]
- Egorov, L.; Alekseev, S.; Ruchin, A.; Sazhnev, A.; Artaev, O.; Esin, M.; Lobachev, E.; Lukiyanov, S.; Semenov, A.; Lukyanova, Y.; et al. Coleoptera (Insecta) in the Middle and Lower Volga regions (Russia). Joint Directorate of the Mordovia State Nature Reserve and National Park “Smolny”. 2022. Occurrence dataset. Available online: https://doi.org/10.15468/u4c9y5 (accessed on 12 November 2022).
- Orlova-Bienkowskaja, M.J.; Bieńkowski, A.O. Southern Range Expansion of the Emerald Ash Borer, Agrilus planipennis, in Russia Threatens Ash and Olive Trees in the Middle East and Southern Europe. Forests 2022, 13, 541. [Google Scholar] [CrossRef]
- Orlova-Bienkowskaja, M.J. (Ed.) . Inventory on Alien Beetles of European Russia; Mukhametov G.V.: Livny, Russia, 2019; p. 882. [Google Scholar]
- Komarov, E.V. New data on the fauna and distribution of beetles (Coleoptera) in the Lower Volga and Middle Don regions. Cauc. Entomol. Bull. 2020, 16, 35–38. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; Lobachev, E.A.; Lukiyanov, S.V.; Sazhnev, A.S.; Semishin, G.B. Expansion of Harmonia axyridis (Pallas, 1773) (Coleoptera: Coccinellidae) to European part of Russia in 2018–2020. Balt. J. Coleopterol. 2020, 20, 51–60. [Google Scholar]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A. Usage of fermental traps for the study of the species diversity of Coleoptera. Insects 2021, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Milkov, F.N. Middle Volga region. In Physical and Geographical Description; Publishing House of the USSR Academy of Sciences: Moscow, Russia, 1953; p. 262. [Google Scholar]
- Golub, V.B.; Tsurikov, M.N.; Prokin, A.A. Insect Collections: Collection, Processing and Storage of Material; KMK Scientific Press Ltd.: Moscow, Russia, 2021; p. 358. [Google Scholar]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A.; Vikhrev, N.E.; Esin, M.N. The use of simple crown traps for the insects collection. Nat. Conserv. Res. 2020, 5, 87–108. [Google Scholar] [CrossRef]
- Cai, C.; Tihelka, E.; Giacomelli, M.; Lawrence, J.F.; Ślipiński, A.; Kundrata, R.; Yamamoto, S.; Thayer, M.K.; Newton, A.F.; Leschen, R.A.B.; et al. Integrated phylogenomics and fossil data illuminate the evolution of beetles. R. Soc. Open Sci. 2022, 9, 211771. [Google Scholar] [CrossRef]
- McKenna, D.D.; Shin, S.; Ahrens, D.; Balke, M.; Beza-Beza, C.; Clarke, D.J.; Donath, A.; Escalona, H.E.; Friedrichh, F.; Letsch, H.; et al. The evolution and genomic basis of beetle diversity. Proc. Natl. Acad. Sci. USA 2019, 116, 24729–24737. [Google Scholar] [CrossRef]
- Löbl, I.; Smetana, A. (Eds.) . Catalogue of Palaearctic Coleoptera, Curculionoidea I.; Apollo Books: Stenstrup, Denmark, 2011; Volume 7, p. 373. [Google Scholar]
- Löbl, I.; Smetana, A. (Eds.) . Catalogue of Palaearctic Coleoptera, Curculionoidea II.; Apollo Books: Stenstrup, Denmark, 2013; Volume 8, p. 707. [Google Scholar]
- Löbl, I.; Löbl, D. (Eds.) Catalogue of Palaearctic Coleoptera, Revised and Updated Version; Hydrophiloidea–Staphylinoidea; Brill: Leiden, The Netherlands; Boston, MA, USA, 2015; Volume 2/1, p. 1702. [Google Scholar]
- Löbl, I.; Löbl, D. (Eds.) Catalogue of Palaearctic Coleoptera, Revised and Updated Version; Scarabaeoidea–Scirtoidea–Dascilloidea–Buprestoidea–Byrrhoidea; Brill: Leiden, The Netherlands; Boston, MA, USA, 2016; Volume 3, p. 983. [Google Scholar]
- Löbl, I.; Löbl, D. (Eds.) Catalogue of Palaearctic Coleoptera, Revised and Updated Version; Archostemata–Adephaga–Myxophaga; Brill: Leiden, The Netherlands; Boston, MA, USA, 2017; Volume 1, p. 1443. [Google Scholar]
