Aquatic Plant Invasion and Management in Riverine Reservoirs: Proactive Management via a Priori Simulation of Management Alternatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Target Species
2.2. Model Description
2.3. Model Calibration
2.4. Model Evaluation
2.5. Model Application
3. Results
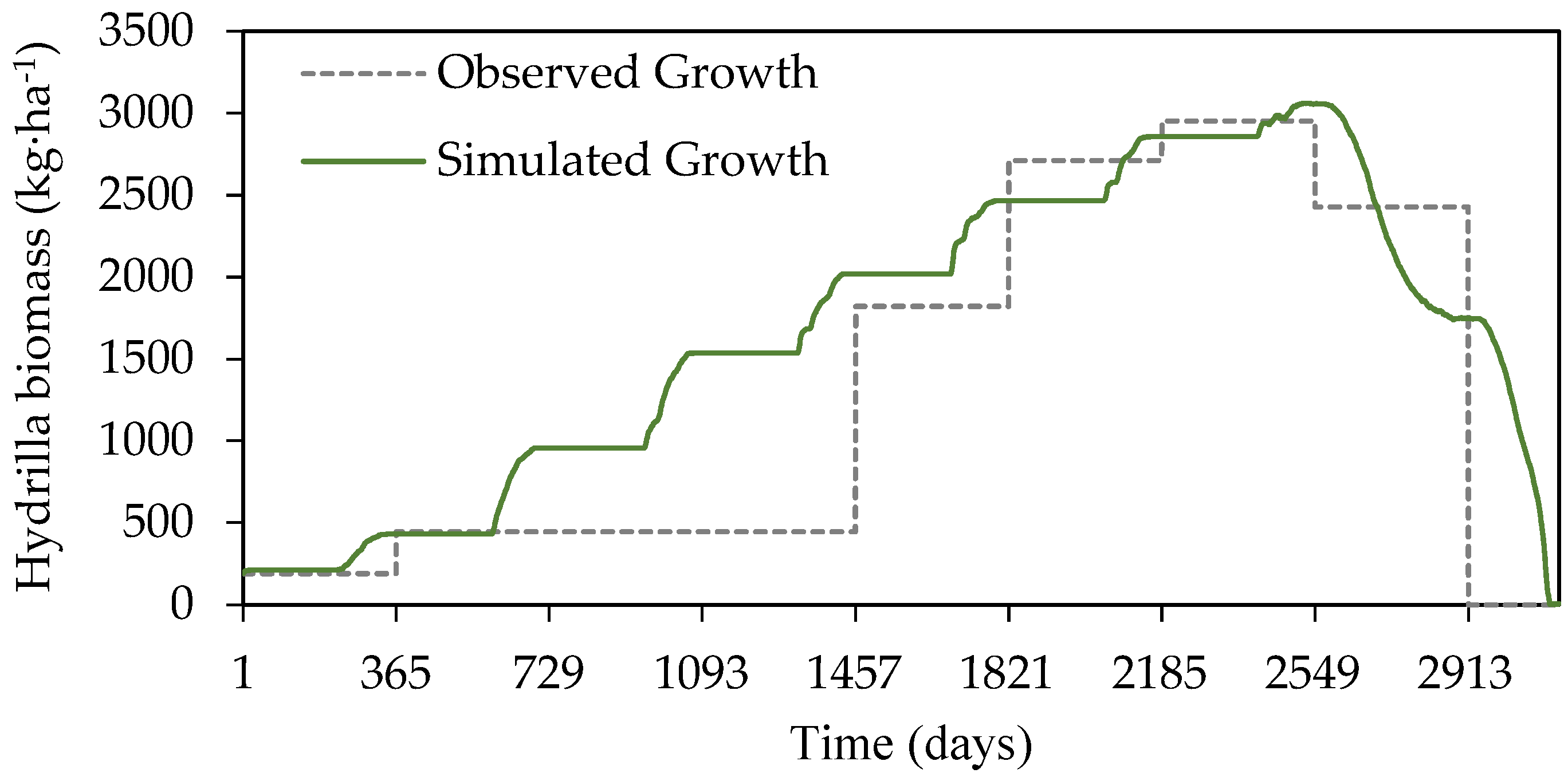
3.1. Model Calibration and Evaluation
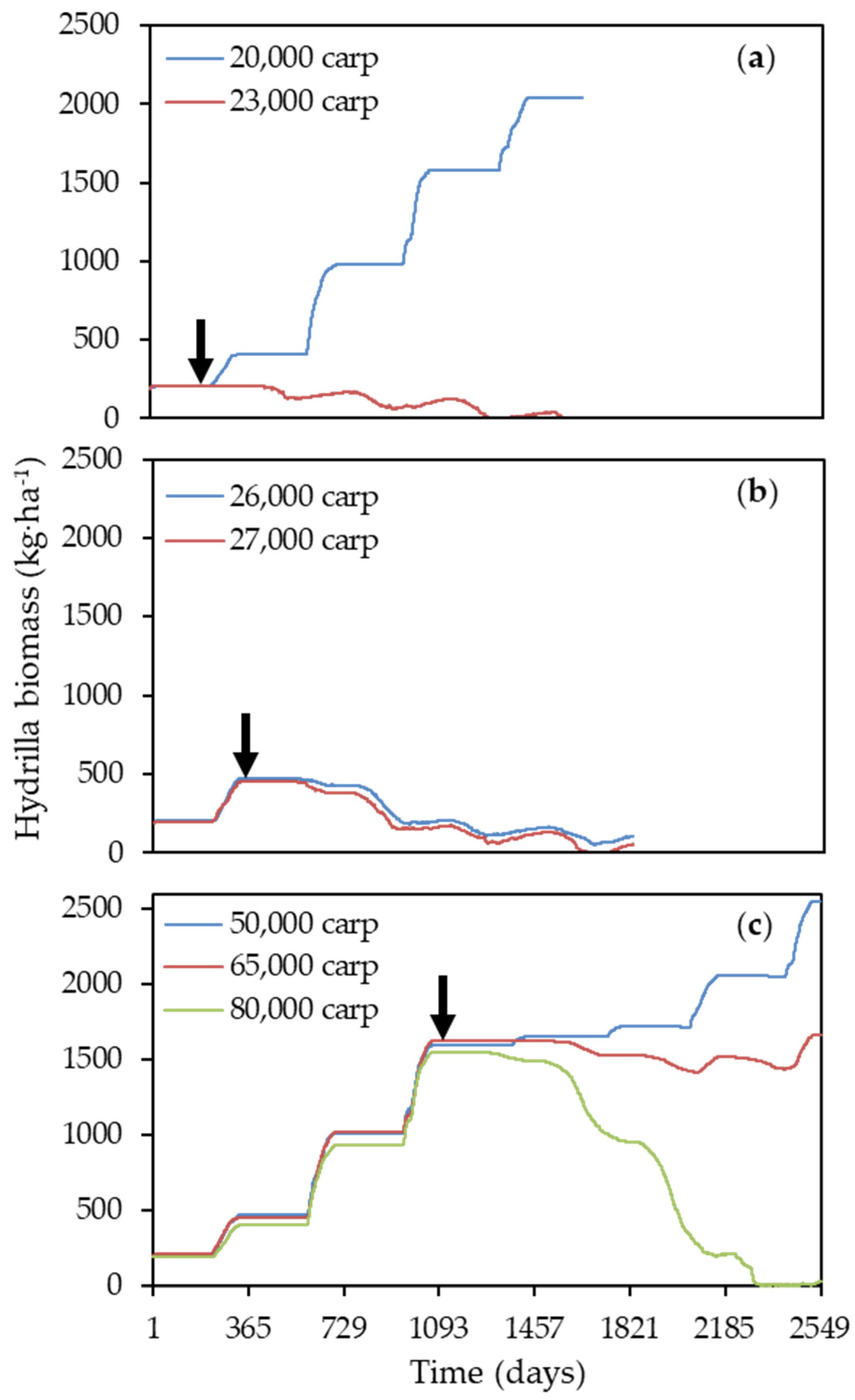
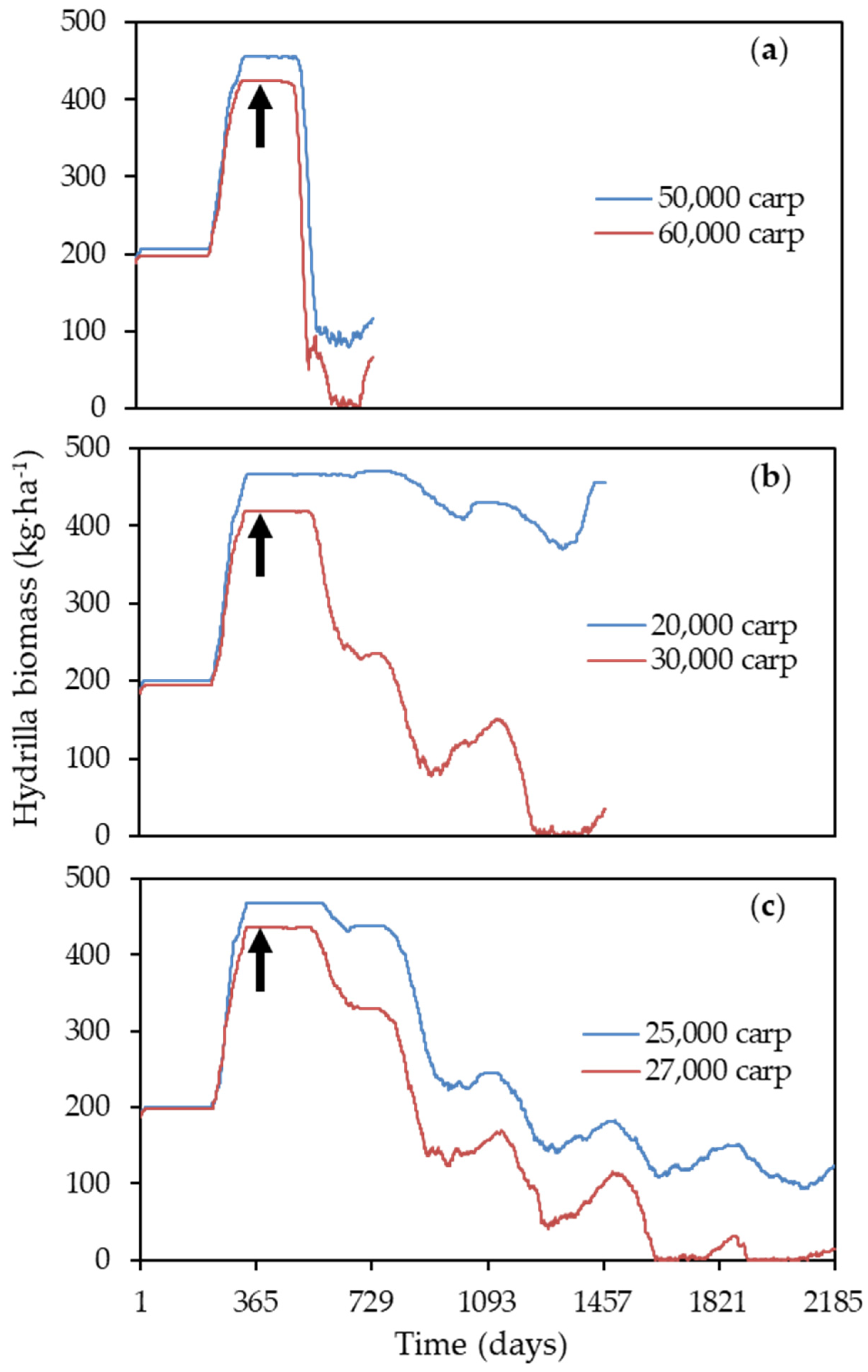
3.2. Model Application
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simberloff, D.; Martin, J.-L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.-R.; Chen, Y.-J.; Wang, H.-H.; Grant, W.E. Predicted range expansion of the invasive plant Leucaena leucocephala in the Hengchun peninsula, Taiwan. Biol. Invasions 2016, 18, 381–394. [Google Scholar] [CrossRef]
- Wang, H.-H.; Koralewski, T.E.; McGrew, E.K.; Grant, W.E.; Byram, T.D. Species Distribution Model for Management of an Invasive Vine in Forestlands of Eastern Texas. Forests 2015, 6, 4374–4390. [Google Scholar] [CrossRef]
- Wang, H.-H.; Grant, W.E.; Teel, P.D.; Lohmeyer, K.H.; Pérez de León, A.A. Enhanced biosurveillance of high-consequence invasive pests: Southern cattle fever ticks, Rhipicephalus (Boophilus) microplus, on livestock and wildlife. Parasites Vectors 2020, 13, 487. [Google Scholar] [CrossRef]
- Luque, G.M.; Bellard, C.; Bertelsmeier, C.; Bonnaud, E.; Genovesi, P.; Simberloff, D.; Courchamp, F. The 100th of the world’s worst invasive alien species. Biol. Invasions 2014, 16, 981–985. [Google Scholar] [CrossRef]
