Opportunistic Macroalgae as a Component in Assessment of Eutrophication
Abstract
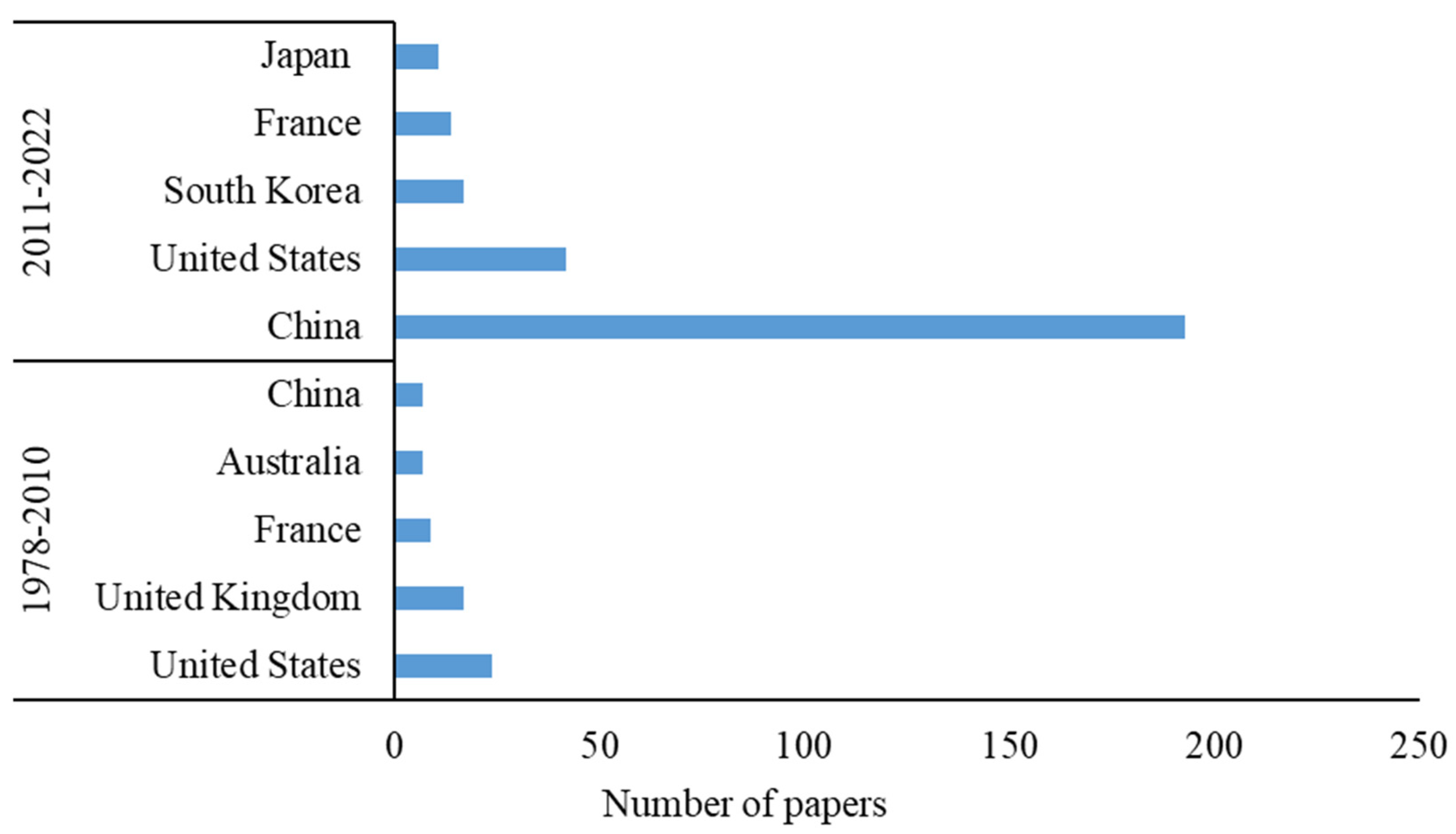
1. Introduction
2. Material and Methods
3. Results
3.1. Green Tides Studies
3.2. Cladophora spp. Studies in the Context of the Green Tides
3.3. Spirogyra spp. Studies in the Context of Eutrophication
3.4. Ecological Assessment, Based on Opportunistic Macroalgae
4. Application of Opportunistic Macroalgae in Ecological Assessment
4.1. Freshwater Ecosystems
4.1.1. Cladophora spp. Blooms
4.1.2. Spirogyra spp. Blooms
4.1.3. Rivers and Streams
4.1.4. Macroalgae as a Tool for Sanitary Assessment of a Recreational Area
4.2. Assessment of the “Green Tides” and Algal Mats in Brackish Water Ecosystems in a Case of the Baltic Sea
4.2.1. Coastal Habitats with Monodominant Communities
4.2.2. Coastal Habitats with Perennial Species
4.3. Marine and Estuarine Ecosystems
4.3.1. Main Principles of Assessment in Marine and Estuarine Habitats
4.3.2. Ecological Evaluation Index
4.3.3. CFR and CCO Indices
4.3.4. Opportunistic Macroalgal Blooming Tool
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonsdorff, E.; Blomqvist, E.M.; Mattila, J.; Norkko, A. Long-term changes and coastal eutrophication. Examples from the Åland Islands and the Archipelago Sea, northern Baltic Sea. Oceanol. Acta 1997, 20, 319–329. [Google Scholar]
- Kraufvelin, P.; Salovius, S. Animal diversity in Baltic rocky shore macroalgae: Can Cladophora glomerata compensate for lost Fucus vesiculosus? Estuar. Coast. Shelf Sci. 2004, 61, 369–378. [Google Scholar] [CrossRef]
- Valiela, I.; McClelland, J.; Hauxwell, J.; Behr, P.J.; Hersh, D.; Foreman, K. Macroalgal blooms in shallow estuaries: Controls and ecophysiological and ecosystem consequences. Limnol. Oceanogr. 1997, 42, 1105–1118. [Google Scholar] [CrossRef]
- Smetacek, V.; Zingone, A. Green and golden seaweed tides on the rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef]
- Higgins, S.N.; Malkin, S.Y.; Howell, E.T.; Guildford, S.J.; Campbell, L.; Hiriart-Bayer, V.; Hecky, R.E. An ecological review of Cladophora glomerata (Chlorophyta) in the Laurentian Great Lakes. J. Phycol. 2008, 44, 839–854. [Google Scholar] [CrossRef] [PubMed]
- Kravtsova, L.S.; Izhboldina, L.A.; Khanaev, I.V.; Pomazkina, G.V.; Rodionova, E.V.; Domysheva, V.M.; Sakirko, M.V.; Tomberg, I.V.; Kostornova, T.Y.; Kravchenko, O.S.; et al. Nearshore benthic blooms of filamentous green algae in Lake Baikal. J. Gt. Lakes Res. 2014, 40, 441–448. [Google Scholar] [CrossRef]
- Directive 2000/60/EC of the European Parliament and the Council Establishing a Framework for Community Action in the Field of Water Policy. 2000. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32000L0060:en:NOT (accessed on 11 December 2022).
- Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive). 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008L0056 (accessed on 11 December 2022).
- D’Archino, R.; Piazzi, L. Macroalgal assemblages as indicators of the ecological status of marine coastal systems: A review. Ecol. Indic. 2021, 129, 107835. [Google Scholar] [CrossRef]
- Gubelit Yu, I.; Kovalchuk, N.A. Macroalgal blooms and species diversity in the Transition Zone of the eastern Gulf of Finland. Hydrobiologia 2010, 656, 83–86. [Google Scholar] [CrossRef]
- Chakraborty, S.; Bhattacharya, T.; Singh, G.; Maity, J.P. Benthic macroalgae as biological indicators of heavy metal pollution in the marine environments: A biomonitoring approach for pollution assessment. Ecotoxicol. Environ. Saf. 2014, 100, 61–68. [Google Scholar] [CrossRef]