- Iwan, D.; Löbl, I. (Eds.) Catalogue of Palaearctic Coleoptera; Revised and Updated Second Edition; Tenebrionoidea; Brill: Leiden, The Netherlands; Boston, MA, USA, 2020; Volume 5, p. 945. [Google Scholar]
- Danilevsky, M. (Ed.) Catalogue of Palaearctic Coleoptera; Updated and Revised Second Edition; Chrysomeloidea I (Vesperidae, Disteniidae, Cerambycidae); Brill: Leiden, The Netherlands; Boston, MA, USA, 2020; Volume 6/1, p. 712. [Google Scholar]
- Löbl, I.; Smetana, A. (Eds.) . Catalogue of Palaearctic Coleoptera, Elateroidea–Derodontoidea–Bostrichoidea–Lymexyloidea–Cleroidea–Cucujoidea; Apollo Books: Stenstrup, Denmark, 2007; Volume 4, p. 935. [Google Scholar]
- Löbl, I.; Smetana, A. (Eds.) . Catalogue of Palaearctic Coleoptera, Chrysomeloidae; Apollo Books: Stenstrup, Denmark, 2010; Volume 6, p. 924. [Google Scholar]
- Robertson, J.; Ślipiński, A.; Moulton, M.; Shockley, F.W.; Giorgi, A.; Lord, N.P.; McKenna, D.D.; Tomaszewska, W.; Forrester, J.; Miller, K.B.; et al. Phylogeny and classification of Cucujoidea and the recognition of a new superfamily Coccinelloidea (Coleoptera: Cucujiformia). Syst. Entomol. 2015, 40, 745–778. [Google Scholar] [CrossRef]
- Alonso-Zarazaga, M.A.; Barrios, H.; Borovec, R.; Bouchard, P.; Caldara, R.; Colonnelli, E.; Gültekin, L.; Hlaváč, P.; Korotyaev, B.; Lyal, C.H.C.; et al. Cooperative Catalogue of Palaearctic Coleoptera Curculionoidea. Monogr. ElectrÓN. SEA 2017, 8, 1–729. [Google Scholar]
- Bousquet, Y. Litteratura Coleopterologica (1758–1900): A guide to selected books related to the taxonomy of Coleoptera with publication dates and notes. ZooKeys 2016, 583, 1–776. [Google Scholar] [CrossRef]
| Column Label | Column Description |
|---|---|
| occurrenceID | An identifier for the occurrence (as opposed to a particular digital record of the occurrence) |
| basisOfRecord | The specific nature of the data record: HumanObservation |
| scientificName | The full scientific name, including the genus name and the lowest level of taxonomic rank with the authority |
| kingdom | The full scientific name of the kingdom in which the taxon is classified |
| decimalLatitude | The geographic latitude of location in decimal degree |
| decimalLongitude | The geographic longitude of location in decimal degrees |
| geodeticDatum | The ellipsoid, geodetic datum or spatial reference system (SRS) upon which the geographic coordinates given in decimalLatitude and decimalLongitude are based |
| country | The name of the country in which the location is found |
| countryCode | The standard code for the country in which the location is found |
| individualCount | The number of individuals present at the time of the occurrence |
| eventDate | The date when material from the trap was collected or the range of dates during which the trap collected material |
| year | The integer day of the month on which the event occurred |
| month | The ordinal month in which the event occurred |
| day | The integer day of the month on which the event occurred |
| recordedBy | A person, group or organization responsible for recording the original occurrence |
| identifiedBy | A list of names of the people who assigned the taxon to the subject |
| bibliographicCitation | A related resource that is referenced or pointed to by the described resource |
| Family | Number of Species | Number of Individuals |
|---|---|---|
| Gyrinidae | 5 | 49 |