- Zedler, J.B.; Kercher, S. Causes and consequences of invasive plants in wetlands: Opportunities, opportunists, and outcomes. Crit. Rev. Plant Sci. 2004, 23, 431–452. [Google Scholar] [CrossRef]
- Lovell, S.J.; Stone, S.F.; Fernandez, L. The economic impacts of aquatic invasive species: A review of the literature. Agric. Resour. Econ. Rev. 2006, 35, 195–208. [Google Scholar] [CrossRef]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Eiswerth, M.E.; Donaldson, S.G.; Johnson, W.S. Potential environmental impacts and economic damages of Eurasian watermilfoil (Myriophyllum spicatum) in western Nevada and northeastern California. Weed Technol. 2000, 14, 511–518. [Google Scholar] [CrossRef]
- Adams, D.C.; Lee, D.J. Estimating the value of invasive aquatic plant control: A bioeconomic analysis of 13 public lakes in Florida. J. Agric. Appl. Econ. 2007, 39, 97–109. [Google Scholar] [CrossRef]
- Bianchini, I.; Cunha-Santino, M.B.d.; Milan, J.; Rodrigues, C.; Dias, J. Growth of Hydrilla verticillata (Lf) Royle under controlled conditions. Hydrobiologia 2010, 644, 301–312. [Google Scholar] [CrossRef]
- Owens, C.S.; Madsen, J.D.; Smart, R.M.; Stewart, R.M. Dispersal of native and nonnative aquatic plant species in the San Marcos River, Texas. J. Aquat. Plant Manag. 2001, 39, 75–79. [Google Scholar]
- Mony, C.; Koschnick, T.; Haller, W.; Muller, S. Competition between two invasive Hydrocharitaceae (Hydrilla verticillata (Lf)(Royle) and Egeria densa (Planch)) as influenced by sediment fertility and season. Aquat. Bot. 2007, 86, 236–242. [Google Scholar] [CrossRef]
