Symbiotic Culture of Three Closely Related Dendrobium Species Reveals a Growth Bottleneck and Differences in Mycorrhizal Specificity at Early Developmental Stages
Abstract
1. Introduction
2. Materials and Method
2.1. Sample Collection
2.2. Fungal Isolation and Molecular Identification
2.3. In Vitro Symbiotic Germination
2.4. In Vitro Symbiotic Culture of Seedlings
2.5. Statistical Analysis
3. Results
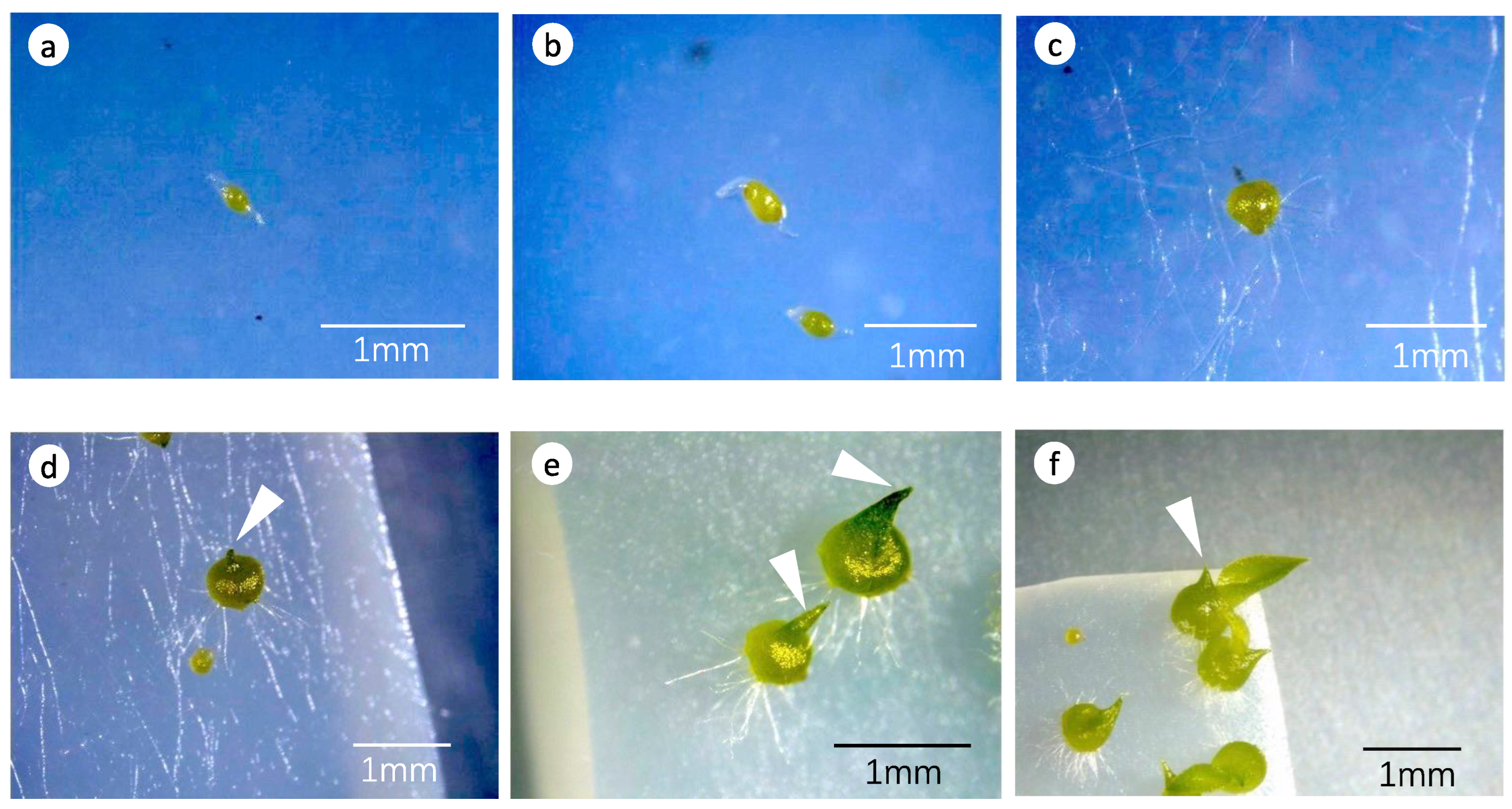
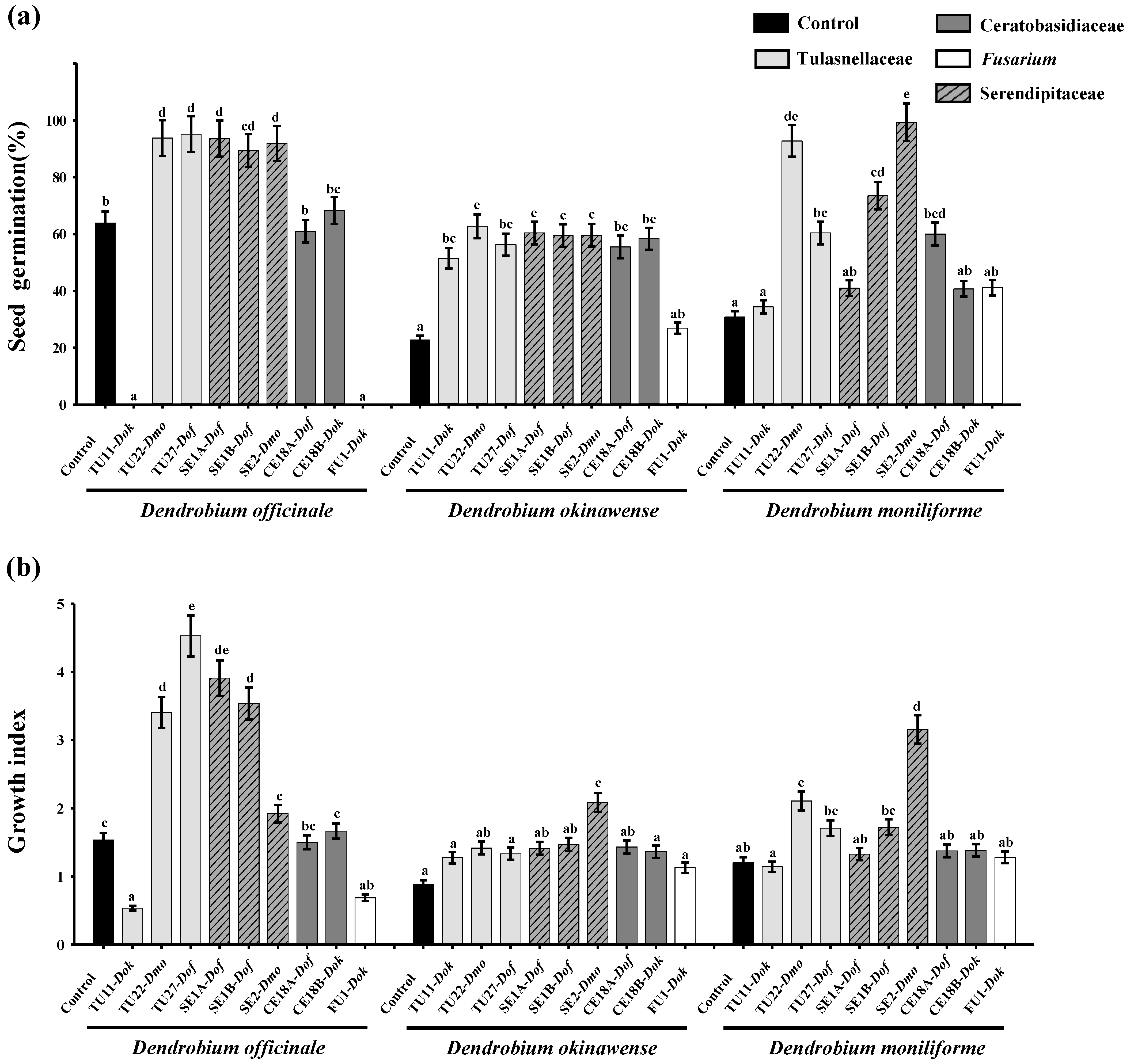
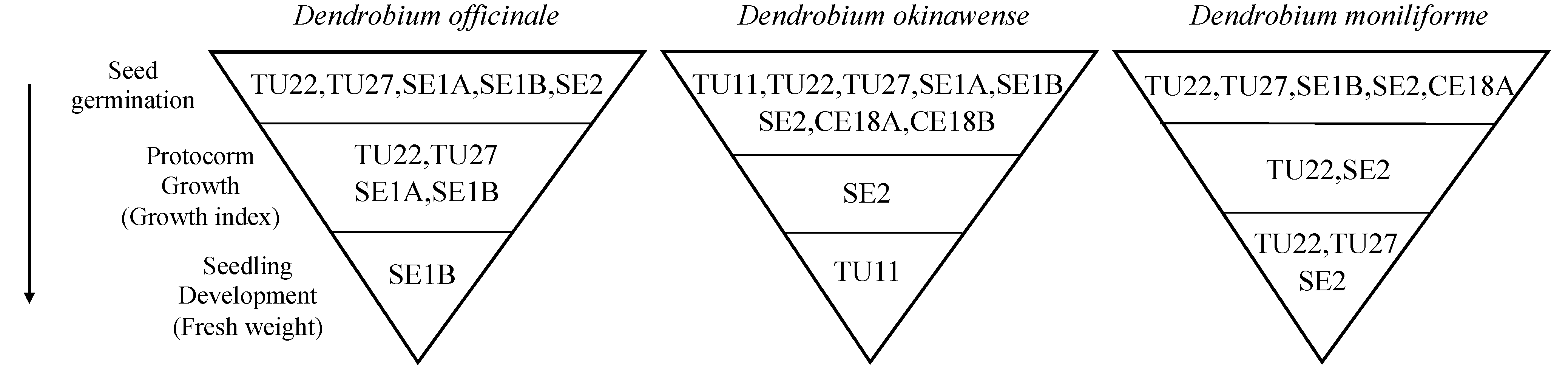
3.1. Effects of Fungal Strains on Symbiotic Germination
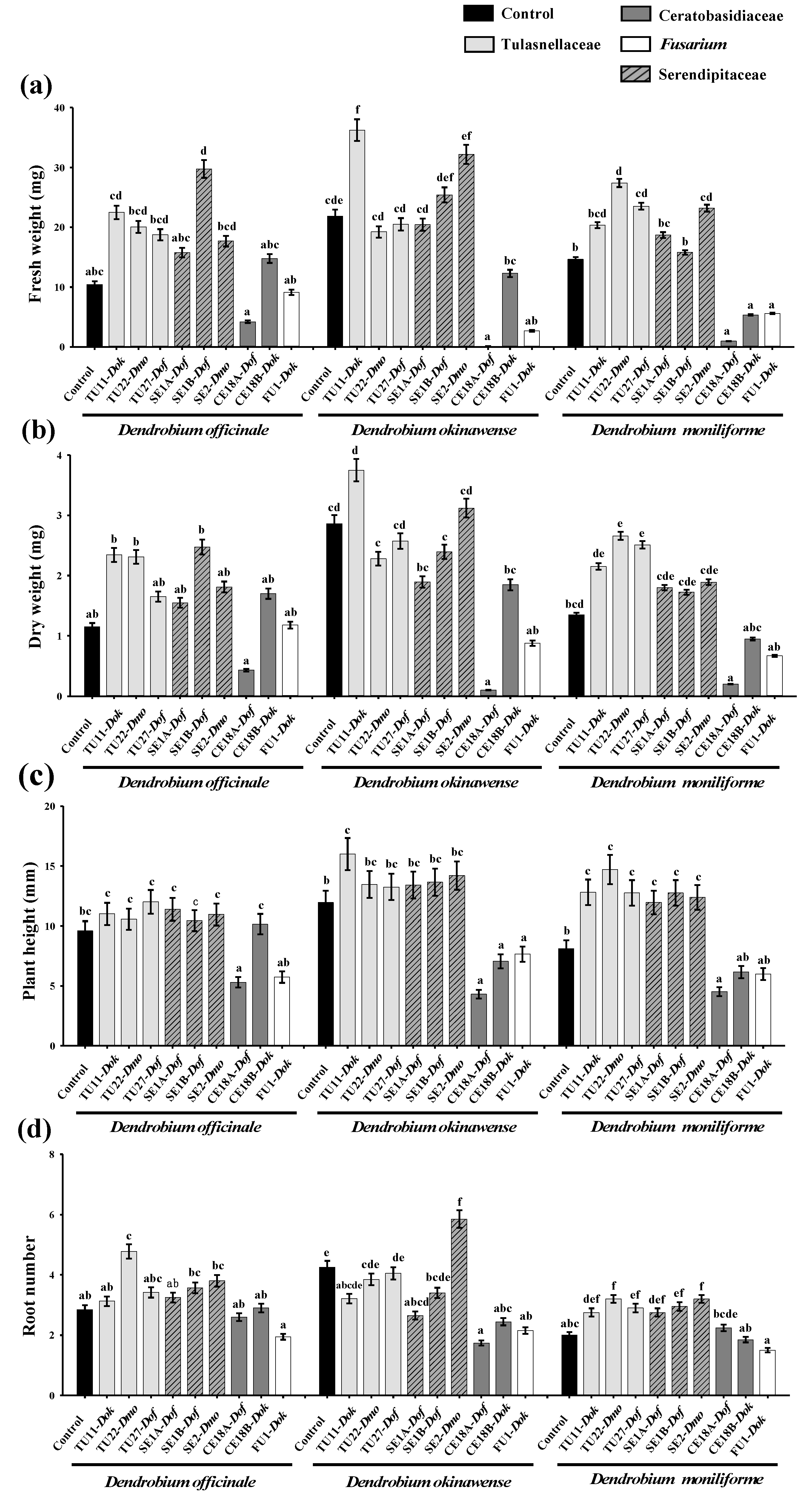
3.2. Effects of Fungal Strains on Symbiotic Cultures of Seedling
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Rasmussen, H.N. Terrestrial Orchids: From Seed to Mycotrophic Plant; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Rasmussen, H.N.; Rasmussen, F.N. Orchid mycorrhiza: Implications of a mycophagous life style. Oikos 2009, 118, 334–345. [Google Scholar] [CrossRef]
