Ecological Distribution Patterns and Indicator Species Analysis of Climber Plants in Changa Manga Forest Plantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study site
2.2. Sampling Design
2.3. Soil Analysis
2.4. Indicator Species Analysis
2.5. Data Analysis
3. Results
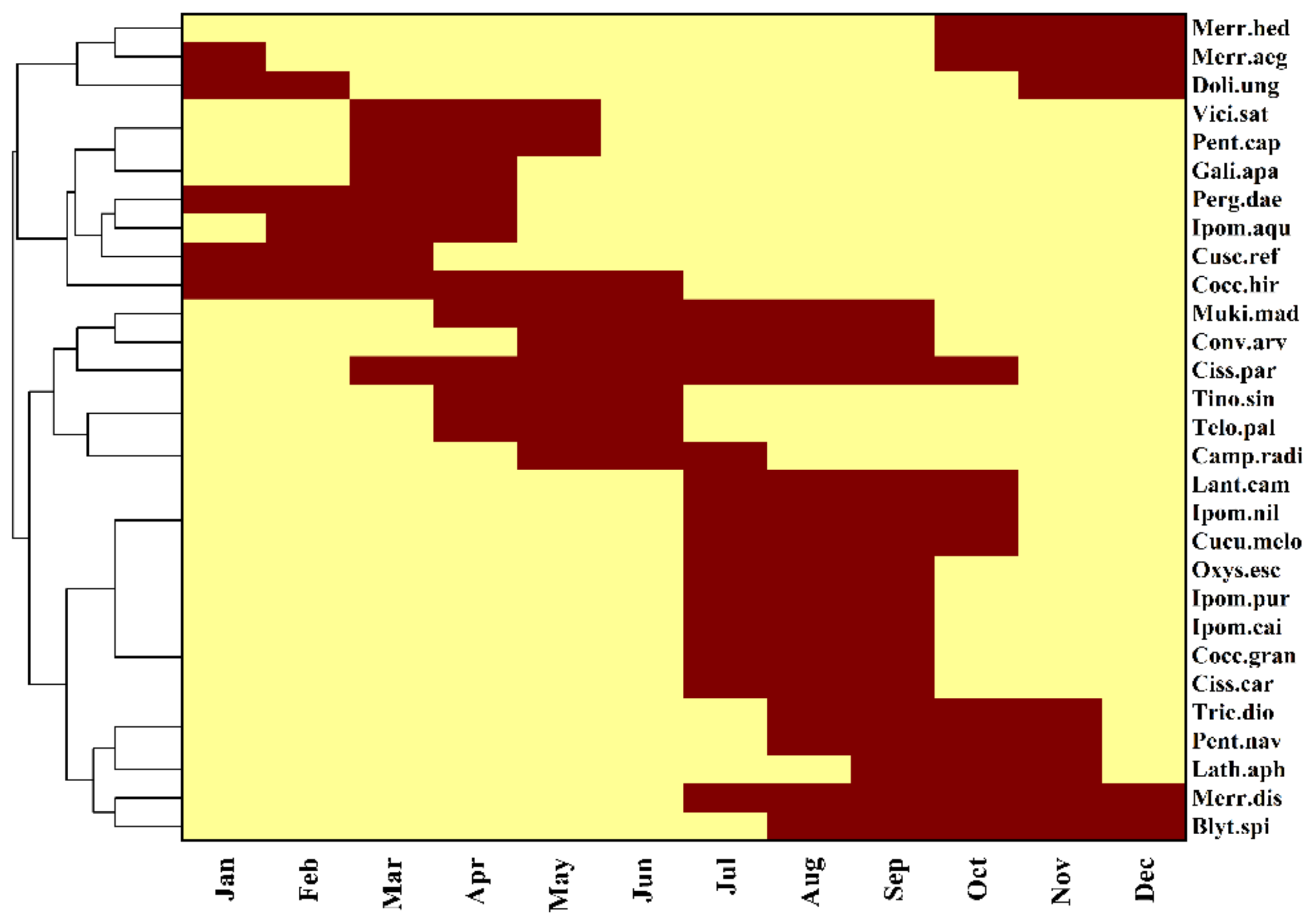
3.1. Taxonomic Diversity and Flowering Phenology
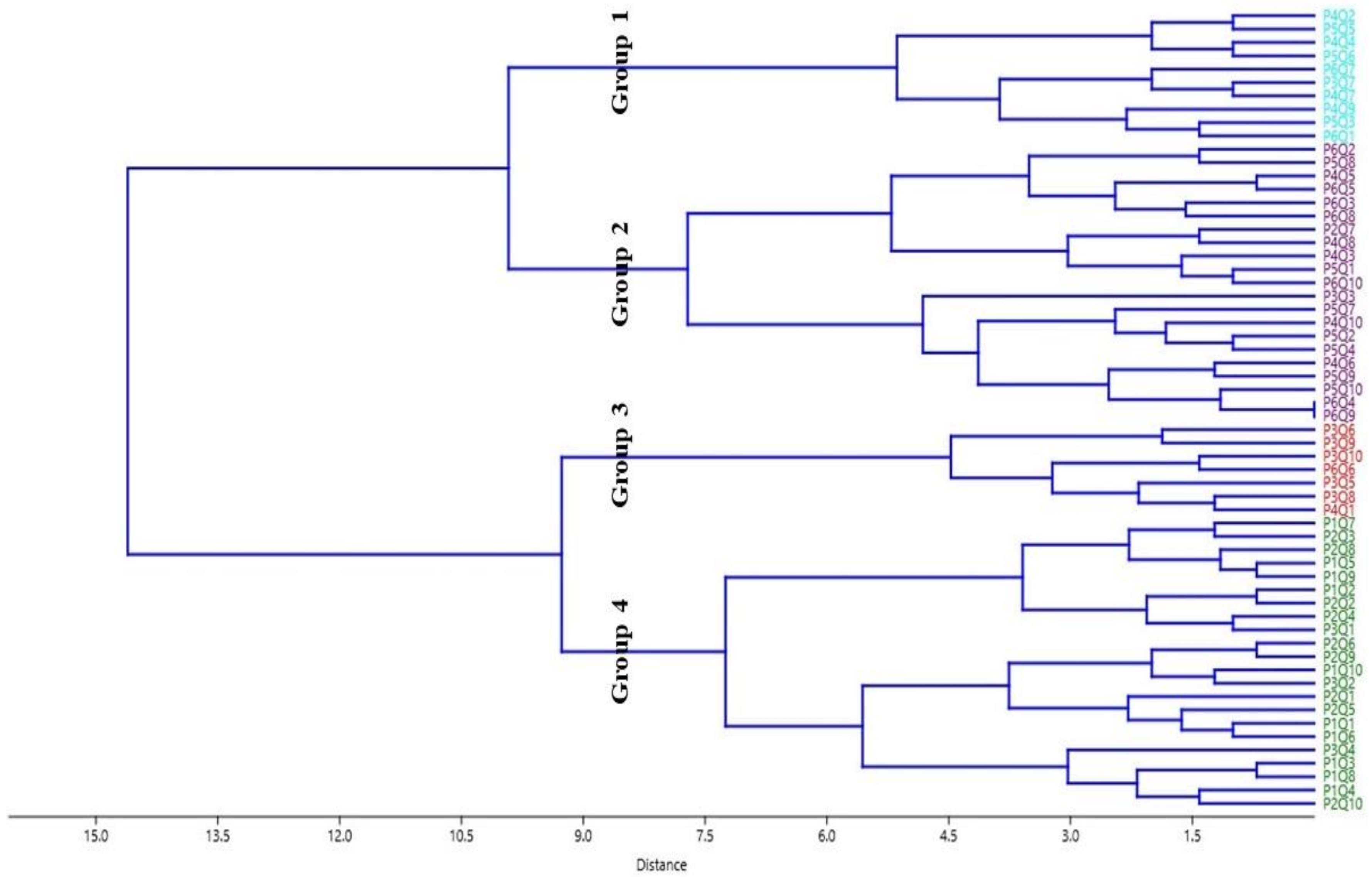
3.2. Cluster Analysis
3.2.1. Group 1
3.2.2. Group 2
3.2.3. Group 3
3.2.4. Group 4
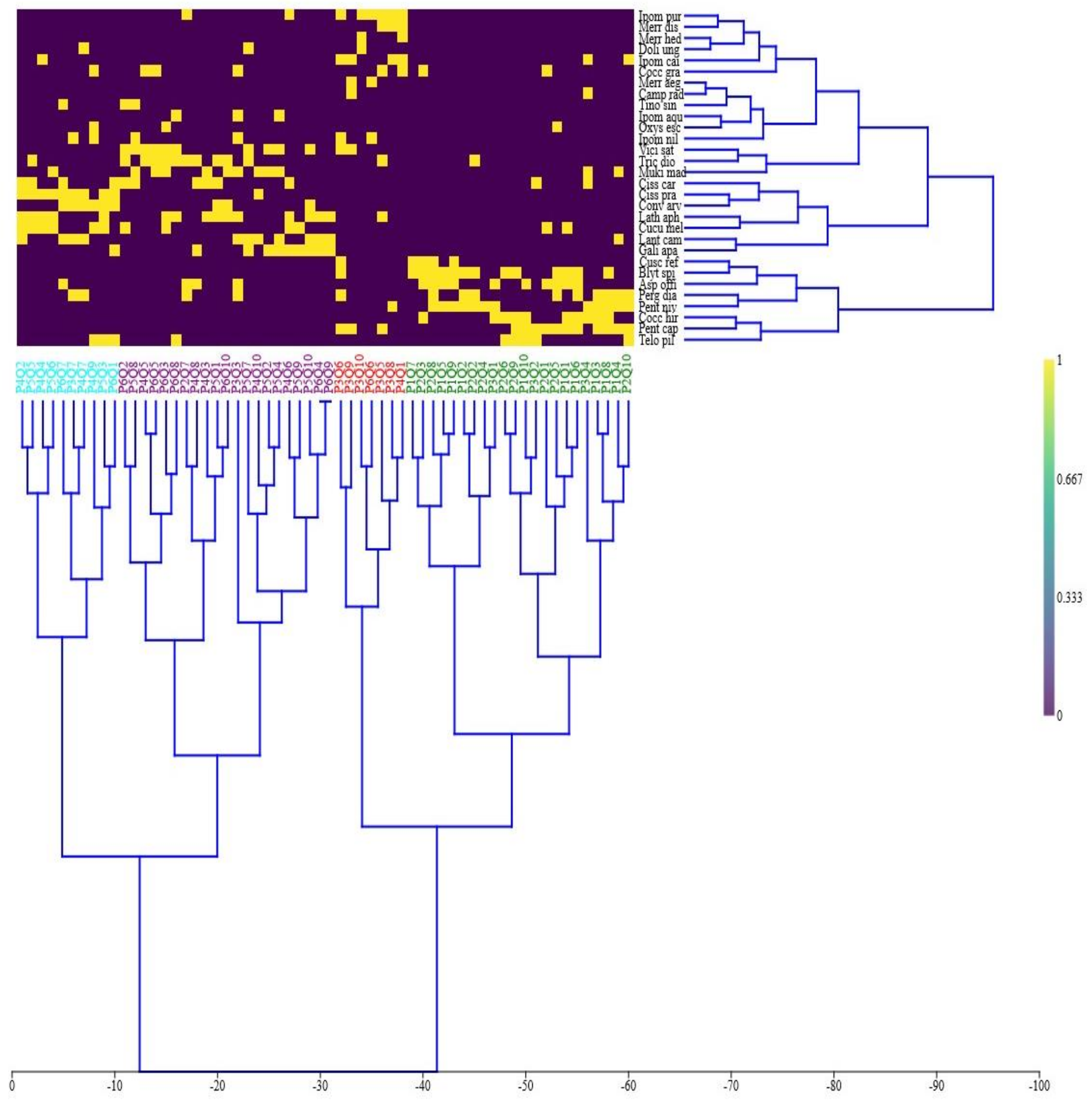
3.3. Indicator Species Analysis
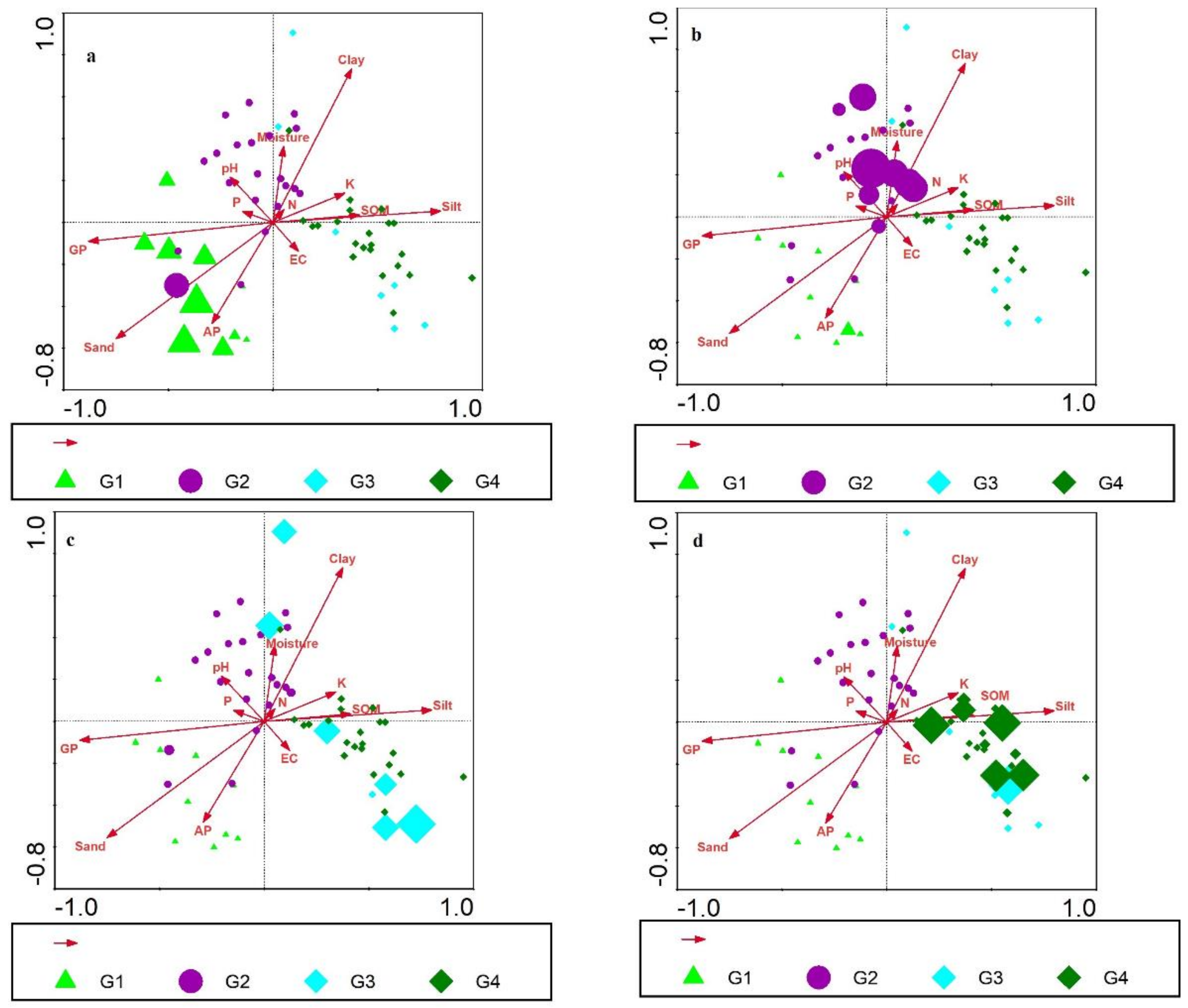
3.4. Edaphic Parameters and Climber Distribution
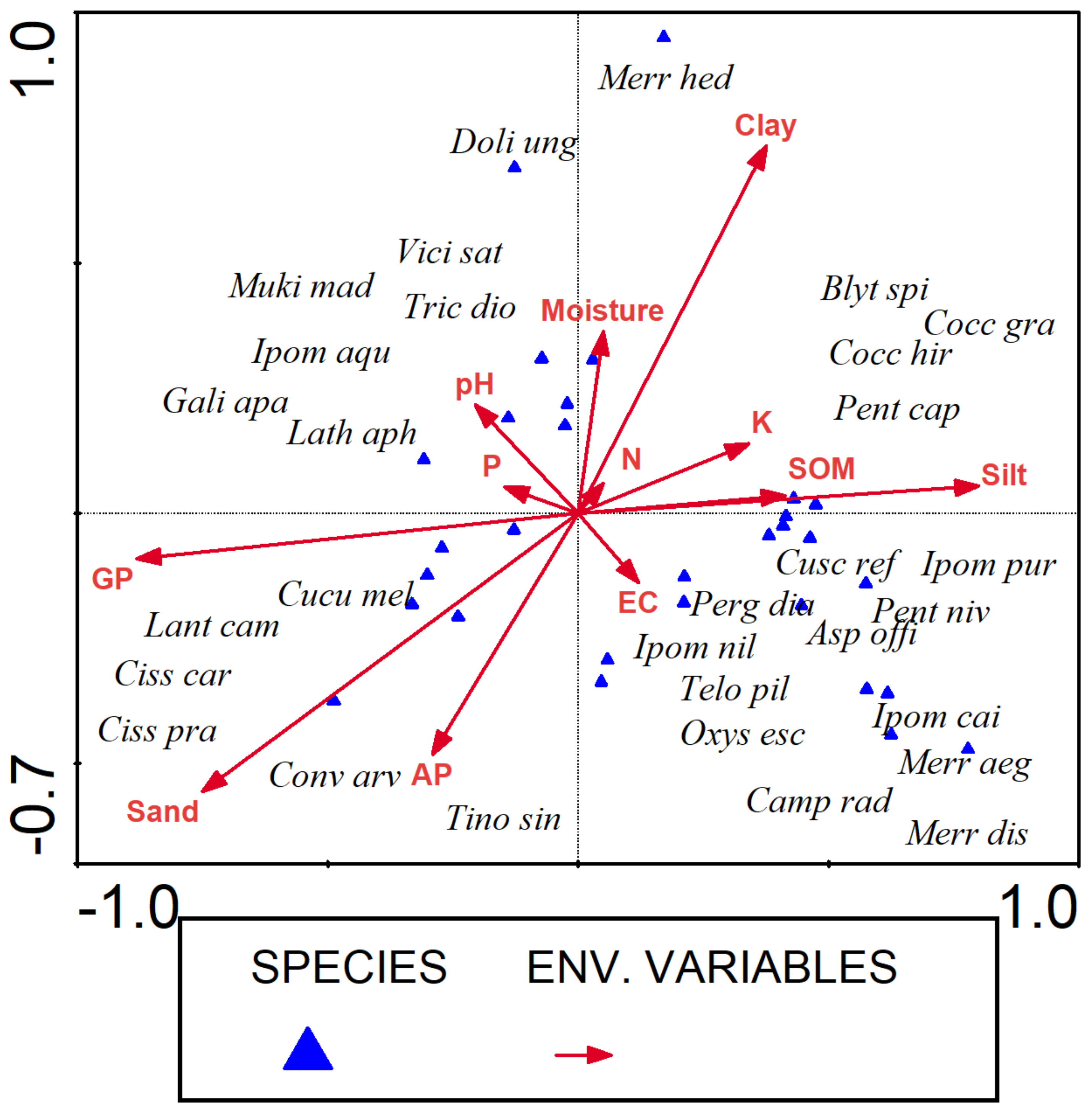
3.5. Canonical Correspondence Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parthasarathy, N.; Muthuramkumar, S.; Muthumperumal, C.; Vivek, P.; Ayyappan, N.; Sridhar, R.M. Liana composition and diversity among tropical forest types of peninsular India. In Ecology of Lianas; Schnitzer, S.A., Bongers, F., Burnham, R.J., Putz, F.E., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 36–49. [Google Scholar]
- Estrada-Villegas, S.; Pedraza Narvaez, S.S.; Sanchez, A.; Schnitzer, S.A. Lianas Significantly Reduce Tree Performance and Biomass Accumulation Across Tropical Forests: A Global Meta-Analysis. Front. For. Glob. Chang. 2022, 4, 812066. [Google Scholar] [CrossRef]
- Schnitzer, S.A.; Bongers, F. The ecology of lianas and their role in forests. Trends Ecol. Evol. 2002, 17, 223–230. [Google Scholar] [CrossRef]
- Sundarapandian, S.M.; Subbiah, S. Diversity and tree population structure of tropical dry evergreen forests in Sivagangai district of Tamil Nadu, India. Trop. Plant Res. 2015, 2, 36–46. [Google Scholar]
- Gentry, A.H. The distribution and evolution of climbing plants. In The Biology of Vines; Putz, F.E., Mooney, H.A., Eds.; Cambridge University Press: Cambridge, UK, 1991; pp. 3–49. [Google Scholar]
- Pérez-Salicrup, D.R.; Sork, V.L.; Putz, F.E. Lianas and trees in a liana forest of amazonian Bolivia. Biotropica 2001, 33, 34–47. [Google Scholar] [CrossRef]
- Santos, K.D.; Kinoshita, L.S.; Rezende, A.A. Species composition of climbers in seasonal semi-deciduous forest fragments of Southeastern Brazil. Biota Neotrop. 2009, 9, 175–188. [Google Scholar] [CrossRef]
- Isnard, S.; Silk, W.K. Moving with climbing plants from Charles Darwin’s time into the 21st century. Am. J. Bot. 2009, 96, 1205–1221. [Google Scholar] [CrossRef]
- Schnitzer, S.A.; Putz, F.E.; Bongers, F.; Kroening, K. The past, present, and potential future of liana ecology. In Ecology of Lianas; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 1–10. [Google Scholar]
- Fiorello, I.; Del Dottore, E.; Tramacere, F.; Mazzolai, B. Taking inspiration from climbing plants: Methodologies and benchmarks—A review. Bioinspir. Biomim. 2020, 15, 031001. [Google Scholar] [CrossRef]
