Associations between Epiphytic Bryophyte and Woody Plant Species in a Temperate Deciduous Broad-Leaved Forest
Abstract
1. Introduction
2. Materials and Methods
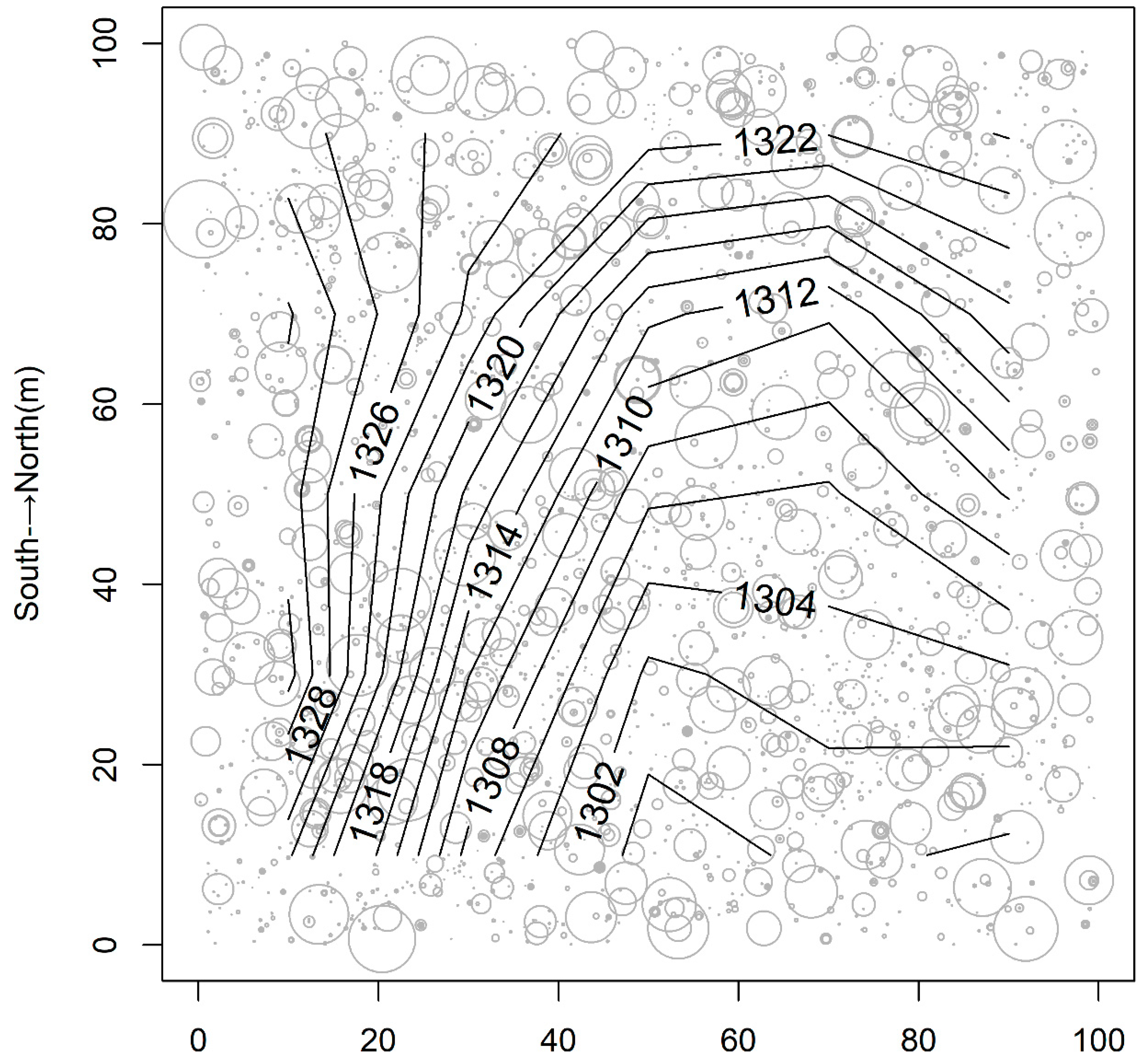
2.1. Study Site and Sampling
2.2. Data Analysis
3. Results
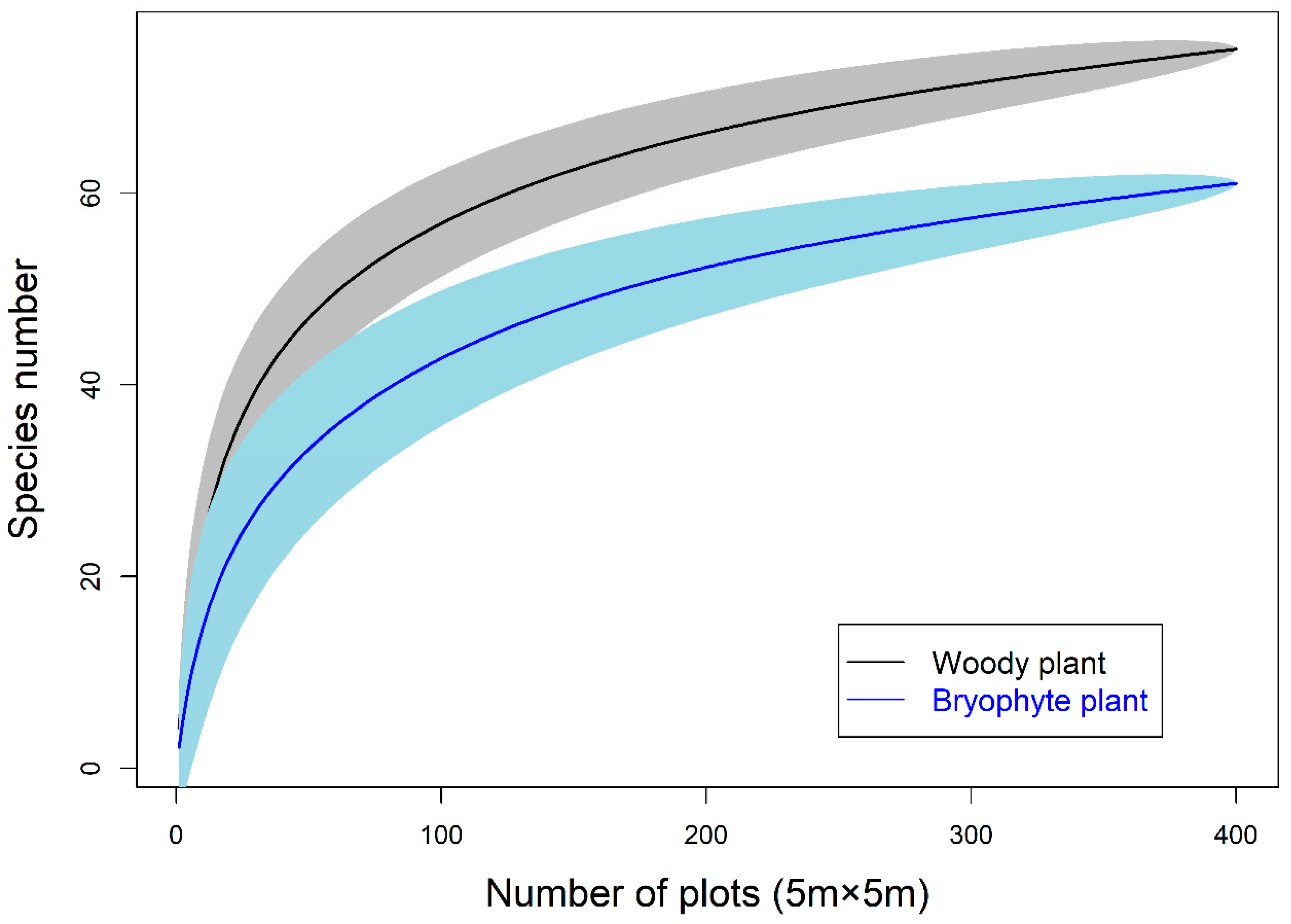
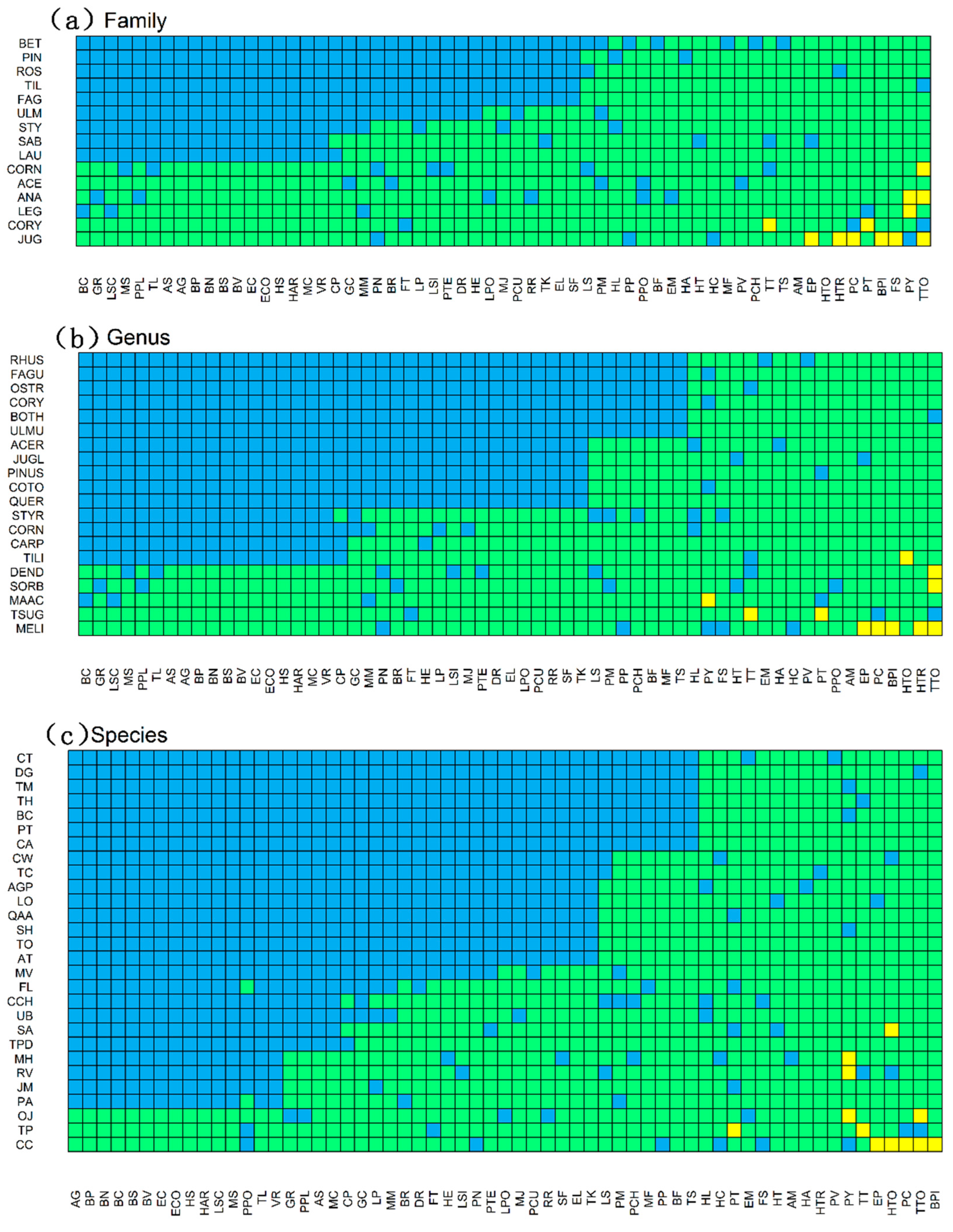
3.1. Diversity within the Network and Connectance
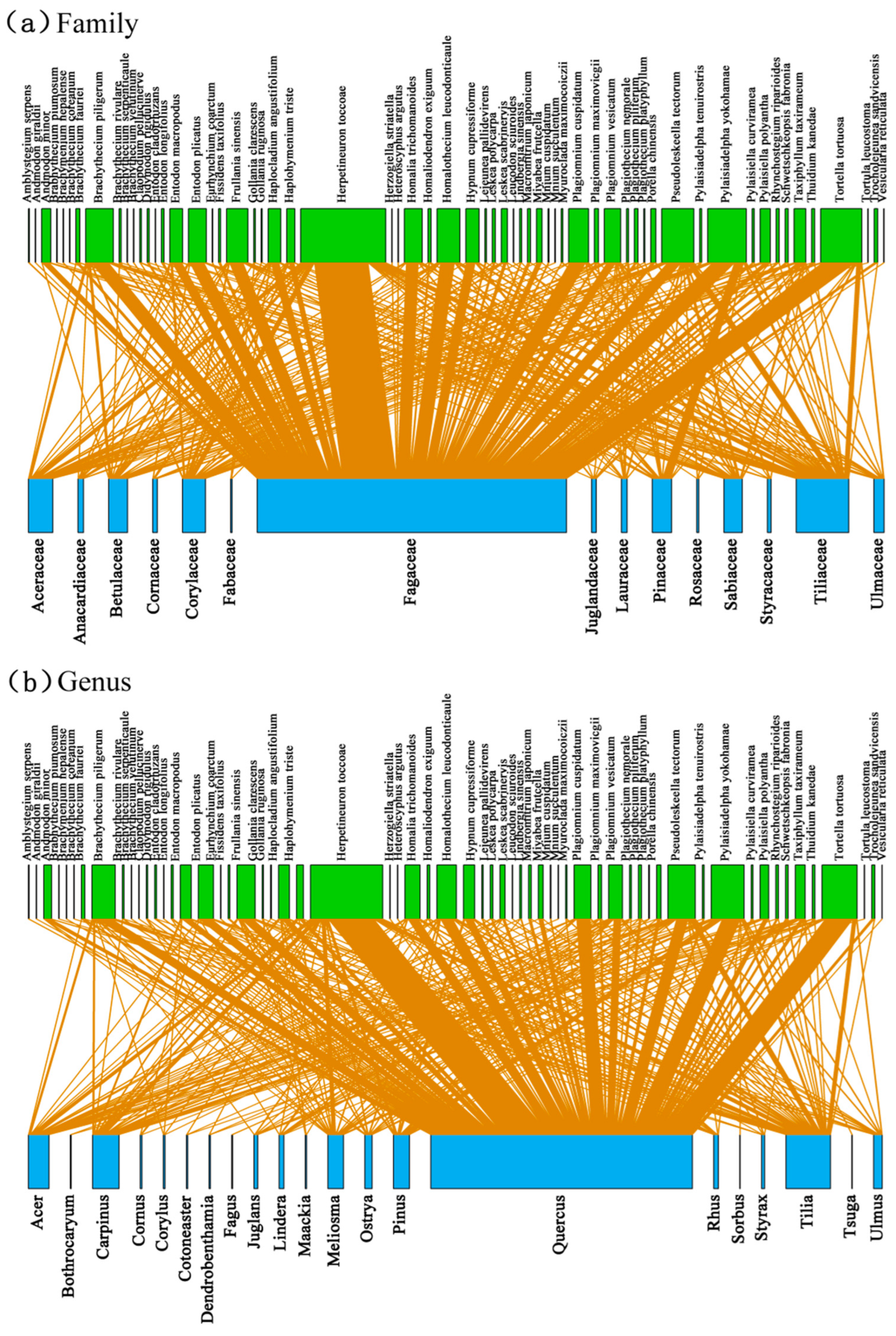
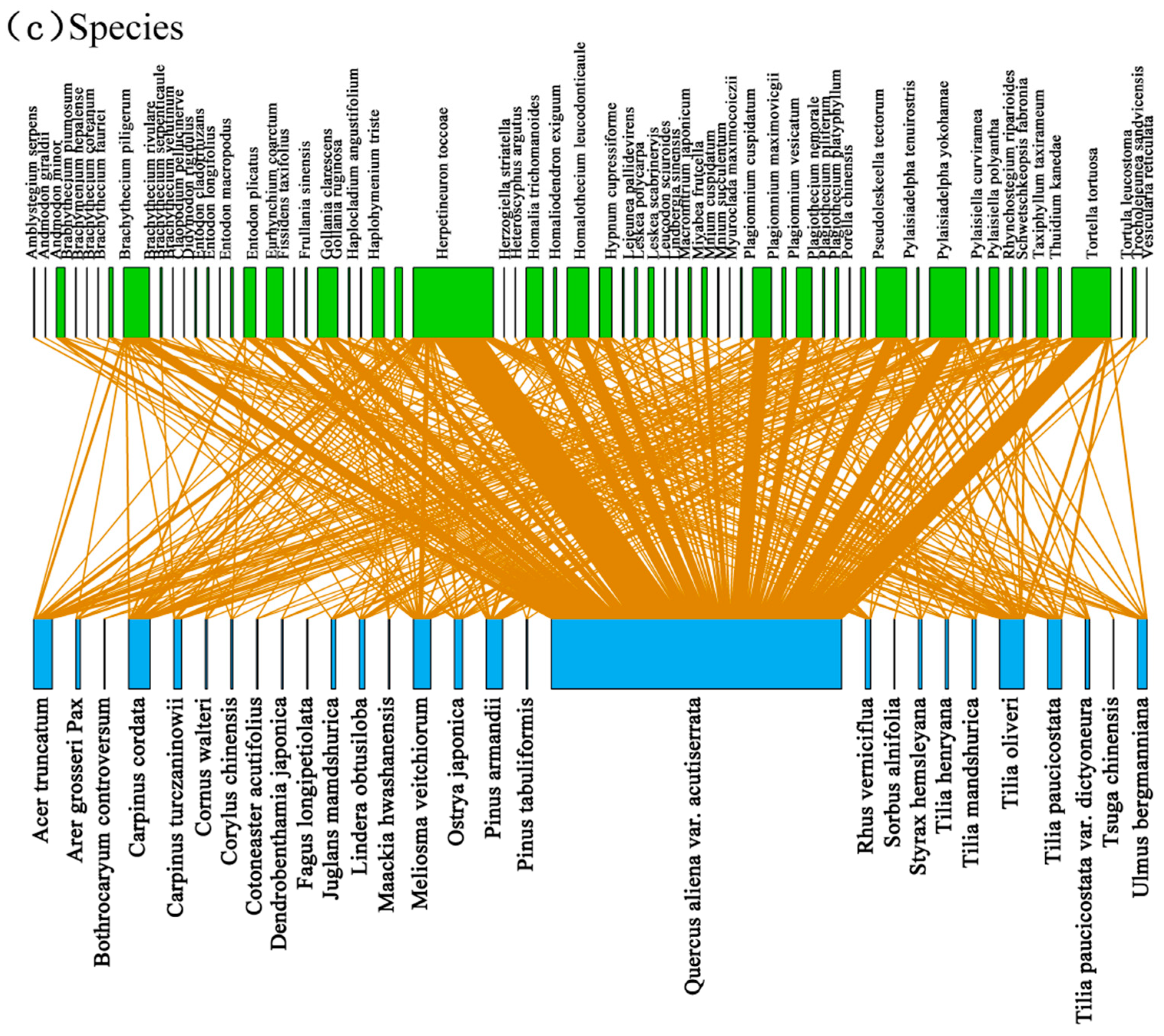
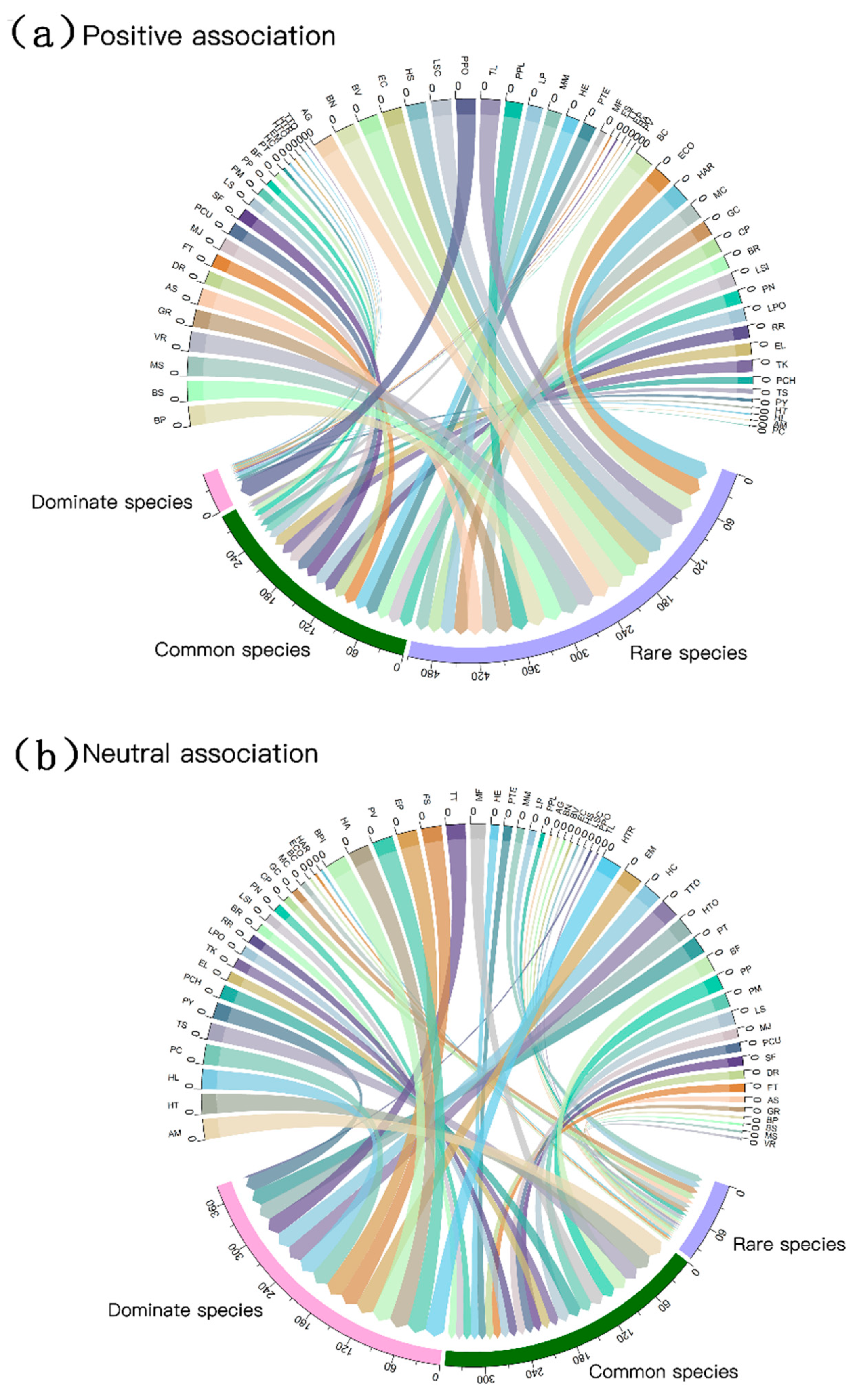
3.2. Associations between Bryophyte and Plant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data availability statement
Acknowledgments
Conflicts of Interest
References
- Frego, K.A. Bryophytes as potential indicators of forest integrity. For. Ecol. Manag. 2007, 242, 65–75. [Google Scholar] [CrossRef]