- Rasmussen, H.N. Recent developments in the study of orchid mycorrhiza. Plant Soil 2002, 244, 149–163. [Google Scholar] [CrossRef]
- Dearnaley, J.D.W.; Martos, F.; Selosse, M.A. Orchid mycorrhizas: Molecular ecology, physiology, evolution and conservation aspects. In The Mycota Vol. 9 Fungal Associations; Hock, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 207–230. [Google Scholar]
- Ventre Lespiaucq, A.; Jacquemyn, H.; Rasmussen, H.N.; Méndez, M. Temporal turnover in mycorrhizal interactions: A proof of concept with orchids. New Phytol. 2021, 230, 1690–1699. [Google Scholar] [CrossRef]
- Yukawa, T.; Ogura-Tsujita, Y.; Shefferson, R.P.; Yokoyama, J. Mycorrhizal diversity in Apostasia (Orchidaceae) indicates the origin and evolution of orchid mycorrhiza. Am. J. Bot. 2009, 96, 1997–2009. [Google Scholar] [CrossRef]
- McCormick, M.K.; Whigham, D.F.; Canchani-Viruet, A. Mycorrhizal fungi affect orchid distribution and population dynamics. New Phytol. 2018, 219, 1207–1215. [Google Scholar] [CrossRef]
- Koopowitz, H. Orchids and Their Conservation; Timber Press: Portland, OR, USA, 2001. [Google Scholar]
- Arditti, J. Fundamentals of Orchid Biology; John Wiley & Sons: New York, NY, USA, 1992. [Google Scholar]
- Meng, Y.Y.; Fan, X.L.; Zhou, L.R.; Shao, S.C.; Liu, Q.; Selosse, M.A.; Gao, J.Y. Symbiotic fungi undergo a taxonomic and functional bottleneck during orchid seeds germination: A case study on Dendrobium moniliforme. Symbiosis 2019, 79, 205–212. [Google Scholar] [CrossRef]
- Warcup, J. Symbiotic germination of some Australian terrestrial orchids. New Phytol. 1973, 72, 387–392. [Google Scholar] [CrossRef]
- Bonnardeaux, Y.; Brundrett, M.; Batty, A.; Dixon, K.; Koch, J.; Sivasithamparam, K. Diversity of mycorrhizal fungi of terrestrial orchids: Compatibility webs, brief encounters, lasting relationships and alien invasions. Mycol. Res. 2007, 111, 51–61. [Google Scholar] [CrossRef]
- Otero, J.T.; Flanagan, N.S.; Herre, E.A.; Ackerman, J.D.; Bayman, P. Widespread mycorrhizal specificity correlates to mycorrhizal function in the neotropical, epiphytic orchid Ionopsis utricularioides (Orchidaceae). Am. J. Bot. 2007, 94, 1944–1950. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.Y.; Chen, X.M.; Guo, S.X.; Lee, Y.I. Effect of different mycobionts on symbiotic germination and seedling growth of Dendrobium officinale, an important medicinal orchid. Bot. Stud. 2020, 61, 2. [Google Scholar] [CrossRef]
- Frodin, D.G. History and concepts of big plant genera. Taxon 2004, 53, 753–776. [Google Scholar] [CrossRef]
- World Flora Online. Available online: http://www.worldfloraonline.org/ (accessed on 30 September 2022).
