Abstract
There is an abundance of bird species in subtropical areas, but studies on the vocal behavior of non-passerines in subtropical regions are limited. In this study, passive acoustic monitoring was used to investigate the temporal acoustic patterns of the vocal activities of the Oriental Turtle Dove (Streptopelia orientalis) in Yaoluoping National Nature Reserve (YNNR) in eastern China. The results show that the vocal production of the Oriental Turtle Dove exhibited a seasonal variation, peaking in the period April–August. Additionally, its diurnal vocal activity displayed a bimodal pattern in late spring and summer, with the first peak in the morning and a secondary peak at dusk. Among weather factors, temperature significantly affected the temporal sound pattern of the Oriental Turtle Dove, instead of humidity and precipitation. This study, which was focused on sound monitoring technology, provides knowledge for further research on bird behavior and ecology. In the future, long-term sound monitoring could be used for managing and conserving bird biodiversity.
1. Introduction
There are many vocal organisms in nature, such as birds, insects, bats, and fish, among which bird sounds occupy an essential part of the acoustic environment for a terrestrial ecosystem [1,2,3]. The vocal activities of birds provide information on the status of the monitored species [4] and are closely related to the rhythm of biological activities and the life cycle [1]. Bird calls and songs are fundamental behaviors for specific sexual and individual recognition, advertising individual condition, mate attraction, territory defense, feeding, predator alarm, or coordination of activities, among others [5,6]. Furthermore, bird vocalizations are easily affected by many factors, including endogenous factors (such as body size, breeding status, and hormones) and exogenous factors (such as light, noise effects, and weather conditions) [7,8], and are often used as indicators to track environmental change [1]. Therefore, the study of bird acoustics has attracted the attention of ornithologists and some ecologists, and has been utilized in research, such as analyzing the biological rhythm, living habits, and social interactions of particular species through their acoustic patterns [4,9], or ascertaining the variety of bird vocal activities according to environmental factors [10,11]. Therefore, monitoring the diel and seasonal patterns of birds’ vocal activity can provide basic knowledge about the behavioral ecology of birds, and may help us to gain a deeper understanding of the relationship between organisms and the environment.
The sound of passerines is characterized by high variability in its components [12,13]. For example, the sound of the Brownish-flanked Bush Warbler (Horornis forties) is composed of one or more whistled notes on an even pitch, followed by one to four more complex notes [14], while the Yellow-streaked Warbler (Phylloscopus armandii) has dozens of syllable types [15]. Conversely, the sound types of non-passerines are usually relatively simple [4,12], such as the monotonous sound of Common Cuckoos (Cuculus canorus) [16]. The difference in the variability of sound types of passerines and non-passerines may be affected by factors such as body size and phylogeny [17], which make them perform differently in the acoustic environment. For example, passerines in the canopy sing earlier than those feeding near the forest floor, while non-passerines show weak opposite results [8]. Another research shows that passerines occupied more proportion in acoustic space, especially in the morning, and non-passerines were prominent in the dusk hours during the observation windows [18]. Furthermore, some non-passerines, which produce sounds at a low frequency, are more vulnerable to the geographical environment and artificial sounds, while passerines may be more sensitive to interference from other species that produce sounds at similar frequencies [19,20]. Although vocal communication can play equally significant functions among different bird taxa, the study of the acoustic patterns and ecology of vocal activity in non-passerines has received much less attention than in passerine species [21].
The species existing in different climatic zones are different [22,23]. Tropical areas have higher net primary production, environmentally stable conditions, and higher biological and evolutionary rates, so the tropics have more biodiversity and vocal species than mid-latitude areas [24,25,26,27]. Due to the differential geographical locations and environmental conditions [28,29], birds exhibit a variety of vocal structures and activities in tropical and temperate zones [30]. Researchers have conducted many studies on birds’ daily and seasonal patterns in temperate and tropical regions [7,31,32,33]. The subtropical zone is in the middle of the temperate and tropical regions and forms migrating birds’ paths [34]. However, there are few studies on the vocal activity of non-passerines in subtropical zones, despite the high avian diversity and their relevance as migratory species [34]. Therefore, focusing on birds’ vocal features in the sub-tropical zone is essential, particularly in poorly studied non-passerine species.
Passive acoustic monitoring technology has been widely used to study the occurrence and behavior of vocal animals [35]. By deploying autonomous sound recorders, we can record various animals’ calls and investigate species diversity dynamics in remote forests or deep waters [9,36,37,38,39]. This method can enable continuous collection of the vocal activities of animals, and it is not invasive, so it is beneficial for studying shy species [40]. One such species is the Oriental Turtle Dove (Streptopelia orientalis), a small terrestrial non-passeriform broadly distributed in the Himalayas, India, northeast Asia, China, and Japan. The Oriental Turtle Dove is usually resident in these regions, although some northern sub-species are migratory [41]. It mainly feeds on rice, wheat, millet seeds, insects, and gastropods [41,42]. It is widely distributed in forested areas and moves in pairs or small groups [41,42]. It is a shy species, often hidden in canopies and aware of the surroundings, making it difficult to monitor visually. Therefore, passive acoustic monitoring might be a solution for obtaining new vital information on this tropical non-passerine species, showing the potential benefits of indirect sound-based sampling methods on shy and cryptic species.
