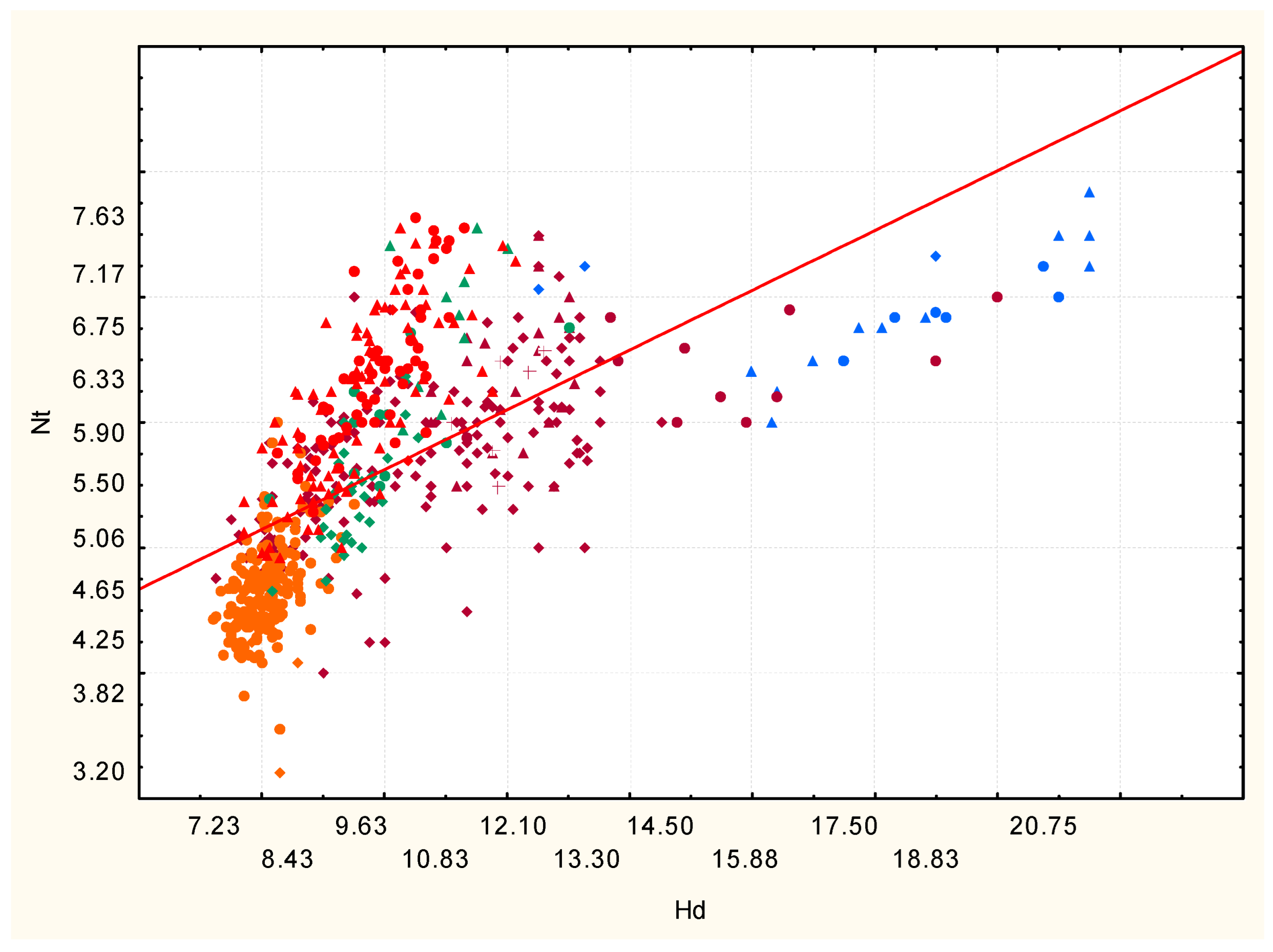
3. Results and Discussion
The vegetation of the Kuyalnik Estuary valley is characterized by a high level of phytocenosis diversity. Its modern territorial and ecological and cenotic differentiation is due to the integrated influence of natural and anthropogenic factors. Amplitudes of syntaxons vary from coenoses of steppe and petrophytic vegetation, formed in dry conditions of the edaphotope, to wetlands, the most moistened and nitrophilous (
Figure 7).
Plant communities of the Kuyalnik Estuary valley are in a state of continuous, sequential and catastrophic changes that lead to the complication or simplification of their structure. These changes are caused by natural and anthropogenic factors and characterized by certain spatiotemporal sequences. Catastrophic changes usually occur unexpectedly under the influence of one or more external factors and have both direct and indirect effects on the vegetation cover. Direct ones lead to the total physical destruction of entire plant communities or parts of them. Indirect ones show themselves as a sharp change in the ecological conditions of the biotope, and they affect the direction and time of the course of further post-catastrophic stages of succession [
4].
Catastrophic changes in vegetation are caused primarily by hydrological changes in the Kuyalnik Estuary valley, the main of which are the following:
1. Straightening and blocking with more than 20 locks of the riverbed, conducted in the 1970–80s of the last century, and sewerage and damming of the lower reaches of the Great Kuyalnyk River (annual flow 7.6 million m
3 per year) (
Figure 8). This led to the isolation of underground springs, the interception of river flow, and the disappearance of freshwater wedging points on the slopes of the valley, and siltation of the canalized channel.
2. Random and excessive water withdrawal from the rivers of the basin of the Kuyalnik Estuary, whose area is more than 1860 km2, through the construction of dams. More than 130 artificial reservoirs in the basin accumulate 70% of the natural annual runoff.
3. Plowing in the past and grazing currently have a negative impact on the level, and, most importantly, the mineral composition of groundwater, which is in all areas of the floodplain of the upper reaches of the Kuyalnik Estuary, leads to their secondary salinization (
Figure 9).
4. Sand sampling at the mouth of the Great Kuyalnyk River and shell rock in the upper reaches of the Kuyalnik Estuary led to a decrease in freshwater inflow into the floodplain part of the valley with corresponding negative consequences [
44].
The scale of the impact of hydrological changes is so significant that, due to the weakening and disappearance of the leaching regime, the vegetation of almost the entire floodplain part of the valley has been transformed in the direction of changing moderately saline meadow communities to excessively saline, and, in some places, solonetz and even solonchak. Unique floodplain saline meadows and swamp communities were totally destroyed, and their restoration is now impossible due to a radical violation of the hydrological regime in the valley territory. The loss of meadow phytodiversity is intensified due to overgrazing of cattle in the floodplain areas. A certain influence is exerted by an increase in summer temperatures by 1–2 degrees Celsius and an increase in the alternation of dry years.