- Zhu, G.; Ji, Z.; Wood, J.J.; Wood, H.P. 139. Dendrobium. Flora China 2009, 25, 367–397. [Google Scholar]
- Teixeira da Silva, J.A.; Tsavkelova, E.A.; Ng, T.B.; Parthibhan, S.; Dobránszki, J.; Cardoso, J.C.; Rao, M.V. Asymbiotic in vitro seed propagation of Dendrobium. Plant Cell Rep. 2015, 34, 1685–1706. [Google Scholar] [CrossRef]
- Teoh, E.S. Medicinal Orchids of Asia; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Zi, X.M.; Sheng, C.L.; Goodale, U.M.; Shao, S.C.; Gao, J.Y. In situ seed baiting to isolate germination-enhancing fungi for an epiphytic orchid, Dendrobium aphyllum (Orchidaceae). Mycorrhiza 2014, 24, 487–499. [Google Scholar] [CrossRef]
- Xing, X.; Ma, X.; Deng, Z.; Chen, J.; Wu, F.; Guo, S. Specificity and preference of mycorrhizal associations in two species of the genus Dendrobium (Orchidaceae). Mycorrhiza 2013, 23, 317–324. [Google Scholar] [CrossRef]
- Xing, X.; Ma, X.; Men, J.; Chen, Y.; Guo, S. Phylogenetic constrains on mycorrhizal specificity in eight Dendrobium (Orchidaceae) species. Sci. China Life Sci. 2017, 60, 536–544. [Google Scholar] [CrossRef]
- Meng, Y.Y.; Shao, S.C.; Liu, S.J.; Gao, J.Y. Do the fungi associated with roots of adult plants support seed germination? A case study on Dendrobium exile (Orchidaceae). Glob. Ecol. Conserv. 2019, 17, e00582. [Google Scholar] [CrossRef]
- Wang, X.J.; Wu, Y.H.; Ming, X.J.; Wang, G.; Gao, J.Y. Isolating ecological-specific fungi and creating fungus-seed bags for epiphytic orchid conservation. Glob. Ecol. Conserv. 2021, 28, e01714. [Google Scholar] [CrossRef]
- Huang, H.; Zi, X.M.; Lin, H.; Gao, J.Y. Host-specificity of symbiotic mycorrhizal fungi for enhancing seed germination, protocorm formation and seedling development of over-collected medicinal orchid, Dendrobium devonianum. J. Microbiol. 2018, 56, 42–48. [Google Scholar] [CrossRef]
- Dan, Y.; Meng, Z.X.; Guo, S.X. Effects of forty strains of Orchidaceae mycorrhizal fungi on growth of protocorms and plantlets of Dendrobium candidum and D. nobile. Afr. J. Microbiol. Res. 2012, 6, 34–39. [Google Scholar] [CrossRef]
- Rammitsu, K.; Abe, S.; Abe, T.; Kotaka, N.; Kudaka, M.; Kudaka, N.; Kinoshita, A.; Ogura-Tsujita, Y. The endangered epiphytic orchid Dendrobium okinawense has a highly specific mycorrhizal association with a single Tulasnellaceae fungus. J. Forest Res. 2021, 26, 215–221. [Google Scholar] [CrossRef]
- Takamiya, T.; Wongsawad, P.; Sathapattayanon, A.; Tajima, N.; Suzuki, S.; Kitamura, S.; Shioda, N.; Handa, T.; Kitanaka, S.; Lijima, H.; et al. Molecular phylogenetics and character evolution of morphologically diverse groups, Dendrobium section Dendrobium and allies. AoB. Plants 2014, 6, plu045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Rammitsu, K.; Tetsuka, K.; Yukawa, T.; Ogura-Tsujita, Y. Dominant Dendrobium officinale mycorrhizal partners vary among habitats and strongly induce seed germination in vitro. Front. Ecol. Evol. 2022, 10, 994641. [Google Scholar] [CrossRef]