- Valladares, F.; Gianoli, E.; Saldana, A. Climbing plants in a temperate rainforest understorey: Searching for high light or coping with deep shade? Ann. Bot. 2011, 108, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.U.; Khan, S.M.; Saqib, Z.; Ullah, Z.; Ahmad, Z.; Ekercin, S.; Mumtaz, A.S.; Ahmad, H. Diversity and abundance of climbers in relation to their hosts and elevation in the monsoon forests of Murree in the Himalayas. Pak. J. Bot. 2020, 52, 601–612. [Google Scholar] [CrossRef]
- Reddy, M.S.; Parthasarathy, N. Liana diversity and distribution on host trees in four inland tropical dry evergreen forests of peninsular India. Trop. Ecol. 2006, 47, 109–123. [Google Scholar]
- Addo-Fordjour, P.; Anning, A.K.; Larbi, J.A.; Akyeampong, S. Liana species richness, abundance and relationship with trees in the Bobiri forest reserve, Ghana: Impact of management systems. For. Ecol. Manag. 2009, 257, 1822–1828. [Google Scholar] [CrossRef]
- Addo-Fordjour, P.; Rahmad, Z.B. Environmental factors associated with liana community assemblages in a tropical forest reserve. Ghana. J. Trop. Ecol. 2015, 31, 69–79. [Google Scholar] [CrossRef]
- Uwalaka, N.O.; Khapugin, A.A.; Muoghalu, J.I. Effect of some environmental factors on liana abundance in a regenerating secondary lowland rainforest in Nigeria three decades after a ground fire. Ecol. Quest. 2020, 31, 27–38. [Google Scholar] [CrossRef]
- Munoz, A.A.; Chacon, P.; Perez, F.; Barnert, E.S.; Armesto, J.J. Diversity and host tree preferences of vascular epiphytes and climbers in a temperate rainforest in southern Chile. Aust. J. Bot. 2003, 51, 381–391. [Google Scholar] [CrossRef]
- Durigon, J.; Miotto, S.T.; Gianoli, E. Distribution and traits of climbing plants in subtropical and temperate South America. J. Veg. Sci. 2014, 25, 1484–1492. [Google Scholar] [CrossRef]
- Dewalt, S.J.; Schnitzer, S.A.; Denslow, J.S. Density and diversity of lianas along a chronosequence in a central Panamanian lowland forest. J. Trop. Ecol. 2000, 16, 1–19. [Google Scholar] [CrossRef]
- Chittibabu, C.V.; Parthasarathy, N. Liana diversity and host relationships in a tropical evergreen forest in the Indian Eastern Ghats. Ecol. Res. 2001, 16, 519–529. [Google Scholar] [CrossRef]
- Kammesheidt, L.; Berhaman, A.; Tay, J.; Abdullah, G.; Azwal, M. Liana abundance, diversity and tree infestation in the Imbak Canyon conservation area, Sabah, Malaysia. J. Trop. Sci. 2009, 21, 265–271. [Google Scholar]
- Ghollasimood, S.; Faridah-Hanum, I.; Nazre, M.; Kamziah, A.K. Abundance and Distribution of Climbers in a Coastal Hill Forest in Perak, Malaysia. J. Agric. Sci. 2012, 4, 245–254. [Google Scholar] [CrossRef]
- Anbarashan, M.; Parthasarathy, N. Diversity and ecology of lianas in tropical dry evergreen forests on the Coromandel Coast of India under various disturbance regimes. Flora-Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 22–32. [Google Scholar] [CrossRef]
- Vivek, P.; Parthasarathy, N. Liana community and functional trait analysis in the tropical dry evergreen forest of India. J. Plant Ecol. 2014, 8, 501–512. [Google Scholar] [CrossRef]
