Spatial Distribution Pattern and Risk Assessment of Invasive Alien Plants on Southern Side of the Daba Mountain Area
Abstract
1. Introduction
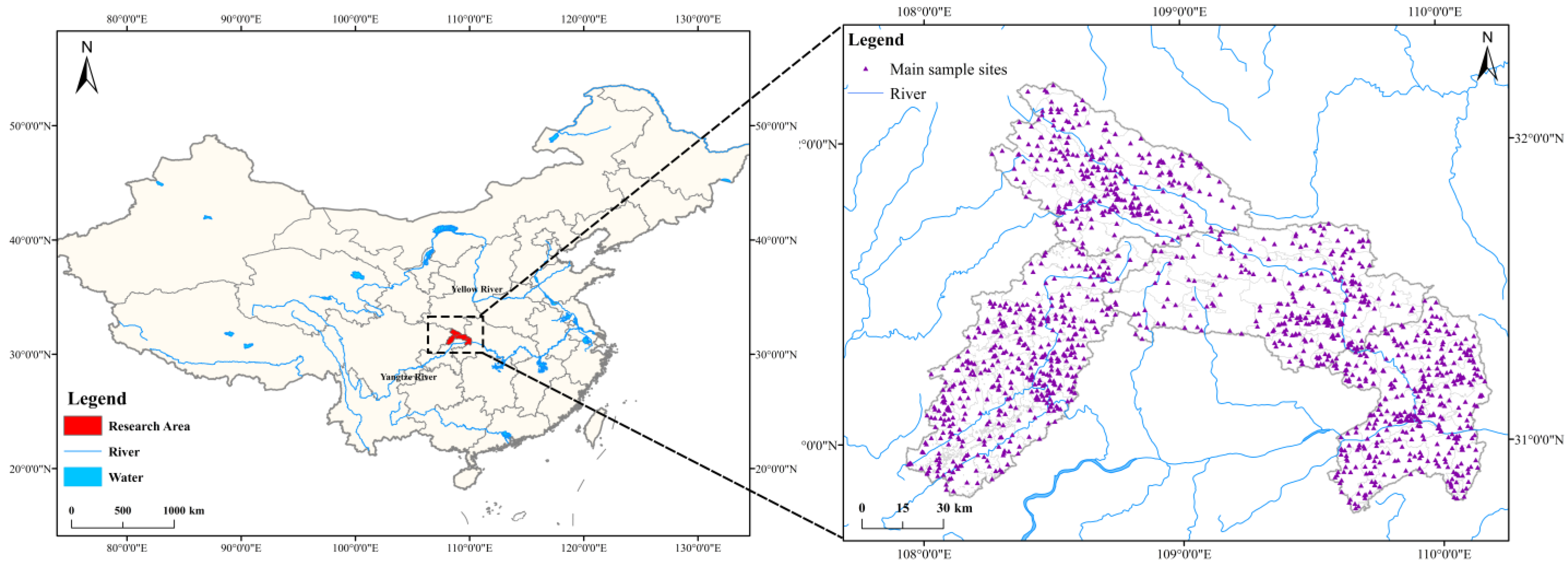
2. Materials and Methods
3. Results
3.1. Taxonomic Composition
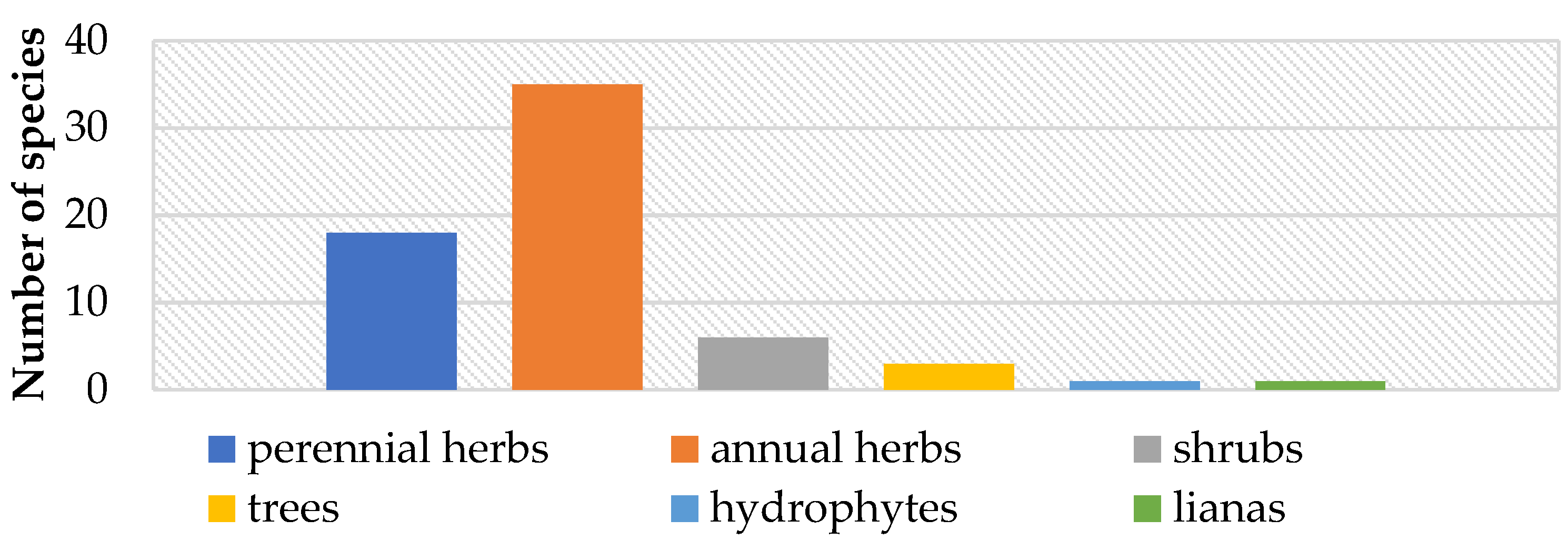
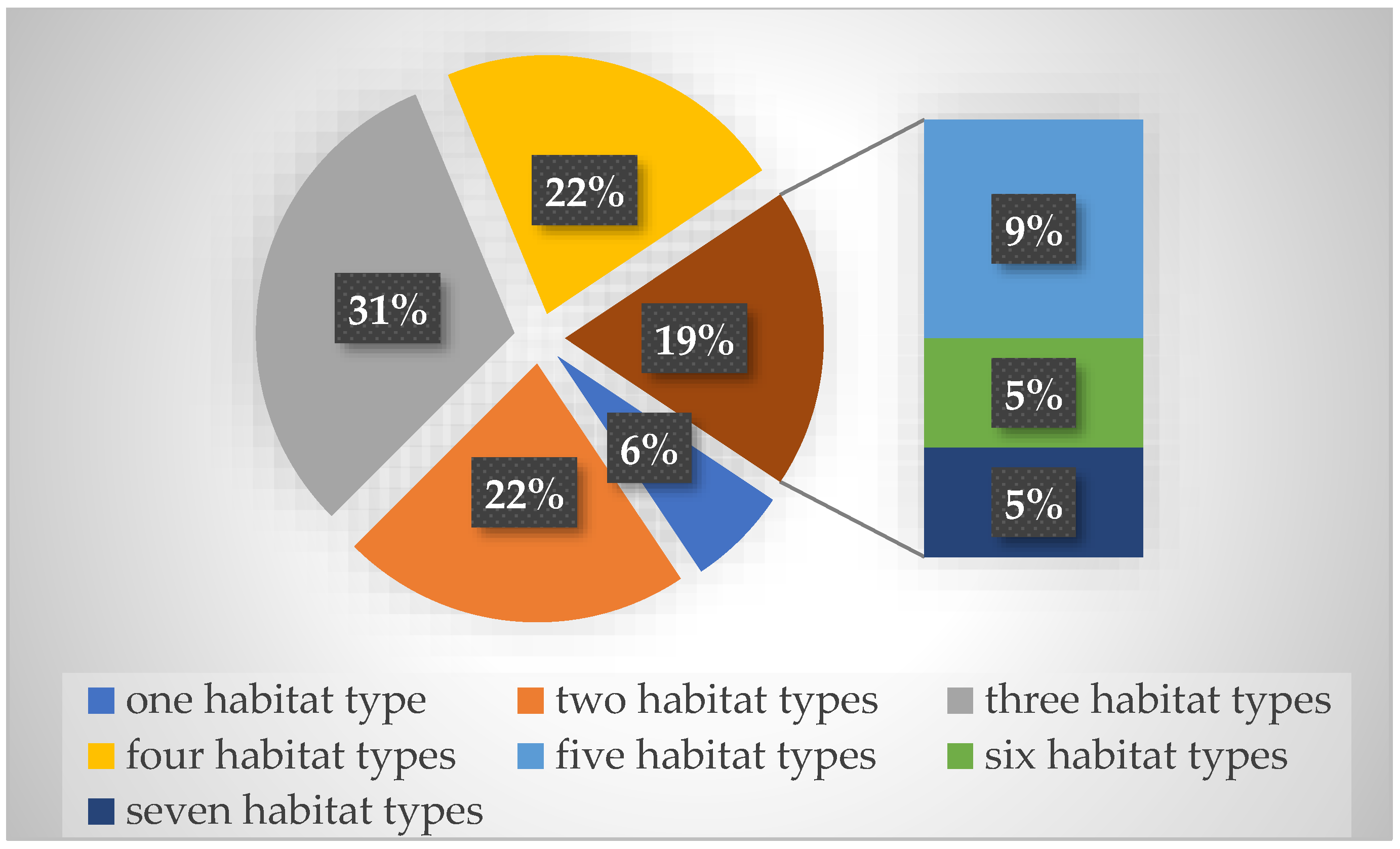
3.2. Life Forms and Habitats
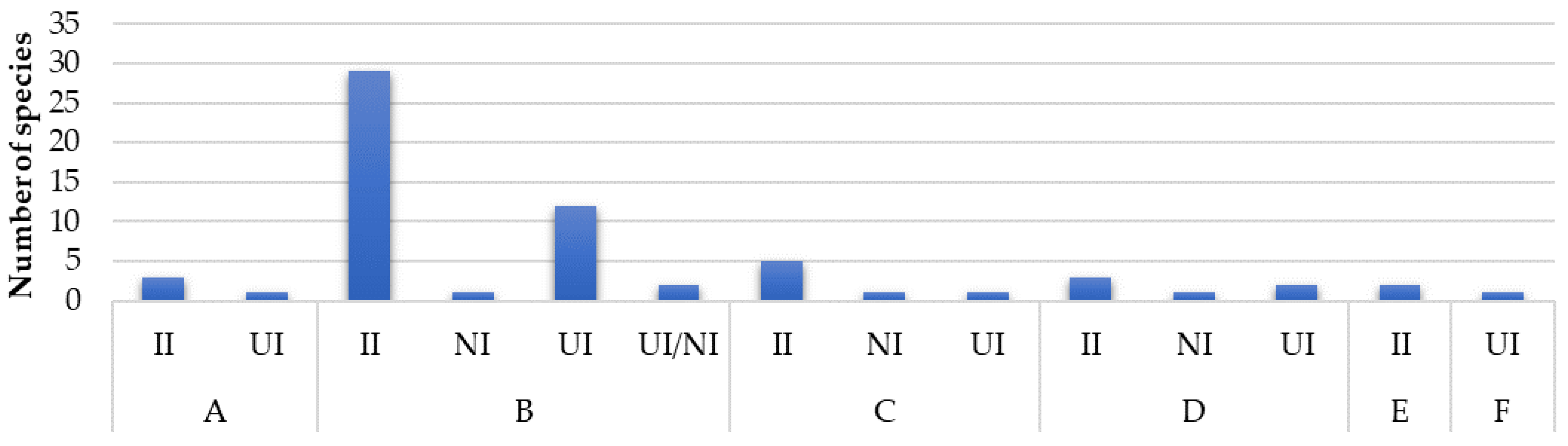
3.3. Origin and Pathways of Introduction
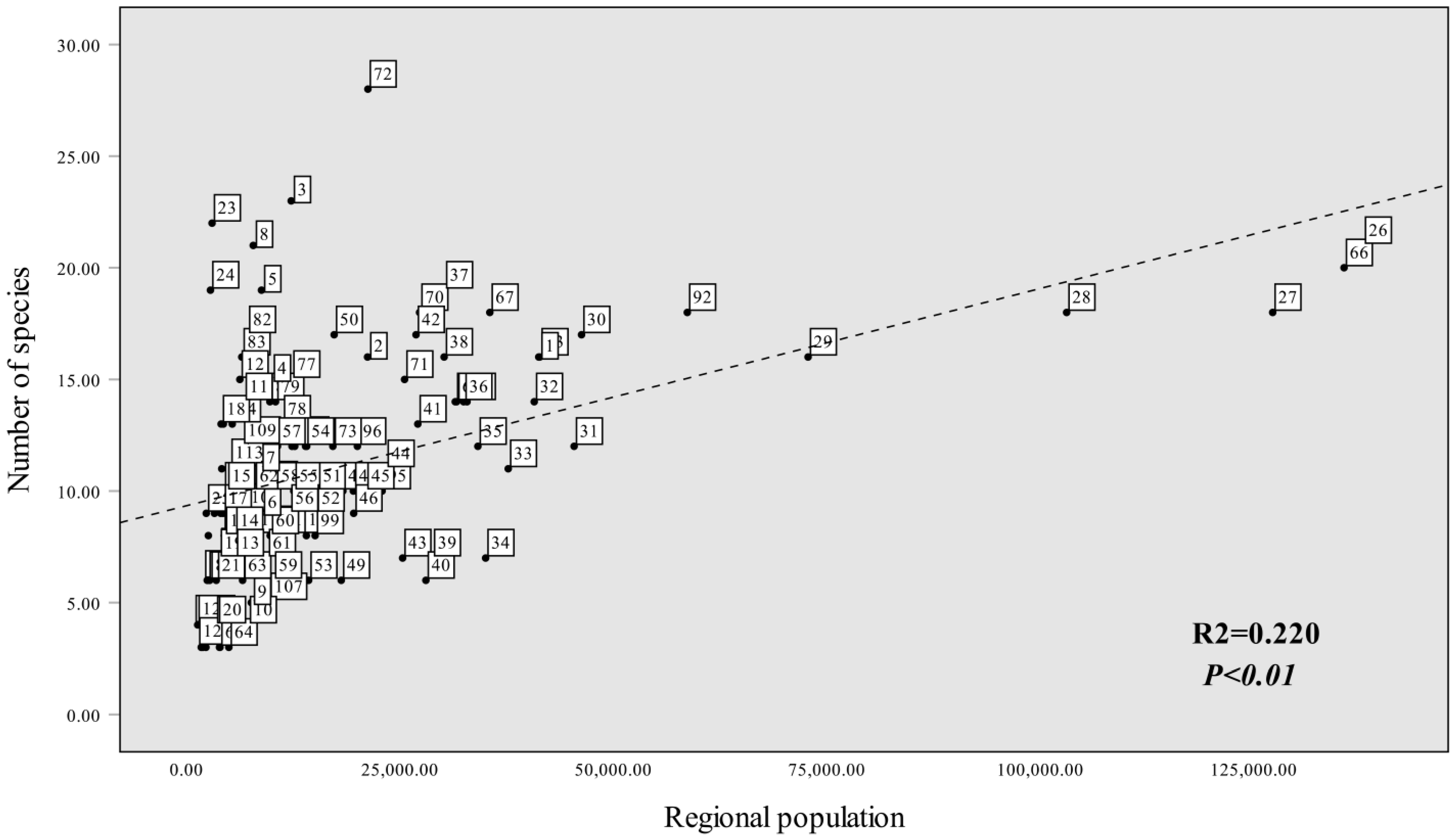
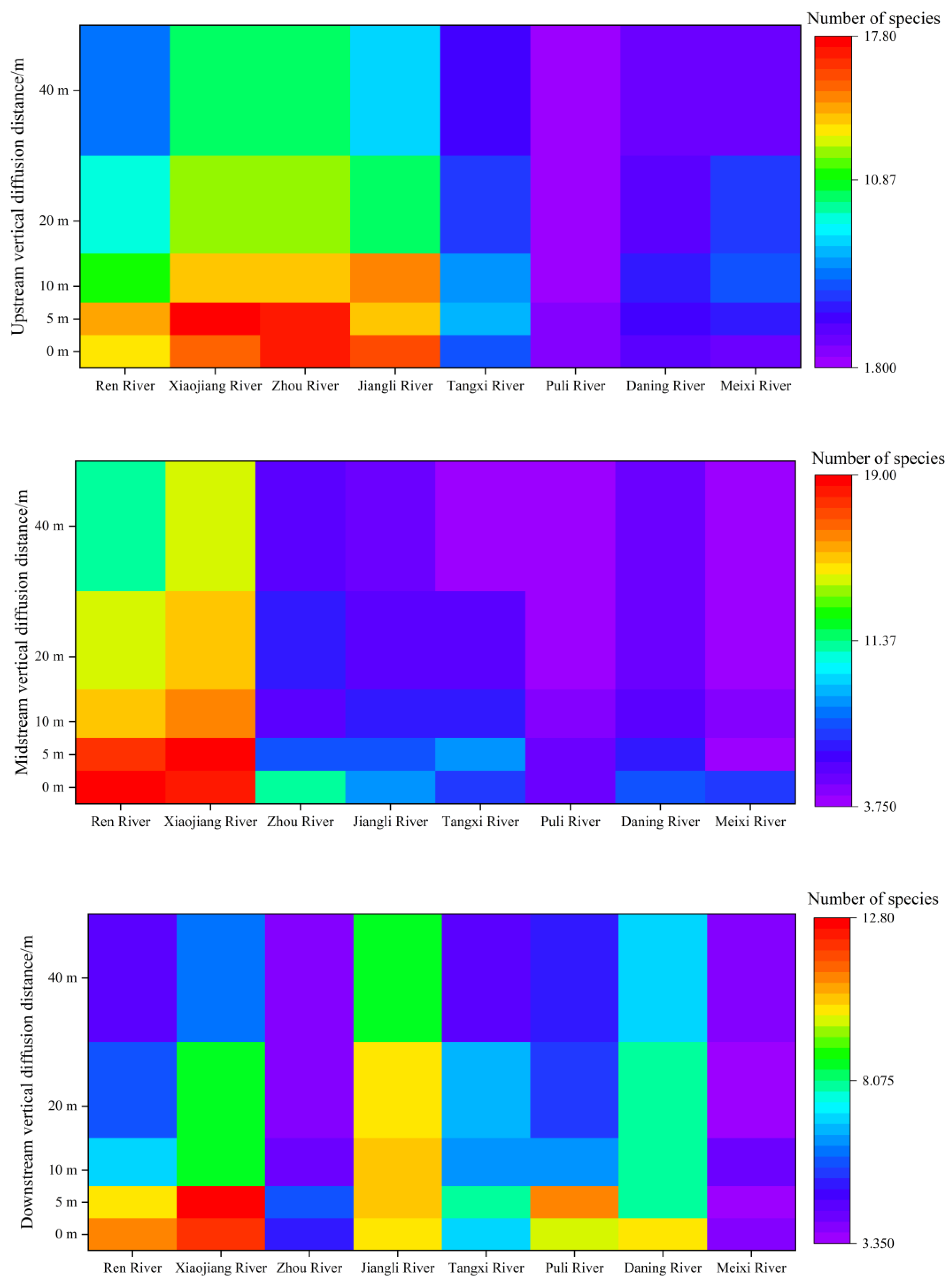
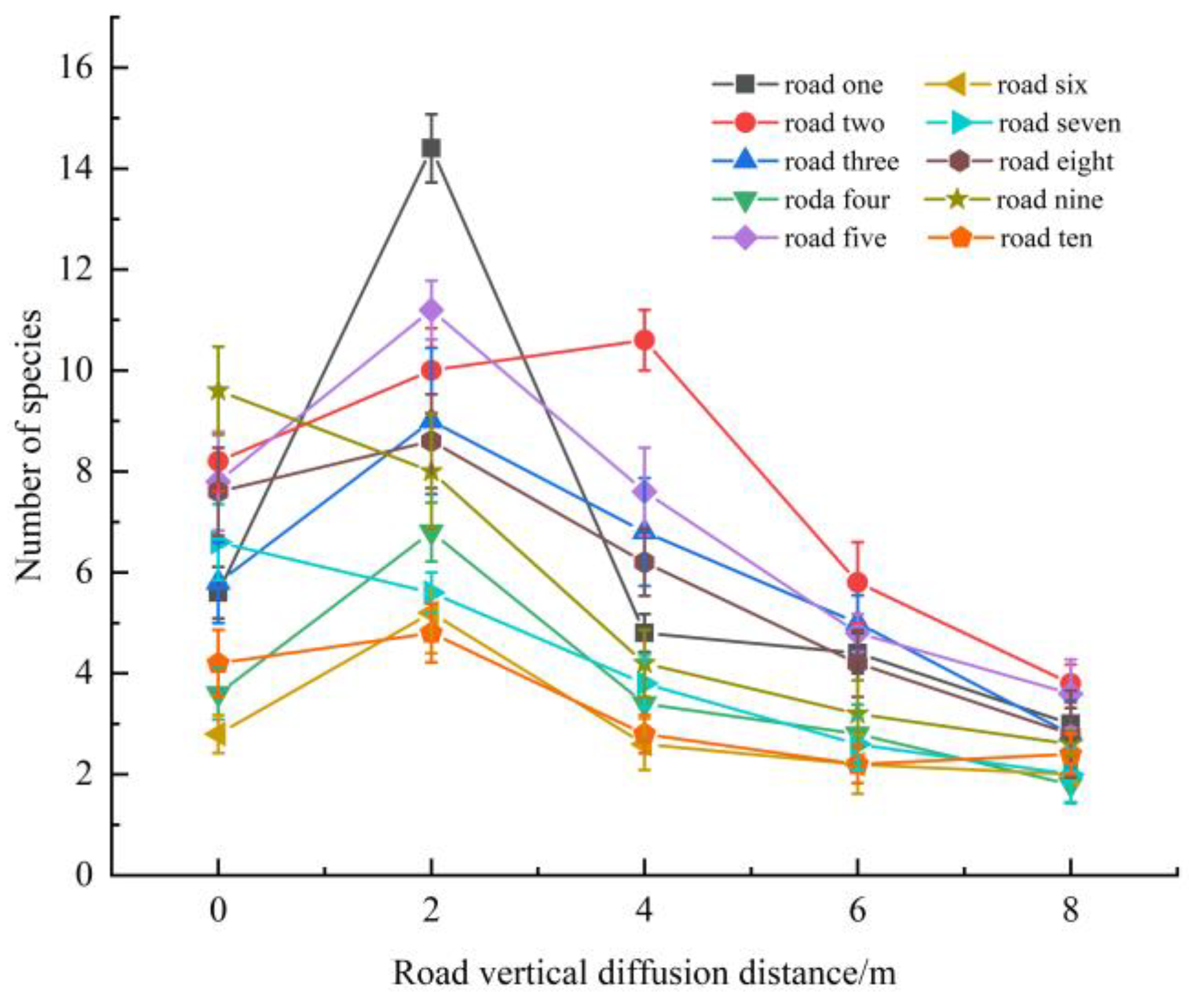
3.4. Spatial Distribution Pattern
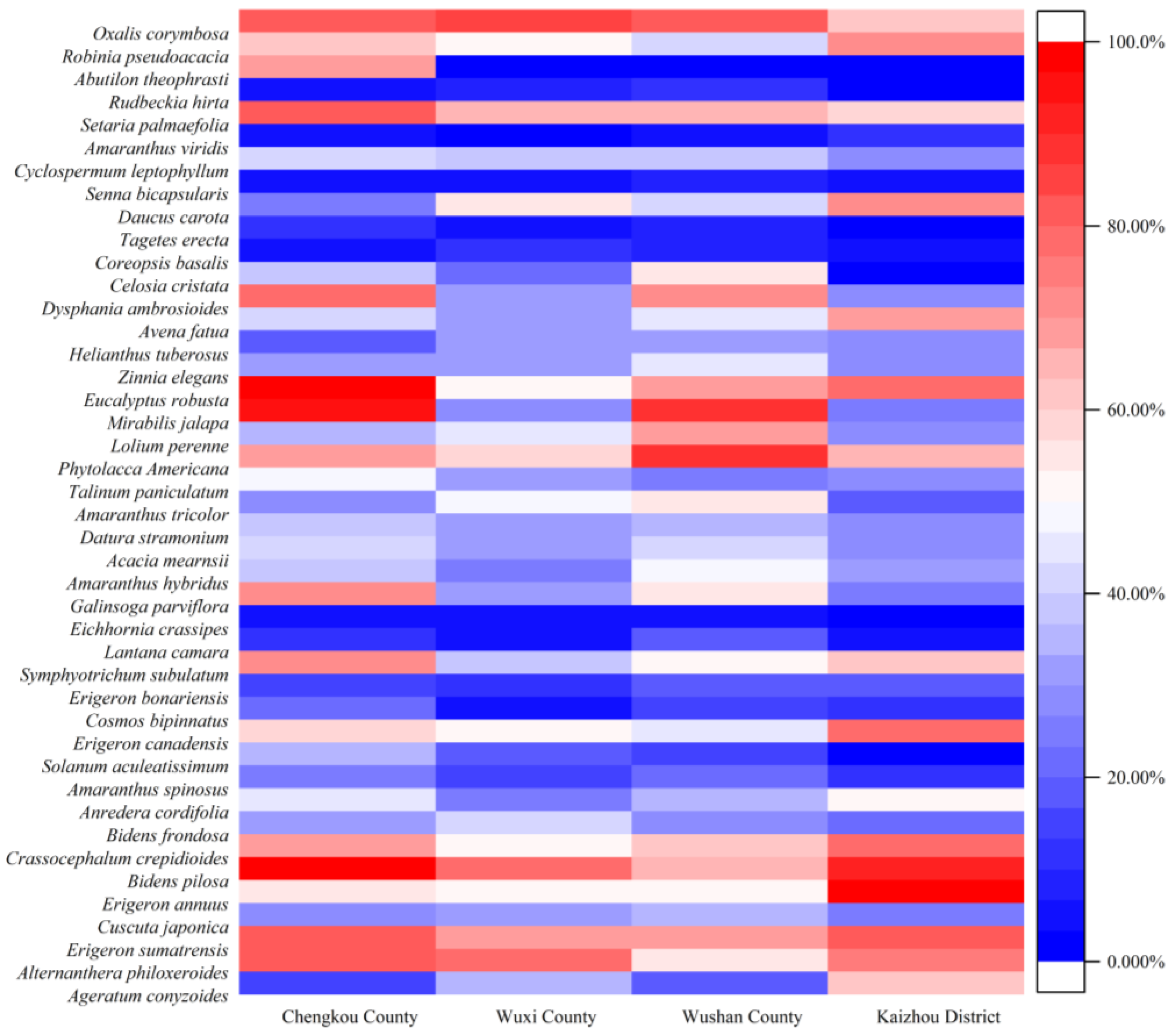
3.5. Construction and Application of Risk Assessment System
4. Discussion
4.1. The High Level of Invasion on the Southern Side of the Daba Mountain Area
4.2. Distribution Patterns and Influencing Factors
4.3. Invasion Risk Assessment