Catastrophic changes in vegetation (salt marsh, saline, saline-meadow steppe, and tree-shrubby) also occurred in part of the valley on which a product pipeline was built connecting the right and left banks of the estuary (
Figure 10).
Significant territories were exposed to such changes in the places of current quarries where sand and limestone are extracted. Quarry sand extraction not only destroyed the natural vegetation cover but also radically changed the topography of the territory (
Figure 11,
Figure 12 and
Figure 13).
Large areas filled with mineralized water, depressions that alternate with striped sand mounds and shell rock have been formed. In such areas, the native vegetation was totally destroyed, or is at its final stage of degradation, and the expansion of ruderal species is observed. In subsidence areas, these species are Xanthium albinum, Carduus acanthoides, Grindelia squarrosa, Artemisia santonica, Conyza canadensis, Iva xanthiifolia, and others; in elevated areas, these are: Elytrigia repens, Bassia sedoides, Bromus squarrosus, and Agropyron pectinatum involved in weediness. In the final stages of overgrowth, shrubby vegetation is developed in these areas, mainly consisting of Elaeagnus angustifolia and E. commutata.
The vegetation in the territory between the water areas of the Kuyalnik Estuary and the Black Sea has undergone the greatest catastrophic changes. In historical times, it was represented by sandspits (bay bars) occupied by coastal psammophytic vegetation (
Ammophiletea,
Cakiletea maritimae, and
Crithmo-Staticetea), and its flat sites were covered with halophytic vegetation (
Therosalicornietea,
Festuco-Puccinellietea,
Juncetea maritimi, and
Bolboschoenetea maritimi). At the beginning of the 20th century, the sandspits were built-up, and the flat areas were turned into filtration fields for water purification from oil contamination. Later, this area was transformed into a cascade of ponds separated from each other by dams. An international highway passes through this part of the valley (
Figure 14).
The vegetation of dams is represented by a ruderal one. In adjacent areas, closed high-grass overgrowth, consisting of
Atriplex tatarica,
Artemisia annua,
Calamagrostis epigeios,
Iva xanthiifolia etc., with the participation of
Phragmites australis, was formed (
Figure 15).
Catastrophic changes in vegetation also occurred on the sandy-shell spits of the Kuyalnik Estuary water area and on the adjacent sloping territories with limestone outcrops that act as centers of growth of plant species belonging to psamophytic-shell and cretophytic floral complexes characterized by a richness of endemic and neoendemic species [
28]. These geosystems have historically played an extremely important role as biogenetic reserves and, at the same time, they act as ecological corridors for the exchange of diasporas of florocenotic complexes in the western and eastern areas of the northern Black Sea region. Their destruction and, accordingly, the destruction of vegetation has become a disaster not only for this local territory, but it also negatively affected the state of vegetation cover of the entire region because of the interruption of biogeographic links. The disruption caused by sand and shell rock quarry extraction in this area also breaks the biogenetic links of coastal littoral vegetation of the Black Sea-Caspian region. Ruderal species, such as
Tribulus terrestris,
Medicago minima,
Melilotus albus,
Xanthium albinum, and others, are expanding within the transformed territories. Subsidence areas are occupied by seedlings of
Elaeagnus angustifolia. Newly formed reservoirs filled with water are overgrown with
Potamogeton pectinatus and
Zannichellia palustris.
Ploughed areas located in the upper part of the estuary are also areas with catastrophically destroyed native vegetation cover. The total area of the floodplain of the mouth area of the Great Kuyalnyk River turned into agricultural land is about 6 km
2, and the amount of the slopes of the Estuary turned into agriculture land is 0.5–1.0 km
2. During the exploitation of agricultural lands, the soil physical and chemical characteristics have significantly changed, and the composition of diasporas and soil microflora have also changed [
45]. A decrease in the river flow, up to its complete cessation, and prolonged summer droughts resulted in their excessive drainage and salinization. These lands, with a loss of fertility, were abandoned and turned into low-productive pastures. Restorative successions of their vegetation occur in the direction of the formation of salt meadows. Field areas abandoned one or two years ago are overgrown with ruderal species
Atriplex tatarica,
A. hortensis,
Amaranthus retroflexus, and others, which later form communities of associations
Amarantho retroflexi-Setarietum glaucae,
Atriplicetum tataricae, and
Atriplicetum hastatae. The next demutation stage is characterized by the introduction and growth of annual cereals, such as
Bromus arvensis,
B. squarrosus, and others, which together with
Atriplex tatarica and
Bassia sedoides form a vegetation cover on middle-aged fallows. These groupings are also unstable. In two or three years,
Atriplex tatarica are removed from the vegetation cover, and
Artemisia santonica appeared instead, and the cenotic role of
Bassia sedoides is enhanced. Communities of
Artemisietum santonicae var.
Bromus japonicus typical of the saline soil of the estuary valley are being formed. Such communities can maintain their phytocenotic positions under these conditions for 10 or more years.
Catastrophic changes in vegetation occur in the sloping areas of the estuary valley covered by salt-meadow and salt-steppe vegetation, where seasonal unpaved car roads are laid annually. At the same time, vegetation is destroyed in the entire area occupied by such roads and is disturbed on the roadside. Laying transport routes in coastal areas is harmful due to their constant expansion in areas where water is released. Their expansion toward the water area is caused by the breaking up of previously laid roads. Their total width often reaches 200–300 m or more by the end of the season (
Figure 16). At the same time, the soil is compacted, and deep ruts are formed with this condition. As stationary observations have shown, the overgrowing of roads with the complete removal of the influence of technology begins in the third year, and less often in the second year. It has been established that wheels compact the soil surface, and its salinity increases even more. These are critical conditions even for succulent saltwort species starting syngenetic processes (
Salicornia perennans,
Bassia hirsuta, etc.). In addition, the wheels destroy the habitats of the larvae of the mosquito
Chironomus plumosus, the waste products of which are in the form of mounds, which are the places for the germination of seeds of succulent soleros. The development of these species contributes to a decrease in salinity and triggers changes in vegetation towards the formation of saline meadows. As observations have shown, the overgrowing of rutted areas on the coast occurs within 5–7 years.
The overgrowing of roads and bicycle roads on the slopes is even slower and is restrained by the influence of storm flows, which intensify the erosion processes of disturbed areas. With a weakening of this influence, overgrowing begins with the formation of ruderal communities, which are dominated by invasive communities (such as Melilotetum albo-officinalis, Chamaeplietum officinalis, Anisantho-Artemisietum austriacae, and Brometum tectorum). Their replacement by quasi-indigenous communities of steppe or solonetz-meadow vegetation, provided there are no repeated disturbances, occurs within 13–20 years.