- Mastan, T.; Parveen, S.N.; Reddy, M.S. Liana species inventory in a tropical dry forest of Sri Lanka Malla Wildlife Sanctuary, Andhra Pradesh, India. J. Environ. Res. Dev. 2015, 9, 1024–1030. [Google Scholar]
- Shukla, G.; Rai, P.; Manohar, K.A.; Chakravarty, S. Quantification of diversity, biomass and carbon storage of climber and liana community in a foothill forest of Indian eastern Himalayas. Acta Ecol. Sin. 2020, 40, 478–482. [Google Scholar] [CrossRef]
- Bongers, F.; Parren, M.P.; Traoré, D. Forest Climbing Plants of West Africa: Diversity, Ecology and Management; CABI Publishing: Oxford, UK, 2005; p. 288. [Google Scholar]
- Ward, E.B.; Pregitzer, C.C.; Kuebbing, S.E.; Bradford, M.A. Invasive lianas are drivers of and passengers to altered soil nutrient availability in urban forests. Biol. Invasions 2020, 22, 935–955. [Google Scholar] [CrossRef]
- Hussain, M.; Khan, S.M.; Ab-Allah, E.F.; Ul Haq, Z.; Alshahrani, T.S.; Alqarawi, A.A.; Ur Rahman, I.; Iqbal, M.; Abdullah Ahmad, H. Assessment of plant communities and identification of indicator species of an ecotonal forest zone at Durand line, District Kurram, Pakistan. Appl. Ecol. Environ. Res. 2019, 17, 6375–6396. [Google Scholar] [CrossRef]
- Saima, S.; Dasti, A.A.; Hussain, F.; Wazir, S.M.; Malik, S.A. Floristic compositions along an 18-km long transect in Ayubia National Park district Abbottabad, Pakistan. Pak. J. Bot. 2009, 41, 2115–2127. [Google Scholar]
- Ali, S.I.; Qaiser, M.A. Phytogeographical analysis of the phanerogams of Pakistan and Kashmir. Proc. R. Soc. Edinb. Sect. B Biol. Sci. 1986, 89, 89–101. [Google Scholar] [CrossRef]
- Ali, S.M.; Qaiser, M. Flora of Pakistan; Fakhri Printing Press: Karachi, Pakistan, 1995. [Google Scholar]
- Ramírez, N.; Dezzeo, N.; Chacon, N. Floristic composition, plant species abundance, and soil properties of montane savannas in the Gran Sabana, Venezuela. Flora-Morphol. Distrib. Funct. Ecol. Plants 2007, 202, 316–327. [Google Scholar] [CrossRef]
- Day, P.R. Particle Fractionation and Particle-Size Analysis. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling, 9.1; Black, C.A., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1965; pp. 543–567. [Google Scholar]
- Nowak, A.S.; Nobis, M. Distribution, floristic structure and habitat requirements of the riparian forest community Populetum talassicae ass. nova in the Central Pamir-Alai Mts (Tajikistan, Middle Asia). Acta Soc. Bot. Pol. 2013, 82, 47–55. [Google Scholar] [CrossRef]
- Abbas, Z.; Khan, S.M.; Alam, J.; Abideen, Z.; Ullah, Z. Plant communities and anthropo-natural threats in the Shigar Valley, (Central Karakorum) Baltistan-Pakistan. Pak. J. Bot. 2012, 52, 987–994. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Yun, C.W.; Abdiyani, S. Assessing vegetation composition and the indicator species around water source areas in a pine forest plantation: A case study from Watujali and Silengkong Catchments, Kebumen, Indonesia. Forests 2019, 10, 825. [Google Scholar]
- Ter Braak, C.J.; Barendregt, L.G. Weighted averaging of species indicator values: Its efficiency in environmental calibration. Math. Biosci. 1986, 78, 57–72. [Google Scholar] [CrossRef]
