Abstract
Mycorrhizal specificity, i.e., the range of fungi allowing mycorrhizal partnerships, differs among orchid species, but that at early developmental stages is unclear. We investigated whether mycorrhizal specificity during seed germination and seedling development differs among three Dendrobium species, D. officinale, D. okinawense and D. moniliforme, in vitro. Nine mycorrhizal fungal strains were obtained from the roots of these species and cultured with a seed of each Dendrobium species. Five to eight fungal strains stimulated seed germination, whereas one to four fungal isolates significantly promoted protocorm development in the three species. To evaluate effects on leafy seedling growth, seedlings obtained from asymbiotic culture were cultured with nine fungal isolates. D. officinale and D. okinawense showed specificity for a single Serendipitaceae or Tulasnellaceae isolate, whereas D. moniliforme exhibited specificity for three isolates of Serendipitaceae and Tulasnellaceae. Therefore, the three Dendrobium species had a growth bottleneck from seed germination to the protocorm stage, and mycorrhizal specificity of protocorm growth and seedling development in vitro varied among the species. Our findings imply divergent mycorrhizal specificity in Dendrobium species at early developmental stages. This study provides insights into the diversity of orchid mycorrhizal specificity, as well as valuable information for conservation of endangered orchids.
1. Introduction
Orchidaceae is one of the largest plant families with >28,000 species []. All orchids need mycorrhizal fungi to provide nutrients for seed germination and early seedling development because orchid seeds are tiny, lack endosperm, and have minimal nutrient reserves []. Most orchids establish photosynthesis after leafing but remain reliant on mycorrhizal fungi for nutrients []. Most orchids form associations with three basidiomycete families: Tulasnellaceae, Serendipitaceae, and Ceratobasidiaceae []. Mycorrhizal specificity, i.e., the range of fungi allowing mycorrhizal partnerships, differs among orchid species from low (generalist; many mycorrhizal fungal taxa) to high (specialist; few taxa) []. The mycorrhizal specificity of orchids ontogenetically changes from juvenile to adult []. Although mycorrhizal specificity is diverse in adult-stage orchids [], in early developmental stages, it is unclear. Orchid seeds germinate and continue growing in the presence of suitable mycorrhizal fungi; therefore, fungi that promote seed germination and seedling growth mediate orchid establishment and survival []. Clarifying the mycorrhizal association at early developmental stages would provide insight into the diversity of orchid mycorrhizal specificity and elucidate orchid distributions. In addition, many orchid species are endangered due to habitat destruction and over-exploitation []. Understanding the mycorrhizal fungi involved in seed germination and seedling growth can provide valuable information for orchid conservation.
During orchid germination, the swollen embryo develops into a unique globular structure called the protocorm, which in turn develops into plantlets with leaves and roots (seedlings) in green orchids []. A variety of fungi can stimulate seed germination, but few provide sufficient nutrients for protocorm development []. This imposes a growth bottleneck between seed germination and the protocorm stage. Orchid-seed germination is dependent on mycorrhizal fungi for carbon resources []. However, after leaves develop, plants obtain carbon by photosynthesis, but depend on mycorrhizal fungi for other nutrients such as minerals and moisture from soil []. Because plants’ physiological needs differ from the protocorm to the leafy seedling stages, the fungi required for development may also change []. Thus, orchid mycorrhizal specificity during early developmental stages needs to be evaluated individually at the seed germination, protocorm growth, and leafy seedling development stages. In vitro seed germination tests, i.e., pure cultures of sterilized seeds and a fungal isolate, are used to identify fungal taxa linked to seed germination and protocorm growth []. Furthermore, inoculation of a fungal isolate on an asymbiotic leafy seedling enables identification of fungal taxa that promote seedling development. Although in vitro seed germination tests have been applied to a variety of orchid species [,], few studies have evaluated leafy seedling development [].
The genus Dendrobium, one of the most species-rich genera of vascular plants [], comprises 1826 species of epiphytic orchids distributed across Asia and Australia [,]. Dendrobium species have high medicinal and ornamental value [,], and some are extremely endangered []. Mycorrhizal specificity varies among adult individuals of Dendrobium species; populations of D. fimbriatum are associated with a single fungal group, and those of D. officinale with four to five fungal groups []. Eight coexisting Dendrobium species showed distinct mycorrhizal communities []. However, the extent of the differences in mycorrhizal specificity among Dendrobium species at early developmental stages is unclear. Although numerous studies have evaluated strains that promote Dendrobium seed germination [,], most examined a single Dendrobium species [,]; few evaluated the mycorrhizal specificity of multiple Dendrobium species []. Because Dendrobium is a highly diversified plant genus, comparison with closely related species will provide insight into the diversification of mycorrhizal specificity during plant speciation.
In this study, we evaluated mycorrhizal specificity during in vitro seed germination and advanced seedling development in three Dendrobium species (D. officinale, D. okinawense, and D. moniliforme) native to Japan. D. officinale is distributed from southern China to southern Japan, mostly in subtropical regions []. D. moniliforme is widely distributed from the Himalayas to Indochina, and from southern China to Japan; it is mostly found in warm temperate regions []. By contrast, D. okinawense is distributed only in Okinawa and Taiwan Islands, in subtropical regions []. Takamiya et al. (2014) demonstrated that these three species belong to the Dendrobium section Dendrobium, and that D. okinawense and D. moniliforme are sister taxa; D. officinale are closely related to these two taxa []. This allows investigation of mycorrhizal specificity among closely related species. Adult plants of D. okinawense have a highly specific association with a single Tulasnellaceae group [], whereas those of D. officinale are associated with multiple Tulasnellaceae and Serendipitaceae groups []. Because mycorrhizal specificity at the adult stage differs between the two Dendrobium species, their specificity at early developmental stages may also differ.