Forest reclamation was the most destructive anthropogenic factor that led to catastrophic changes. Large-scale terracing of slopes, mainly the right-bank, and their afforestation was conducted in the 1960–70s of the last century. Artificial terraces were arranged with a width of 2–5 m and a depth of 0.5–1 m (
Figure 17). Terracing of slopes and formation of tree and shrub plantations was conducted only on the right bank of the valley in an area of 25–30 km
2.
Elaeagnus angustifolia,
E. commutata,
Juglans regia,
Malus domestica,
Ulmus laevis,
Rosa canina,
Crataegus monogyna,
Berberis vulgaris,
Armeniaca vulgaris,
Rhamnus cathartica,
Ligustrum vulgare,
Cotinus coggygria, and introduced species
Celtis occidentalis,
Robinia pseudoacacia,
Gleditsia triacanthos,
Styphnolobium japonicum,
Pinus pallasiana,
Amygdalus communis, and some others were planted in the relief depressions. This activity not only simultaneously destroyed the native steppe and petrophytic vegetation, but also radically changed the topographic characteristics and the soil cover structure of slopes.
A survey of terraced slopes in 2016–2017 showed that a satisfactory condition of tree stands was observed only on the slopes of some gullies, particularly Kovalivska. On the parent slopes of the valley, the loss of trees and shrubs in large areas were recorded in. In wooded areas, a sharp decrease in the types of sod and mixed grasses typical of steppe vegetation was observed. The herbaceous layer was almost completely absent in the plantings of trees with high crown closure. Since forest vegetation is not native to the steppe zone, its existence is maintained by the implementation of special measures and the planning of new plantings. This contradicts the natural and historical development of the steppe biota. Therefore, the creation of forest stands, particularly on sloping areas, even without terracing, is environmentally unjustified and harmful since it resulted in the impoverishment of biodiversity. Artificially created forest communities are vulnerable to natural environmental factors. They are affected by pests and diseases. Seed renewal is practically not observed, so they are characterized by a constant tendency to self-destruction. Afforestation also causes fragmentation of native steppe vegetation communities and violation of their natural integrity. At the same time, endemic and relict florocenotic complexes are exposed to significant transformations.
Forest reclamation of slopes resulted in the formation of transformed ecotopes and causes the development of new successive series of vegetation not inherent in this territory. The direction of post-catastrophic recovery processes directly depends on the conditions of newly formed ecotopes. On stabilized slope screes, native grass, or shrubby plant communities that are typical for these conditions often developed rapidly. In particular, O.V. Kostylov recorded the distribution of communities formed by
Crataegus praearmata and
Prunus spinosa on the slopes in the 19680s [
35]. The plantings of
Berberis vulgaris,
Cotinus coggigria,
Rosa canina,
Crataegus monogyna, and
Ligustrum vulgare were successfully naturalized in the estuary valley (
Figure 18). They bear abundant seeds, reproduce by seed, and even form a number of morphotypes.
Native species, such as
Ulmus laevis,
Pyrus communis, differ do better when they are separate individuals and in plantings in general, while introduced species, especially
Pinus pallasiana, are characterized by doing worse (
Figure 19).
Ulmus laevis and
Elaeagnus angustifolia are naturally restored in gullies and depressions on the slopes, and
E. angustifolia forms a strip of vegetation around the estuary. In their grass cover, demutation processes of natural steppe vegetation occur (
Figure 20).
In some places, communities of
Salvio nemorosae-Festucetum valesiacae associations are developing on terraces with sparse or degraded tree and shrubby stands on chernozem low-humus soils (
Figure 21), and
Pimpinello titanophilae-Thymetum dimorphi or
Ephedro distachyae-Stipetum capillatae are developing on crushed stone washed-out slopes. However, the rate of recovery succession and its outcome depends on the strength of the influence of anthropogenic factors (pastoral, pyrogenic, etc.). When these factors are strong and frequent, further restorative changes in vegetation are very rarely completed with the formation of indigenous plant communities. More often, quasi-native phytocenoses are formed with a large participation of adventitious and ruderal species, particularly
Aegilopsetum cylindricae,
Anisantho-Artemisietum austriacae, and
Melilotetum albo-officinalis communities.
Spontaneous fires caused by non-compliance with fire safety are another destructive anthropogenic factor. The catastrophic consequences of fires in the Kuyalnik Estuary valley were primarily associated with their scale and great power. Given the geomorphological features of the territory, their scale and power are determined by the overlap of top and bottom fires. At the same time, the combustion temperature rises sharply, and the sod of cereals, both in the underground and the surface rhizomatous layer, and tree and shrubby vegetation, are totally burned. During the survey period, particularly in 2016, large areas of the coast, right-bank and left-bank slopes turned into a black desert. In August 2017, the vegetation on the left-bank slopes was burnt in an area of about 5 km in length and in some areas of the right-bank slopes. Fires begin at the end of summer with the appearance of dead wood and at the end after the onset of autumn rains. Fires are intense, especially if they start at the bottom of slopes. In almost all cases, they reach the upper slopes. In other cases, the upper part of the slopes burns out, the fire does not fall into the lower part. The magnitude of the fires is so large that firefighters are often unsuccessful is putting out the fire due to the steepness of the slopes. Their areas are in different years from 200 to 1000 m2.
Dried herbage of savanoid communities on the estuary coasts formed by annual cereals (
Aegilops cylindrica,
Hordeum murinum,
Bromus squarrosus,
B. mollis,
B. japonicus, and
Anisantha tectorum) and tall grass weeds (
Centaurea sterilis,
C. diffusa,
Grindelia squarrosa, and others) create an extremely high fire hazard. These species also form the grass layer in
Elaeagnus angustifolia comm. and
Elaeagnus commutata comm. Namely, the pyrogenic factor superimposed on global trends in climate change resulted in the emergence and spread of savanoid communities in the territories previously occupied by saline meadows. The combined effect of these two factors was indicated by multiple researchers [
46,
47,
48].
Tree and shrubby stands on terraced slopes and on the coast are subjected to catastrophic pyrogenic effects. Almost all plantings of
Gleditsia triacanthos,
Robinia pseudoacacia,
Elaeagnus angustifolia and
E. commutata, a significant part of
Pinus pallasiana plantings, and curtains and separate bushes of
Crataegus monogyna and
Rosa canina were affected by consequences of frequent fires. Artificial tree and shrubby stands (primarily
Pinus pallasiana) are quickly degraded, and later deciduous species are removed. Continuous shrubby thickets formed by deciduous shrubs
Berberis vulgaris,
Cerasus mahaleb,
Ligustrum vulgare, and
Cotinus coggygria are less exposed to the pyrogenic factor than sparse thickets formed by
Crataegus monogyna and
Rosa canina. Fires cause the destruction and/or suppression of thickets and separate plants. We associate the effect of the pyrogenic factor with the almost complete absence of communities of shrubby steppes in the estuary valley with the dominant
Amygdalus nana and
Caragana frutex widely distributed in other regions of the steppe zone. The first species occurs only on the edges of shrubby thickets, while the second species has a low projective cover (up to 15%), although it grows in steppe communities. The height of individual plants barely reaches 20–25 cm.