- Ter Braak, C.J.; Prentice, I.C. A theory of gradient analysis. Adv. Ecol. Res. 1988, 18, 271–317. [Google Scholar]
- Ward, J.H., Jr. Hierarchical grouping to optimize an objective function. JASA 1963, 58, 236–244. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MJM Software Design: Corvallis, OR, USA, 2002. [Google Scholar]
- Langer, E.R.L.; Steward, G.A.; Kimberley, M.O. Vegetation structure, composition and effect of pine plantation harvesting on riparian buffers in New Zealand. For. Ecol. Manag. 2008, 256, 949–957. [Google Scholar] [CrossRef]
- Jadán, O.; Donoso, D.A.; Cedillo, H.; Bermúdez, F.; Cabrera, O. Floristic groups, and changes in diversity and structure of trees, in tropical montane forests in the Southern Andes of Ecuador. Diversity 2021, 13, 400. [Google Scholar] [CrossRef]
- Sarvalingam, A.; Rajendran, A. Rare, Endangered and Threatened (RET) climbers of Southern Western Ghats, India. Rev. Chil. de Hist. Nat. 2016, 89, 1822. [Google Scholar] [CrossRef]
- Muthumperumal, C.; Parthasarathy, N. Angiosperms, climbing plants in tropical forests of southern Eastern Ghats, Tamil Nadu, India. Check List 2009, 5, 92–111. [Google Scholar] [CrossRef]
- Bovini, M.G.; Faria, M.; Oliveira, R.R.; Kurtz, B.R. Floristic diversity of the Cagarras Islands Natural Monument, Rio de Janeiro, Brazil. Check List 2014, 10, 366–373. [Google Scholar] [CrossRef]
- Araujo, D.; Alves, M. Climbing plants of a fragmented area of lowland Atlantic Forest, Igarassu, Pernambuco (Northeastern Brazil). Phytotaxa 2010, 8, 1–24. [Google Scholar] [CrossRef]
- Srinivas, K.; Sundarapandian, S. Diversity and composition of climbing plants in tropical dry forests of Northern Andhra Pradesh, India. Int. Res. J. Nat. Appl. Sci. 2017, 4, 31–47. [Google Scholar]
- Lü, X.T.; Tang, J.W.; Feng, Z.L.; Li, M.H. Diversity and aboveground biomass of lianas in the tropical seasonal rain forests of Xishuangbanna, SW China. Rev. Biol. Trop. 2009, 57, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Muoghalu, J.I.; Okeesan, O.O. Climber species composition, abundance and relationship with trees in a Nigerian secondary forest. Afr. J. Ecol. 2005, 43, 258–266. [Google Scholar] [CrossRef]
- Nabe-Nielsen, J. Diversity and distribution of lianas in a neotropical rain forest, Yasuní National Park, Ecuador. J. Trop. Ecol. 2001, 17, 1–19. [Google Scholar] [CrossRef]
- Qasem, J.R.; Foy, C.L. Weed allelopathy, its ecological impacts and future prospects: A review. J. Plant Prod. 2001, 4, 43–119. [Google Scholar] [CrossRef]
- Haq, S.M.; Singh, B.; Bashir, F.; Farooq, A.J.; Singh, B.; Calixto, E.S. Exploring and understanding the floristic richness, life-form, leaf-size spectra and phenology of plants in protected forests: A case study of Dachigam National Park in Himalaya, Asia. Acta Ecol. Sin. 2021, 41, 479–490. [Google Scholar] [CrossRef]