- Rinne, H.; Korpinen, S.; Mattila, J.; Salovius-Laurén, S. Functionality of potential macroalgal indicators in the northern Baltic Sea. Aquat. Bot. 2018, 149, 52–60. [Google Scholar] [CrossRef]
- Bach, S.D.; Josselyn, M.N. Mass blooms of the alga Cladophora in Bermuda. Mar. Pollut. Bull. 1978, 9, 34–37. [Google Scholar] [CrossRef]
- Bach, S.D.; Josselyn, M.N. Production and biomass of Cladophora prolifera (Chlorophyta, Cladophorales) in Bermuda. Bot. Mar. 1979, 22, 163–168. [Google Scholar] [CrossRef]
- Schramm, W.; Booth, W. Mass bloom of the alga Cladophora prolifera in Bermuda: Productivity and phosphorus accumulation. Bot. Mar. 1981, 24, 419–426. [Google Scholar] [CrossRef]
- Lapointe, B.E.; O’Connell, J. Nutrient-enhanced growth of Cladophora prolifera in Harrington Sound, Bermuda: Eutrophication of a confined, phosphorus-limited marine ecosystem. Estuar. Coast. Shelf Sci. 1989, 28, 347–360. [Google Scholar] [CrossRef]
- Shellem, B.H.; Josselyn, M.N. Physiological ecology of Enteromorpha clathrata (Roth) Grev. on a salt marsh mudflat. Bot. Mar. 1982, 25, 541–549. [Google Scholar] [CrossRef]
- Soulsby, P.G.; Lowthion, D.; Houston, M. Effects of macroalgal mats on the ecology of intertidal mudflats. Mar. Pollut. Bull. 1982, 13, 162–166. [Google Scholar] [CrossRef]
- Tubbs, C.R.; Tubbs, J.M. Macroalgal mats in Langstone Harbour, Hampshire, England. Mar. Pollut. Bull. 1983, 14, 148–149. [Google Scholar] [CrossRef]
- Hull, S.C. Macroalgal mats and species abundance: A field experiment. Estuar. Coast. Shelf Sci. 1987, 25, 519–532. [Google Scholar] [CrossRef]
- Mazé, J.; Morand, P.; Potoky, P. Stabilisation of “Green tides” Ulva by a method of composting with a view to pollution limitation. J. Appl. Phycol. 1993, 5, 183–190. [Google Scholar] [CrossRef]
- Viaroli, P.; Naldi, M.; Bondavalli, C.; Bencivelli, S. Growth of the seaweed Ulva rigida C. Agardh in relation to biomass densities, internal nutrient pools and external nutrient supply in the Sacca di Goro lagoon (Northern Italy). Hydrobiologia 1996, 329, 93–103. [Google Scholar] [CrossRef]
- Liu, D.; Keesing, J.K.; Xing, Q.; Shi, P. World’s Largest Macroalgal Bloom Caused by Expansion of Seaweed Aquaculture in China. Mar. Pollut. Bull. 2009, 58, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiao, J.; Fan, S.; Li, Y.; Liu, X.; Liu, D. Who made the world’s largest green tide in china?—An integrated study on the initiation and early development of the green tide in yellow sea. Limnol. Oceanogr. 2015, 60, 1105–1117. [Google Scholar] [CrossRef]
- Nelson, T.; Haberlin, K.; Nelson, A.V.; Ribarich, H.; Hotchkiss, R.; Van Alstyne, K.; Buckingham, L.; Simunds, D.J.; Fredrickson, K. Ecological and physiological controls of species composition in green macroalgal blooms. Ecology 2008, 89, 1287–1298. [Google Scholar] [CrossRef]
- Liu, F.; Pang, S.; Chopin, T.; Gao, S.; Shan, T.; Zhao, X.; Li, J. Understanding the recurrent large-scale green tide in the Yellow Sea: Temporal and spatial correlations between multiple geographical, aquacultural and biological factors. Mar. Environ. Res. 2013, 83, 38–47. [Google Scholar] [CrossRef]
- Watson, S.B.; Miller, C.; Arhonditsis, G.; Boyer, G.L.; Carmichael, W.; Charlton, M.N.; Confesor, R.; Depew, D.C.; Höök, T.O.; Ludsin, S.A.; et al. The re-eutrophication of Lake Erie: Harmful algal blooms and hypoxia. Harmful Algae 2016, 56, 44–66. [Google Scholar] [CrossRef]