Asparagus verticillatus is the only species of tree and shrubby communities less affected by fires. Due to its powerful root system with many underground renewal buds, individuals of this species quickly increase their vegetative mass after a fire (
Figure 22).
In the literature, the influence of fires on the dynamics of grass communities, especially of steppe ones, is covered ambiguously. Issues of pyrogenic successions have been of interest to researchers since the second half of the 20th century [
49,
50,
51,
52,
53,
54,
55,
56]. Authors consider the steppe as a fire-type ecosystem adapted to the powerful flow of energy passing through food chains. Landscapes of true meadow steppes were defined as pyrogenic cyclical [
57]. The works of L.Yu. Rodin [
58] were devoted to the study of the effect of fires on steppe vegetation; he distinguished three directions of the effect of burning: positive, neutral, and negative. The researcher identified 13 positive aspects in vegetation changes under the influence of burning (65% of the studies analyzed were considered as positive), 6 as neutral (21%) and only 4 as negative (14%). Although the author later acknowledged that catastrophic-scale fires are mostly harmful to steppe biotic diversity, and the use of controlled pasture burning should be strictly regulated [
59]. The favorable moderate effect of the pyrogenic factor on the plant communities of protected zonal steppes has been proven by many steppe scientists [
55,
60,
61,
62,
63,
64]. The observations proved that controlled fires, which occur once every 5–10 years, contribute to the normal functioning of zonal steppe groupings by disposing of accumulated litter, suppressing shrubby vegetation, stimulating vegetative, and seed renewal of sod cereals and xerophytic mixed grasses [
65]. Regulated burning of steppe herbage in protected and other natural areas (when an organization of regulated grazing or planned mowing is not possible) is one of the methods of managing steppe communities and preventing the occurrence of reservatogenic successions [
66]. However, frequent and severe fires cause changes in the ratio of chemical elements in the matter-cycling system of the communities, increasing the temperature regime and reducing soil moisture. Bare soil surface, particularly on slopes, is more vulnerable to wind and water erosion because of the changes in its trophic levels and hydrothermal regime. This, accordingly, worsens the conditions for the existence of soil microorganisms and affects the restoration processes in above-ground plant parts and seed germination. In frequently burned areas, an expansion of pyrophytes (mostly annual ruderal and adventitious species) was observed, which maintain cenotic positions for a long time and inhibit the steppe herbage restoration. Regular fires dramatically negatively affect the development of shrub, moss, and lichen layers in plant communities.
On the gentle slopes of the left-bank in the upper part of the estuary valley, near the product pipeline, a strong fire occurred in autumn 2016 covering about 200 hectares. As a result of the fire, the above-ground plant phytomass and litter were burned completely. The year following the fire, communities of associations of
Stipo lessingianae-Salvietum nutantis and
Stipo ucrainicae-Agropyretum pectinati typical for zonal steppe generally restored their floral structure (
Figure 23). However, the total projective cover of grass stands in these communities was 20–30% less than in areas not affected by fire. In addition, the patches of bare, later washed-out soil (30–40%) and the total absence of steppe litter, which reduces the soil water evaporation, were observed.
The strong thinning of the grass stand affected competitive relations between plant species. The released econishes were filled with vegetatively mobile species of the steppe complex that have a broad ecological amplitude (Galatella villosa, Elytrigia repens, E. intermedia, Carex praecox, Poa angustifolia, and others), and explerent (ruderal) species (Centaurea solstitialis, C. diffusa, Sisymbrium orientale, Falcaria vulgaris, and others). Single bushes of Crataegus monogyna and Rosa canina that grew in the study area had the remains of dead burnt skeletal branches and trunks, and the height of living ones reached only 50–80 cm. The projective cover of Caragana frutex did not exceed 1–5%, and the height of individual plants reached 15–20 cm. Vegetation of the left-bank slopes located opposite the Kovalivka village is also often affected by the pyrogenic factor. On the area of slopes below the power line, after the August and September fires in 2017, a total burnout of above-ground phytomass and steppe litter was observed, and a decrease in the area of living parts of sod cereals Festuca valesiaca, Stipa lessingiana, and S. capillata by 50% was observed. Restoration of the grass stand began with the appearance of young vegetative shoots of Phragmites australis (at the bottom of the slopes), Asparagus verticillatus, and leaf rosettes of taproot mixed grasses: Taraxacum bessarabicum, Limonium platyphyllum, Seseli campestre, Verbascum phoeniceum, Goniolimon tataricum, and others.
The left-bank slopes above the Novokubanskaya Balka were dominated by steppe communities with the predominance of
Stipa lessingiana in 2009 [
67]. Although sod grasses (which include the above-mentioned species) have a fairly high pyrogenic resistance, they could not withstand regular burning on poor and dry stony soils. Currently, due to frequent fires, feather grass communities have been replaced by digressive plant groups of semi-desert type with a dominance of
Festuca valesiaca,
Agropyron pectinatum,
Kochia prostrata, and
Ephedra distachya (
Salvio nemorosae-Festucetum valesiacae var.
Artemisia austriaca,
Astero oleifolii-Ephedretum distachyae,
Ephedra distachya comm.), and
Elytrigia repens and
E. intermedia (
Salvio nemorosae-Elytrigietum intermediae) on erosive areas of slopes. Fires that occur on the left-bank loess slopes contribute to increased erosion and landslide processes. The combined effect of pyrogenic and geomorphogenic factors directs the succession of steppe vegetation in the slope areas of the estuary valleys towards the development of desertified erosiophilic communities, in which the cenotic role of steppe sod grasses is decreased, and the role of rhizomatous grasses is increased (in particular,
Elytrigia repens,
E. intermedia,
Agropyron pectinatum). The phytodiversity and richness of the plant communities are generally reduced. Both rare and common species of steppe mixed herbaceous vegetation were removed from the composition of these communities. It was established that the current state of vegetation cover of steppe communities in the Kuyalnik Estuary valley (especially its left-bank slopes) is adversely affected not only by tree and shrubby communities, but also by grass ones.
The steppe communities developed on the slopes of the Kuyalnik Estuary valley are significantly vulnerable to the pyrogenic effect than the watershed ones since the conditions of the slope ecotopes (especially of the southern-, southwestern- and western-faced slopes) are significantly different from the watershed conditions and become similar to the deserted ones. The consequence of combined effect of geomorphogenic and anthropic factors is a change in zonal steppe communities (with the participation of Stipa pulcherrima and S. ucrainica) on derivative communities more resistant to many factors of anthropic influence: Stipa lessingiana and S. capillata, and the absence of shrubby steppes with the dominance of Amygdalus nana and a minor participation of Caragana frutex in steppe communities.