- Mežaka, A.; Brūmelis, G.; Piterāns, A. Tree and stand-scale factors affecting richness and composition of epiphytic bryophytes and lichens in deciduous woodland key habitats. Biodivers. Conserv. 2012, 21, 3221–3241. [Google Scholar] [CrossRef]
- Szövényi, P.; Hock, Z.S.; Tóth, Z. Phorophyte preferences of epiphytic bryophytes in a stream valley in the Carpathian Basin. J. Bryol. 2004, 26, 137–146. [Google Scholar] [CrossRef]
- Chen, Y.; Niu, S.; Li, P.; Jia, H.; Wang, H.; Ye, Y.; Yuan, Z. Stand structure and substrate diversity as two major drivers for bryophyte distribution in a temperate montane ecosystem. Front Plant Sci. 2017, 8, 874. [Google Scholar]
- Chen, Y.; Jia, H.R.; Niu, S.; Zhang, X.; Wang, H.L.; Ye, Y.Z.; Yuan, Z.L. Effects of Topographical Heterogeneity and Dispersal Limitation on Species Turnover in a Temperate Mountane Ecosystem: A Case Study in the Henan Province, China. Russ. J. Ecol. 2018, 49, 40–46. [Google Scholar] [CrossRef]
- Chiarucci, A.; D’auria, F.; Bonini, I. Is vascular plant species diversity a predictor of bryophyte species diversity in Mediterranean forests? Biodivers. Conserv. 2007, 16, 525–545. [Google Scholar] [CrossRef]
- Spitale, D.; Petraglia, A.; Tomaselli, M. Structural equation modelling detects unexpected differences between bryophyte and vascular plant richness along multiple environmental gradients. J. Biogeogr. 2009, 36, 745–755. [Google Scholar] [CrossRef]
- Fritz, Ö.; Niklasson, M.; Churski, M. Tree age is a key factor for the conservation of epiphytic lichens and bryophytes in beech forests. Appl. Veg. Sci. 2009, 12, 93–106. [Google Scholar] [CrossRef]
- Madžule, L.; Brūmelis, G.; Tjarve, D. Structures determining bryophyte species richness in a managed forest landscape in boreo-nemoral Europe. Biodivers. Conserv. 2012, 21, 437–450. [Google Scholar] [CrossRef]