- Thomaz, S.M.; Mormul, R.P.; Michelan, T.S. Propagule pressure, invasibility of freshwater ecosystems by macrophytes and their ecological impacts: A review of tropical freshwater ecosystems. Hydrobiologia 2015, 746, 39–59. [Google Scholar] [CrossRef]
- Anderson, L.G.; Dunn, A.M.; Rosewarne, P.J.; Stebbing, P.D. Invaders in hot water: A simple decontamination method to prevent the accidental spread of aquatic invasive non-native species. Biol. Invasions 2015, 17, 2287–2297. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-H.; Grant, W.E.; Swannack, T.M.; Gan, J.; Rogers, W.E.; Koralewski, T.E.; Miller, J.H.; Taylor, J.W. Predicted range expansion of Chinese tallow tree (Triadica sebifera) in forestlands of the southern United States. Divers. Distrib. 2011, 17, 552–565. [Google Scholar] [CrossRef]
- Wang, H.-H.; Grant, W.E.; Gan, J.; Rogers, W.E.; Swannack, T.M.; Koralewski, T.E.; Miller, J.H.; Taylor, J.W. Integrating spread dynamics and economics of timber production to manage Chinese tallow invasions in southern U.S. forestlands. PLoS ONE 2012, 7, e33877. [Google Scholar] [CrossRef]
- Wang, H.-H.; Grant, W.E.; Koralewski, T.E.; Elliott, N.C.; Brewer, M.J.; Westbrook, J.K. Where do all the aphids go? A series of thought experiments within the context of area-wide pest management. Agric. Syst. 2020, 185, 102957. [Google Scholar] [CrossRef]