In this study, we monitored the vocal behavior of the Oriental Turtle Dove at six sites for one year in the Yaoluoping National Nature Reserve (YNNR), a subtropical forest area in eastern China. Using passive acoustic techniques, we aimed to do the following; (1) reveal seasonal and diurnal vocalization patterns and determine the temporal peak of vocal activity, and (2) evaluate the effect of weather parameters on the vocal activity of the Oriental Turtle Dove. Previous studies have discussed the relationship between calling activity and temporal patterns of important behaviors in some non-Passeriformes (e.g., [4]). Based on this, we predicted that the Oriental Turtle Dove would show significant seasonal and daily changes in its vocal activity thorough the year related to mate attraction and territory defense [40,43]. It was found that the relative air humidity was positively associated with vocal activity in the study of another member of Columbidae in the Neotropics [21]. Due to the differences in climate in the regions, we speculated that the vocal activity of the Oriental Turtle Dove in subtropical areas would be affected by other weather factors. By depicting the unique vocal patterns of non-passerines in subtropical areas, we expect to contribute to the study of bird behavior and ecology.
2. Materials and Methods
2.1. Study Area and Data Acquisition
Yaoluoping National Nature Reserve (116°02′ E~116°11′ E, 30°57′ N~31°06′ N; YNNR) is in the north subtropical monsoon climatic zone, with humid air, a low temperature, and abundant rain and snow [44,45]. It is an important water conservation forest in the Ta-pieh Mountain area and a typical forest ecosystem. More than 100 species of birds [46] and 200 insect species [44,47,48] live in the YNNR, constituting a unique soundscape for ecoacoustic research. We selected six sampling sites in the YNNR (Figure 1), which were distributed throughout the buffer (N = 3) and experimental zones (N = 3) of the protected area. In the nature reserve, human development is allowed within the experimental area, while the buffer area allows only a limited amount of human activity, such as some scientific research, and the core area does not allow human access [49]. The altitude range of our sampling was 800–1400 m. The details of the sampling sites are provided within the Supplementary Files (Table S1).
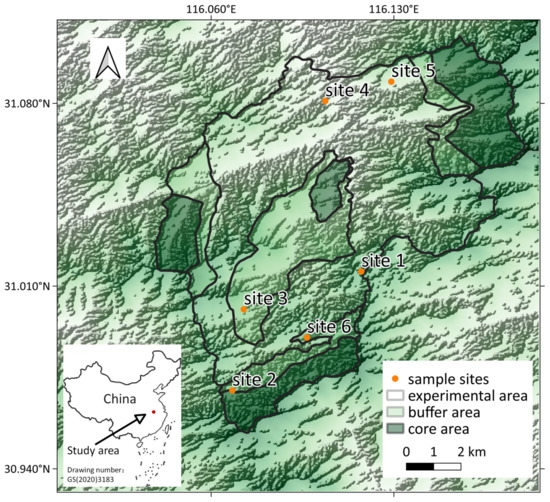
Figure 1.
Study area with the geographical location of the six sampling sites in the YNNR (China).
We placed one Song Meter SM4 recorder (Song Meter SM4, Wildlife Acoustics, Maynard, MA, USA) at each sampling site on a tree trunk, approximately 1.5 m above the ground. All recordings were saved in WAV stereo format on Secure Digital (SD) cards with 16-bit precision. Periods of 5 min every half an hour were recorded between 0 and 24 h every day, from 6 April 2019 to 5 April 2020. This recording interval could record sound more efficiently to save memory. Due to battery replacement and equipment damage, the total number of recording days during which data were collected was 1927 (Table S1).
Annual data from the Huoshan weather station were downloaded from the China Meteorological Data Service Center (http://data.cma.cn/, accessed on 22 April 2022), including daily precipitation, average temperature, and average relative humidity. Huoshan is the nearest meteorological station, about 17–28 km away from the research area [50].
2.2. Acoustic Analysis
The sound of the Oriental Turtle Dove is composed mainly of four syllables, with two hoarse and two clear tones [51]. The acoustic spectrum of the Oriental Turtle Dove is shown in Figure 2, exhibiting its three calls. Using Raven Pro 1.5 software [52], the spectrum was obtained based on the Hanning window fast Fourier transform (FFT) (FFT = 512; overlap: 50%; and frame size: 100%). The vocal activity of the Oriental Turtle Dove was quantified by scanning the spectrum of each 5 min recording period and counting the number of individual calls. The number of calls per unit of time of a species is related to the population abundance [53,54]. Therefore, according to the vocal activity at different times, the temporal activity patterns of the Oriental Turtle Dove could be indicated.
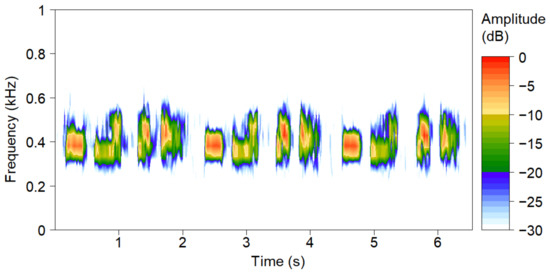
Figure 2.
The acoustic spectrum of a typical call of the Oriental Turtle Dove.