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Yin, W.; Wu, M.; Tian, B.; Yu, H.; Wang, Q.; Ding, J. Effects of bio-invasion on the Yellow River basin ecosystem and its countermeasures. Biodivers. Sci. 2020, 28, 1533–1545. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y.; Li, X.; Liu, Z.; Wu, J.; Musa, A.; Ma, Q.; Yu, H.; Cui, X.; Wang, L. Geographical distribution and determining factors of different invasive ranks of alien species across China. Sci. Total Environ. 2020, 722, 137929. [Google Scholar] [CrossRef] [PubMed]
- Egoh, B.N.; Ntshotsho, P.; Maoela, M.A.; Blanchard, R.; Ayompe, L.M.; Rahlao, S. Setting the scene for achievable post-2020 convention on biological diversity targets: A review of the impacts of invasive alien species on ecosystem services in Africa. J. Environ. Manag. 2020, 261, 110171. [Google Scholar] [CrossRef]
- Bhatta, S.; Joshi, L.R.; Shrestha, B.B. Distribution and impact of invasive alien plant species in Bardia National Park, western Nepal. Environ. Conserv. 2020, 47, 197–205. [Google Scholar] [CrossRef]
- Chichizola, G.A.; Gonzalez, S.L.; Rovere, A.E. Alien plant species on roadsides of the northwestern Patagonian steppe (Argentina). PLoS ONE 2021, 16, e0246657. [Google Scholar] [CrossRef]
- Pysek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists′ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Powell, K.I.; Chase, J.M.; Knight, T.M. A Synthesis of Plant Invasion Effects on Biodiversity Across Spatial Scales. Am. J. Bot. 2011, 98, 539–548. [Google Scholar] [CrossRef]
- Hastings, A.; Cuddington, K.; Davies, K.F.; Dugaw, C.J.; Elmendorf, S.; Freestone, A.; Harrison, S.; Holland, M.; Lambrinos, J.; Malvadkar, U.; et al. The spatial spread of invasions: New developments in theory and evidence. Ecol. Lett. 2005, 8, 91–101. [Google Scholar] [CrossRef]
- Pysek, P.; Hulme, P.E. Spatio-temporal dynamics of plant invasions: Linking pattern to process. Ecoscience 2005, 12, 302–315. [Google Scholar] [CrossRef]
- Stricker, K.B.; Hagan, D.; Flory, S.L. Improving methods to evaluate the impacts of plant invasions: Lessons from 40 years of research. Aob Plants 2015, 7, plv028. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Wu, J.M.; Bai, Y.Y.; Zhou, L.; Wang, G.X. Identifying the most noxious invasive plants in China: Role of geographical origin, life form and means of introduction. Biodivers. Conserv. 2009, 18, 305–316. [Google Scholar] [CrossRef]
- Liu, M.; Li, D.; Hu, J.; Liu, D.; Ma, Z.; Cheng, X.; Zhao, C.; Liu, Q. Altitudinal pattern of shrub biomass allocation in Southwest China. PLoS ONE 2020, 15, e0240861. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhou, S.; Yin, X.; Zhang, C.; Li, R.; Chen, J.; Ma, D.; Wang, Y.; Yu, Z.; Chen, Y. Patterns of tree species richness in Southwest China. Environ. Monit. Assess. 2021, 193, 97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-G.; Slik, J.W.F.; Ma, K.-P. Priority areas for the conservation of perennial plants in China. Biol. Conserv. 2017, 210, 56–63. [Google Scholar] [CrossRef]
- Resource Environmental Science and Data Center, C.A.o.S. Available online: http://www.resdc.cn/Default.aspx (accessed on 19 November 2022).