In this study, to investigate whether mycorrhizal specificity at early developmental stages differs among D. officinale, D. okinawense, and D. moniliforme, seeds of the three species were cultured with nine fungal isolates obtained from these three species. Furthermore, to evaluate the effect of fungal isolates on seedling growth after leafing, seedlings from asymbiotic culture were cultured with nine fungal isolates. We examined compatible fungal isolates during three early developmental stages—seed germination, protocorm growth and seedling development—and compared in vitro mycorrhizal specificity among the three Dendrobium species.
2. Materials and Method
2.1. Sample Collection
Roots of D. officinale, D. okinawense, and D. moniliforme were collected from natural habitats or cultivated plants in Japan. We sampled three individuals from two wild populations of D. officinale in Kagoshima Prefecture, and one wild individual of D. okinawense in Okinawa Prefecture. Roots of D. moniliforme were collected from one individual from a wild population in Kagoshima Prefecture and one cultivated individual from Shizuoka Prefecture. Samples were stored in polyethylene bags mixed with humid moss/paper with labels and transferred to the laboratory for fungal isolation.
Seeds of D. officinale were obtained from three mature capsules from three individuals in Kagoshima Prefecture. Seeds of D. okinawense were collected from two mature capsules from one individual cultivated in a greenhouse at Okinawa Churashima Foundation. Seeds of D. moniliforme were obtained from three mature capsules from three individuals cultivated in a private garden in Shizuoka Prefecture. Capsules of the same species were mixed and used for symbiotic germination testing.
2.2. Fungal Isolation and Molecular Identification
Collected roots were washed with tap water, and mycorrhizal colonization was evaluated by microscopy using hand sections of roots. Colonized cortex layers of mycorrhizal roots were excised under a stereomicroscope, rinsed three times with sterile water, and cut open under sterile water to release the fungal pelotons. Sterile water mixed with pelotons was dropped onto water agar medium with 50 ppm each of streptomycin and tetracycline. Plates were incubated at 25 ± 1 °C in darkness for 3 days. Fungal colonies from actively growing isolates were subcultivated onto a potato dextrose agar (PDA) dish for purification.
DNA was extracted from the fungal isolates following the method of Izumitsu et al. (2012) []. Internal transcribed spacer (ITS) sequences of nuclear ribosomal DNA were amplified using the primer pairs ITS5/TW13. PCR amplification and sequencing were carried out as described by Rammitsu et al. (2021) []. PCR products were analyzed using the BigDye v. 3.1 terminator system [] and 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. ITS sequences were analyzed by BLAST search against the GenBank sequence database to find the closest matching sequence. The ITS sequences of fungal isolates were submitted to the DNA Data Bank of Japan (DDBJ) (Table 1). All ITS sequences were assigned to operational taxonomic units (OTUs) defined by 97% sequence similarity. A total of nine isolates (including seven OTUs) were obtained (Table 1). To compare the effects of different isolates within the same OTU, two isolates were used for SE1 and CE18. SE1A and CE18A have 99.0% and 99.4% sequence similarity with SE1B and CE18B, respectively. FU1 showed 97% sequence similarity with Fusarium oxysporum (MT447552), which forms fungal coils in Dendrobium candidum root cells []. The isolates were deposited in the Biological Resource Center of the National Institute of Technology and Evaluation (NBRC) (Table 1).

Table 1.
Fungal isolates from Dendrobium officinale, D. okinawense, and D. moniliforme used for symbiotic culture.
2.3. In Vitro Symbiotic Germination
Mature capsules were sterilized using 75% ethanol and dried for 1 week using silica gel desiccant until nearly ruptured. Only seeds from nearly dehisced capsules were collected, to ensure that they were mature, and stored at 5°C until use. Prior to use, the seeds were evaluated using the 2,3,5-triphenyl tetrazolium chloride (TTC) test to ensure high viability (>90%). A PDA medium in a 9-cm-diameter Petri dish was inoculated with a 5 × 5 mm agar cube of fungal inoculum in the middle of the Petri dish as a pre-culture and incubated in darkness at 25 ± 1 °C for 7 days. Seeds were sterilized for 3 min with 1% sodium hypochlorite solution, and about 100 seeds were sown onto the surface of an oatmeal agar medium (OMA; 2.5 g/L oatmeal and 15 g/L agar) in Petri dishes and incubated at 25 ± 1 °C for 1 week to check for contamination. Next, 1 × 1 cm discs were cut off (5–10 seeds per disc) and transplanted to a new OMA medium. A total of 20 seeds on 2–4 discs were placed on the OMA medium. Discs were placed at the same distance from the center, so that the seeds contacted the mycelium elongating from the center at the same time. Five replicates (100 seeds) were used for each fungal treatment. Petri dishes without a fungal inoculum were the control. A 6 mm plug of fungal culture was inoculated at the center of the Petri dish with the OMA medium and incubated under a 12/12 h light/dark photoperiod at 25 ± 1 °C.