2.3. Data Analysis
A generalized additive mixed model (GAMM) was used to study the time dimension of the vocal activity of the Oriental Turtle Dove. This method can deduce nonlinear fitting to the temporal changes in hours and months. We used the function “gamm” of the mgcv package in R version 3.5.3 [55,56]. We calculated the average value of the number of calls for about five days of each month (the 1st–5th, 6th–10th, 11th–15th, 16th–20th, 21st–25th, and 26th–29th/30th/31st) at each hour, to eliminate the error caused by different actual sampling days and also to reduce the dataset for computational speed. A simplified data format, 24 (h) * 72 (six times a month) * 6 (sites), was used for modeling. The mean number of calls detected per unit of time was the response variable. The corresponding hour, month, and the interaction of hour and month were included as predictors. Site was included as a random effects factor to control for spatial pseudoreplication of repeated measures. We used a cyclic cubic regression spline for the hour smooth term and a spline function by default for the month. We tested different distribution families (Gaussian, Poisson, quasi-Poisson, and negative binomial), calculated the Akaike Information Criterion (AIC), and visually checked the residuals. The Gaussian family was finally selected, since it had the lowest AIC. We also checked the residual temporal autocorrelation and tested three models with different autoregressive orders of the ARMA structure. After analysis of variance, we fit an ARMA process to the residuals with one-order autoregression.
To study the influence of weather factors on the vocal activity of the Oriental Turtle Dove, we used a linear mixed model (LMM) using the “lmer” function of the lmerTest package [57]. We used the total number of vocalizations per day (log-10 transformed) as the response variable, and daily precipitation, average temperature, and average relative humidity as the predictor weather variables. A quadratic term for the season (fitted as the day of the year) was also used as a predictor variable, considering that seasonal trends may affect the studied relationship. Site was again included as a random effects factor. Since the Oriental Turtle Dove called very little in winter in the YNNR, we only focused on the relationship between the calls and weather factors in the first half of the year from 6 April 2019 to 6 October 2019. The Q–Q plots, the histogram of residuals, and the plots of fitted values against residuals were inspected to evaluate the normality of the residuals.
3. Results
Based on the 5 min audio samples over a complete annual cycle, we identified a total of 18,476 individual calls for the Oriental Turtle Dove, including 3808 for site 1, 426 for site 2, 7271 for site 3, 1086 for site 4, 2297 for site 5, and 3588 for site 6.
3.1. Temporal Acoustic Pattern
The vocal activity of the Oriental Turtle Dove showed significant seasonal differences and daily variations conditional on season (Table 1), with different hourly acoustic patterns among months (Figure 3).

Table 1.
Summary of a Gaussian GAMM performed to test the effects of hour, month, and their interaction, on the calling activity of the Oriental Turtle Dove in the YNNR.
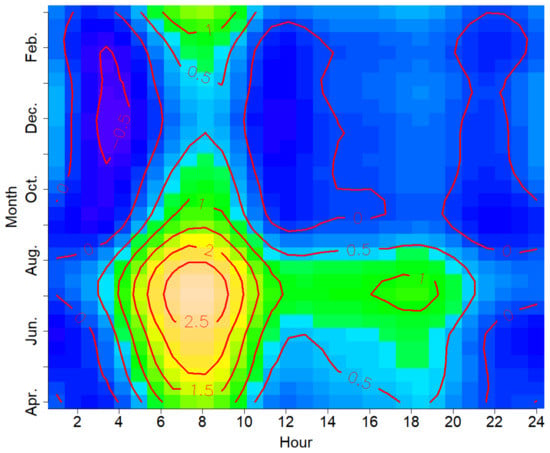
Figure 3.
Combined seasonal and daily patterns of vocal activity in the Oriental Turtle Dove as the five-day average number of individual calls per hour in the annual cycle 2019–2020.
The Oriental Turtle Dove’s vocal activity was more frequent from April to August (Figure 3 and Figure 4), and the activity intensity was the strongest in July (Table S2). Our results indicated no vocal activity from November to February.
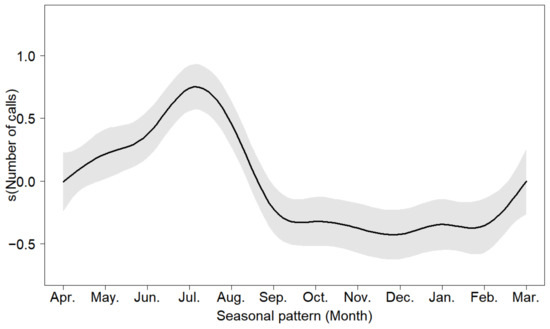
Figure 4.
Seasonal vocal activity of the Oriental Turtle Dove. The gray shaded part represents the associated 95% confidence intervals.
The daily activity showed a bimodal pattern in those months when the Oriental Turtle Dove was vocally active, with a maximum activity from 06:00 to 08:00 and a lower secondary peak from 16:00 to 18:00 (Figure 3). For example, there was a prominent morning peak and a secondary peak in July, while the morning peak in April was lower than in July and the secondary peak was almost invisible (Figure 5). The vocal activity from 06:00 to 08:00 accounted for 51.3% of the whole day, and that from 16:00 to 18:00 accounted for 15.5% (Table S3). No calls were detected between 20:00 and 03:00.
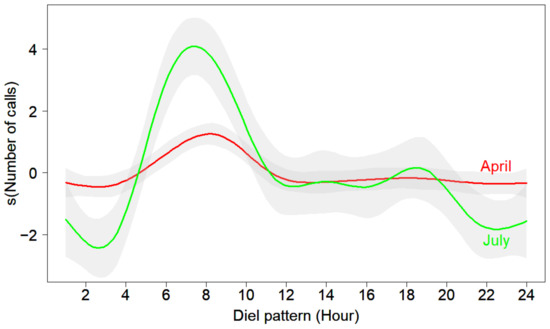
Figure 5.
Daily vocal activity of the Oriental Turtle Dove in April and July. The gray shaded part represents the associated 95% confidence intervals.