- Xu, Y.; Huang, J.; Lu, X.; Ding, Y.; Zang, R. Priorities and conservation gaps across three biodiversity dimensions of rare and endangered plant species in China. Biol. Conserv. 2019, 229, 30–37. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, R.; Zhang, E.; Yang, H.; Xu, M. Declining chironomid diversity in relation to human influences in southwest China. Anthropocene 2021, 36, 100308. [Google Scholar] [CrossRef]
- Bai, F.; Chisholm, R.; Sang, W.; Dong, M. Spatial Risk Assessment of Alien Invasive Plants in China. Environ. Sci. Technol. 2013, 47, 7624–7632. [Google Scholar] [CrossRef]
- Yang, Q.; Jin, B.; Zhao, X.; Chen, C.; Cheng, H.; Wang, H.; He, D.; Zhang, Y.; Peng, J.; Li, Z.; et al. Composition, Distribution, and Factors Affecting Invasive Plants in Grasslands of Guizhou Province of Southwest China. Diversity 2022, 14, 167. [Google Scholar] [CrossRef]
- Xiaoling, Y.; Quanru, L.; Haiyang, S.; Xianfeng, Z.; Yong, Z.; Li, C.; Yan, L.; Haiying, M.; Shuyan, Q.; Jinshuang, M. The categorization and analysis on the geographic distribution patterns of Chinese alien invasive plants. Biodivers. Sci. 2014, 22, 667. [Google Scholar] [CrossRef]
- Li, W.; Zheng, Y.; Zhang, L.; Lei, Y.; Li, Y.; Liao, Z.; Li, Z.; Feng, Y. Postintroduction evolution contributes to the successful invasion of Chromolaena odorata. Ecol. Evol. 2020, 10, 1252–1263. [Google Scholar] [CrossRef]
- Shu-Gang, L.U.; Cheng-Dong, X.U.; Xiao-Dong, D.; Yu-Qing, D.; Yi, W. The Impacts of the Alien Invasive Plants on Biodiversity in Longitudinal Range-Gorge Region of Southwest China. Acta Bot. Yunnanica 2006, 28, 607. [Google Scholar]
- Sang, W.; Zhu, L.; Axmacher, J.C. Invasion pattern of Eupatorium adenophorum Spreng in southern China. Biol. Invasions 2010, 12, 1721–1730. [Google Scholar] [CrossRef]
- Coffin, A.W. From roadkill to road ecology: A review of the ecological effects of roads. J. Transp. Geogr. 2007, 15, 396–406. [Google Scholar] [CrossRef]
- Mola, I.; Jimenez, M.D.; Lopez-Jimenez, N.; Casado, M.A.; Balaguer, L. Roadside Reclamation Outside the Revegetation Season: Management Options under Schedule Pressure. Restor. Ecol. 2011, 19, 83–92. [Google Scholar] [CrossRef]
- Christen, D.; Matlack, G. The role of roadsides in plant invasions: A demographic approach. Conserv. Biol. 2006, 20, 385–391. [Google Scholar] [CrossRef]
- Gelbard, J.L.; Belnap, J. Roads as conduits for exotic plant invasions in a semiarid landscape. Conserv. Biol. 2003, 17, 420–432. [Google Scholar] [CrossRef]
- Valladares, F.; Tena, D.; Matesanz, S.; Bochet, E.; Balaguer, L.; Costa-Tenorio, M.; Tormo, J.; Garcia-Fayos, P. Functional traits and phylogeny: What is the main ecological process determining species assemblage in roadside plant communities? J. Veg. Sci. 2008, 19, 381–392. [Google Scholar] [CrossRef]
- McDougall, K.L.; Lembrechts, J.; Rew, L.J.; Haider, S.; Cavieres, L.A.; Kueffer, C.; Milbau, A.; Naylor, B.J.; Nunez, M.A.; Pauchard, A.; et al. Running off the road: Roadside non-native plants invading mountain vegetation. Biol. Invasions 2018, 20, 3461–3473. [Google Scholar] [CrossRef]
- Von der Lippe, M.; Kowarik, I. Interactions between propagule pressure and seed traits shape human-mediated seed dispersal along roads. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 123–130. [Google Scholar] [CrossRef]
- Pauchard, A.; Alaback, P.B. Influence of elevation, land use, and landscape context on patterns of alien plant invasions along roadsides in protected areas of south-central Chile. Conserv. Biol. 2004, 18, 238–248. [Google Scholar] [CrossRef]
- Egizi, A.; Kiser, J.; Abadam, C.; Fonseca, D.M. The hitchhiker′s guide to becoming invasive: Exotic mosquitoes spread across a US state by human transport not autonomous flight. Mol. Ecol. 2016, 25, 3033–3047. [Google Scholar] [CrossRef] [PubMed]
- Von der Lippe, M.; Bullock, J.M.; Kowarik, I.; Knopp, T.; Wichmann, M. Human-Mediated Dispersal of Seeds by the Airflow of Vehicles. PLoS ONE 2013, 8, e52733. [Google Scholar] [CrossRef]
- Daniels, M.K.; Iacona, G.D.; Armsworth, P.R.; Larson, E.R. Do roads or streams explain plant invasions in forested protected areas? Biol. Invasions 2019, 21, 3121–3134. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Ma, M.; Ding, Z.; Wu, S.; Jia, W.; Chen, Q.; Yi, X.; Zhang, J.; Li, X.; et al. Dam-induced difference of invasive plant species distribution along the riparian habitats. Sci. Total Environ. 2022, 808, 152103. [Google Scholar] [CrossRef] [PubMed]
- Andelkovic, A.A.; Pavlovic, D.M.; Marisavljevic, D.P.; Zivkovic, M.M.; Novkovic, M.Z.; Popovic, S.S.; Cvijanovic, D.L.; Radulovic, S.B. Plant invasions in riparian areas of the Middle Danube Basin in Serbia. Neobiota 2022, 71, 23–48. [Google Scholar] [CrossRef]
- Cuda, J.; Rumlerova, Z.; Bruna, J.; Skalova, H.; Pysek, P. Floods affect the abundance of invasive Impatiens glandulifera and its spread from river corridors. Divers. Distrib. 2017, 23, 342–354. [Google Scholar] [CrossRef]
- Holestova, A.; Douda, J. Plant species over-occupancy indicates river valleys are natural corridors for migration. Plant Ecol. 2022, 223, 71–83. [Google Scholar] [CrossRef]
- Marinsek, A.; Kutnar, L. Occurrence of invasive alien plant species in the floodplain forests along the Mura River in Slovenia. Period. Biol. 2017, 119, 251–260. [Google Scholar] [CrossRef]
- Zelnik, I. The presence of invasive alien plant species in different habitats: Case study from Slovenia. Acta Biol. Slov. 2012, 55, 25–38. [Google Scholar]
- Catford, J.A.; Morris, W.K.; Vesk, P.A.; Gippel, C.J.; Downes, B.J. Species and environmental characteristics point to flow regulation and drought as drivers of riparian plant invasion. Divers. Distrib. 2014, 20, 1084–1096. [Google Scholar] [CrossRef]
- Mortenson, S.G.; Weisberg, P.J. Does river regulation increase the dominance of invasive woody species in riparian landscapes? Glob. Ecol. Biogeogr. 2010, 19, 562–574. [Google Scholar] [CrossRef]
- Catford, J.A.; Downes, B.J.; Gippel, C.J.; Vesk, P.A. Flow regulation reduces native plant cover and facilitates exotic invasion in riparian wetlands. J. Appl. Ecol. 2011, 48, 432–442. [Google Scholar] [CrossRef]
- Hua, F.; Guo, X.; Ling, L.; Sen, L.; Tan, G.; Xuemei, L. Invasion Patterns of Alternanthera philoxeroides Along Riverside in Chengdu. Chin. Agric. Sci. Bull. 2021, 37, 78–85. [Google Scholar]
- Zelnik, I.; Mavric Klenovsek, V.; Gaberscik, A. Complex Undisturbed Riparian Zones Are Resistant to Colonisation by Invasive Alien Plant Species. Water 2020, 12, 345. [Google Scholar] [CrossRef]