After 3 months of incubation, the number of seeds was counted under a stereomicroscope. Seed development was classified as follows: stage, 0 no germination (Figure 1a); stage 1, an enlarged embryo with ruptured seed coat (Figure 1b); stage 2, a globular embryo (protocorm) with rhizoids (Figure 1c); stage 3, a protocorm with an apical meristem (Figure 1d); stage 4, emergence of the first leaf (seedling formation) (Figure 1e); and stage 5, a seedling with a second leaf and further development (Figure 1f). The seed germination (%) per stage was calculated using the following formula: Percentage seed germination = (number of seeds per germination stage ÷ total number of viable seeds) × 100. The germination stage corresponded to stage 2 and above. The standardized growth index (GI) modified from Spoerl (1948) [] was calculated as follows: GI = (N1 + N2 × 2 + N3 × 3 + N4 × 4 + N5 × 5) ÷ (N0 + N1 +N2 + N3 + N4 + N5), where N0 is the number of seeds at stage 0, N1 is the number at stage 1, etc. []. The GI can range from 0 (no seeds germinated) to 5 (all seeds reached the seedling stage).
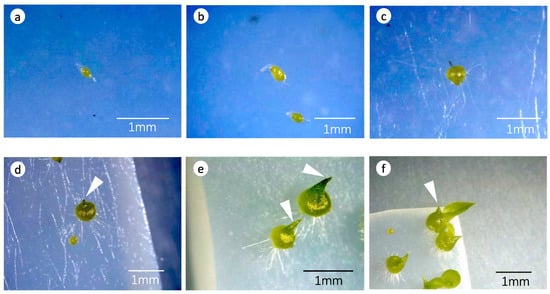
Figure 1.
The six developmental stages of germinating Dendrobium officinale seeds. (a) Stage 0: no germination; (b) stage 1: an enlarged embryo with ruptured seed coat; (c) stage 2: a globular embryo (protocorm) with rhizoids; (d) stage 3: a protocorm with an apical meristem (arrow); (e) stage 4: emergence of the first leaf (arrow) (seedling formation); and (f) stage 5: a seedling with a second leaf (arrow) and further development.
2.4. In Vitro Symbiotic Culture of Seedlings
Seedlings after leafing obtained from asymbiotic culture were cultured with the nine fungal isolates. Seeds were sterilized with 1% sodium hypochlorite solution for 3 min and transferred to a new Dogashima medium (NDM) []. Seedlings at stage 5 were used for symbiotic culture. Fungal isolates were pre-cultured on PDA in darkness for 10 days. Four seedlings and one colonized agar plug (diameter = 6 mm) from the edge of the fungal colony were transferred to a modified oatmeal agar medium (ONY; 0.38 g/L NH4NO3, 0.2 g/L KH2PO4, 0.1 g/L MgSO4·7H2O, 0.1 g/L KCl, 0.1 g/L yeast extract, 2.5 g/L oatmeal, and 15 g/L agar). Each glass bottle (450 mL) contained 50 mL of ONY medium, and five glass bottles were used per treatment. The control lacked a fungal isolate. The cultures were grown at 25 ± 1 °C under a 12 h/12 h light/dark photoperiod. After 3 months, the plant height, the longest root length, and the number of roots, leaves, and tillers were measured per plant. Seedlings were dried at 50°C for 48 h for fresh and dry weight measurements [].
2.5. Statistical Analysis
The effects of fungal isolates on seed germination and seedling development (GI, fresh and dry weight, plant height, root number, longest root, leaf number and tiller number) were statistically compared within each Dendrobium species using SPSS Statistics 27.0.1 (IBM Corp., Armonk, NY, USA) software. For seed germination testing, each treatment was replicated on five plates. Twenty plants per treatment were used for seedling growth testing. The data were analyzed by one-way analysis of variance (ANOVA), followed by the Tukey-Kramer test (p < 0.05).
3. Results
3.1. Effects of Fungal Strains on Symbiotic Germination
Seeds of D. officinale, D. okinawense, and D. moniliforme were cultured symbiotically with nine fungal isolates (three Tulasnellaceae, three Serendipitaceae, two Ceratobasidiaceae, and one Fusarium) (Table 1). Several OTU isolates promoted germination (Figure 2a), but fewer significantly increased the GI value in all three Dendrobium species (Figure 2b). For D. officinale, the TU22, TU27, SE1A, SE1B, and SE2 isolates promoted seed germination (Figure 2a), four of which significantly increased the GI value (Figure 2b). Seed germination of D. okinawense was significantly promoted by all isolates except for FU1 (Figure 2a), but only isolate SE2 significantly increased the GI value (Figure 2b). In D. moniliforme, the TU22, TU27, SE1B, SE2, and CE18A isolates promoted seed germination (Figure 2a), two of which (TU22 and SE2) had high GI values (Figure 2b).
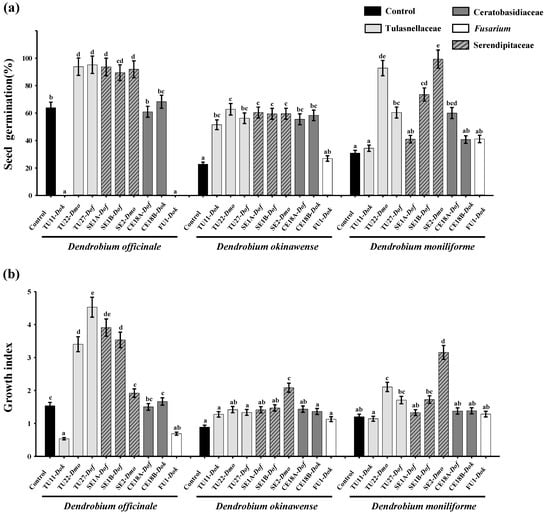
Figure 2.