3.2. Climate Predictors
The Oriental Turtle Dove’s vocal activity was positively and significantly correlated with the average temperature value during the analyzed period (from 6 April 2019 to 6 October 2019) among different weather factors (Table 2 and Figure 6). The species called more when the average temperature was high. At the same time, the seasonal effect had a weak impact on vocal activity. Precipitation and average relative humidity had no significant effects on the activities of the Oriental Turtle Dove.

Table 2.
Results of the LMM analyzing the effects of weather parameters on the daily vocal activity of the Oriental Turtle Dove in the YNNR.
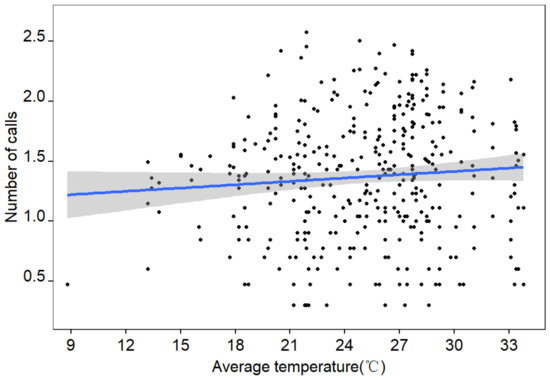
Figure 6.
Scatterplot showing the variation in the daily vocal activity (after log-10 transformation) of the Oriental Turtle Dove as a function of the daily average temperature during the period 6 April 2019 to 6 October 2019. The linear regression line is shown in blue, and the corresponding 95% confidence intervals in gray.
4. Discussion
To the best of our knowledge, this is the first study to monitor the daily and seasonal vocal activities of the Oriental Turtle Dove in the YNNR, during a whole annual cycle. This study provides useful data for the ecological monitoring and management of the acoustic activity patterns of the species in subtropical Asia. Our analysis showed that the vocal activity of the Oriental Turtle Dove varied at different times of the day and among seasons. We also found that the daily pattern of the call activity varied seasonally, with maximum activity in the early morning, which was much higher between April and August, and a secondary evening peak, only present in late spring and summer. Among the weather factors, temperature was significantly associated with the acoustic activity of the Oriental Turtle Dove.
The daily and seasonal patterns of the Oriental Turtle Dove were not independent, and there was an apparent interaction between hour and month, showing that the daily pattern was affected by seasonal factors. The seasonal acoustic pattern showed that the Oriental Turtle Dove was more active in spring and summer, especially in July. Previous studies showed a positive correlation between the number of birds around the recorder and vocal activity [53]. Although there was no visual data of occurrence and activity for the Oriental Turtle Dove for 2019, previous annual field surveys in the YNNR showed that the species was present during the period April-July from 2011 to 2015 [46], which was consistent with the seasonal pattern of the vocal activity found in the present study, and supported the reliability of passive acoustic methods for the monitoring of the species. It also showed a clear seasonal activity pattern in the study of the White-Tipped Dove in Neotropical region [21], which was consistent with the results of this paper. However, the White-Tipped Dove made sounds each month, and we did not detect the Oriental Turtle Dove in winter (from December to February). This result might be related to regional differences in climate conditions and resource availability. The YNNR is in the north of the subtropical region, where evergreen and deciduous broad-leaved forests are mixed [45]. In winter, the vegetation and temperature conditions are not as suitable as in summer [58,59]. However, the tropical zone is still relatively warm in winter and suitable for species activities [26]. Although the Oriental Turtle Dove is a resident bird in the Ta-pieh Mountains [46], the activities of the Oriental Turtle Dove are difficult to monitor in winter, and these birds might appear in other warmer places within the same mountain range and surrounding lower areas. In addition, the vocal activity of birds is generally closely associated with breeding cycles and reaches a peak when attracting mates and establishing territories [40]. If the purpose of a vocal is related to guarding the territory, then the vocal activity should keep constant throughout the year and become higher when other males are present [60]. On the other hand, if the vocal is central to attracting mates, more males make sounds during the breeding season for pairing [61]. So, the seasonal acoustic pattern of the Oriental Turtle Dove indicated that the species’ vocal activity might be related to its breeding behaviors. In the YNNR, a subtropical seasonal zone in the northern hemisphere, the species’ breeding season coincides with the peak of resources in late spring and summer.
In the months when the Oriental Turtle Dove was vocal, there was a daily pattern with maximum activity in the early morning, which was much higher between May and August, and a secondary evening peak, only present in late spring and summer. This result was consistent with the daily activity patterns of members of the same family, such as the Neotropical White-Tipped Dove (Leptotila verreauxi), the Pale-Vented Pigeon (Patagioenas cayennensis), and the Scaled Pigeon (Patagioenas speciosa) [21,32]. We found that the singing activities of the Oriental Turtle Dove from 06:00 to 08:00 accounted for approximately half of the total vocal activities, which was in agreement with a previous description of the first three hours after sunrise of the White-Tipped Dove being defined as a time of high calling activity [21]. Our results were consistent with the diurnal activity patterns of other non-Passeriformes [62,63,64] and Passeriformes [65], displaying a peak in vocal activity in the morning and a secondary lower activity peak toward sunset [65,66]. Many birds inspect vacant territories for occupation in the early morning [67], and the Oriental Turtle Dove might vocalize more at this time to guard its territories. Additionally, increased vocal production in the morning may also suggest its function in intersexual contexts [6]. For example, females choose males who sing more in the morning [68].