- Izumitsu, K.; Hatoh, K.; Sumita, T.; Kitade, Y.; Morita, A.; Gafur, A.; Ohta, A.; Kawai, M.; Yamanaka, T.; Neda, H.; et al. Rapid and simple preparation of mushroom DNA directly from colonies and fruiting bodies for PCR. Mycoscience 2012, 53, 396–401. [Google Scholar] [CrossRef]
- Rosenblum, B.B.; Lee, L.G.; Spurgeon, S.L.; Khan, S.H.; Menchen, S.M.; Heiner, C.R.; Chen, S.M. New dye-labeled terminators for improved DNA sequencing patterns. Nucleic Acids Res. 1997, 25, 4500–4504. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, K.; Cheng, S.; Nie, Q.; Zhou, S.X.; Chen, Q.; Zhou, J.; Zhen, X.; Li, X.T.; Zhen, T.W.; et al. Fusarium oxysporum KB-3 from Bletilla striata: An orchid mycorrhizal fungus. Mycorrhiza 2019, 29, 531–540. [Google Scholar] [CrossRef]
- Spoerl, E. Amino acids as sources of nitrogen for orchid embryos. Am. J. Bot. 1948, 35, 88–95. [Google Scholar] [CrossRef]
- Otero, J.T.; Ackerman, J.D.; Bayman, P. Differences in mycorrhizal preferences between two tropical orchids. Mol. Ecol. 2004, 13, 2393–2404. [Google Scholar] [CrossRef]
- Tokuhara, K.; Mii, M. Micropropagation of Phalaenopsis and Doritaenopsis by culturing shoot tips of flower stalk buds. Plant Cell Rep. 1993, 13, 7–11. [Google Scholar] [CrossRef]
- Tan, X.M.; Wang, C.L.; Chen, X.M.; Zhou, Y.Q.; Wang, Y.Q.; Luo, A.X.; Liu, Z.H.; Guo, S.X. In vitro seed germination and seedling growth of an endangered epiphytic orchid, Dendrobium officinale, endemic to China using mycorrhizal fungi (Tulasnella sp.). Sci. Hortic. 2014, 165, 62–68. [Google Scholar] [CrossRef]
- Tang, Y.J.; Zhou, D.Y.; Dai, J.; Li, Y.; Xing, Y.M.; Guo, S.X.; Chen, J. Potential specificity between mycorrhizal fungi isolated from widespread Dendrobium spp. and rare D. huoshanense seeds. Curr. Microbiol. 2022, 79, 264. [Google Scholar] [CrossRef]
- Bidartondo, M.I.; Read, D.J. Fungal specificity bottlenecks during orchid germination and development. Mol. Ecol. 2008, 17, 3707–3716. [Google Scholar] [CrossRef]
- Fuji, M.; Miura, C.; Yamamoto, T.; Komiyama, S.; Suetsugu, K.; Yagame, T.; Yamato, M.; Kaminaka, H. Relative effectiveness of Tulasnella fungal strains in orchid mycorrhizal symbioses between germination and subsequent seedling growth. Symbiosis 2020, 81, 53–63. [Google Scholar] [CrossRef]
- Pereira, M.C.; Rocha, D.I.; Veloso, T.G.R.; Pereira, O.L.; Francino, D.M.T.; Meira, R.M.S.A.; Kasuya, M.C.M. Characterization of seed germination and protocorm development of Cyrtopodium glutiniferum (Orchidaceae) promoted by mycorrhizal fungi Epulorhiza spp. Acta Bot. Brasilica 2015, 29, 567–574. [Google Scholar] [CrossRef]
- Shao, S.C.; Xi, H.P.; Mohandass, D. Symbiotic mycorrhizal fungi isolated via ex situ seed baiting induce seed germination of Dendrobium catenatum Lindl. (Orchidaceae). Appl. Ecol. Environ. Res. 2019, 17, 9753–9771. [Google Scholar] [CrossRef]