Changes in vegetation as a result of pasture impact on the territory of the Kuyalnik Estuary valley have both positive and negative effects. At all stages of the evolution of herbaceous plant communities, their vegetation cover served as a food reserve for many species of wild animals. Ungulates act as invariable components of the steppes [
68]. Moderate livestock grazing maintains the species and cenotic diversity in grass ecosystems, while grazing of ungulates in protected steppes is an effective and natural way to manage plant communities [
69]. Overgrazing has a profound destructive effect on steppe ecosystems [
70]. The massive grazing of sheep and goats is especially harmful for grass communities of pastures, the sheep and goats destroy the sod plants with their sharp hooves and cause a greater negative impact than eating of aboveground plant parts (
Figure 24). Direct, excessive livestock grazing affects the steppe vegetation negatively and causes the complete destruction of the native grass cover. It also affects it indirectly through soil compaction [
71,
72]. In addition, on the slopes of the Kuyalnik Estuary valley, in stock driving roads, numerous trails are formed, which can be eroded by storm and meltwater thereby increasing soil erosion and landslide phenomena.
In the process of pasqual changes, zonal saline-meadow, and steppe communities are changed to derivatives ones that are at the different stages of degradation. This leads to a reduction in the area of natural grass vegetation, a destruction of many steppe plant species (primarily rare ones listed in the Red Book of Ukraine), an increase in the number of weeds, poisonous, and inedible species for livestock, and an increase in their cenotic role. Areas of the coast of the Kuyalnik Estuary, the lower gentle parts of the slopes, the bottoms, and the slopes of large gullies located near settlements are intensively used as pastures. In recent years, there has been an increase in the grazing pressure on plant communities due to an increase in the number of sheep and goats. Such pressure exceeds the production capabilities of herbaceous plant communities in the Kuyalnik Estuary valley, which causes their degradation and development of series of successive phytocenoses.
Cattle grazing, particularly in coastal areas occupied by groups of pioneer halophytes of the class Therosalicornietea, is conducted in the second half of summer and autumn. During this period, a seasonal reduction in the surface of the estuary water area and the emergence of substantial coastal areas occurs. Grazing animals partially consume the aboveground parts of plants, but more often they trample, compact the soil, and change the microrelief of pastures. It was established that livestock grazing in areas occupied by communities of associations Salicornietum prostratae and Bassietum hirsutae does not cause significant violations in their structure, since they are restored annually as soon as ecotope conditions make it possible. Animals contribute to the spread of seeds of these species and their colonization of newly formed land areas. For a replacement of the above-mentioned groupings, associations of Halimionetum pedunculatae or Salicornio perennantis-Suaedetum salsae are developed on the heavily compacted soils only in some areas where coastal pastures are intensively used.
Degradation of communities of saline-steppe and saline vegetation under the influence of grazing is characterized by an increase in the number of ruderal species, such as Atriplex prostrata, Melilotus albus, Grindelia squarrosa, Centaurea solstitialis, Lactuca tatarica, Consolida regalis, Bromus squarrosus, Lepidium ruderale, and others. Moderate grazing pressure resulted in the development of communities of Artemisietum santonicae var. Bromus japonicus. Pasture intensification and associated xerophitization of environmental conditions cause the formation of communities of Poo bulbosae-Artemisietum santonicae and Taraxaco bessarabicae-Artemisietum santonicae associations. In addition to representatives of saline-meadow vegetation, xerophytic synanthropic species Achillea setacea, Artemisia austriaca, Carduus acanthoides, Centaurea adpressa, Grindelia squarrosa, Lactuca tatarica, Taraxacum bessarabicum, and others take a significant part in the communities of the latter association.
In the floodplain of the Great Kuyalnik River valley, livestock grazing is conducted (as already mentioned) on the fallows, causing the development of low-productive digressive communities of Taraxaco bessarabicae-Artemisietum santonicae association with a substantial presence of Bassia sedoides and Grindelia squarrosa. Ruderal communities of Onopordetum acanthii, Carduo acanthoidis-Onopordetum acanthii, Hyoscyamo nigri-Conietum maculati are being formed in the sites of camps and sheepfolds. In areas of fallows subjected to intensive grazing, the herbage degrades and specific succession communities of Poo bulbosae-Artemisietum santonicae and Taraxaco bessarabicae-Artemisietum santonicae are developed. Poa sterilis, P. bulbosa and other cereals are resistant to grazing pressure predominated in these communities, which cover large territories in the floodplain part of the valley. In some areas around low aeolian hills, in conditions of sufficient flushing, processes of formation of columnar saline soil covered with tall thickets of Atriplex tatarica are observed.
It was established that the floral richness of steppe communities Is directly related to the grazing intensity and duration and decreases with the prolongation of the grazing duration. In the estuary valley, five stages of pasqual digression were identified using the example of steppe communities with the association
Stipo lessingianae-Salvietum nutantis, which are also typical for other regions of the Northern Black Sea region [
71,
73]. At the first stage (weak grazing), communities in associations of
Stipo lessingianae-Salvietum nutantis followed the typical structure: the projective cover is 80–90%, the herbage includes two or three sub-layers, and the maximal height of which reaches 80 cm. A sparse (0.2–0.3) layer of tall bushes with
Crataegus monogyna and
Rosa canina or low bushes with
Caragana frutex is quite often observed.
Stipa lessingiana and
S. capillata dominated,
S. ucrainica occasionally occurs; the floral composition of the communities includes 50–60 species. Plant communities on this stage are still floristically rich and quite high-productive. However, there is a certain decrease in their vitality, and sometimes a decrease in the number of coenopopulations of
Stipa lessingiana and xeromesophytic mixed grass species.
At the second stage, along with an increase in grazing pressure or in the case of sheep and goat grazing, a change in the floristic composition of communities is manifested by a decrease in the number of mixed grass species (primarily legumes). Species of the genus
Stipa degrade much more slowly. After grazing cessation, the steppe restores relatively quickly, up to 5 years. At the third stage of pasqual digression accompanied by prolonged grazing of significant livestock numbers, the dominant role passes to
Agropyron pectinatum (gentle slopes with degraded chernozems) or
Bothriochloa ischaemum (steeper slopes covered with washed-out gravel or clay soils). The role of annual cereals
Bromus japonicus,
B. squarrosus,
Anisantha tectorum, and other species:
Euphorbia seguieriana,
Salvia nemorosa,
Kochia prostrata,
Koeleria cristata,
Artemisia austriaca, and others is enhanced. Typical mixed grass steppe species (
Salvia nutans,
Astragalus albidus,
A. austriacus,
A. onobrychis,
Phlomis pungens, and many others) fall from the grass stands. The total projective cover of communities is reduced. At this stage of digression,
Salvio nemorosae-Festucetum valesiacae var.