In respect of weather factors, the Oriental Turtle Dove in subtropical areas is more sensitive to temperature than humidity, while the White-Tipped Dove, another member of Columbidae, is mainly affected by humidity in the Neotropical region [21]. The differences found among studies might be due to regional climate differences. In the case of the YNNR, with fresh subtropical weather, the photoperiod and temperature are probably the limiting factors. Instead, in the tropics, with relatively homogenous temperatures throughout the year, rainfall and humidity are usually the dominant factors [21,69]. Studies have shown that birds adjust their reproductive phenology to cope with climate variability, and the phenological sensitivity to temperature may vary between species [70]. Resident birds are more sensitive to climate variability than migratory species [70]. The Oriental Turtle Dove is a migratory bird in some places and a resident bird in others [51], which may make it more selective in respect of temperature. We also observed the influence of seasonal effects on vocal activity, simultaneously. It is worth noting that if the calling activity is related to reproductive purposes, the maximum calling activity would be synchronized with the peak of resources (i.e., late spring and summer) when temperatures are also higher, which means that the seasonal activity of the Oriental Turtle Dove may mediate the significant impact of temperature. In addition, previous studies have shown that the influence of weather factors on the vocal activity of birds varies with dry and wet seasons [71]. However, we only studied the relationship between the vocal activities of the Oriental Turtle Dove and the weather factors during the period April-October, so this study did not fully indicate the relationship between weather factors and the seasonal cycle of activities in the dove.
This study only covered a limited number of sampling sites, which limited the analysis of daily and seasonal patterns and weather factors. Therefore, some of our profiles may require further research at more testing sites to obtain more reliable conclusions. Further research should add geographical factors, vegetation conditions, and the interference of other vocal species, such as birds or insects, at the different sampling sites [72,73], in order to improve our methods and increase our understanding of the vocal behavior of non-Passeriformes tropical birds.
In conclusion, this study provides important insights into the daily and seasonal changes in the Oriental Turtle Dove from the acoustic perspective, as well as into the relationship between bird activities and weather factors. These results can help us better understand the vocal activity and behavioral ecology of non-passerines and the interaction between biology and the environment in subtropical areas. In the future, long-term acoustic monitoring, based on passive automatized technologies, could be used as an effective tool to track population trends, increase knowledge regarding the ecology of cryptic and shy birds, and improve the management of nature reserves and the protection of avian diversity.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/d14121043/s1. Table S1. Spatial location and collection days of the sampling sites; Table S2. The number of calls (based on the mean number of each 1/6 month) of the Oriental Turtle Dove was detected per month at six sites. The total number and percentage of calls detected per month, with respect to the total number of calls, are also shown; Table S3. The number of calls (based on the mean number of each 1/6 month) of the Oriental Turtle Dove was detected per hour at six sites. The total number and percentage of calls detected per hour, with respect to the total number of calls, are also shown.
Author Contributions
Conceptualization, M.W., J.M. and F.L.; methodology, M.W., J.M. and F.L. investigation, M.W., J.M. and F.L.; writing—original draft preparation, M.W.; supervision, F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Hefei Institutes of Physical Science, the Chinese Academy of Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Field surveys were conducted in the Yaoluoping National Nature Reserve under the instruction of Jun Chu and the forest manager, to whom the authors are most grateful. The authors thank the anonymous reviewers for helping to improve the manuscript. They also thank Sabah Mushtaq Puswal and Muhammad Zahid Sharif for their helpful suggestions on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gasc, A.; Francomano, D.; Dunning, J.B.; Pijanowski, B.C. Future directions for soundscape ecology: The importance of ornithological contributions. Auk 2017, 134, 215–228. [Google Scholar] [CrossRef]
- Wiley, R.H. Bird Song, Biological Themes and Variations. Condor 1996, 98, 670. [Google Scholar] [CrossRef]