- Wang, H.-H.; Grant, W.E.; Teel, P.D.; Lohmeyer, K.H.; Pérez de León, A.A. Simulated dynamics of southern cattle fever ticks (Rhipicephalus (Boophilus) microplus) in south Texas, USA: Investigating potential wildlife-mediated impacts on eradication efforts. Parasites Vectors 2021, 14, 231. [Google Scholar] [CrossRef]
- Van Nes, E.H.; Scheffer, M.; van den Berg, M.S.; Coops, H. Charisma: A spatial explicit simulation model of submerged macrophytes. Ecol. Model. 2003, 159, 103–116. [Google Scholar] [CrossRef]
- Santha, C.R.; Grant, W.; Neill, W.H.; Strawn, R.K. Biological control of aquatic vegetation using grass carp: Simulation of alternative strategies. Ecol. Model. 1991, 59, 229–245. [Google Scholar] [CrossRef]
- Sintes, T.; Moragues, E.; Traveset, A.; Rita, J. Clonal growth dynamics of the invasive Carpobrotus affine acinaciformis in Mediterranean coastal systems: A non-linear model. Ecol. Model. 2007, 206, 110–118. [Google Scholar] [CrossRef]
- Kelly, R.; Lundy, M.G.; Mineur, F.; Harrod, C.; Maggs, C.A.; Humphries, N.E.; Sims, D.W.; Reid, N. Historical data reveal power-law dispersal patterns of invasive aquatic species. Ecography 2014, 37, 581–590. [Google Scholar] [CrossRef]
- Jacobs, M.J.; Macisaac, H.J. Modelling spread of the invasive macrophyte Cabomba caroliniana. Freshw. Biol. 2009, 54, 296–305. [Google Scholar] [CrossRef]
- Van den Brink, P.J.; Baveco, J.M.; Verboom, J.; Heimbach, F. An individual-based approach to model spatial population dynamics of invertebrates in aquatic ecosystems after pesticide contamination. Environ. Toxicol. Chem. 2007, 26, 2226–2236. [Google Scholar] [CrossRef]