(a) Seed germination (%) and (b) seedling growth index (GI) of fungal isolates on Dendrobium officinale (Dof), D. okinawense (Dok) and D. moniliforme (Dmo) at 3 months after symbiotic culture. The host orchid is indicated after the fungal isolate name. Data are means of five replicates; bars indicate standard errors. Different letters denote significant differences according to the Tukey-Kramer test at the p < 0.05 level.
In D. officinale and D. moniliforme, the highest GI values resulted from the fungal isolates from their own roots (Figure 2b), whereas the isolate with the highest GI in D. okinawense, SE2, was isolated from D. moniliforme. In D. officinale, among four isolates with a high GI value (TU22, TU27, SE1A, and SE1B), three (TU27, SE1A, and SE1B) were from D. officinale; the isolate with the highest GI was TU27. D. moniliforme had two isolates with high GI values (TU22 and SE2), both of which were isolated from D. moniliforme. The effects of fungal isolates within the same OTU were compared between SE1 and CE18. Isolates within the same OTU showed similar effects, except that SE1B promoted seed germination of D. moniliforme more so than SE1A (Figure 2a). FU1 showed no positive effect in any of the Dendrobium species. In the control, no development beyond stage 2 was observed in any of the three Dendrobium species.
3.2. Effects of Fungal Strains on Symbiotic Cultures of Seedling
Seedling growth of D. officinale, D. okinawense, and D. moniliforme seedlings differed according to fungal strain (Figure 3 and Supplementary Figure S1). In D. officinale, seedlings inoculated with SE1B showed the highest fresh and dry weights with a significant difference in fresh weight seen compared with the other types of seedlings (Figure 3a,b). The number of roots was greatest after TU22 inoculation (Figure 3d). For D. okinawense, TU11 inoculation significantly increased the fresh weight and plant height and resulted in the highest dry weight (Figure 3a–c). Seedlings cultured with SE2 had the largest number of roots (Figure 3d). In D. moniliforme, TU22 significantly increased fresh weight, dry weight, plant height, and root number (Figure 3). TU27 and SE2 also increased seedling fresh weight, plant height, and leaf number (Figure 3a,c and Supplementary Figure S1).
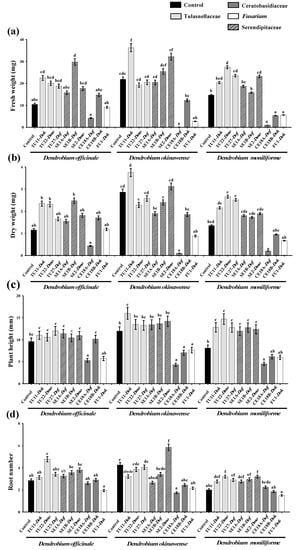
Figure 3.
Effect of fungal isolates on the growth of asymbiotic cultured seedlings of Dendrobium officinale (Dof), D. okinawense (Dok), and D. moniliforme (Dmo) at three months after symbiotic culture. Fresh weight (a) and dry weight (b), plant height (c), and number of roots (d). Host orchid is indicated after the fungal isolate name. Bars show standard errors (n = 20 plants per treatment). Mean values with different letters are significantly different at p < 0.05 (Tukey-Kramer test).
Fungal isolates with an increased fresh/dry weight varied in whether they promoted above-or belowground growth. In D. okinawense, TU11, which showed the highest fresh/dry weight, markedly increased plant height but other indices were not promoted (Figure 3 and Supplementary Figure S1). In D. moniliforme, TU22 and TU27 significantly promoted all indices, including plant height, root number, longest roots, leaf number, and tiller number. On the other hand, SE1B, which induced the highest fresh/dry weight, showed no promotive effects on any of the other indices in D. officinale.
The most effective fungal isolates for D. officinale, D. okinawense, and D. moniliforme originated from their own roots. SE1B isolated from D. officinale increased the fresh weight of D. officinale (Figure 3a). TU11 from D. okinawense had the greatest effect on the fresh weight and plant height of D. okinawense (Figure 3a,c). Two fungal strains, TU22 and SE2, from D. moniliforme significantly increased the fresh weight of D. moniliforme (Figure 3a). Fungal isolates within the same OTU had similar growth-promoting effects, except that SE1B exerted a greater effect on D. officinale growth than SE1A (Figure 3). Also, CE18 and FU1 showed no positive effect, and sometimes a negative effect, on seedling development. The inoculation of CE18A caused seedling death in D. okinawense and D. moniliforme.