- Mölder, A.; Schmidt, M.; Engel, F.; Schönfelder, E.; Schulz, F. Bryophytes as indicators of ancient woodlands in Schleswig-Holstein (Northern Germany). Ecol. Indic. 2015, 54, 12–30. [Google Scholar] [CrossRef]
- Ódor, P.; Király, I.; Tinya, F.; Bortignon, F.; Nascimbene, J. Patterns and drivers of species composition of epiphytic bryophytes and lichens in managed temperate forests. For. Ecol. Manag. 2013, 306, 256–265. [Google Scholar] [CrossRef]
- Bates, J.W. Influence of chemical and physical factors on Quercus and Fraxinus epiphytes at Loch Sunart, western Scotland: A multivariate analysis. J. Ecol. 1992, 80, 163–179. [Google Scholar] [CrossRef]
- Gustafsson, L.; Eriksson, I. Factors of importance for the epiphytic vegetation of Populus tremula with special emphasis on bark chemistry and soil. J. Appl. Ecol. 1995, 32, 412–424. [Google Scholar] [CrossRef]
- Moe, B.; Botnen, A. Epiphytic vegetation on pollarded trunks of Fraxinus excelsior in four different habitats at Grinde, Leikanger, western Norway. Plant Ecol. 2000, 151, 143–159. [Google Scholar] [CrossRef]
- Thomas, S.C.; Liguori, D.A.; Halpern, C.B. Corticolous bryophytes in managed douglas-fir forests: Habitat differentiation and response to thinning and fertilization. Can. J. Bot. 2001, 79, 886–896. [Google Scholar]
- Plášek, V.; Nowak, A.; Nobis, M.; Kusza, G.; Kochanowska, K. Effect of 30 years of road traffic abandonment on epiphytic moss diversity. Environ. Monit. Assess. 2014, 186, 8943–8959. [Google Scholar] [CrossRef] [PubMed]