- Zelnik, I.; Haler, M.; Gaberscik, A. Vulnerability of a riparian zone towards invasion by alien plants depends on its structure. Biologia 2015, 70, 869–878. [Google Scholar] [CrossRef]
- Chen, X.; Wang, R.; Cau, Q.; Zhang, H.; Ge, X.; Liu, J. The Relationship between the Distribution of Invasive Plant Alternanthera philoxeroides and Soil Properties is Scale-Dependent. Pol. J. Environ. Stud. 2015, 24, 1931–1938. [Google Scholar] [CrossRef]
- Reynolds, L.V.; Perry, L.G.; Shafroth, P.B.; Katz, G.; Norton, A. Invasion of Siberian Elm (Ulmus pumila) Along the South Platte River: The Roles of Seed Source, Human Influence, and River Geomorphology. Wetlands 2022, 42, 10. [Google Scholar] [CrossRef]
- Greenwood, H.; O′Dowd, D.J.; Lake, P.S. Willow (Salix × rubens) invasion of the riparian zone in south-eastern Australia: Reduced abundance and altered composition of terrestrial arthropods. Divers. Distrib. 2004, 10, 485–492. [Google Scholar] [CrossRef]
- Castro-Diez, P.; Alonso, A. Effects of non-native riparian plants in riparian and fluvial ecosystems: A review for the Iberian Peninsula. Limnetica 2017, 36, 525–541. [Google Scholar] [CrossRef]
- Fonseca, E.; Both, C.; Cechin, S.Z. Introduction pathways and socio-economic variables drive the distribution of alien amphibians and reptiles in a megadiverse country. Divers. Distrib. 2019, 25, 1130–1141. [Google Scholar] [CrossRef]
- Spear, D.; Foxcroft, L.C.; Bezuidenhout, H.; McGeoch, M.A. Human population density explains alien species richness in protected areas. Biol. Conserv. 2013, 159, 137–147. [Google Scholar] [CrossRef]
- Thuiller, W.; Richardson, D.M.; Pysek, P.; Midgley, G.F.; Hughes, G.O.; Rouget, M. Niche-based modelling as a tool for predicting the risk of alien plant invasions at a global scale. Global. Change. Biol. 2005, 11, 2234–2250. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, F.; Zhang, Y.; Wang, C.; Xu, H. Spatial distribution patterns of invasive alien species in China. Glob. Ecol. Conserv. 2021, 26, e01432. [Google Scholar] [CrossRef]
- Bertelsmeier, C.; Ollier, S.; Liebhold, A.; Keller, L. Recent human history governs global ant invasion dynamics. Nat. Ecol. Evol. 2017, 1, 0184. [Google Scholar] [CrossRef] [PubMed]
- Thuiller, W.; Richardson, D.M.; Rouget, M.; Procheş, S.; Wilson, J.R. Interactions between environment, species traits, and human uses describe patterns of plant invasions. Ecology 2006, 87, 1755–1769. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, M.; Miao, S.L.; Li, Z.Y.; Song, M.H.; Wang, R.Q. Invasive alien plants in China: Role of clonality and geographical origin. Biol. Invasions 2006, 8, 1461–1470. [Google Scholar] [CrossRef]
- Saaty, T.L. HIGHLIGHTS AND CRITICAL-POINTS IN THE THEORY AND APPLICATION OF THE ANALYTIC HIERARCHY PROCESS. Eur. J. Oper. Res. 1994, 74, 426–447. [Google Scholar] [CrossRef]
- Saaty, R.W. The Analytic Hierarchy Process—What It Is and How It Is Used. Math. Model. 1987, 9, 161–176. [Google Scholar] [CrossRef]
- Nielsen, A.M.; Fei, S.L. Assessing the flexibility of the Analytic Hierarchy Process for prioritization of invasive plant management. Neobiota 2015, 27, 25–36. [Google Scholar] [CrossRef]
- Potgieter, L.J.; Gaertner, M.; Irlich, U.M.; O′Farrell, P.J.; Stafford, L.; Vogt, H.; Richardson, D.M. Managing Urban Plant Invasions: A Multi-Criteria Prioritization Approach. Environ. Manag. 2018, 62, 1168–1185. [Google Scholar] [CrossRef]
- Huang, J.H.; Huang, J.H.; Liu, C.R.; Zhang, J.L.; Lu, X.H.; Ma, K.P. Diversity hotspots and conservation gaps for the Chinese endemic seed flora. Biol. Conserv. 2016, 198, 104–112. [Google Scholar] [CrossRef]
- Lei, F.M.; Wei, G.A.; Zhao, H.F.; Yin, Z.H.; Lu, J.L. China subregional avian endemism and biodiversity conservation. Biodivers. Conserv. 2007, 16, 1119–1130. [Google Scholar] [CrossRef]
- Dazhi, C. Research on the Present Situation of Biodiversity Conservation in Daba Mountain and its Developent. Hubei For. Sci. Technol. 2017, 46, 68–70+83. [Google Scholar]
- Chunyan, T.; Haoyang, X.; Bin, C. Investigation and Fauna Analysis of Cerambycoidea in Daba Mountain of Chongqing. J. Chongqing Norm. Univ. (Nat. Sci.) 2020, 37, 72–85. [Google Scholar]
- Editorial Committee of Flora of China. Flora Reipublicae Popularis Sinicae; Science Press: Beijing, China, 2004. [Google Scholar]
- Sichuan Flora Editorial Committee. Flora of Sichuan; Sichuan Science and Technology Press: Sichuan, China, 1988. [Google Scholar]
- Yu, J. Catalogue of Higher Plants in Daba Mountain Area; Science Press: Beijing, China, 2014. [Google Scholar]
- Jinshuang, M.; Xiaoling, Y.; Jin, Y.; Zhanghua, W.; Huiru, L. Invasive Flora of China; Shanghai Jiao Tong University Press: Shanghai, China, 2020. [Google Scholar]
- Hui, G. Establishment of Invasive Risk Assessment System for Alien Introduced Terrestrial Plants in East China. Master’s Thesis, Nanjing Forestry University, Nanjing, China, 2014. [Google Scholar]
- Holt, J. Score averaging for alien species risk assessment: A probabilistic alternative. J. Environ. Manag. 2006, 81, 58–62. [Google Scholar] [CrossRef]
- Gordon, D.R.; Gantz, C.A. Risk assessment for invasiveness differs for aquatic and terrestrial plant species. Biol. Invasions 2011, 13, 1829–1842. [Google Scholar] [CrossRef]
- Andreu, J.; Vila, M. Risk analysis of potential invasive plants in Spain. J. Nat. Conserv. 2010, 18, 34–44. [Google Scholar] [CrossRef]
- Luo, M.; Xiao, L.; Chen, X.; Lin, K.; Liu, B.; He, Z.; Liu, J.; Zheng, S. Invasive Alien Plants and Invasion Risk Assessment on Pingtan Island. Sustainability 2022, 14, 923. [Google Scholar] [CrossRef]
- Jianmeng, F.; Xiaodong, D.; Chengdong, X.; Fengshu, C. Risk Assessment of Alien Invasive Plants in China and ITS Spatial Distribution Patterns. J. Southwest Univ. (Nat. Sci. Ed.) 2011, 33, 57–63. [Google Scholar] [CrossRef]
- Yan, W. Risk Assessment for the Imported Plants and Their Carried Harmful Insects and Pathogens; Shanghai Science and Technology Press: Shanghai, China, 2017. [Google Scholar]
- Hu, D.; Ye, H.; Qiu, Y.; Hou, J.; Bai, J.; He, T.; Tan, Y.; Liu, M.; Ye, P. Invasive risk assessment of alien plants along Wolong subalpine highway. J. Sichuan Univ. (Nat. Sci. Ed.) 2020, 57, 1002–1008. [Google Scholar]
- Wei, Z.; Zhu, J.; Pan, C.; Wang, Y.; Hu, Q.; Zhou, Y.; Jin, S. Investigation and risk assessment of alien invasive plants in Ningbo, Zhejiang Province. J. Zhejiang AF Univ. 2021, 38, 552–559. [Google Scholar]
- Geospatial Data Cold. Available online: http://www.gscloud.cn/sources/ (accessed on 19 November 2021).