- Norkko, J.; Bonsdorff, E.; Norkko, A. Drifting algal mats as an alternative habitat for benthic invertebrates: Species specific response to a transient resource. J. Exp. Mar. Biol. Ecol. 2000, 248, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Fletcher, R.L.; Raven, J.A. Preliminary studies on the growth of selected ‘green tide’ algae in laboratory culture: Effects of irradiance, temperature, salinity and nutrients on growth rate. Bot. Mar. 2001, 44, 327–336. [Google Scholar] [CrossRef]
- Lavery, P.S.; Lukatelich, R.J.; McComb, A.J. Changes in the biomass and species composition of macroalgae in a eutrophic estuary. Estuar. Coast. Shelf Sci. 1991, 33, 1–22. [Google Scholar] [CrossRef]
- Smith, R.F.; Ludwig, H.F. Eutrophication mechanisms at Lake Tahoe-I. Source of an exogenous Spirogyra bloom on the south shore, January 1967. Water Res. 1968, 2, 615–624. [Google Scholar] [CrossRef]
- Nozaki, K.; Mitsuhashi, H.; Tuji, A. Preliminary report on diel changes in dissolved oxygen concentration in filamentous green algal mats in littoral zone of Lake Biwa. Jpn. J. Limnol. 1998, 59, 211–213. [Google Scholar] [CrossRef]
- Pillsbury, R.W.; Lowe, R.L.; Yang, D.P.; Greenwood, J.L. Changes in the benthic algal community and nutrient limitation in Saginaw Bay, Lake Huron, during the invasion of the zebra mussel (Dreissena polymorpha). J. N. Am. Benthol. Soc. 2002, 21, 238–252. [Google Scholar] [CrossRef][Green Version]
- Timoshkin, O.A.; Moore, M.V.; Kulikova, N.N.; Tomberg, I.V.; Malnik, V.V.; Shimaraev, M.N.; Troitskaya, E.S.; Shirokaya, A.A.; Sinyukovich, V.N.; Zaitseva, E.P.; et al. Groundwater contamination by sewage causes benthic algal outbreaks in the littoral zone of lake Baikal (East Siberia). J. Gt. Lakes Res. 2018, 44, 230–244. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Gubelit Yu, I. Green Tides: New Consequences of the Eutrophication of Natural Waters (Invited Review). Contemp. Probl. Ecol. 2019, 12, 109–125. [Google Scholar] [CrossRef]
- Dahl, A.L. Surface area in ecological analysis: Quantification of benthic coral-reef algae. Mar. Biol. 1973, 23, 239–249. [Google Scholar] [CrossRef]
- Borja, A.; Dauer, D.M. Assessing the environmental quality status in estuarine and coastal systems: Comparing methodologies and indices. Ecol. Indic. 2008, 8, 331–337. [Google Scholar] [CrossRef]
- Deegan, L.A.; Finn, J.T.; Ayvazian, S.G.; Ryder-Kieffer, C.A.; Buonaccorsi, J. Development and validation of an estuarine biotic integrity index. Estuaries 1997, 20, 601–617. [Google Scholar] [CrossRef]
- Sfriso, A.; Facca, C. Ecological indices based on macroalgae and angiosperms in the mediterranean eco-region: An overview. In Life in the Mediterranean Sea: A Look at Habitat Changes; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 483–503. [Google Scholar]
- Simboura, N.; Panayotidis, P.; Papathanassiou, E. A synthesis of the biological quality elements for the implementation of the European Water Framework Directive in the Mediterranean ecoregion: The case of Saronikos Gulf. Ecol. Indic. 2005, 5, 253–266. [Google Scholar] [CrossRef]
- Ballesteros, E.; Torras, X.; Pinedo, S.; García, M.; Mangialajo, L.; de Torres, M. A new methodology based on littoral community cartography dominated by macroalgae for the implementation of the European Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 172–180. [Google Scholar] [CrossRef]