- Haq, S.M.; Hamid, M.; Lone, F.A.; Singh, B. Himalayan hotspot with Alien Weeds: A case study of biological spectrum, phenology, and diversity of weedy plants of high-altitude mountains in District Kupwara of J&K Himalaya, India. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2021, 91, 139–152. [Google Scholar]
- Hamid, M.; Khuroo, A.A.; Malik, A.H.; Ahmad, R.; Singh, C.P.; Dolezal, J.; Haq, S.M. Early evidence of shifts in alpine summit vegetation: A case study from Kashmir Himalaya. Front. Plant Sci. 2020, 11, 421. [Google Scholar] [CrossRef]
- Haq, S.M.; Malik, A.H.; Khuroo, A.A.; Rashid, I. Floristic composition and biological spectrum of Kerana remote valley of northwestern Himalaya. Acta Ecol. Sin. 2019, 39, 372–379. [Google Scholar] [CrossRef]
- Pandian, E.; Parthasarathy, N. Decadal (2003–2013) changes in liana diversity, abundance and aboveground biomass in four inland tropical dry evergreen forest sites of peninsular India. J. For. Res. 2016, 27, 133–146. [Google Scholar] [CrossRef]
- Pandian, E.; Ravichandran, P. Diversity and Threatened Climber Plants in Tropical Forests of Courtallam Hills, Southern Western Ghats, India. J. Trop. For. Environ. 2019, 9. [Google Scholar] [CrossRef]
- Putz, F.E.; Chai, P. Ecological studies of lianas in Lambir national park, Sarawak, Malaysia. J. Ecol. 1987, 75, 523–531. [Google Scholar] [CrossRef]
- Campbell, E.J.F.; Newbery, D.M. Ecological relationships between lianas and trees in lowland rain forest in Sabah, East Malaysia. J. Trop. Ecol. 1993, 9, 469–490. [Google Scholar] [CrossRef]
- Muthuramkumar, S.; Parthasarathy, N. Tree-liana relationships in a tropical evergreen forest at Varagalaiar, Anamalais, Western Ghats, India. J. Trop. Ecol. 2001, 17, 395–409. [Google Scholar] [CrossRef]
- Iqbal, M.; Khan, S.M.; Ahmad, Z.; Hussain, M.; Shah, S.N.; Kamran, S.; Manan, F.; Ul-Haq, Z.; Ullah, S. Vegetation classification of the Margalla Foothills, Islamabad under the influence of edaphic factors and anthropogenic activities using modern ecological tools. Pak. J. Bot. 2021, 53, 1831–1843. [Google Scholar] [CrossRef]
- Carter, G.A.; Teramura, A.H. Climber photosynthesis and relations to climbing mechanics in a forest understory. Am. J. Bot. 1988, 75, 1011–1018. [Google Scholar] [CrossRef]
- Leicht-Young, S.A.; Pavlovic, N.B.; Frohnapple, K.J.; Grundel, R. Liana habitat and host preferences in northern temperate forests. Ecol. Manag. 2010, 260, 1467–1477. [Google Scholar] [CrossRef]
- Ding, Y.; Zang, R. Effects of logging on the diversity of lianas in a lowland tropical rain forest in Hainan Island, South China. Biotropica 2009, 41, 618–624. [Google Scholar] [CrossRef]
- Haq, S.M.; Yaqoob, U.; Calixto, E.S.; Kumar, M.; Rahman, I.U.; Hashem, A.; Abd_Allah, E.F.; Alakeel, M.A.; Alqarawi, A.A.; Abdalla, M.; et al. Long-term impact of transhumance pastoralism and associated disturbances in high-altitude forests of Indian Western Himalaya. Sustainability 2021, 13, 12497. [Google Scholar] [CrossRef]