- Miller, J.K.; Roketenetz, L.; Garris, H. Modeling the interaction between the exotic invasive aquatic macrophyte Myriophyllum spicatum and the native biocontrol agent Euhrychiopsis lecontei to improve augmented management programs. BioControl 2011, 56, 935–945. [Google Scholar] [CrossRef]
- Herbert, M.E.; Gelwick, F.P.; Montgomery, W.L. Spatial variation of headwater fish assemblages explained by hydrologic variability and upstream effects of impoundment. Copeia 2003, 2003, 273–284. [Google Scholar] [CrossRef]
- Chilton, E.W.; Webb, M.A.; Ott, R.A. Hydrilla management in Lake Conroe, Texas: A case history. In Proceedings of the American Fisheries Society Symposium, Ottawa, ON, Canada, 18 August 2008; pp. 247–257. [Google Scholar]
- Smart, R.M.; Dick, G.; Doyle, R. Techniques for establishing native aquatic plants. J. Aquat. Plant Manag. 1998, 36, 44–49. [Google Scholar]
- Chadwell, T.B.; Engelhardt, K.A. Effects of pre-existing submersed vegetation and propagule pressure on the invasion success of Hydrilla verticillata. J. Appl. Ecol. 2008, 45, 515–523. [Google Scholar] [CrossRef]
- Barrat-Segretain, M.H. Biomass allocation in three macrophyte species in relation to the disturbance level of their habitat. Freshw. Biol. 2001, 46, 935–945. [Google Scholar] [CrossRef]
- Efremov, A.; Bolotova, Y.; Mesterházy, A.; Toma, C. Features of Distribution of Hydrilla verticillata (L. fil.) Royle (Hydrocharitaceae) in North Eurasia. J. Coast. Res. 2007, 34, 675–686. [Google Scholar] [CrossRef]