| Haliplidae | 12 | 65 |
| Noteridae | 2 | 18 |
| Dytiscidae | 76 | 613 |
| Carabidae | 145 | 1441 |
| Scirtidae | 11 | 336 |
| Eucinetidae | 1 | 4 |
| Dascillidae | 1 | 1 |
| Byrrhidae | 3 | 11 |
| Buprestidae | 18 | 139 |
| Dryopidae | 2 | 4 |
| Elmidae | 3 | 23 |
| Limnichidae | 1 | 1 |
| Heteroceridae | 12 | 621 |
| Throscidae | 2 | 2 |
| Eucnemidae | 10 | 31 |
| Lycidae | 5 | 69 |
| Cantharidae | 20 | 402 |
| Elateridae | 50 | 869 |
| Drilidae | 1 | 3 |
| Lampyridae | 1 | 37 |
| Histeridae | 25 | 143 |
| Georissidae | 1 | 1 |
| Helophoridae | 4 | 12 |
| Hydrochidae | 4 | 8 |
| Hydrophilidae | 49 | 909 |
| Ptiliidae | 2 | 2 |
| Hydraenidae | 6 | 20 |
| Leiodidae | 16 | 58 |
| Staphylinidae | 74 | 719 |
| Trogidae | 1 | 2 |
| Lucanidae | 5 | 214 |
| Bolboceratidae | 1 | 5 |
| Geotrupidae | 3 | 38 |
| Scarabaeidae | 67 | 3963 |
| Dermestidae | 16 | 345 |
| Ptinidae | 14 | 234 |
| Byturidae | 2 | 30 |
| Biphyllidae | 1 | 3 |
| Cleridae | 6 | 57 |
| Trogossitidae | 4 | 11 |
| Melyridae | 9 | 282 |
| Lymexylidae | 1 | 1 |
| Mordellidae | 6 | 165 |
| Ripiphoridae | 1 | 1 |
| Scraptiidae | 5 | 169 |
| Oedemeridae | 8 | 184 |
| Mycteridae | 1 | 1 |
| Aderidae | 3 | 24 |
| Boridae | 1 | 2 |
| Pythidae | 1 | 1 |
| Salpingidae | 4 | 8 |
| Pyrochroidae | 3 | 41 |
| Meloidae | 6 | 13 |
| Anthicidae | 7 | 19 |
| Melandryidae | 15 | 83 |
| Zopheridae | 3 | 5 |
| Ciidae | 21 | 75 |
| Tetratomidae | 1 | 2 |
| Mycetophagidae | 10 | 139 |
| Tenebrionidae | 33 | 744 |
| Bothrideridae | 1 | 4 |
| Cerylonidae | 5 | 65 |
| Latridiidae | 18 | 839 |
| Corylophidae | 3 | 16 |
| Endomychidae | 4 | 27 |
| Coccinellidae | 43 | 674 |
| Erotylidae | 11 | 179 |
| Sphindidae | 3 | 69 |
| Monotomidae | 8 | 85 |
| Kateretidae | 5 | 12 |
| Nitidulidae | 25 | 7988 |
| Cryptophagidae | 7 | 63 |
| Cucujidae | 3 | 38 |
| Silvanidae | 6 | 18 |
| Phalacridae | 6 | 12 |
| Laemophloeidae | 5 | 9 |
| Orsodacnidae | 1 | 25 |
| Cerambycidae | 84 | 3359 |
| Chrysomelidae | 142 | 1194 |
| Cimberididae | 1 | 2 |
| Anthribidae | 6 | 18 |
| Attelabidae | 8 | 83 |
| Brentidae | 51 | 683 |
| Curculionidae | 202 | 2499 |
| Total | 1469 | 31,433 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egorov, L.V.; Alekseev, S.K.; Ruchin, A.B.; Sazhnev, A.S.; Artaev, O.N.; Esin, M.N.; Lobachev, E.A.; Lukiyanov, S.V.; Semenov, A.V.; Lukyanova, Y.A.; et al. Biodiversity of Coleoptera (Insecta) in the Middle and Lower Volga Regions (Russia). Diversity 2022, 14, 1128. https://doi.org/10.3390/d14121128
Egorov LV, Alekseev SK, Ruchin AB, Sazhnev AS, Artaev ON, Esin MN, Lobachev EA, Lukiyanov SV, Semenov AV, Lukyanova YA, et al. Biodiversity of Coleoptera (Insecta) in the Middle and Lower Volga Regions (Russia). Diversity. 2022; 14(12):1128. https://doi.org/10.3390/d14121128
Chicago/Turabian StyleEgorov, Leonid V., Sergei K. Alekseev, Alexander B. Ruchin, Aleksey S. Sazhnev, Oleg N. Artaev, Mikhail N. Esin, Evgeniy A. Lobachev, Sergei V. Lukiyanov, Anatoliy V. Semenov, Yulia A. Lukyanova, and et al. 2022. "Biodiversity of Coleoptera (Insecta) in the Middle and Lower Volga Regions (Russia)" Diversity 14, no. 12: 1128. https://doi.org/10.3390/d14121128
APA StyleEgorov, L. V., Alekseev, S. K., Ruchin, A. B., Sazhnev, A. S., Artaev, O. N., Esin, M. N., Lobachev, E. A., Lukiyanov, S. V., Semenov, A. V., Lukyanova, Y. A., Shulaev, N. V., & Litvinov, K. V. (2022). Biodiversity of Coleoptera (Insecta) in the Middle and Lower Volga Regions (Russia). Diversity, 14(12), 1128. https://doi.org/10.3390/d14121128