- Patrício, J.; Neto, J.M.; Teixeira, H.; Marques, J.C. Opportunistic macroalgae metrics for transitional waters. Testing tools to assess ecological quality status in Portugal. Mar. Pollut. Bull. 2007, 54, 1887–1896. [Google Scholar] [CrossRef][Green Version]
- Hu, L.; Hu, C.; He, M.-X. Remote estimation of biomass of Ulva prolifera macroalgae in the Yellow Sea. Remote Sens Environ. 2017, 192, 217–227. [Google Scholar] [CrossRef]
- Neil, J.H.; Owen, G.E. Distribution, environmental requirements and significance of Cladophora in the Great Lakes. In Proceedings of the 7th Conference on Great Lakes Research, Toronto ON, Canada, 6–7 April 1964; Great Lakes Research Division, University of Michigan: Ann Arbor, MI, USA, 1964; pp. 113–121. [Google Scholar]
- Auer, M.T.; Canale, R.P. Ecological and mathematical modeling of Cladophora in Lake Huron: 3. The dependence of growth rates on internal phosphorus pool size. J. Gt. Lakes Res. 1982, 8, 93–99. [Google Scholar] [CrossRef]
- Millner, G.C.; Sweeney, R.A.; Frederick, V.R. Biomass and Distribution of Cladophora glomerata in relation to some physical-chemical variables at two sites in Lake Erie. J. Gt. Lakes Res. 1982, 8, 35–41. [Google Scholar] [CrossRef]
- Dodds, W.K. Factors associated with dominance of the filamentous green alga Cladophora glomerata. Water Res. 1991, 25, 1125–1132. [Google Scholar] [CrossRef]
- Auer, M.T.; McDonald, C.P.; Kuczynski, A.; Huang, C.; Xue, P. Management of the phosphorus–Cladophora dynamic at a site on Lake Ontario using a multi-module bioavailable P Model. Water 2021, 13, 375. [Google Scholar] [CrossRef]
- Higgins, S.N.; Howell, E.T.; Hecky, R.E.; Guilford, S.J.; Smith, R.E. The wall of green: The status of Cladophora glomerata on the northern shores of Lake Erie’s eastern basin, 1995–2002. J. Gt. Lakes Res. 2005, 31, 547–563. [Google Scholar] [CrossRef]
- Auer, M.T.; Tomlinson, L.M.; Higgins, S.N.; Malkin, S.Y.; Howell, E.T.; Bootsma, H.A. Great Lakes Cladophora in the 21st century: Same algae—Different ecosystem. J. Gt. Lakes Res. 2010, 36, 248–255. [Google Scholar] [CrossRef]
- Greb, S.; Garrison, P.; Pfeiffer, S. Cladophora and water quality of Lake Michigan: A systematic survey of Wisconsin nearshore areas. In Special Report 2005-01; Bootsma, H.A., Jensen, E.T., Young, E.B., Berges, J.A., Eds.; Cladophora research and management in the Great Lakes; Great Lakes Water Institute. University of Wisconsin: Milwaukee, WI, USA, 2004; pp. 73–80. [Google Scholar]
- Higgins, S.N.; Christopher, M.; Pennuto, C.M.; Howell, T.; Lewis, T.W.; Makarewicz, J.C. Urban influences on Cladophora blooms in Lake Ontario. J. Gt. Lakes Res. 2012, 38, 116–123. [Google Scholar] [CrossRef]
- Kravtsova, L.S.; Mizandrontsev, I.B.; Vorobyova, S.S.; Izhboldina, L.A.; Mincheva, E.V.; Potyomkina, T.G.; Golobokova, L.P.; Sakirko, M.V.; Triboy, T.I.; Khanaev, I.V.; et al. Influence of water motion on the spatial distribution of Spirogyra in Lake Baikal. J. Gt. Lakes Res. 2020, 46, 29–40. [Google Scholar] [CrossRef]
- Kobanova, G.I.; Takhteev, V.V.; Rusanovskaya, O.O.; Timofeyev, M.A. Lake Baikal ecosystem faces the threat of eutrophication. Int. J. Ecology. 2016, 2016, 6058082. [Google Scholar] [CrossRef]
- Kravtsova, L.S.; Izhboldina, L.A.; Khanaev, I.V.; Pomazkina, G.V.; Domysheva, V.M.; Kravchenko, O.S.; Grachev, M.A. Disturbances of the vertical zoning of green algae in the coastal part of the Listvennichnyi Gulf of Lake Baikal. Dokl. Biol. Sci. 2012, 447, 350–352. [Google Scholar] [CrossRef]