- Poesel, A.; Kunc, H.P.; Foerster, K.; Johnsen, A.; Kempenaers, B. Early birds are sexy: Male age, dawn song and extrapair paternity in blue tits, Cyanistes (formerly Parus) caeruleus. Anim. Behav. 2006, 72, 531–538. [Google Scholar] [CrossRef]
- Mei, J.; Puswal, S.M.; Wang, M.; Liu, F. Diurnal and Seasonal Patterns of Calling Activity of Seven Cuculidae Species in a Forest of Eastern China. Diversity 2022, 14, 249. [Google Scholar] [CrossRef]
- Whytock, R.C.; Fuentes-Montemayor, E.; Watts, K.; Macgregor, N.A.; Call, E.; Mann, J.A.; Park, K.J. Regional land-use and local management create scale-dependent ‘landscapes of fear’ for a common woodland bird. Landsc. Ecol. 2020, 35, 607–620. [Google Scholar] [CrossRef]
- Gil, D.; Llusia, D. The Bird Dawn Chorus Revisited. In Coding Strategies in Vertebrate Acoustic Communication; Aubin, T., Mathevon, N., Eds.; Springer: Cham, Switzerland, 2020; pp. 45–90. [Google Scholar] [CrossRef]
- Bruni, A.; Mennill, D.J.; Foote, J.R. Dawn chorus start time variation in a temperate bird community: Relationships with seasonality, weather, and ambient light. J. Ornithol. 2014, 155, 877–890. [Google Scholar] [CrossRef]
- Berg, K.S.; Brumfield, R.T.; Apanius, V. Phylogenetic and ecological determinants of the neotropical dawn chorus. Proc. Biol. Sci. 2006, 273, 999–1005. [Google Scholar] [CrossRef]
- Xia, C.; Lloyd, H.; Shi, J.; Wei, C.; Zhang, Y.; Manser, M. Dawn singing of the Brownish-flanked Bush Warbler influences dawn chorusing in a bird community. Ethology 2018, 124, 400–409. [Google Scholar] [CrossRef]
- Hasan, N.M. Comparison of the onset of dawn chorus of bulbuls and house sparrows in two different geographical locations: Effect of climate, noise and light pollution. Res.Opin. Anim. Vet. Sci. 2010, 1, 220–225. [Google Scholar]
- Marín-Gómez, O.H.; Dáttilo, W.; Sosa-López, J.R.; Santiago-Alarcon, D.; MacGregor-Fors, I. Where has the city choir gone? Loss of the temporal structure of bird dawn choruses in urban areas. Landsc. Urban Plan. 2020, 194, 3665. [Google Scholar] [CrossRef]
- Potamitis, I.; Ntalampiras, S.; Jahn, O.; Riede, K. Automatic bird sound detection in long real-field recordings: Applications and tools. Appl. Acoust. 2014, 80, 1–9. [Google Scholar] [CrossRef]
- Goretskaia, M.I. Song structure variability in passerine birds: Random variation or direct informative changes. Biol. Bull. 2013, 40, 748–759. [Google Scholar] [CrossRef]
- Xia, C.; Xiao, H.; Zhang, Y. Individual Variation in Brownish-Flanked Bush Warbler Songs. Condor 2010, 112, 591–595. [Google Scholar] [CrossRef]
- Li, J.; Lv, N.; Jochen, M.; Sun, Y. Primary Research on Geographical Variance of Song Spectrum of Yellow-streaked Warbler. Chin. J. Zool. 2009, 44, 122–127. [Google Scholar] [CrossRef]
- Zsebők, S.; Moskát, C.; Bán, M. Individually distinctive vocalization in Common Cuckoos (Cuculus canorus). J. Ornithol. 2016, 158, 213–222. [Google Scholar] [CrossRef]
- Jurisevic, M.A.; Sanderson, K.J. A Comparative Analysis of Distress Call Structure in Australian Passerine and Non-Passerine Species: Influence of Size and Phylogeny. J. Avian Biol. 1998, 29, 61–71. [Google Scholar] [CrossRef]
- Divyapriya, C.; Pramod, P. Ornithophony in the soundscape of Anaikatty Hills, Coimbatore, Tamil Nadu, India. J. Threat. Taxa 2019, 11, 14471–14483. [Google Scholar] [CrossRef]
- Gage, S.H.; Axel, A.C. Visualization of temporal change in soundscape power of a Michigan lake habitat over a 4-year period. Ecol. Inform. 2014, 21, 100–109. [Google Scholar] [CrossRef]
- Brumm, H.; Slabbekoorn, H. Acoustic Communication in Noise. Adv. Study Behav. 2005, 35, 151–209. [Google Scholar]
- Pérez-Granados, C.; Schuchmann, K.-L. Diel and Seasonal Variations of Vocal Behavior of the Neotropical White-Tipped Dove (Leptotila verreauxi). Diversity 2020, 12, 402. [Google Scholar] [CrossRef]
- Miles, L.; Newton, A.C.; DeFries, R.S.; Ravilious, C.; May, I.; Blyth, S.; Kapos, V.; Gordon, J.E. A global overview of the conservation status of tropical dry forests. J. Biogeogr. 2006, 33, 491–505. [Google Scholar] [CrossRef]
- Rodríguez, A.; Gasc, A.; Pavoine, S.; Grandcolas, P.; Gaucher, P.; Sueur, J. Temporal and spatial variability of animal sound within a neotropical forest. Ecol. Inform. 2014, 21, 133–143. [Google Scholar] [CrossRef]
- Kelt, D.A.; Brown, J.H. Community Structure of Desert Small Mammals: Comparisons across Four Continents. Ecology 1996, 77, 746–761. [Google Scholar] [CrossRef]
- Farina, A.; Pieretti, N. Biodiversity Assessment in Temperate Biomes using Ecoacoustics. In Ecoacoustics: The Ecological Role of Sounds; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Brown, J.H. Why are there so many species in the tropics? J. Biogeogr. 2014, 41, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Romdal, T.S.; Araújo, M.B.; Rahbek, C. Life on a tropical planet: Niche conservatism and the global diversity gradient. Glob. Ecol. Biogeogr. 2013, 22, 344–350. [Google Scholar] [CrossRef]