Artemisia austriaca communities (on degraded chernozems),
Salvio nemorosae-Elytrigietum intermediae (on saline soils and landslide steep slopes), and
Bothriochloetum ischaemi (on washed-out gravel soils) are developing on pastures [
74]. A special feature of the 3rd-stage phytocenoses is their mosaic horizontal structure characterized by an appearance of different-sized patches formed by fragments of ruderal communities with a predominance of
Polygonum aviculare,
Trifolium campestre,
Medicago minima,
Erodium cicutarium, and others.
At the second and third stages of pasqual digression, steppe communities still retain the main characteristic of their structure, and a sufficient seed reserve. After the cessation or substantial reduction in the grazing pressure, the recovery of feather grass steppes occurs only after 5–8 years, as confirmed by long-term studies.
The fourth stage of excessive grazing pressure (extreme overstocking) is typical for the most intensively used areas. At this stage, sod grasses disappear from the herbage. Instead, annual and short-lived Roa bulbosa, Bromus japonicus, B. squarrosus, Anisantha tectorum, and Aegilops cylindrica dominate. Low-productive and floristically poor communities of Poo bulbosae-Artemisietum santonicae, Anisantho-Artemisietum austriacae, Aegilopsetum cylindricae, Artemisia absinthium-Bromus squarrosus comm., and other associations are formed. Only the most xerophytic species of steppe mixed grasses, such as Marrubium peregrinum, Euphorbia seguierana, Potentilla supina, Eryngium campestre, and Falcaria vulgaris, are still presented in the vegetation cover. Ruderal and poisonous plant species predominate. Restoration of the primary vegetation cover of poor pastures subjected to grazing cessation requires a longer time period: 9–15 years.
The fifth stage is represented by communities of ruderal species (Conium maculatum, Heliotropium europaeum, Onopordum acanthium, Chondrilla juncea, Grindelia squarrosa, Lactuca serriola, Carduus crispus, Xanthium albinum, Centaurea solstitialis, Polygonum aviculare, and annual and minor species of cereals). They are formed in places of massive and long-term stay of livestock near barns, farms, and watering holes. It was found that compaction of the surface soil horizon is a specific characteristic of intensively used pastures and sites of long-term livestock keeping. This causes an increase in its capillarity, deterioration of aeration and water permeability, and surface overheating in summer and overcooling in winter. Secondary salinization and, as a result, an increased risk of erosion are also observed. The grass cover of such areas is sparse. The vegetation of paddocks is practically not restored naturally and requires special remedial measures.
On the territory of the estuary valley, the strongest pasqual degradation of steppe vegetation is observed on the slopes of the valleys of the Novokubanska, Kubanska and Ilinska gullies, and on the main slopes of the estuary valley near the villages of Stara Emetivka, Kovalivka, Ilinka, Lativka, and Krasnosilka. The state of the steppe sites in these areas corresponds to the fourth and sometimes fifth stages of pasture digression. On the part of the slopes below the Novokubanska gully, in the direction of the Krasnosilka village, moderate livestock grazing takes place. The state of steppe areas corresponds to the second and third stages of pasture digression. A cattle drive through loess slopes intensifies erosion processes. An increase in the number of goats grazing on the territory of the locality of
Glycyrrhiza glabra (species listed in the Red Book of Ukraine, unique in the region) poses, as already noted, a real threat of its complete destruction [
75].
Mowing-induced vegetation changes depend on the mowing timing and frequency, the cutting height, and type of haymaking management measures. In the estuary valley, only some coastal territories are subjected to annual mowing, and areas of plateaus and gentle slopes near settlements with digressive-demutational steppe communities. Haymaking is not performed on the slopes.
Areas of fallows and abandoned pastures on the left-bank estuary valley are regularly mowed down. Their vegetation cover is represented by communities with a prevalence of Aegilops cylindrica, Anisantha tectorum, Bromus squarrosus, and other annual cereals. Haymaking of the areas is performed once a year, mechanically, in early summer, in the pre-seeding stage. This influence causes gradual positive changes in the structure of vegetation cover toward the formation of meadow-steppe and steppe communities. In some cases, the phenisicial factor, especially in combination with pyrogenic and pasqual factors, can have a catastrophic effect on vegetation. Research on the location of the rare species Glycyrrhiza glabra growth has shown that its cenopopulations tend to degrade as a result of annual mowing of these areas.
On the slopes of the left-bank estuary, on large areas of fallows, quasi-savanoid communities of
Aegilopsetum cylindricae association develop (
Figure 25). Along with a high projective cover (up to 80%), they are dominated by
Aegilops cylindrica, and the floral composition is dominated by annual cereals (such as
Hordeum murinum,
Bromus squarrosus,
B. japonicus,
Anisantha tectorum) and ruderal, mostly annual or biennial species (such as
Centaurea adpressa,
Chenopodium urbicum,
Cirsium setosum,
Convolvulus arvensis,
Crepis tectorum,
Papaver rhoeas,
Grindelia squarrosa,
Lepidium ruderale,
Melilotus officinalis, and others). Sod cereals (such as
Stipa lessingiana,
S. capillata,
Festuca valesiaca, and
Agropyron pectinatum), and steppe mixed grass species (such as
Salvia nemorosa,
S. nutans,
Eryngium campestre, etc.) are presented sporadically. Their presence indicates the beginning of the development of demutational steppe communities on the next successive stage. Moderate anthropic influence (such as periodic mowing and burning) contributes to the increase in the role of steppe species, primarily sod cereals, as a result of which the demutation of steppe vegetation here occurs without the participation of rhizomatous cereals. This stage is typical for areas of recently abandoned fields that are not mowed or grazed, but only spontaneously burned.
Demutation of steppe vegetation occurs, according to the classical scheme, through the stages of ruderal vegetation and rhizomatous cereals. The ruderal stage is characterized by the formation of communities of Artemisietum annuae, Onopordetum acanthii associations, or non-ranking groupings dominated by tall species such as Descurainia sophia, Centaurea solstitialis, Melilotus albus, and so on. Over time the participation of rhizomatous cereals Elytrigia repens, Poa angustifolia, and Bromopsis riparia and types of steppe mixed grasses, such as Seseli campestre, Salvia nemorosa, Teucrium chamaedrys, Plantago lanceolata, Tragopogon major, and others, increases in herbage evidencing the beginning of the stage of rhizomatous cereals.