- Volkova, E.A.; Bondarenko, N.A.; Timoshkin, O.A. Morphotaxonomy, distribution and abundance of Spirogyra (Zygnematophyceae, Charophyta) in Lake Baikal, East Siberia. Phycologia 2018, 57, 298–308. [Google Scholar] [CrossRef]
- Entwisle, T.J. Phenology of Cladophora-Stigeoclonium community in two urban creeks of Melbourne. Aust. J. Mar. Freshw. Res. 1989, 40, 471–489. [Google Scholar] [CrossRef]
- Kelly, M.G.; Krokowsky, J.; Harding, J.P.C. A new method for rapid assessment of macroalgae as a complement to diatom-based assessments of ecological status. Sci. Total Environ. 2016, 568, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Byappanahalli, M.N.; Shively, D.A.; Nevers, M.B.; Sadowsky, M.J.; Whitman, R.L. Growth and survival of Escherichia coli and enterococci populations in the macro-alga Cladophora (Chlorophyta). FEMS Microbiol. Ecol. 2003, 46, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Chun, C.L.; Peller, J.R.; Shively, D.; Byappanahalli, M.N.; Whitman, R.L.; Staley, C.; Zhange, Q.; Ishii, S.; Sadowsky, M.J. Virulence and biodegradation potential of dynamic microbial communities associated with decaying Cladophora in Great Lakes. Sci. Total Environ. 2017, 574, 872–880. [Google Scholar] [CrossRef]
- Englebert, E.T.; McDermott, C.; Kleinheinz, G.T. Effects of the nuisance algae, Cladophora, on Escherichia coli at recreational beaches in Wisconsin. Sci. Total Environ. 2008, 404, 10–17. [Google Scholar] [CrossRef]
- Gubelit, Y.I.; Vainshtein, M.B. Growth of Enterobacteria on Algal Mats in the Eastern Part of the Gulf of Finland. Inland Water Biol. 2011, 4, 132–136. [Google Scholar] [CrossRef]
- Horsney, I.S.; Hide, D. The Production of Antimicrobial Compounds by British Marine Algae. I. Antibiotic Producing Marine Algae. Eur. J. Phycol. 1974, 9, 353–361. [Google Scholar]
- Gubelit, Y.I.; Berezina, N.A. The causes and consequences of algal blooms: The Cladophora glomerata bloom and the Neva estuary (eastern Baltic Sea). Mar. Pollut. Bull. 2010, 61, 183–188. [Google Scholar] [CrossRef]
- Lehvo, A.; Bäck, S. Survey of macroalgal mats in the Gulf of Finland, Baltic Sea. Aquat. Conserv. Mar. Freshw. Ecosyst. 2001, 1, 11–18. [Google Scholar] [CrossRef]
- Lauringson, V.; Kotta, J. Influence of the thin drift algal mats on the distribution of macrozoobenthos in Kõiguste Bay, NE Baltic Sea. Hydrobiologia 2006, 554, 97–105. [Google Scholar] [CrossRef]
- Berezina, N.A.; Gubelit, Y.I.; Polyak, Y.M.; Sharov, A.N.; Kudryavtseva, V.A.; Lubimtsev, V.A.; Petukhov, V.A.; Shigaeva, T.D. An integrated approach to the assessment of the eastern Gulf of Finland health: A case study of coastal habitats. J. Mar. Syst. 2017, 171, 159–171. [Google Scholar] [CrossRef]
- Nielsen, R.; Kristiansen, A.; Mathiesen, L.; Mathiesen, H. Distributional index of the benthic macroalgae of the Baltic Sea area. Acta Bot. Fenn. 1995, 155, 51. [Google Scholar]
- Kiirikki, M.; Lehvo, A. Life strategies of filamentous algae in the northern Baltic proper. Sarsia 1997, 82, 259–267. [Google Scholar] [CrossRef]
- Cossellu, M.; Nordberg, K. Recent environmental changes and filamentous algal mats in shallow bays on the Swedish west coast—A result of climate change? J. Sea Res. 2010, 63, 202–212. [Google Scholar] [CrossRef]
- Gubelit, Y.I. Climatic impact on community of filamentous macroalgae in the Neva estuary (eastern Baltic Sea). Mar. Pollut. Bull. 2015, 91, 166–172. [Google Scholar] [CrossRef]