- EDDMapS. Hydrilla, Hydrilla verticillata (L. f.) Royle. In Early Detection & Distribution Mapping System; The University of Georgia: Athens, GA, USA, 2017; Available online: http://www.eddmaps.org/ (accessed on 30 July 2016).
- Michel, A.; Arias, R.S.; Scheffler, B.E.; Duke, S.O.; Netherland, M.; Dayan, F.E. Somatic mutation-mediated evolution of herbicide resistance in the nonindigenous invasive plant Hydrilla (Hydrilla verticillata). Mol. Ecol. 2004, 13, 3229–3237. [Google Scholar] [CrossRef] [PubMed]
- Dibble, E.D.; Kovalenko, K. Ecological impact of grass carp: A review of the available data. J. Aquat. Plant Manag. 2009, 47, 1–15. [Google Scholar]
- Chilton, E.W.; Muoneke, M.I. Biology and management of grass carp (Ctenopharyngodon idella, Cyprinidae) for vegetation control: A North American perspective. Rev. Fish Biol. Fish. 1992, 2, 283–320. [Google Scholar] [CrossRef]
- Cuda, J.; Charudattan, R.; Grodowitz, M.; Newman, R.; Shearer, J.; Tamayo, M.; Villegas, B. Recent advances in biological control of submersed aquatic weeds. J. Aquat. Plant Manag. 2008, 46, 15–32. [Google Scholar]
- Wilensky, U. NetLogo; Center for Connected Learning and Computer-Based Modeling, Northwestern University: Evanston, IL, USA, 1999; Available online: http://ccl.northwestern.edu/netlogo/ (accessed on 26 July 2016).
- TWDB. Volumetric Survey of Lake Conroe. Available online: http://www.twdb.texas.gov/hydro_survey/conroe/1996-04/ (accessed on 6 September 2016).
- USNO. Sun or Moon Rise/Set Table for One Year: U.S. Cities and Towns. Available online: http://aa.usno.navy.mil/data/index.php (accessed on 6 September 2016).