- Morelli, F.; Pruscini, F.; Santolini, R.; Perna, P.; Benedetti, Y.; Sisti, D. Landscape heterogeneity metrics as indicators of bird diversity: Determining the optimal spatial scales in different landscapes. Ecol. Indic. 2013, 34, 372–379. [Google Scholar] [CrossRef]
- Farina, A.; Ceraulo, M.; Bobryk, C.; Pieretti, N.; Quinci, E.; Lattanzi, E. Spatial and temporal variation of bird dawn chorus and successive acoustic morning activity in a Mediterranean landscape. Bioacoustics 2015, 24, 269–288. [Google Scholar] [CrossRef]
- Eldridge, A.; Guyot, P.; Moscoso, P.; Johnston, A.; Eyre-Walker, Y.; Peck, M. Sounding out ecoacoustic metrics: Avian species richness is predicted by acoustic indices in temperate but not tropical habitats. Ecol. Indic. 2018, 95, 939–952. [Google Scholar] [CrossRef]
- Depraetere, M.; Pavoine, S.; Jiguet, F.; Gasc, A.; Duvail, S.; Sueur, J. Monitoring animal diversity using acoustic indices: Implementation in a temperate woodland. Ecol. Indic. 2012, 13, 46–54. [Google Scholar] [CrossRef]
- Luther, D. The influence of the acoustic community on songs of birds in a neotropical rain forest. Behav. Ecol. 2009, 20, 864–871. [Google Scholar] [CrossRef]
- Hart, P.J.; Hall, R.; Ray, W.; Beck, A.; Zook, J. Cicadas impact bird communication in a noisy tropical rainforest. Behav. Ecol. 2015, 26, 839–842. [Google Scholar] [CrossRef]
- Nores, M. On the status of forest birds in tropical and subtropical South America. Clim. Chang. 2011, 108, 387–390. [Google Scholar] [CrossRef]
- Sueur, J.; Farina, A. Ecoacoustics: The Ecological Investigation and Interpretation of Environmental Sound. Biosemiotics 2015, 8, 493–502. [Google Scholar] [CrossRef]
- Wimmer, J.D. Acoustic Sensing: Roles and Applications in Monitoring Avian Biodiversity. Ph.D. Thesis, Queensland University of Technology, Brisbane, Australia, 2015. [Google Scholar]
- Wall, C.C.; Simard, P.; Lembke, C.; Mann, D.A. Large-scale passive acoustic monitoring of fish sound production on the West Florida Shelf. Mar. Ecol. Prog. Ser. 2013, 484, 173–188. [Google Scholar] [CrossRef]
- Xia, C.; Wei, C.; Lloyd, H.; Liu, J.; Wu, Q.; Zhang, Y.; Manser, M. Dawn Singing Intensity of the Male Brownish-Flanked Bush Warbler: Effects of Territorial Insertions and Number of Neighbors. Ethology 2014, 120, 324–330. [Google Scholar] [CrossRef]
- Gibb, R.; Browning, E.; Glover-Kapfer, P.; Jones, K.E.; Börger, L. Emerging opportunities and challenges for passive acoustics in ecological assessment and monitoring. Methods Ecol. Evol. 2018, 10, 169–185. [Google Scholar] [CrossRef]
- Yoo, S.; Kim, H.N.; Lee, J.W.; Yoo, J.C. Seasonal and diurnal patterns of population vocal activity in avian brood parasites. Ibis 2019, 162, 1001–1011. [Google Scholar] [CrossRef]
- Mohan, S.K.; Shyamkumar, P.; Harish Babu, M. Rare Sighting of Oriental Turtle Dove Streptopelia Orientalis from Plachikkara. Pioneer 2020, 37–39. [Google Scholar]
- Wilson, M.G.; Korovin, V.A. Oriental Turtle Dove breeding in the Western Palearctic. Br. Birds 2003, 96, 234–241. [Google Scholar]
- Todt, D.; Naguib, M. Vocal Interactions in Birds: The Use of Song as a Model in Communication. In Advances in the Study of Behavior; Academic Press: Cambridge, MA, USA, 2000; pp. 247–296. [Google Scholar] [CrossRef]
- Jingmin, L.; Demin, H.; Jie, F.; Chengbo, G. A study on fauna and diversity of Odonata in Yaoluoping Nature Reserve. J. Biol. 2013, 30, 73. [Google Scholar] [CrossRef]
- Xie, Z.; Wu, G. The vegetation types and their distributions in Yaoluoping Natural Reserve of Anhui province. J. East China Norm. Univ. 1995, 3, 93–100. [Google Scholar]
- Li, L.; Peng, C.; Fan, Y.; Haigen, X.; Yinxu, H.; Haohao, M.; Yaqiong, W.; Lei, Y. A Comparative Study of Bird Species Diversity in Breeding Season at Anhui Yaoluoping National Nature Reserve. Chin. J. Wildl. 2017, 38, 052–062. [Google Scholar] [CrossRef]
- Shu, Z.; De-Min, H.; Jie, F.; Xia, W.; Jie, F. The fauna and diversity of Heteroptera insects in Yaoluoping Nature Reserve. Chin. Bull. Entomol. 2008, 45, 799–805. [Google Scholar]
- Naiyi, L.; Zihao, L.; Lei, Y.; Yucai, Z.; Jun, C.; Ruochun, Y.; Jie, F. Diversity and Faunal Composition of Butterflies in Yaoluoping National Nature Reserve. Genom. Appl. Biol. 2018, 37, 1941–1946. [Google Scholar] [CrossRef]
- Song, K.; Mi, C.-R.; Zhao, Y.-Z.; Yang, N.; Sun, Y.-H.; Xu, J.-L. Zonation of nature reserve according to the habitat requirement of conservation target: A case study on the endangered Brown Eared-Pheasant at Baihuashan Nature Reserve. Glob. Ecol. Conserv. 2021, 32, e01941. [Google Scholar] [CrossRef]
- Puswal, S.M.; Mei, J.; Wang, M.; Liu, F. Daily and Seasonal Patterns in the Singing Activity of Birds in East China. Ardea 2022, 110, 5–17. [Google Scholar] [CrossRef]