4. Discussion
Three Dendrobium species showed a growth bottleneck during seed germination, and the fungal isolates that affected protocorm growth and seedling development varied among them (Figure 4). A variety of fungal isolates stimulated seed germination, but few promoted protocorm and seedling development (Figure 4). Five isolates (TU22, TU27, SE1A, SE1B, and SE2) contributed to D. officinale seed germination, four of which (TU22, TU27, SE1A, and SE1B) increased the GI value (Figure 2). However, only SE1B significantly increased seedling fresh weight (Figure 3a). Decreasing fungal diversity during development was found in D. moniliforme: five isolates (TU22, TU27, SE1B, SE2, and CE18A) promoted seed germination, whereas two (TU22, and SE2) and three (TU22, TU27, and SE2) isolates promoted protocorm growth and seedling development, respectively (Figure 2, Figure 3 and Figure 4). The largest bottleneck was in D. okinawense: eight fungi (TU11, TU22, TU27, SE1A, SE1B, SE2, CE18A, CE18B) promoted seed germination, but only one (SE2 or TU11) accelerated protocorm and seedling development (Figure 2, Figure 3 and Figure 4).
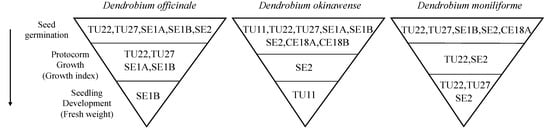
Figure 4.
Fungal strains that affect early developmental stages (seed germination, protocorm growth, and seedling development) of Dendrobium officinale, D. okinawense, and D. moniliforme. Fungal isolates that significantly increased seed germination (%) and growth index (GI) values are classified as fungi promoting seed germination and protocorm growth, respectively. For the seedling developmental stage, fungal isolate(s) that significantly increased fresh weight are shown.
A similar bottleneck was found in previous studies on Dendrobium species. Only two of seven Tulasnella isolates promoted seedling development in D. moniliforme []. Two of six fungal strains, Serendipitaceae and Tulasnella calospora, promoted rapid seed germination up to the seedling stage in D. officinale []. Similar bottlenecks have been reported in D. aphyllum [], D. devonianum [], D. exile [], and D. huoshanense []. Therefore, bottlenecking may be common among Dendrobium species. A taxonomic bottleneck during seed germination has been documented in other orchids, such as Cephalanthera [], Bletilla striata [], and Cyrtopodium glutiniferum []. Because orchid seeds have scant nutrient reserves, orchid-seed germination depends on mycorrhizal fungi for mineral and carbon resources []. A variety of fungi can stimulate seed germination, but few provide sufficient nutrients for subsequent protocorm growth, resulting in a major bottleneck. Stimulation of seed germination by diverse fungi may increase plant fitness. Swelling of an embryo and the development of rhizoids expand the surface area, resulting in a higher probability of encountering a growth-promoting fungus.
The same fungal OTUs contributed to D. officinale and D. moniliforme development at three developmental phases, whereas the fungal strain shifted from SE2 to TU11 during seedling development in D. okinawense (Figure 4). In D. officinale, SE1B supports plant growth from seed germination to seedling development. This fungal isolate shared 97% ITS sequence similarity to the fungal isolate Thanatephorus sp. SSCDO-8 (MH348617), which reportedly promotes seed germination, protocorm growth, and seedling development in D. officinale []. In D. moniliforme, TU22 and SE2 showed positive effects in all three developmental phases (Figure 4). The fungal OTU, SE1, is a dominant fungal partner for adult D. officinale individuals in wild populations [], and associations with fungal strains that contribute to growth in the early developmental stage may remain throughout the orchid life cycle. In D. okinawense, the effective fungal strain shifted from SE2 to TU11 during protocorm and seedling development (Figure 4). Eleven mature plants of D. okinawense from natural habitats were predominantly associated with a single Tulasnellaceae OTU, TU11 []. Therefore, the association of D. okinawense with TU11 continues to the adult stage. Switching of mycorrhizal fungi between juvenile to adult plants has been reported in other orchid species, such as Oeceoclades maculata []. Orchid-seed germination is dependent on mycorrhizal fungi for carbon resources, but plants obtain carbon by photosynthesis after leaf development []. Because plant physiological needs differ from the juvenile to adult stage, the fungi required for development may also change. In D. okinawense, SE2 likely contributes to carbon supply during early developmental stages, whereas TU11 provides the nutrients needed for post-leafing seedling development. Orchids with fungal switching may have a higher risk of mortality than non-switching orchids because they encounter other compatible fungi during growth. D. okinawense is distributed only on the islands of Okinawa and Taiwan. In addition, adult individuals of D. okinawense have a highly specific association with TU11 []. Fungal switching and narrow specificity might explain the rarity of D. okinawense.
Our findings imply that D. okinawense and D. officinale, adult plants which differ in mycorrhizal specificity, also differ in specificity at early developmental stages. At the adult stage, D. okinawense shows high mycorrhizal specificity toward TU11 [], whereas D. officinale is associated with multiple Tulasnellaceae and Serendipitaceae fungi []. In this study, D. okinawense showed higher specificity in vitro than D. officinale at early developmental stages. Only one fungal isolate significantly promoted protocorm growth in D. okinawense, compared to four in D. officinale (Figure 4). Dendrobium species with high mycorrhizal specificity at the mature stage could also have high specificity at juvenile stages. The effective fungal isolate at the seedling development stage differed between D. officinale and D. okinawense (Figure 4). SE1 and TU11 were the dominant fungal partners for adult plants of D. officinale and D. okinawense, respectively [,]. Fungal strains that promote the development of leafy seedlings may also dominate during adult stages.