- Pipalova, I. A review of grass carp use for aquatic weed control and its impact on water bodies. J. Aquat. Plant Manag. 2006, 44, 1–12. [Google Scholar]
- Langeland, K.A. Hydrilla verticillata (LF) Royle (Hydrocharitaceae), “The Perfect Aquatic Weed”. Castanea 1996, 61, 293–304. [Google Scholar]
- Bowes, G.; Holaday, A.S.; Haller, W.T. Seasonal variation in the biomass, tuber density, and photosynthetic metabolism of Hydrilla in three Florida lakes. J. Aquat. Plant Manag. 1979, 17, 61–65. [Google Scholar]
- Bain, M.B.; Webb, D.H.; Tangedal, M.D.; Mangum, L.N. Movements and habitat use by grass carp in a large mainstream reservoir. Trans. Am. Fish. Soc. 1990, 119, 553–561. [Google Scholar] [CrossRef]
- Cassani, J.R.; Maloney, D. Grass carp movement in two morphologically diverse reservoirs. J. Aquat. Plant Manag. 1991, 29, 83–88. [Google Scholar]
- Klussmann, W.G.; Noble, R.L.; Martyn, R.D.; Clark, W.J.; Betsill, R.K.; Bettoli, P.W.; Cichra, M.F.; Campbell, J.M. Control of Aquatic Macrophytes by Grass Carp in Lake Conroe, Texas, and the Effects on the Reservoir Ecosystem; Texas Agricultural Experiment Station: College Station, TX, USA, 1983. [Google Scholar]
- Clapp, D.; Hestand, R., III; Thompson, B. Hauling and post-stocking mortality of triploid grass carp. J. Aquat. Plant Manag. 1994, 32, 41–43. [Google Scholar]
- Colle, D.E.; Shireman, J.V.; Rottmann, R.W. Food selection by grass carp fingerlings in a vegetated pond. Trans. Am. Fish. Soc. 1978, 107, 149–152. [Google Scholar] [CrossRef]




| Growth of Hydrilla (Gh; kg fresh weight per ha per day): where Bh is the biomass of Hydrilla (kg fresh weight per ha), and c is a plant growth temperature coefficient: when day length is increasing when day length is decreasing where T is the mean daily air temperature (°C). |
| Senescence of Hydrilla (Sh; kg fresh weight per ha per day): where d is the degree–day senescence coefficient, and e is the senescence temperature coefficient: when degree days accumulated since 1 April < 525 when degree days accumulated since 1 April ≥ 525 |
| Consumption (herbivory, H) of Hydrilla by grass carp (kg fresh weight of Hydrilla per grass carp per day): × GC where W is the live weight (kg) of an individual grass carp, GC is the number of grass carp in the system, and tch is an herbivory rate temperature coefficient: when day length is increasing when day length is decreasing |
| Growth (Gc) of grass carp (kg live weight per day): when T ≥ 11 °C when T < 11 °C Where M is maintenance costs (kg live weight per day): |
| Date | Number of Grass Carp Stocked | Cumulative Number Stocked | Number of Surviving Fish | Stocking Rate (N/ha) |
|---|---|---|---|---|
| March 2006 | 4330 | 4330 | 4300 | 22.5 |
| August 2006 | 9311 | 13,641 | 13,064 | 36.8 |
| October 2006 | 13,800 | 27,441 | 26,168 | 56.6 |
| February 2007 | 10,000 | 37,441 | 33,376 | 70.7 |
| August 2007 | 23,386 | 60,827 | 54,983 | 72.6 |
| August 2007 | 25,364 | 86,191 | 71,735 | 99.8 |
| November 2007 | 15,575 | 101,766 | 81,564 | 103.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edgerton, E.; Wang, H.-H.; Grant, W.E.; Masser, M. Aquatic Plant Invasion and Management in Riverine Reservoirs: Proactive Management via a Priori Simulation of Management Alternatives. Diversity 2022, 14, 1113. https://doi.org/10.3390/d14121113
Edgerton E, Wang H-H, Grant WE, Masser M. Aquatic Plant Invasion and Management in Riverine Reservoirs: Proactive Management via a Priori Simulation of Management Alternatives. Diversity. 2022; 14(12):1113. https://doi.org/10.3390/d14121113
Chicago/Turabian StyleEdgerton, Elizabeth, Hsiao-Hsuan Wang, William E. Grant, and Michael Masser. 2022. "Aquatic Plant Invasion and Management in Riverine Reservoirs: Proactive Management via a Priori Simulation of Management Alternatives" Diversity 14, no. 12: 1113. https://doi.org/10.3390/d14121113
APA StyleEdgerton, E., Wang, H.-H., Grant, W. E., & Masser, M. (2022). Aquatic Plant Invasion and Management in Riverine Reservoirs: Proactive Management via a Priori Simulation of Management Alternatives. Diversity, 14(12), 1113. https://doi.org/10.3390/d14121113