- Clements, J.F.; Schulenberg, T.S.; Iliff, M.J.; Roberson, D.; Fredericks, T.A.; Sullivan, B.L.; Wood, C.L. The eBirdClements Checklist of Birds of the World v2021; Cornell Univeristy: Ithaca, NY, USA, 2021. [Google Scholar]
- Bioacoustics Research Program. Raven Pro. Interactive Sound Analysis Software, Version 1.5 Computer Software; The Cornell Lab of Ornithology: Ithaca, NY, USA, 2014. [Google Scholar]
- Pérez-Granados, C.; Bota, G.; Giralt, D.; Barrero, A.; Gómez-Catasús, J.; Bustillo-De La Rosa, D.; Traba, J. Vocal activity rate index: A useful method to infer terrestrial bird abundance with acoustic monitoring. Ibis 2019, 161, 901–907. [Google Scholar] [CrossRef]
- Pérez-Granados, C.; Traba, J. Estimating bird density using passive acoustic monitoring: A review of methods and suggestions for further research. Ibis 2021, 163, 765–783. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Lin, X.; Zhang, D. Inference in Generalized Additive Mixed Models by Using Smoothing Splines. J. R. Stat. Society. Ser. B Stat. Methodol. 1999, 61, 381–400. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, L.-Z.; Wang, W.-G.; Shen, S.-B.; Han, D.-M. Seasonal Dynamics of the Avian Guild Structure of Mountain Secondary Forest in Dabieshan Mountain. Zool. Res. 2009, 30, 277–287. [Google Scholar] [CrossRef]
- Hongqun, Z.; Yuanjian, Y.; Shangpei, X.; Binfan, H.; Aimin, Z.; Wenyu, W. Seasonal and Spatial Variability of Vegetation and Land Surface Temperature in Anhui Province. J. Appl. Meteorol. Sci. 2011, 22, 232–240. [Google Scholar]
- Tobias, J.A.; Gamarra-Toledo, V.; Garcia-Olaechea, D.; Pulgarin, P.C.; Seddon, N. Year-round resource defence and the evolution of male and female song in suboscine birds: Social armaments are mutual ornaments. J. Evol. Biol. 2011, 24, 2118–2138. [Google Scholar] [CrossRef]
- Odom, K.J.; Omland, K.E.; McCaffrey, D.R.; Monroe, M.K.; Christhilf, J.L.; Roberts, N.S.; Logue, D.M. Typical Males and Unconventional Females: Songs and Singing Behaviors of a Tropical, Duetting Oriole in the Breeding and Non-Breeding Season. Front. Ecol. Evol. 2016, 4, 14. [Google Scholar] [CrossRef]
- Hadjikyriakou, T.G.; Kassara, C.; de Roland, L.-A.R.; Giokas, S.; Tsiopelas, N.; Evangelidis, A.; Thorstrom, R.; Kirschel, A.N.G. Phenology, variation in habitat use, and daily activity patterns of Eleonora’s falcon overwintering in Madagascar. Landsc. Ecol. 2019, 35, 159–172. [Google Scholar] [CrossRef]
- Pérez-Granados, C.; Schuchmann, K.-L. Passive Acoustic Monitoring of Chaco Chachalaca (Ortalis canicollis) Over a Year: Vocal Activity Pattern and Monitoring Recommendations. Trop. Conserv. Sci. 2021, 14, 1–11. [Google Scholar] [CrossRef]
- Lafleur, L.; Pardo, L.; Spínola, R.M.; Saénz, J.; Cove, M.V. Notes on Plumage Patterns and activity of the great Currasow (Crax rubra) in northeastern Costa rica. Cracid News 2014, 36, 17–19. [Google Scholar]
- Sosa-López, J.R.; Mennill, D.J. The vocal behavior of the Brown-throated Wren (Troglodytes brunneicollis): Song structure, repertoires, sharing, syntax, and diel variation. J. Ornithol. 2014, 155, 435–446. [Google Scholar] [CrossRef]
- Voigt, C.; Leitner, S.; Gahr, M.; Ter Maat, A. Seasonal and diurnal variation of vocal behaviour in duetting White-browed Sparrow Weavers. J. Ornithol. 2021, 162, 1163–1172. [Google Scholar] [CrossRef]
- Amrhein, V.; Kunc, H.P.; Naguib, M. Non-territorial nightingales prospect territories during the dawn chorus. Proc. Biol. Sci. 2004, 271 (Suppl. S4), S167–S169. [Google Scholar] [CrossRef]
- Beaulieu, M.; Sockman, K.W. Song in the cold is ‘hot’: Memory of and preference for sexual signals perceived under thermal challenge. Biol. Lett. 2012, 8, 751–753. [Google Scholar] [CrossRef]
- Opaev, A.; Gogoleva, S.; Palko, I.; Nguyen, V.T.; Rozhnov, V. Annual acoustic dynamics are associated with seasonality in a monsoon tropical forest in South Vietnam. Ecol. Indic. 2021, 122, 107269. [Google Scholar] [CrossRef]
- Samplonius, J.M.; Bartosova, L.; Burgess, M.D.; Bushuev, A.V.; Eeva, T.; Ivankina, E.V.; Kerimov, A.B.; Krams, I.; Laaksonen, T.; Magi, M.; et al. Phenological sensitivity to climate change is higher in resident than in migrant bird populations among European cavity breeders. Glob. Chang. Biol. 2018, 24, 3780–3790. [Google Scholar] [CrossRef]
- Pérez-Granados, C.; Schuchmann, K.-L. Seasonal Climate Impacts on Vocal Activity in Two Neotropical Nonpasserines. Diversity 2021, 13, 319. [Google Scholar] [CrossRef]
- Lin, T.-H.; Tsao, Y.; Wang, Y.-H.; Yen, H.-W.; Lu, S.-S. Computing biodiversity change via a soundscape monitoring network. In Proceedings of the 2017 Pacific Neighborhood Consortium Annual Conference and Joint Meetings (PNC), Tainan, Taiwan, 7–9 November 2017; pp. 128–133. [Google Scholar]
- Quintero, I.; Jetz, W. Global elevational diversity and diversification of birds. Nature 2018, 555, 246–250. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).