- Rahman, I.U.; Hart, R.E.; Ijaz, F.; Afzal, A.; Iqbal, Z.; Calixto, E.S.; Abd_Allah, E.F.; Alqarawi, A.A.; Hashem, A.; Al-Arjani, A.B.F.; et al. Environmental variables drive plant species composition and distribution in the moist temperate forests of Northwestern Himalaya, Pakistan. PLoS ONE 2022, 17, e0260687. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.M.; Tariq, A.; Li, Q.; Yaqoob, U.; Majeed, M.; Hassan, M.; Fatima, S.; Kumar, M.; Bussmann, R.W.; Moazzam, M.F.U.; et al. Influence of Edaphic Properties in Determining Forest Community Patterns of the Zabarwan Mountain Range in the Kashmir Himalayas. Forests 2022, 13, 1214. [Google Scholar] [CrossRef]
- Haq, S.M.; Yaqoob, U.; Hamid, S.; Hassan, M.; Bashir, F.; Waheed, M.; Bussmann, R.W. Localized impact of livestock settlements on vegetation patterns in fir forests of Kashmir Himalaya. Acta Ecol. Sin. 2022, 42, 407–416. [Google Scholar] [CrossRef]
- Woods, C.L.; Cardelús, C.L.; DeWalt, S.J. Microhabitat associations of vascular epiphytes in a wet tropical forest canopy. J. Ecol. 2015, 103, 421–430. [Google Scholar] [CrossRef]
- Wang, X.; Long, W.; Schamp, B.S.; Yang, X.; Kang, Y.; Xie, Z.; Xiong, M. Vascular epiphyte diversity differs with host crown zone and diameter, but not orientation in a tropical cloud forest. PLoS ONE 2016, 11, e0158548. [Google Scholar] [CrossRef]
- Dale, V.H.; Beyeler, S.C. Challenges in the development and use of ecological indicators. Ecol. Indic. 2001, 1, 3–10. [Google Scholar] [CrossRef]
- Bauer, G.; Klein, M.C.; Gorb, S.N.; Speck, T.; Voigt, D.; Gallenmüller, F. Always on the bright side: The climbing mechanism of Galium aparine. Proc. R. Soc. B Biol. Sci. 2011, 278, 2233–2239. [Google Scholar] [CrossRef]
- Goodman, A.M. Mechanical adaptations of cleavers (Galium aparine). Ann. Bot. 2005, 95, 475–480. [Google Scholar] [CrossRef]
- Chaney, L.; Baucom, R.S. The costs and benefits of tolerance to competition in Ipomoea purpurea, the common morning glory. Evolution 2014, 68, 1698–1709. [Google Scholar] [CrossRef]
- Senbeta, F.; Schmitt, C.; Denich, M.; Demissew, S.; Velk, P.L.; Preisinger, H.; Woldemariam, T.; Teketay, D. The diversity and distribution of lianas in the Afromontane rain forests of Ethiopia. Divers. Distrib. 2005, 11, 443–452. [Google Scholar] [CrossRef]


| Species | Species Code | Climber Type | Climbing Mode | Group 1 | Group 2 | Group 3 | Group 4 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IndVal (%) | p-Value | IndVal (%) | p-Value | IndVal (%) | p-Value | IndVal (%) | p-Value | ||||
| Blyttia spiralis (Forssk.) D.V. Field & J.R.I. Wood | Blyt.spi | Herbaceous climber | Twiner | 0 | 1 | 0 | 1 | 0.33 | 0.56 | 14.77 | p < 0.1 |
| Campsis radicans (L.) Seem. | Camp.radi | Woody Climber | Twiner | 0 | 1 | 0 | 1 | 4.37 | 0.33 | 2.46 | 0.32 |
| Cissampelos pareira L. | Ciss.car | Herbaceous climber | Tendril | 26.7 | p < 0.05 | 1.59 | 0.32 | 0 | 1 | 0 | 1 |
| Cissus carnosa Lam. | Ciss.par | Woody Climber | Tendril | 6.2 | 0.22 | 6.54 | 0.22 | 0 | 1 | 0.56 | 0.54 |
| Coccinia grandis (L.) Voigt | Cocc.gran | Herbaceous climber | Twiner | 0.28 | 0.55 | 1.29 | 0.36 | 4.71 | 0.26 | 0.75 | 0.46 |