- Bayman, P.; Mosquera-Espinosa, A.T.; Saladini-Aponte, C.M.; Hurtado-Guevara, N.C.; Viera-Ruiz, N.L. Age-dependent mycorrhizal specificity in an invasive orchid, Oeceoclades maculata. Am. J. Bot. 2016, 103, 1880–1889. [Google Scholar] [CrossRef]
- Veldre, V.; Abarenkov, K.; Bahram, M.; Martos, F.; Selosse, M.A.; Tamm, H.; Kõljalg, U.; Tedersoo, L. Evolution of nutritional modes of Ceratobasidiaceae (Cantharellales, Basidiomycota) as revealed from publicly available ITS sequences. Fungal Ecol. 2013, 6, 256–268. [Google Scholar] [CrossRef]
- Laurence, M.H.; Summerell, B.A.; Burgess, L.W.; Liew, E.C. Genealogical concordance phylogenetic species recognition in the Fusarium oxysporum species complex. Fungal Biol. 2014, 118, 374–384. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Brys, R.; Merckx, V.; Waud, M.; Lievens, B.; Wiegand, T. Coexisting orchid species have distinct mycorrhizal communities and display strong spatial segregation. New Phytol. 2014, 202, 616–627. [Google Scholar] [CrossRef]
| Fungal Genus | Fungal Isolates | Isolate ID | Host Plants | NCBI Accession No. | NBRC Accession No. |
|---|---|---|---|---|---|
| Tulasnellaceae | TU11 | F833 | D. okinawense | LC683207 | NBRC 115273 |
| TU22 | F761 | D. moniliforme | LC683208 | NBRC 115545 | |
| TU27 | F763 | D. officinale | LC683202 | NBRC 115262 | |
| Serendipitaceae | SE1A | F358 | D. officinale | LC683209 | NBRC 114327 |
| SE1B | F809 | D. officinale | LC683203 | NBRC 115270 | |
| SE2 | F444 | D. moniliforme | LC683210 | NBRC 115521 | |
| Ceratobasidiaceae | CE18A | F356 | D. officinale | LC597346 | NBRC 114326 |
| CE18B | F828 | D. okinawense | LC683211 | NBRC 115272 | |
| Fusarium | FU1 | F830 | D. okinawense | LC683212 | NBRC 115531 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Rammitsu, K.; Kinoshita, A.; Tokuhara, K.; Yukawa, T.; Ogura-Tsujita, Y. Symbiotic Culture of Three Closely Related Dendrobium Species Reveals a Growth Bottleneck and Differences in Mycorrhizal Specificity at Early Developmental Stages. Diversity 2022, 14, 1119. https://doi.org/10.3390/d14121119
Zhang L, Rammitsu K, Kinoshita A, Tokuhara K, Yukawa T, Ogura-Tsujita Y. Symbiotic Culture of Three Closely Related Dendrobium Species Reveals a Growth Bottleneck and Differences in Mycorrhizal Specificity at Early Developmental Stages. Diversity. 2022; 14(12):1119. https://doi.org/10.3390/d14121119
Chicago/Turabian StyleZhang, Liyue, Kento Rammitsu, Akihiko Kinoshita, Ken Tokuhara, Tomohisa Yukawa, and Yuki Ogura-Tsujita. 2022. "Symbiotic Culture of Three Closely Related Dendrobium Species Reveals a Growth Bottleneck and Differences in Mycorrhizal Specificity at Early Developmental Stages" Diversity 14, no. 12: 1119. https://doi.org/10.3390/d14121119
APA StyleZhang, L., Rammitsu, K., Kinoshita, A., Tokuhara, K., Yukawa, T., & Ogura-Tsujita, Y. (2022). Symbiotic Culture of Three Closely Related Dendrobium Species Reveals a Growth Bottleneck and Differences in Mycorrhizal Specificity at Early Developmental Stages. Diversity, 14(12), 1119. https://doi.org/10.3390/d14121119