- Tu, W.; Xiong, Q.; Qiu, X.; Zhang, Y. Dynamics of invasive alien plant species in China under climate change scenarios. Ecol. Indic. 2021, 129, 107919. [Google Scholar] [CrossRef]
- Wang, C.-J.; Li, Q.-F.; Wan, J.-Z. Potential invasive plant expansion in global ecoregions under climate change. Peerj 2019, 7, e6479. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, X.; Wei, H.; Wang, D.; Chen, R.; Wang, L.; Gu, W. Predicting the invasive trend of exotic plants in China based on the ensemble model under climate change: A case for three invasive plants of Asteraceae. Sci. Total Environ. 2021, 756, 143841. [Google Scholar] [CrossRef] [PubMed]
- Inderjit; Pergl, J.; van Kleunen, M.; Hejda, M.; Babu, C.R.; Majumdar, S.; Singh, P.; Singh, S.P.; Salamma, S.; Rao, B.R.P.; et al. Naturalized alien flora of the Indian states: Biogeographic patterns, taxonomic structure and drivers of species richness. Biol. Invasions 2018, 20, 1625–1638. [Google Scholar] [CrossRef]
- Pysek, P. Is there a taxonomic pattern to plant invasions? Oikos 1998, 82, 282–294. [Google Scholar] [CrossRef]
- Weber, E.; Sun, S.G.; Li, B. Invasive alien plants in China: Diversity and ecological insights. Biol. Invasions 2008, 10, 1411–1429. [Google Scholar] [CrossRef]
- Corli, A.; Sheppard, C.S. Effects of Residence Time, Auto-Fertility and Pollinator Dependence on Reproductive Output and Spread of Alien and Native Asteraceae. Plants 2019, 8, 108. [Google Scholar] [CrossRef]
- Fenesi, A.; Sandor, D.; Pysek, P.; Dawson, W.; Ruprecht, E.; Essl, F.; Kreft, H.; Pergl, J.; Weigelt, P.; Winter, M.; et al. The role of fruit heteromorphism in the naturalization of Asteraceae. Ann. Bot. 2019, 123, 1043–1052. [Google Scholar] [CrossRef]
- Assad, R.; Reshi, Z.A.; Jan, S.; Rashid, I. Biology of Amaranths. Bot. Rev. 2017, 83, 382–436. [Google Scholar] [CrossRef]
- Dong, M.; Lu, J.Z.; Zhang, W.J.; Chen, J.K.; Li, B. Canada goldenrod (Solidago canadensis): An invasive alien weed rapidly spreading in China. Acta Phytotaxon. Sin. 2006, 44, 72–85. [Google Scholar] [CrossRef]
- Eisaguirre, J.M.; Williams, P.J.; Lu, X.Y.; Kissling, M.L.; Beatty, W.S.; Esslinger, G.G.; Womble, J.N.; Hooten, M.B. Diffusion modeling reveals effects of multiple release sites and human activity on a recolonizing apex predator. Mov. Ecol. 2021, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Bar-Massada, A.; Radeloff, V.C.; Stewart, S.I. Biotic and Abiotic Effects of Human Settlement in the wildland-Urban Interface. Bioscience 2014, 64, 429–437. [Google Scholar] [CrossRef]
- Lutscher, F.; Seo, G. The effect of temporal variability on persistence conditions in rivers. J. Theor. Biol. 2011, 283, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Buchan, L.A.J.; Padilla, D.K. Estimating the probability of long-distance overland dispersal of invading aquatic species. Ecol. Appl. 1999, 9, 254–265. [Google Scholar] [CrossRef]
- Alston, K.P.; Richardson, D.M. The roles of habitat features, disturbance, and distance from putative source populations in structuring alien plant invasions at the urban/wildland interface on the Cape Peninsula, South Africa. Biol. Conserv. 2006, 132, 183–198. [Google Scholar] [CrossRef]
- Sullivan, J.J.; Timmins, S.M.; Williams, P.A. Movement of exotic plants into coastal native forests from gardens in northern New Zealand. N. Z. J. Ecol. 2005, 29, 1–10. [Google Scholar]
- Feng, J.; Zhang, Z.; Nan, R. The roles of climatic factors in spatial patterns of alien invasive plants from America into China. Biodivers. Conserv. 2011, 20, 3385–3391. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; Bowler, P.A.; Xiong, W. Invasive aquatic plants in China. Aquat. Invasions. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, L.; Jiang, Z.; Wu, J.; Cui, X.; Li, X.; Liu, Z.; Musa, A.; Ma, Q.; Yu, H.; et al. Effects of climatic and social factors on dispersal strategies of alien species across China. Sci. Total Environ. 2020, 749, 141443. [Google Scholar] [CrossRef]
- Chengxu, W.; Mingxing, Z.; Xuhui, C.; Bo, Q. Review on Allelopathy of Exotic Invasive Plants. Procedia Eng. 2011, 18, 240–246. [Google Scholar] [CrossRef]
- Fassoni, A.C.; Martins, M.L. Mathematical analysis of a model for plant invasion mediated by allelopathy. Ecol. Complex. 2014, 18, 49–58. [Google Scholar] [CrossRef]
- Wang, S.; Wei, M.; Wu, B.; Cheng, H.; Wang, C. Combined nitrogen deposition and Cd stress antagonistically affect the allelopathy of invasive alien species Canada goldenrod on the cultivated crop lettuce. Sci. Hortic. 2020, 261, 108955. [Google Scholar] [CrossRef]
- Dimitrakopoulos, P.G.; Koukoulas, S.; Galanidis, A.; Delipetrou, P.; Gounaridis, D.; Touloumi, K.; Arianoutsou, M. Factors shaping alien plant species richness spatial patterns across Natura 2000 Special Areas of Conservation of Greece. Sci. Total Environ. 2017, 601–602, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Guohuan, W.; Bai, F.; Weiguo, S. Spatial distribution of invasive alien animal and plant species and its influencing factors in China. Plant Sci. J. 2017, 35, 513–524. [Google Scholar]
- Caiyun, Z.; Xiaoyan, L.; Feifei, L.; Jinfang, Z.; Chaodan, G.; Junsheng, L. The distribution pattern and determinant factors of the main invasive alien plants in nation nature reserve in China. Acta Ecol. Sin. 2022, 42, 1–10. [Google Scholar]
- Guangyao, X.; Hongyuan, L.; Xunqiang, M.; Weiqing, M. Composition and spatial-temporal distribution of Chinese naturalized plants. Chin. J. Plant Ecol. 2019, 43, 601–610. [Google Scholar]
- Pysek, P.; Jarosik, V.; Hulme, P.E.; Kuehn, I.; Wild, J.; Arianoutsou, M.; Bacher, S.; Chiron, F.; Didziulis, V.; Essl, F.; et al. Disentangling the role of environmental and human pressures on biological invasions across Europe. Proc. Natl. Acad. Sci. USA 2010, 107, 12157–12162. [Google Scholar] [CrossRef]
- Le Roux, P.C.; Ramaswiela, T.; Kalwij, J.M.; Shaw, J.D.; Ryan, P.G.; Treasure, A.M.; McClelland, G.T.W.; McGeoch, M.A.; Chown, S.L. Human activities, propagule pressure and alien plants in the sub-Antarctic: Tests of generalities and evidence in support of management. Biol. Conserv. 2013, 161, 18–27. [Google Scholar] [CrossRef]
- Dyer, E.E.; Cassey, P.; Redding, D.W.; Collen, B.; Franks, V.; Gaston, K.J.; Jones, K.E.; Kark, S.; Orme, C.D.L.; Blackburn, T.M. The Global Distribution and Drivers of Alien Bird Species Richness. PLoS Biol. 2017, 15, e2000942. [Google Scholar] [CrossRef]
- Arianoutsou, M.; Delipetrou, P.; Vila, M.; Dimitrakopoulos, P.G.; Celesti-Grapow, L.; Wardell-Johnson, G.; Henderson, L.; Fuentes, N.; Ugarte-Mendes, E.; Rundel, P.W. Comparative Patterns of Plant Invasions in the Mediterranean Biome. PLoS ONE 2013, 8, e79174. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakopoulos, P.G.; Koukoulas, S.; Michelaki, C.; Galanidis, A. Anthropogenic and environmental determinants of alien plant species spatial distribution on an island scale. Sci. Total Environ. 2022, 805, 150314. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Yang, L.; Deng, H.; Qi, X.; Ding, Y.; Zou, J.; Tian, C. On Risk Assessment of Invasive Plants in Beibei District of Chongqing. J. Southwest Norm. Univ. (Nat. Sci. Ed.) 2016, 41, 76–80. [Google Scholar] [CrossRef]