The results of our symbiotic seedling culture imply that an increase in above- or below-ground biomass is different among fungal species. In D. okinawense, TU11 promoted only plant height, while TU22 and TU27 facilitated all indices in D. moniliforme (Figure 3 and Supplementary Figure S1). The lack of increase in any indices except fresh/dry weight caused by the inoculation of D. officinale with SE1B may be due to an increase in other indices that were not examined in this study, such as root or stem thickness. An increase in aboveground biomass may increase photosynthesis while an increase in belowground biomass may expand nutrient acquisition from the roots and/or mycorrhizal associations. It is possible that fungal species mediate above- and belowground biomass ratios; further study is required to resolve this issue.
Under control conditions, seeds of the three Dendrobium species showed no seedling development, but they reached stage 2 on poor nutrient medium. The enlarged embryos quickly turned green, suggesting that nutrient acquisition through photosynthesis may have allowed them to develop to stage 2. Seed germination in D. devonianum was significantly delayed under dark compared to light conditions []. These results indicate that photosynthesis contributes to the initial germination of Dendrobium. Inoculation with Ceratobasidiaceae and Fusarium fungi often inhibited growth more than that with controls. Because these are also known plant pathogens [,], they may behave in a parasitic way during the juvenile stage in Dendrobium species.
Mycorrhizal specificity at early developmental stages in vitro differs among the three closely related Dendrobium species analyzed in this study. Divergence of mycorrhizal specificity at the adult stage has been observed in Dendrobium species, ranging from dominance by a single fungus to diverse mycobionts [,,,]. Our results imply diversity of mycorrhizal specificity during seed germination and seedling development in Dendrobium species. D. okinawense and D. moniliforme are sister taxa within the section Dendrobium [], but their mycorrhizal specificity differed markedly. At the seedling development stage, D. okinawense had high specificity for TU11, and D. moniliforme for TU22, TU27, and SE2 (Figure 4). Therefore, the mycorrhizal specificity of the genus Dendrobium became highly diversified during speciation. Distinct mycorrhizal communities might contribute to plant niche differentiation and reduce species competition among orchids []. The genus Dendrobium comprises 1826 species [] and is one of the most species-rich genera of vascular plants []. Highly divergent mycorrhizal specificity could contribute to this extreme diversification of the genus Dendrobium. Our study elucidates the diversity of orchid mycorrhizal specificities and provides valuable information for conservation of endangered Dendrobium species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14121119/s1, Figure S1: Effects of fungal isolates on the seedling growth of Dendrobium officinale, D. okinawense, and D. moniliforme at three months after symbiotic culture. Longest root (a), leaf number (b), and tiller number (c).
Author Contributions
Y.O.-T. were involved in the study conception and design. Sampling, experiments, data collection and analysis were performed by L.Z. and K.R., A.K., K.T. and T.Y. The manuscript was written by L.Z. and Y.O.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by JSPS KAKENHI grant numbers 21K06306 to Y.O.-T, Environment Research and Technology Development Fund (JPMEERF20184004) of the Environmental Restoration and Conservation Agency of Japan, and Research Grant from Yakushima Environmental and Cultural Foundation to K.R.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Sequence data have been deposited in DDBJ under accession numbers (LC597346, LC683202–683203 and LC597307–597312).
Acknowledgments
The authors would like to thank A. Abe, S. Abe, T. Abe, M. Amano, M. Goto, N. Kotaka, M. Kudaka, N. Kudaka, Y. Ikeda, T. Saito, H. Sato, K. Suzuki, K. Tetsuka, T. Tetsuka and Y. Yamashita for sampling and field work. The DNA sequencing analyses were made using a Genetic Analyzer at Analytical Research Center for Experimental Sciences, Saga University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Rasmussen, H.N. Terrestrial Orchids: From Seed to Mycotrophic Plant; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Rasmussen, H.N.; Rasmussen, F.N. Orchid mycorrhiza: Implications of a mycophagous life style. Oikos 2009, 118, 334–345. [Google Scholar] [CrossRef]
- Rasmussen, H.N. Recent developments in the study of orchid mycorrhiza. Plant Soil 2002, 244, 149–163. [Google Scholar] [CrossRef]