| Cocculus hirsutus (L.) W. Theob. | Cocc.hir | Woody Climber | Scrambler | 0 | 1 | 0 | 1 | 0 | 1 | 5.49 | 0.23 |
| Convolvulus arvensis L. | Conv.arv | Herbaceous climber | Scrambler | 8.22 | 0.16 | 2.7 | 0.30 | 0 | 1 | 0 | 1 |
| Cucumis melo subsp. agrestis (Naudin) Pangalo | Cucu.mel | Herbaceous climber | Scrambler | 18.33 | p < 0.05 | 4.03 | 0.27 | 0 | 1 | 2.46 | 0.32 |
| Cuscuta reflexa Roxb. | Cusc.ref | Parasite climber | Twiner | 0 | 1 | 0 | 1 | 1.01 | 0.49 | 2.65 | 0.26 |
| Dolichandra unguis-cati (L.) L.G. Lohmann | Doli.ung | Woody Climber | Tendril | 0.43 | 0.53 | 0.15 | 0.63 | 2.35 | 0.36 | 0 | 1 |
| Galium aparine L | Gali.apa | Herbaceous climber | Scrambler | 2.59 | 0.33 | 20.15 | p < 0.05 | 0 | 1 | 0 | 1 |
| Ipomoea aquatica Forssk. | Ipom.aqu | Herbaceous climber | Scrambler | 0 | 1 | 0.60 | 0.47 | 0 | 1 | 0 | 1 |
| Ipomoea cairica (L.) Sweet | Ipom.cai | Herbaceous climber | Twiner | 0.43 | 0.53 | 0 | 1 | 6.06 | 0.19 | 0.94 | 0.39 |
| Ipomoea nil (L.) Roth | Ipom.nil | Herbaceous climber | Twiner | 0.72 | 0.36 | 0.22 | 0.57 | 1.34 | 0.46 | 0 | 1 |
| Ipomoea purpurea (L.) Roth | Ipom.pur | Herbaceous climber | Twiner | 0 | 1 | 0.22 | 0.56 | 30.3 | p < 0.05 | 0 | 1 |
| Lantana camara L. | Lant.cam | Climbing shrub | Scrambler | 6.78 | 0.20 | 7.3 | 0.19 | 0 | 1 | 0.37 | 0.56 |
| Lathyrus aphaca L. | Lath.aph | Herbaceous climber | Tendril | 11.26 | p < 0.1 | 9.35 | p < 0.1 | 2.02 | 0.40 | 0 | 1 |
| Merremia aegyptia (L.) Urb. | Merr.aeg | Herbaceous climber | Twiner | 0 | 1 | 0 | 1 | 7.071 | p < 0.1 | 0 | 1 |
| Merremia dissecta (Jacq.) Hallier f. | Merr.dis | Herbaceous climber | Twiner | 0 | 1 | 0 | 1 | 5.38 | 0.23 | 0 | 1 |
| Merremia hederacea (Burm. f.) Hallier f. | Merr.hed | Herbaceous climber | Twiner | 0 | 1 | 0 | 1 | 4.37 | 0.33 | 0 | 1 |
| Mukia maderaspatana (L.) M. Roem. | Muki.mad | Herbaceous climber | Tendril | 0.43 | 0.53 | 11.03 | p < 0.1 | 0 | 1 | 0.75 | 0.45 |
| Oxystelma esculentum (L. f.) Sm. | Oxys.esc | Herbaceous climber | Twiner | 0.14 | 0.59 | 0.15 | 0.62 | 0 | 1 | 0.56 | 0.53 |
| Pentatropis capensis (L. f.) Bullock | Pent.cap | Herbaceous climber | Twiner | 0 | 1 | 0 | 1 | 6.06 | 0.20 | 12.88 | 0.13 |
| Pentatropis nivalis (J.F. Gmel.) D.V. Field & J.R.I. Wood | Pent.nav | Herbaceous climber | Twiner | 0 | 1 | 0 | 1 | 6.06 | 0.20 | 17.05 | p < 0.05 |
| Pergularia daemia (Forssk.) Chiov. | Perg.dae | Herbaceous climber | Twiner | 3.75 | 0.26 | 1.21 | 0.40 | 1.34 | 0.46 | 12.5 | 0.16 |
| Telosma pallida (Roxb.) Craib | Telo.pal | Woody Climber | Twiner | 10.1 | 0.13 | 0.07 | 0.66 | 0 | 1 | 11.17 | 0.18 |
| Tinospora sinensis (Lour.) Merr. | Tino.sin | Woody Climber | Twiner | 0.43 | 0.52 | 0.30 | 0.49 | 0 | 1 | 0 | 1 |
| Trichosanthes dioica Roxb. | Tric.dio | Herbaceous climber | Tendril | 2.74 | 0.29 | 13.99 | p < 0.1 | 0.67 | 0.53 | 0.94 | 0.40 |
| Vicia sativa L. | Vici.sa | Herbaceous climber | Tendril | 0 | 1 | 18.4 | p < 0.1 | 16.5 | p < 0.1 | 0 | 1 |
| Soil Characteristics | Variable Name | Group 1 | Group 2 | Group 3 | Group 4 | H-Values | p-Values |
|---|---|---|---|---|---|---|---|