Regular frequent mowing of meadow and steppe plant communities causes negative changes in the composition and productivity of herbage species [
71,
76,
77,
78]. As a result of annual mowing, plant species that are in the state of flowering or budding during the mowing period fallout from the grass stands. Removal of substantial phytomass volumes leads to soil overheating and water evaporation resulting in xerization of ecotopes, and, subsequently, of plant communities. Frequent mowing of steppe vegetation leads to a decrease in its complexity and mosaicity, simplification of horizontal and vertical structures, and to floristic impoverishment.
Under the influence of recreation, vegetation dynamics are caused by mechanical disturbances of the vegetation cover, soil compaction, changes in its physical and chemical characteristics, water supply, and chemical and mechanical contamination (such as spent fuel, lubricants, waste products, food residues, and household waste). All these factors are associated with the presence of many people in natural ecosystems and spontaneous recreational activities.
In the southern part of the Kuyalnik Estuary valley, there is a large sanatorium-resort complex “Kuyalnik”, which covers an area of about 18 hectares. On its territory, native vegetation is totally destroyed, and largely degraded on beaches and other recreation areas of coastal territories due to direct trampling. By trampling, the continuous vegetation cover is also divided into separate bio-groups by pathways and viewing platforms. The share of ruderal annual cereals (such as Bromus squarrosus, B. mollis, Anisantha tectorum, and Aegilops cylindrica) and adventitious species (such as Xeranthemum annum and Grindelia squarrosa) rises in the structure of communities. Demutations of native vegetation occur extremely slowly in areas of long-term stay of recreationists.
In the rest of the estuary valley, recreational activities are also limited to coastal and slope areas. During the summer period, annual extreme car rallies are performed on the coastal areas released from water. Their route passes through the coast of the estuary covered by halophytic vegetation. Other areas of the route pass along slopes with limestone outcrops occupied by petrophytic vegetation. The total rally distance is more than 100 km. The car wheels destroy and transform the vegetation cover, compact the soil, and leave deep ruts. Additionally, chemical soil contamination also occurs.
Unauthorized bike tracks laid on the right bank of the valley also pose a threat to the steppe and shrubby vegetation of the slopes. Annual autumn bicycle rallies with a length greater than 70 km gather annually about 1500 participants and fans. Deep bicycle tracks serve as channels for temporary watercourses of stormwater and meltwater, increasing erosion processes on the slopes.
Vegetation changes due to invasions of alien species occur throughout the estuary valley. A total of 87 adventive species are distributed here, which is 18% of the total floristic composition [
79]. The expansion of these species causes changes in the structure, functioning, and stability of natural ecosystems, displacement of native plant species, and biological contamination of the gene pool due to the hybridization of close native and non-native taxa [
80]. Especially harmful are transformer plants capable of transforming ecosystems that have overcome the phytocenotic barrier and become a part of the native flora. They radically change the structure, dynamics, and functions of native plant communities or create new cenoses that displace the aboriginal ones.
The most intense changes in vegetation cover due to adventization processes are observed in the coastal territories of the valley in a zone of saline-meadow and meadow-steppe communities. Currently, in territories where communities of alliance Plantagini salsae-Artemision santonicae should prevail, substantial areas are occupied by phytocenoses dominated by transformer species Elaeagnus angustifolia and E. commutata. The grass cover is dominated by archaeophytes and kenophytes: Bromus squarrosus, Grindelia squarrosa, Atriplex prostrata, Cichorium intybus, Carduus acanthoides, Anisantha tectorum, and A. sterilis, which displace the species of native flora. The source of their appearance and distribution within the region is primarily ruderal phytocenoses.
A total of 15 species have the highest invasive capacity: Anisantha tectorum, Echinochloa crusgalli, Capsella bursa-pastoris, Ambrosia artemisiifolia, Conyza canadensis, Bromus squarrosus, Centaurea diffusa, Carduus acanthoides, Grindelia squarrosa, Xanthium albinum, Elaeagnus angustifolia, Iva xanthiifolia, Brachyactis ciliata, Hordeum murinum, and Papaver rhoeas, and transformer species occur among them. Descurainia sophia, Portulaca oleracea, Onopordum acanthium, Artemisia absinthium, Atriplex prostrata, A. tatarica, Cichorium intybus, Lactuca serriola, Sclerochloa dura, Senecio vulgaris, Setaria viridis, Sonchus arvensis, S. oleraceus, Amaranthus blitoides, A. retroflexus show moderate activity.
A great hazard for the native communities of the valley is the spread of such transformer species as
Elaeagnus angustifolia,
E. commutata, and
Cotinus coggygria. These species form a community with a gradually increasing area due to the transformation of grass steppe vegetation. These results are consistent with the results of studies conducted in native steppe reserves (“Streltsivsky steppe”, “Stone Grave”, etc.). A characteristic feature of modern processes associated with the increasing anthropogenic transformation of the vegetation cover in the steppe zone is the formation of thickets of foreign tree species at the early stages of succession (from 10 years), most often with the participation of
Ulmus pumila,
Fraxinus lanceolata, and
Acer negundo [
81,
82]. The succession course typical of the steppe zone is observed only in areas where moderate grazing and/or haymaking is performed.
In the Kuyalnik Estuary valley, plantings of Celtis occidentalis inhibit the development of all other species, especially native flora due to the strong allelopathic effect. Rooting of individual plants of C. occidentalis to shrub groupings of Crataegus monogyna-Cotinus coggygria comm. and Crataegus monogyna-Rosa canina comm. located near their parent plantings was recorded. Cotinus coggygria has also demonstrated the tendency to spread on the right bank slopes of the valley. This species is well restored by seeds and forms thickets characterized by high crown closure and lack of grass cover. C. coggygria is an expansive species in the estuary valley and inhabits ecotopes occupied by native steppe and shrubby communities. Lycium barbarum is much less common, and it forms thickets mainly in disturbed areas in the vicinity of settlements and is not introduced into native groupings.
Identifying historical changes in vegetation cover, particularly caused by human activity, including quantifying their scales in space and time, is crucial for assessing long-term impacts on biodiversity and ecosystem functioning [
83]. Understanding the processes underlying historical responses will also contribute to the development of appropriate mitigation strategies to reduce the harmful effects of human activity on natural systems [
84]. This is of particular importance for arid regions of the planet [
85].
Multiple works were devoted to historical changes in the vegetation of the Black Sea region [
71,
86,
87,
88,
89,
90]. For thousands of years, changes in climatic conditions have caused corresponding historical transformations of plant communities. In the early Holocene, periglacial steppes were transformed into meadow, true, and desert steppes occupied most of the territory of modern Ukraine [
86]. In the middle and late Holocene, substantial areas were covered by woody vegetation. It was at this time that the border of the steppe zone came as close as possible to the modern one, and the forests along the river valleys reached the Black Sea. The steppes of Ukraine 4–5 millennia ago were distinguished by a wide variety of herbage, substantial spatial differentiation, widely distributed shrubby communities, and greater afforestation than what currently there. The forests stretched into valleys of rivers, estuaries, and gullies. In some places, they reached the watershed areas [
88].