- Salo, T.; Salovius-Lauren, S. Green algae as bioindicators for long-term nutrient pollution along a coastal eutrophication gradient. Ecol. Indic. 2022, 140, 109034. [Google Scholar] [CrossRef]
- Neto, J.M.; Gaspar, R.; Pereira, L.; Marques, J.C. Marine Macroalgae AssessmentTool (MarMAT) for intertidal rocky shores. Quality assessment under the scope of the European Water Framework Directive. Ecol. Indic. 2012, 19, 39–47. [Google Scholar] [CrossRef]
- Wells, E.; Wood, P.; Wilkinson, M.; Scanlan, C. The use of macroalgal species richness and composition on intertidal rocky seashores in the assessment of ecological quality under the European Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 151–161. [Google Scholar] [CrossRef]
- Wallenstein, F.M.; Neto, A.I.; Patarra, R.F.; Prestes, A.C.L.; Alvaro, N.V.; Rodrigues, A.S.; Wilkinson, M. Indices to monitor coastal ecological quality of rocky shores based on seaweed communities: Simplification for wide geographical use. Rev. Gest. Costeira Integr. 2013, 13, 15–25. [Google Scholar] [CrossRef]
- De Casamajor, M.N.; Lalanne, Y.; Derrien-Courtel, S.; Le Gal, A.; Quintano, E.; Lissardy, M. Cystoseira baccata meadows along the French Basque coast (Bay of Biscay) as a reference for the implementation of the Water Framework and Marine Strategy EU directives. Cont. Shelf Res. 2019, 182, 12–21. [Google Scholar] [CrossRef]
- Littler, M.M.; Littler, D.S. The evolution of thallus form and survival strategiesin benthic marine macroalgae: Field and laboratory tests of a functional formmodel. Am. Nat. 1980, 116, 25–44. [Google Scholar] [CrossRef]
- Littler, M.M.; Littler, D.S. Relationships between macroalgal functional formgroups and substrata stability in a subtropical rocky-intertidal system. J. Exp. Mar. Biol. Ecol. 1984, 74, 13–34. [Google Scholar] [CrossRef]
- Orfanidis, S.; Panayotidis, P.; Stamatis, N. Ecological evaluation of transitional and coastal waters: A marine benthic macrophytes-based model. Mediterr. Mar. Res. 2001, 2, 45–65. [Google Scholar] [CrossRef]
- Orfanidis, S.; Panayotidis, P.; Stamatis, N. An insight to the ecological evaluation index (EEI). Ecol. Indic. 2003, 3, 27–33. [Google Scholar] [CrossRef]
- Guinda, X.; Juanes, J.A.; Puente, A.; Revilla, J.A. Comparison of two methods forquality assessment of macroalgae assemblages, under different pollution types. Ecol. Indic. 2008, 8, 743–753. [Google Scholar] [CrossRef]
- Guinda, X.; Juanes, J.A.; Puente, A. The Quality of Rocky Bottoms index (CFR):a validated method for the assessment of macroalgae according to the European Water Framework Directive. Mar. Environ. Res. 2014, 102, 3–10. [Google Scholar] [CrossRef]
- Ar Gall, E.; Le Duff, M.L.; Sauriau, P.-G.; De Casamajor, M.-N.; Gevaert, F.; Poisson, E.; Hacquebart, P.; Joncourt, Y.; Barillé, A.-L.; Buchet, R.; et al. Implementation of a new index to assess intertidal seaweed communities as bioindicators for the European Water Framework Directory. Ecol. Indic. 2016, 60, 162–173. [Google Scholar] [CrossRef]
- Foden, J.; Wells, E.; Scanlan, C.; Best, M.A. Water Framework Directive Development of Classification Tools for Ecological Assessment: Opportunistic Macroalgae Blooming; TAG Report for Marine Plants Task Team, January 2010; Water Framework Directive—United Kingdom Advisory Group (WFD-UKTAG): Stirling, Scotland, UK, 2010. [Google Scholar]

| Paper | Number of Citations |
|---|---|