| 1. Soil Texture | 1. Sand | 54.96 ± 1.25 | 36.38 ± 1.91 | 25.25 ± 0.95 | 28.62 ± 0.71 | 10.872 | 0.004 ** |
| 2. Silt | 32.79 ± 0.79 | 37.39 ± 0.70 | 45.31 ± 2.61 | 46.78 ± 1.93 | 11.483 | 0.005 ** | |
| 3. Clay | 15.25 ± 0.56 | 26.22 ± 1.31 | 28 ± 3.45 | 26.4 ± 1.23 | 12.972 | 0.003 ** | |
| 2. Nutrients | 1. SOM | 0.69 ± 0.02 | 0.71 ± 0.01 | 0.74 ± 0.01 | 0.74 ± 0.01 | 4.597 | 0.223 |
| 2. Nitrogen | 0.28 ± 0.09 | 0.35 ± 0.07 | 0.27 ± 0.07 | 0.30 ± 0.03 | 2.348 | 0.075 | |
| 3. K | 267.67 ± 26.7 | 294.87 ± 32.1 | 285.94 ± 48.3 | 383.79 ± 41.7 | 7.648 | 0.051 * | |
| 4. P | 3.21 ± 0.6 | 3.01 ± 0.5 | 2.37 ± 1.03 | 2.67 ± 0.5 | 5.476 | 0.136 | |
| 3. Chemical Properties | 1. Moisture | 15.31 ± 3.21 | 18.36 ± 1.95 | 12.92 ± 3.34 | 15.49 ± 6.5 | 7.459 | 0.051 * |
| 2. pH | 6.79 ± 0.19 | 7.02 ± 0.15 | 6.96 ± 0.25 | 6.57±0.13 | 2.195 | 0.532 | |
| 3. EC | 0.91 ± 0.04 | 0.94 ± 0.04 | 0.91 ± 0.08 | 0.96 ± 0.03 | 2.402 | 0.523 |
| Axes | 1 | 2 | 3 | 4 | Total Inertia |
|---|---|---|---|---|---|
| Eigenvalues: | 0.67 | 0.2 | 0.131 | 0.08 | 7.343 |
| Species–environment correlations: | 0.922 | 0.664 | 0.581 | 0.426 | |
| Cumulative % variance of species data | 9.1 | 11.8 | 13.6 | 14.7 | |
| Cumulative % variance of species-environment relation | 56.8 | 73.7 | 84.9 | 91.7 | |
| Summary of Monte Carlo test | |||||
| Test of significance of first canonical axis | Test of significance of all canonical axes | ||||
| Eigen value = 0.67 | Trace = 1.180 | ||||
| F-ratio = 5.322 | F-ratio = 1.691 | ||||
| p-value = 0.0020 | p-value = 0.0020 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waheed, M.; Haq, S.M.; Fatima, K.; Arshad, F.; Bussmann, R.W.; Masood, F.R.; Alataway, A.; Z. Dewidar, A.; F. Almutairi, K.; Elansary, H.O.; et al. Ecological Distribution Patterns and Indicator Species Analysis of Climber Plants in Changa Manga Forest Plantation. Diversity 2022, 14, 988. https://doi.org/10.3390/d14110988
Waheed M, Haq SM, Fatima K, Arshad F, Bussmann RW, Masood FR, Alataway A, Z. Dewidar A, F. Almutairi K, Elansary HO, et al. Ecological Distribution Patterns and Indicator Species Analysis of Climber Plants in Changa Manga Forest Plantation. Diversity. 2022; 14(11):988. https://doi.org/10.3390/d14110988
Chicago/Turabian StyleWaheed, Muhammad, Shiekh Marifatul Haq, Kaniz Fatima, Fahim Arshad, Rainer W. Bussmann, Farhat Rass Masood, Abed Alataway, Ahmed Z. Dewidar, Khalid F. Almutairi, Hosam O. Elansary, and et al. 2022. "Ecological Distribution Patterns and Indicator Species Analysis of Climber Plants in Changa Manga Forest Plantation" Diversity 14, no. 11: 988. https://doi.org/10.3390/d14110988
APA StyleWaheed, M., Haq, S. M., Fatima, K., Arshad, F., Bussmann, R. W., Masood, F. R., Alataway, A., Z. Dewidar, A., F. Almutairi, K., Elansary, H. O., Kassem, H. S., Al-Yafrasi, M., & Yessoufou, K. (2022). Ecological Distribution Patterns and Indicator Species Analysis of Climber Plants in Changa Manga Forest Plantation. Diversity, 14(11), 988. https://doi.org/10.3390/d14110988