With the development and intensification of economic activity, forests were almost totally destroyed, and steppe vegetation was changed toward its xerophytization. In the pre-agricultural period, in the lower part of the Great Kuyalnik River and in the estuary valley, the gentle slopes of the valley side and gullies were occupied by broad-leaved forests, and the steeper slopes were covered by thickets of mesoxerophilic bushes. Steppe and petrophytic-steppe communities were common in places with Pontic limestone outcrops or near-surface limestones, and on steep erosive slopes, the vegetation of which underwent cyclical geomorphogenic changes [
89].
In the process of centuries-old development of the banks of estuaries, river valleys, gullies, and ravines with the retreat of coastal strips, due to landslide processes, the vegetation of newly formed watershed areas with zonal steppes formed on them migrated to the slopes and participated in the formation of the slope plant communities [
34].
At the beginning of the nineteenth century, as previously noted, the active development of the estuary valley and surrounding territories continued. Kubanka, Emetivka, Severinivka, Ilinka, and other villages were built. As a result of intensive economic activity (such as deforestation, grazing, burning of shrubs, grass stands, and ploughing of watershed territories) by the beginning of the 20
th century, the native woody vegetation was almost completely destroyed, and the grass vegetation underwent substantial changes in this area. At the end of the 19
th century, P.S. Shesterikov noted that in the southwestern part of the then Odesa district “every convenient piece of land is used with special diligence” [
32]. Ruderal communities created by members of the genera
Euphorbia,
Onopordum,
Carduus,
Cirsium, and others were formed around the settlements and in grazing sites. Native forests were destroyed, and thickets of shrubs with
Euonymus europaea,
Ligustrum vulgare,
Cerasus mahaleb,
Prunus spinosa, etc., remained on the slopes of the estuary, in the grass layer of which such relict forest elements as
Corydalis solida,
Gymnospermium odessanum,
Anemone sylvestris,
Pulsatilla nigricans, and others were preserved. Native vegetation has been preserved only in gullies, ravines, sands, and salt marshes, particularly in deep gullies between the Severinivka and Ilinka villages. As already noted, some negative changes have also taken place in the estuary valley over the past century. As already noted, a substantial share of the areas on the right-bank slopes was terraced and planted with trees and shrubs with a large participation of introducents, and the proportion of adventive species in the composition of grass communities increased.
A prediction of further changes in vegetation was made based on the analysis of the dynamic trends of the Kuyalnik Estuary valley. There are many publications and models in the literature that predict the response of plant communities related to global changes. These mainly include changes in ecological niches, population dynamics, species interactions, spatial disturbances, ecosystem processes, and plant functional responses [
12]. The leading factors are anthropogenic, which slow down, accelerate, or change the natural processes of functioning of plant communities. Strengthening or weakening of this action (first and foremost, the elimination of any natural factor involved in the formation of plant communities) leads to a disruption of the system of external and internal relationships in vegetation cover and, ultimately, to its transformation.
If the intensity of anthropogenic factors is maintained, the vegetation cover of the Kuyalnik Estuary valley will be exposed to negative consequences. Intensive, debilitating use of natural resources in the Great Kuyalnik River valley, the estuary itself and small rivers within its basin, and watershed adjacent territories, construction and expansion of village areas, resort and recreational pressure will cause a reduction in the volume of river flow, an increasing topsoil wash-out along the valley slopes, an abrasion of the slopes and a secondary salinization of large territories.
Currently, the negative impact of human influence on the ecosystems of the Kuyalnik Estuary valley and adjacent territories is increasing because of global climate change. The water area of the estuary will continue to decrease, while the area of the dry bottom will increase, transforming it into a salt desert (
Figure 26). Slope biotopes will undergo xerophytization and halophytization due to air transport of salts, and steppe communities will continue to change towards desertification. Salinization in the lower parts of the slopes will contribute to the further expansion of small-species halophilic-steppe communities (
Galatella biflora, and others) which will occupy territories previously covered by steppe and shrubby communities. Frequent and severe fires will cause a sharp reduction in the area of shrubby vegetation and a degradation of steppe vegetation.
On the estuary coast and in the floodplain part of the Great Kuyalnik River valley the halophytization processes are intensified. Simultaneously with an increase in pasture load, this will cause a sharp decrease in the number of saline-meadow species and an increase in semi-desert ones (ephemera and ephemeroids), and species indicator of saline and salt marsh soils. The permanent nature of pyrogenic impact on steppe communities will cause even greater desertification and degradation, reducing the role of species of the genus Stipa, and the development of desert-steppe communities with a dominance of Elytrigia repens, Agropyron pectinatum, Kochia prostrata, and Artemisia austriaca. In general, all this can result in the degradation of the unique mega-geosystem of the Kuyalnik Estuary valley.




 —Lemnetea,
—Lemnetea,  —Potamogetonetea,
—Potamogetonetea,  —Phragmito-Magnocaricetea;
—Phragmito-Magnocaricetea;  —Bolboschoenetea maritimi,
—Bolboschoenetea maritimi,  —Therosalicornietea,
—Therosalicornietea,  —Juncetea maritimi,
—Juncetea maritimi,  —Festuco-Puccinellietea;
—Festuco-Puccinellietea;  —Festuco-Brometea,
—Festuco-Brometea,  —Sedo-Scleranthetea;
—Sedo-Scleranthetea;  —Salicetea purpureae,
—Salicetea purpureae,  —Robinietea,
—Robinietea,  —Crataego-Prunetea;
—Crataego-Prunetea;  —Stellarietea mediae,
—Stellarietea mediae,  —Artemisietea vulgaris,
—Artemisietea vulgaris,  —Polygono-Poetea annuae.
—Polygono-Poetea annuae.
 —Lemnetea,
—Lemnetea,  —Potamogetonetea,
—Potamogetonetea,  —Phragmito-Magnocaricetea;
—Phragmito-Magnocaricetea;  —Bolboschoenetea maritimi,
—Bolboschoenetea maritimi,  —Therosalicornietea,
—Therosalicornietea,  —Juncetea maritimi,
—Juncetea maritimi,  —Festuco-Puccinellietea;
—Festuco-Puccinellietea;  —Festuco-Brometea,
—Festuco-Brometea,  —Sedo-Scleranthetea;
—Sedo-Scleranthetea;  —Salicetea purpureae,
—Salicetea purpureae,  —Robinietea,
—Robinietea,  —Crataego-Prunetea;
—Crataego-Prunetea;  —Stellarietea mediae,
—Stellarietea mediae,  —Artemisietea vulgaris,
—Artemisietea vulgaris,  —Polygono-Poetea annuae.
—Polygono-Poetea annuae.