- Dearnaley, J.D.W.; Martos, F.; Selosse, M.A. Orchid mycorrhizas: Molecular ecology, physiology, evolution and conservation aspects. In The Mycota Vol. 9 Fungal Associations; Hock, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 207–230. [Google Scholar]
- Ventre Lespiaucq, A.; Jacquemyn, H.; Rasmussen, H.N.; Méndez, M. Temporal turnover in mycorrhizal interactions: A proof of concept with orchids. New Phytol. 2021, 230, 1690–1699. [Google Scholar] [CrossRef]
- Yukawa, T.; Ogura-Tsujita, Y.; Shefferson, R.P.; Yokoyama, J. Mycorrhizal diversity in Apostasia (Orchidaceae) indicates the origin and evolution of orchid mycorrhiza. Am. J. Bot. 2009, 96, 1997–2009. [Google Scholar] [CrossRef]
- McCormick, M.K.; Whigham, D.F.; Canchani-Viruet, A. Mycorrhizal fungi affect orchid distribution and population dynamics. New Phytol. 2018, 219, 1207–1215. [Google Scholar] [CrossRef]
- Koopowitz, H. Orchids and Their Conservation; Timber Press: Portland, OR, USA, 2001. [Google Scholar]
- Arditti, J. Fundamentals of Orchid Biology; John Wiley & Sons: New York, NY, USA, 1992. [Google Scholar]
- Meng, Y.Y.; Fan, X.L.; Zhou, L.R.; Shao, S.C.; Liu, Q.; Selosse, M.A.; Gao, J.Y. Symbiotic fungi undergo a taxonomic and functional bottleneck during orchid seeds germination: A case study on Dendrobium moniliforme. Symbiosis 2019, 79, 205–212. [Google Scholar] [CrossRef]
- Warcup, J. Symbiotic germination of some Australian terrestrial orchids. New Phytol. 1973, 72, 387–392. [Google Scholar] [CrossRef]
- Bonnardeaux, Y.; Brundrett, M.; Batty, A.; Dixon, K.; Koch, J.; Sivasithamparam, K. Diversity of mycorrhizal fungi of terrestrial orchids: Compatibility webs, brief encounters, lasting relationships and alien invasions. Mycol. Res. 2007, 111, 51–61. [Google Scholar] [CrossRef]
- Otero, J.T.; Flanagan, N.S.; Herre, E.A.; Ackerman, J.D.; Bayman, P. Widespread mycorrhizal specificity correlates to mycorrhizal function in the neotropical, epiphytic orchid Ionopsis utricularioides (Orchidaceae). Am. J. Bot. 2007, 94, 1944–1950. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.Y.; Chen, X.M.; Guo, S.X.; Lee, Y.I. Effect of different mycobionts on symbiotic germination and seedling growth of Dendrobium officinale, an important medicinal orchid. Bot. Stud. 2020, 61, 2. [Google Scholar] [CrossRef]
- Frodin, D.G. History and concepts of big plant genera. Taxon 2004, 53, 753–776. [Google Scholar] [CrossRef]
- World Flora Online. Available online: http://www.worldfloraonline.org/ (accessed on 30 September 2022).
- Zhu, G.; Ji, Z.; Wood, J.J.; Wood, H.P. 139. Dendrobium. Flora China 2009, 25, 367–397. [Google Scholar]
- Teixeira da Silva, J.A.; Tsavkelova, E.A.; Ng, T.B.; Parthibhan, S.; Dobránszki, J.; Cardoso, J.C.; Rao, M.V. Asymbiotic in vitro seed propagation of Dendrobium. Plant Cell Rep. 2015, 34, 1685–1706. [Google Scholar] [CrossRef]
- Teoh, E.S. Medicinal Orchids of Asia; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Zi, X.M.; Sheng, C.L.; Goodale, U.M.; Shao, S.C.; Gao, J.Y. In situ seed baiting to isolate germination-enhancing fungi for an epiphytic orchid, Dendrobium aphyllum (Orchidaceae). Mycorrhiza 2014, 24, 487–499. [Google Scholar] [CrossRef]
- Xing, X.; Ma, X.; Deng, Z.; Chen, J.; Wu, F.; Guo, S. Specificity and preference of mycorrhizal associations in two species of the genus Dendrobium (Orchidaceae). Mycorrhiza 2013, 23, 317–324. [Google Scholar] [CrossRef]
- Xing, X.; Ma, X.; Men, J.; Chen, Y.; Guo, S. Phylogenetic constrains on mycorrhizal specificity in eight Dendrobium (Orchidaceae) species. Sci. China Life Sci. 2017, 60, 536–544. [Google Scholar] [CrossRef]
- Meng, Y.Y.; Shao, S.C.; Liu, S.J.; Gao, J.Y. Do the fungi associated with roots of adult plants support seed germination? A case study on Dendrobium exile (Orchidaceae). Glob. Ecol. Conserv. 2019, 17, e00582. [Google Scholar] [CrossRef]
- Wang, X.J.; Wu, Y.H.; Ming, X.J.; Wang, G.; Gao, J.Y. Isolating ecological-specific fungi and creating fungus-seed bags for epiphytic orchid conservation. Glob. Ecol. Conserv. 2021, 28, e01714. [Google Scholar] [CrossRef]
- Huang, H.; Zi, X.M.; Lin, H.; Gao, J.Y. Host-specificity of symbiotic mycorrhizal fungi for enhancing seed germination, protocorm formation and seedling development of over-collected medicinal orchid, Dendrobium devonianum. J. Microbiol. 2018, 56, 42–48. [Google Scholar] [CrossRef]
- Dan, Y.; Meng, Z.X.; Guo, S.X. Effects of forty strains of Orchidaceae mycorrhizal fungi on growth of protocorms and plantlets of Dendrobium candidum and D. nobile. Afr. J. Microbiol. Res. 2012, 6, 34–39. [Google Scholar] [CrossRef]