| Liu D. et al. World’s largest macroalgal bloom caused by expansion of seaweed aquaculture in China. Mar Pollut Bull. 2009, 58(6), 888–895. | 417 |
| Ye N. et al. ‘Green tides’ are overwhelming the coastline of our blue planet: Taking the world’s largest example. Ecol Res. 2011, 26(3), 477–485. | 278 |
| Watson SB. et al. The re-eutrophication of Lake Erie: Harmful algal blooms and hypoxia. Harmful Algae. 2016, 56, 44–66. | 265 |
| Hu C. et al. On the recurrent Ulva prolifera blooms in the Yellow Sea and East China Sea. J Geophys Res C Oceans. 2010, 115(5) | 248 |
| Liu D. et al. Recurrence of the world’s largest green-tide in 2009 in Yellow Sea, China: Porphyra yezoensis aquaculture rafts confirmed as nursery for macroalgal blooms. Mar Pollut Bull. 2010, 60(9), 1423–1432. | 217 |
| Taylor R et al. Preliminary studies on the growth of selected ‘green tide’ algae in laboratory culture: Effects of irradiance, temperature, salinity and nutrients on growth rate. Bot Mar. 2001, 44(4), 327–336. | 209 |
| Keesing JK et al. Inter- and intra-annual patterns of Ulva prolifera green tides in the Yellow Sea during 2007–2009, their origin and relationship to the expansion of coastal seaweed aquaculture in China. Mar Pollut Bull. 2011, 62(6), 1169–1182. | 206 |
| Norkko J et al. Drifting algal mats as an alternative habitat for benthic invertebrates: Species specific response to a transient resource. J Exp Mar Biol Ecol. 2000, 248(1), 79–104. | 171 |
| Higgins SN. et al. An ecological review of Cladophora glomerata (Chlorophyta) in the Laurentian Great Lakes. J Phycol. 2008, 44(4), 839–854. | 170 |
| Wang Z. et al. Who made the world’s largest green tide in China?—an integrated study on the initiation and early development of the green tide in yellow sea. Limnol Oceanogr. 2015, 60(4), 1105–1117. | 150 |
| Paper | Number of Citations |
|---|---|
| Watson SB., et al. The re-eutrophication of Lake Erie: Harmful algal blooms and hypoxia. Harmful Algae, 2016, 56, 44–66. | 265 |
| Taylor R., et al. Preliminary studies on the growth of selected “green tide” algae in laboratory culture: Effects of irradiance, temperature, salinity and nutrients on growth rate. Bot Mar, 2001, 44(4), 327–336. | 209 |
| Higgins SN. et al. An ecological review of Cladophora glomerata (Chlorophyta) in the Laurentian Great Lakes. J Phycol. 2008, 44(4), 839–854. | 170 |
| Lapointe BE, O’Connell J. Nutrient-enhanced growth of Cladophora prolifera in Harrington sound, Bermuda: Eutrophication of a confined, phosphorus-limited marine ecosystem. Estuar Coast Shelf Sci. 1989, 28(4), 347–360. | 144 |
| Lavery PS., et al. Changes in the biomass and species composition of macroalgae in a eutrophic estuary. Estuar Coast Shelf Sci. 1991, 33(1), 1–22. | 114 |
| Paper | Number of Citations |
|---|---|
| Kravtsova LS et al. Nearshore benthic blooms of filamentous green algae in Lake Baikal. J Great Lakes Res. 2014, 40(2), 441–448. | 89 |
| Timoshkin OA et al. Groundwater contamination by sewage causes benthic algal outbreaks in the littoral zone of Lake Baikal (East Siberia). J Great Lakes Res. 2018, 44(2), 230–244. | 53 |
| Pillsbury RW et al. Changes in the benthic algal community and nutrient limitation in Saginaw Bay, Lake Huron, during the invasion of the zebra mussel (Dreissena polymorpha). J North Am Benthological Soc. 2002, 21(2), 238–252. | 48 |
| Trochine C. et al. Filamentous green algae inhibit phytoplankton with enhanced effects when lakes get warmer. Freshw Biol. 2011, 56(3), 541–553. | 37 |