- Rayment, J.; French, K.; Bedward, M. Understanding patterns and pathways of exotic perennial grass invasion in South-eastern Australian grassy communities. Divers. Distrib. 2022, 28, 1136–1150. [Google Scholar] [CrossRef]
- Yang, X. Risk Assessment and Control Strategies of the Invasive Plants in the National Nature Reserve of Chongqing Jinyun Mountain. Master’s Thesis, Southwest University, El Paso, TX, USA, 2016. [Google Scholar]

| First-Level Index | Second-Level Index | Third-Level Index |
|---|---|---|
| Invasion history (6 points) | Invasion record in Chongqing (2 points) | Yes (2 points); no (0 points) |
| Pathways of introduction (4 points) | Intentional introduction (2 points); unintentional introduction (1 point); natural introduction (0.5 point); unknown (0.5 point) | |
| Acclimatization (17 points) | Origin (7 points) | America (3 points); other areas (2 points) |
| Number of villages and towns distributed (10 points) | 1 ≤ numbers ≤ 3 (2 points); 3 < numbers ≤ 6 (3 points); 6 < numbers ≤ 8 (5 points) | |
| Growth condition (9 points) | Growth state (6 points) | Good (4 points); average (1.5 points); poor (0.5 points) |
| Naturalized (3 points) | Yes (2 points); no (1 point) | |
| Biological characteristics (19 points) | Life forms (10 points) | Annual herb (3 points); perennial herb (2 points); liana (1.5 points); shrub (1.5 points); arbor (1 point); hydrophyte (1 point) |
| Reproduction modes (6 points) | Sexual reproduction (1.5 points); asexual reproduction (1.5 points); both (3 points) | |
| Habitats (3 points) | Specific habitat (0.5 points); minority habitat (1 point); multi-habitat (1.5 points) | |
| Diffusion mode and ability (12 points) | Diffusion mode (3 points) | Wind propagation (0.5 points); flow propagation (0.5 points); self-propagation (0.5 points); animal carrying or feeding propagation (0.5 point); human active communication (1 point) |
| Enemy (3 points) | Effective natural enemy (0 points); non-effective natural enemy (1 point); no natural enemy (2 points) | |
| Diffusion ability (6 points) | Powerful (3 points); general (2 points); poor (1 point) | |
| Harm and influence (12 points) | Economic harm (4 points) | Serious influence (3 points); general influence (1 points); no influence (0 points) |
| Ecological harm (4 points) | Serious influence (3 points); general influence (1 point); no influence (0 points) | |
| Allergic harm (4 points) | Allelopathic toxicity exists (2 points); other harm (2 points); no harm(0 points) | |
| Control and quarantine difficulty (25 points) | Identification difficulty (10 points) | Great difficulty (5 points); intermediate difficulty (3 points); easy (2 points) |
| Control difficulty (15 points) | Effective prevention and control measures exist (4 points); with feasible method and unknown effect (5 points); no feasible way yet (6 points) |
| Latin | Risk Value | Risk Grade | Latin | Risk Value | Risk Grade |
|---|---|---|---|---|---|
| Alternanthera philoxeroides | 48 | high-risk | Ageratum conyzoides | 48 | high-risk |
| Erigeron sumatrensis | 46.5 | high-risk | Cuscuta japonica | 45.5 | high-risk |
| Erigeron annuus | 45.5 | high-risk | Bidens pilosa | 44.5 | high-risk |
| Crassocephalum crepidioides | 44.5 | high-risk | Bidens frondosa | 44 | high-risk |
| Anredera cordifolia | 44 | high-risk | Amaranthus spinosus | 43.5 | high-risk |
| Solanum aculeatissimum | 43.5 | high-risk | Erigeron canadensis | 43.5 | high-risk |
| Cosmos bipinnatus | 43 | high-risk | Erigeron bonariensis | 41.5 | high-risk |
| Symphyotrichum subulatum | 41.5 | high-risk | Lantana camara | 41 | high-risk |
| Eichhornia crassipes | 40 | high-risk | Galinsoga parviflora | 40 | high-risk |
| Amaranthus hybridus | 38.5 | medium-risk | Acacia mearnsii | 38 | medium-risk |
| Datura stramonium | 38 | medium-risk | Amaranthus tricolor | 37.5 | medium-risk |
| Talinum paniculatum | 37 | medium-risk | Phytolacca Americana | 37 | medium-risk |
| Lolium perenne | 37 | medium-risk | Mirabilis jalapa | 36.5 | medium-risk |
| Eucalyptus robusta | 35 | medium-risk | Zinnia elegans | 35 | medium-risk |
| Helianthus tuberosus | 35 | medium-risk | Avena fatua | 35 | medium-risk |
| Dysphania ambrosioides | 34.5 | medium-risk | Celosia cristata | 34 | medium-risk |
| Coreopsis basalis | 34 | medium-risk | Tagetes erecta | 34 | medium-risk |
| Daucus carota | 34 | medium-risk | Senna bicapsularis | 33.5 | medium-risk |
| Cyclospermum leptophyllum | 33.5 | medium-risk | Amaranthus viridis | 33.5 | medium-risk |
| Setaria palmifolia | 33.5 | medium-risk | Rudbeckia hirta | 33 | medium-risk |
| Abutilon theophrasti | 33 | medium-risk | Robinia pseudoacacia | 33 | medium-risk |
| Oxalis corymbosa | 33 | medium-risk | Veronica persica | 32 | low-risk |
| Ipomoea purpurea | 32 | low-risk | Eleusine indica | 31 | low-risk |
| Amaranthus caudatus | 31 | low-risk | Geranium carolinianum | 31 | low-risk |
| Leucaena leucocephala | 30.5 | low-risk | Euphorbia maculata | 30 | low-risk |
| Melilotus officinalis. | 30 | low-risk | Euphorbia hirta | 30 | low-risk |
| Trifolium pratense | 30 | low-risk | Celosia argentea | 29 | low-risk |
| Trifolium repens | 29 | low-risk | Opuntia dillenii | 28.5 | low-risk |
| Verbena bonariensis | 28.5 | low-risk | Salvia splendens | 28 | low-risk |
| Chenopodium ficifolium | 27 | low-risk | Medicago sativa | 24 | low-risk |
| Ulex europaeus | 21.5 | low-risk | Mimosa pudica | 21 | low-risk |
| Oenothera glazioviana | 21 | low-risk | Echinacea purpurea | 21 | low-risk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Deng, H.; Zuo, Y.; Yang, J.; Yang, Y.; Huang, Y.; Qin, Q.; Yang, C. Spatial Distribution Pattern and Risk Assessment of Invasive Alien Plants on Southern Side of the Daba Mountain Area. Diversity 2022, 14, 1019. https://doi.org/10.3390/d14121019
Wang Y, Deng H, Zuo Y, Yang J, Yang Y, Huang Y, Qin Q, Yang C. Spatial Distribution Pattern and Risk Assessment of Invasive Alien Plants on Southern Side of the Daba Mountain Area. Diversity. 2022; 14(12):1019. https://doi.org/10.3390/d14121019
Chicago/Turabian StyleWang, Yuanyuan, Hongping Deng, Youwei Zuo, Jun Yang, Yubing Yang, Yan Huang, Qi Qin, and Chongyi Yang. 2022. "Spatial Distribution Pattern and Risk Assessment of Invasive Alien Plants on Southern Side of the Daba Mountain Area" Diversity 14, no. 12: 1019. https://doi.org/10.3390/d14121019
APA StyleWang, Y., Deng, H., Zuo, Y., Yang, J., Yang, Y., Huang, Y., Qin, Q., & Yang, C. (2022). Spatial Distribution Pattern and Risk Assessment of Invasive Alien Plants on Southern Side of the Daba Mountain Area. Diversity, 14(12), 1019. https://doi.org/10.3390/d14121019