- Rammitsu, K.; Abe, S.; Abe, T.; Kotaka, N.; Kudaka, M.; Kudaka, N.; Kinoshita, A.; Ogura-Tsujita, Y. The endangered epiphytic orchid Dendrobium okinawense has a highly specific mycorrhizal association with a single Tulasnellaceae fungus. J. Forest Res. 2021, 26, 215–221. [Google Scholar] [CrossRef]
- Takamiya, T.; Wongsawad, P.; Sathapattayanon, A.; Tajima, N.; Suzuki, S.; Kitamura, S.; Shioda, N.; Handa, T.; Kitanaka, S.; Lijima, H.; et al. Molecular phylogenetics and character evolution of morphologically diverse groups, Dendrobium section Dendrobium and allies. AoB. Plants 2014, 6, plu045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Rammitsu, K.; Tetsuka, K.; Yukawa, T.; Ogura-Tsujita, Y. Dominant Dendrobium officinale mycorrhizal partners vary among habitats and strongly induce seed germination in vitro. Front. Ecol. Evol. 2022, 10, 994641. [Google Scholar] [CrossRef]
- Izumitsu, K.; Hatoh, K.; Sumita, T.; Kitade, Y.; Morita, A.; Gafur, A.; Ohta, A.; Kawai, M.; Yamanaka, T.; Neda, H.; et al. Rapid and simple preparation of mushroom DNA directly from colonies and fruiting bodies for PCR. Mycoscience 2012, 53, 396–401. [Google Scholar] [CrossRef]
- Rosenblum, B.B.; Lee, L.G.; Spurgeon, S.L.; Khan, S.H.; Menchen, S.M.; Heiner, C.R.; Chen, S.M. New dye-labeled terminators for improved DNA sequencing patterns. Nucleic Acids Res. 1997, 25, 4500–4504. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, K.; Cheng, S.; Nie, Q.; Zhou, S.X.; Chen, Q.; Zhou, J.; Zhen, X.; Li, X.T.; Zhen, T.W.; et al. Fusarium oxysporum KB-3 from Bletilla striata: An orchid mycorrhizal fungus. Mycorrhiza 2019, 29, 531–540. [Google Scholar] [CrossRef]
- Spoerl, E. Amino acids as sources of nitrogen for orchid embryos. Am. J. Bot. 1948, 35, 88–95. [Google Scholar] [CrossRef]
- Otero, J.T.; Ackerman, J.D.; Bayman, P. Differences in mycorrhizal preferences between two tropical orchids. Mol. Ecol. 2004, 13, 2393–2404. [Google Scholar] [CrossRef]
- Tokuhara, K.; Mii, M. Micropropagation of Phalaenopsis and Doritaenopsis by culturing shoot tips of flower stalk buds. Plant Cell Rep. 1993, 13, 7–11. [Google Scholar] [CrossRef]
- Tan, X.M.; Wang, C.L.; Chen, X.M.; Zhou, Y.Q.; Wang, Y.Q.; Luo, A.X.; Liu, Z.H.; Guo, S.X. In vitro seed germination and seedling growth of an endangered epiphytic orchid, Dendrobium officinale, endemic to China using mycorrhizal fungi (Tulasnella sp.). Sci. Hortic. 2014, 165, 62–68. [Google Scholar] [CrossRef]
- Tang, Y.J.; Zhou, D.Y.; Dai, J.; Li, Y.; Xing, Y.M.; Guo, S.X.; Chen, J. Potential specificity between mycorrhizal fungi isolated from widespread Dendrobium spp. and rare D. huoshanense seeds. Curr. Microbiol. 2022, 79, 264. [Google Scholar] [CrossRef]
- Bidartondo, M.I.; Read, D.J. Fungal specificity bottlenecks during orchid germination and development. Mol. Ecol. 2008, 17, 3707–3716. [Google Scholar] [CrossRef]
- Fuji, M.; Miura, C.; Yamamoto, T.; Komiyama, S.; Suetsugu, K.; Yagame, T.; Yamato, M.; Kaminaka, H. Relative effectiveness of Tulasnella fungal strains in orchid mycorrhizal symbioses between germination and subsequent seedling growth. Symbiosis 2020, 81, 53–63. [Google Scholar] [CrossRef]
- Pereira, M.C.; Rocha, D.I.; Veloso, T.G.R.; Pereira, O.L.; Francino, D.M.T.; Meira, R.M.S.A.; Kasuya, M.C.M. Characterization of seed germination and protocorm development of Cyrtopodium glutiniferum (Orchidaceae) promoted by mycorrhizal fungi Epulorhiza spp. Acta Bot. Brasilica 2015, 29, 567–574. [Google Scholar] [CrossRef]
- Shao, S.C.; Xi, H.P.; Mohandass, D. Symbiotic mycorrhizal fungi isolated via ex situ seed baiting induce seed germination of Dendrobium catenatum Lindl. (Orchidaceae). Appl. Ecol. Environ. Res. 2019, 17, 9753–9771. [Google Scholar] [CrossRef]
- Bayman, P.; Mosquera-Espinosa, A.T.; Saladini-Aponte, C.M.; Hurtado-Guevara, N.C.; Viera-Ruiz, N.L. Age-dependent mycorrhizal specificity in an invasive orchid, Oeceoclades maculata. Am. J. Bot. 2016, 103, 1880–1889. [Google Scholar] [CrossRef]
- Veldre, V.; Abarenkov, K.; Bahram, M.; Martos, F.; Selosse, M.A.; Tamm, H.; Kõljalg, U.; Tedersoo, L. Evolution of nutritional modes of Ceratobasidiaceae (Cantharellales, Basidiomycota) as revealed from publicly available ITS sequences. Fungal Ecol. 2013, 6, 256–268. [Google Scholar] [CrossRef]
- Laurence, M.H.; Summerell, B.A.; Burgess, L.W.; Liew, E.C. Genealogical concordance phylogenetic species recognition in the Fusarium oxysporum species complex. Fungal Biol. 2014, 118, 374–384. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Brys, R.; Merckx, V.; Waud, M.; Lievens, B.; Wiegand, T. Coexisting orchid species have distinct mycorrhizal communities and display strong spatial segregation. New Phytol. 2014, 202, 616–627. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).