| Gladyshev MI, Gubelit YI. Green Tides: New Consequences of the Eutrophication of Natural Waters (Invited Review). Contemp Probl Ecol. 2019, 12(2), 109–125. | 31 |
| Paper | Number of Citations |
|---|---|
| Borja A, Dauer DM. Assessing the environmental quality status in estuarine and coastal systems: Comparing methodologies and indices. Ecol Indic. 2008, 8(4), 331–337. | 274 |
| Ballesteros E et al. A new methodology based on littoral community cartography dominated by macroalgae for the implementation of the European Water Framework Directive. Mar Pollut Bull 2007, 55(1–6), 172–180. | 256 |
| Simboura N. et al. A synthesis of the biological quality elements for the implementation of the European Water Framework Directive in the Mediterranean ecoregion: The case of Saronikos Gulf. Ecol Indic. 2005, 5(3), 253–266. | 139 |
| Juanes JA et al. Macroalgae, a suitable indicator of the ecological status of coastal rocky communities in the NE Atlantic. Ecol Indic. 2008, 8(4), 351–359. | 137 |
| Nielsen R et al. Distributional index of the benthic macroalgae of the Baltic Sea area. Acta Bot Fenn. 1995, 155. | 130 |
| Deegan LA et al. Development and validation of an estuarine biotic integrity index. Estuaries. 1997, 20(3), 601–617. | 128 |
| Wells E et al. The use of macroalgal species richness and composition on intertidal rocky seashores in the assessment of ecological quality under the European Water Framework Directive. Mar Pollut Bull. 2007, 55(1–6), 151–161. | 112 |
| Orfanidis S. et al. Ecological Evaluation Index continuous formula (EEI-c) application: A step forward for functional groups, the formula and reference condition values. Mediterr Mar Sci. 2011, 12(1), 199–231. | 101 |
| Dahl AL. Surface area in ecological analysis: Quantification of benthic coral-reef algae. Mar Biol. 1973, 23(4), 239–249. | 87 |
| Hu L et al. Remote estimation of biomass of Ulva prolifera macroalgae in the Yellow Sea. Remote Sens Environ. 2017, 192, 217–227. | 85 |
| Type of Habitat | Freshwater and Brackishwater Ecosystems with a Dominance of One or Few Macroalgae Species | Marine and Estuarine Ecosystems with High Species Diversity |
|---|---|---|
| Metrics | 1. Coverage of opportunistic species (%) | 1. Species composition |
| 2. Biomass | 2. Number of characteristic species | |
| 3. Thickness of algal mats | 3. Total algal coverage (%) | |
| 4. Signs of Hypoxia | 4. Total cover of opportunistic species | |
| 5. Cumulative algal cover | 5. Proportion of opportunistic species | |
| 6. Ratio of opportunistic and perennial species 7. An area covered by algal mats | 6. An area covered by algal mats | |
| Recommended time of sampling | Seasonal peak of biomass | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gubelit, Y.I. Opportunistic Macroalgae as a Component in Assessment of Eutrophication. Diversity 2022, 14, 1112. https://doi.org/10.3390/d14121112
Gubelit YI. Opportunistic Macroalgae as a Component in Assessment of Eutrophication. Diversity. 2022; 14(12):1112. https://doi.org/10.3390/d14121112
Chicago/Turabian StyleGubelit, Yulia I. 2022. "Opportunistic Macroalgae as a Component in Assessment of Eutrophication" Diversity 14, no. 12: 1112. https://doi.org/10.3390/d14121112
APA StyleGubelit, Y. I. (2022). Opportunistic Macroalgae as a Component in Assessment of Eutrophication. Diversity, 14(12), 1112. https://doi.org/10.3390/d14121112