- Číhal, L.; Kaláb, O.; Plášek, V. Modeling the distribution of rare and interesting moss species of the family Orthotrichaceae (Bryophyta) in Tajikistan and Kyrgyzstan. Acta Soc. Bot. Pol. 2017, 86, 3543. [Google Scholar] [CrossRef][Green Version]
- Harms, K.E.; Condit, R.; Hubbell, S.P.; Foster, R.B. Habitat associations of trees and shrubs in a 50-ha neotropical forest plot. J. Ecol. 2001, 89, 947–959. [Google Scholar] [CrossRef]
- Lai, J.; Mi, X.; Ren, H.; Ma, K. Species-habitat associations change in a subtropical forest of China. J. Veg. Sci. 2009, 20, 415–423. [Google Scholar] [CrossRef]
- Svenning, J.C. Microhabitat specialization in a species-rich palm community in Amazonian Ecuador. J. Ecol. 1999, 87, 55–65. [Google Scholar] [CrossRef]
- Chen, Y.; Shao, Y.; Xi, J.; Yuan, Z.; Ye, Y.; Wang, T. Community preferences of woody plant species in a heterogeneous temperate forest, China. Front. Ecol. Evol. 2020, 8, 165. [Google Scholar]
- Nicolai, V. The bark of trees: Thermal properties, microclimate and fauna. Oecologia 1986, 69, 148–160. [Google Scholar] [CrossRef]
- Dray, S.; Legendre, P.; Peres-Neto, P. Spatial modelling: Acomprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecol. Model. 2006, 196, 483–493. [Google Scholar] [CrossRef]
- Yuan, Z.; Gazol, A.; Wang, X.; Lin, F.; Ye, J.; Bai, X.; Hao, Z. Scale specific determinants of tree diversity in an old growth temperate forest in China. Basic Appl. Ecol. 2011, 12, 488–495. [Google Scholar] [CrossRef]
- Brown, J.H. On the relationship between abundance and distribution of species. Am. Nat. 1984, 124, 255–279. [Google Scholar] [CrossRef]
- Gaston, K.J.; Blackburn, T.M.; Lawton, J.H. Interspecific abundance-range size relationships: An appraisal of mechanisms. J. Anim. Ecol. 1997, 66, 579–601. [Google Scholar] [CrossRef]
- Mi, X.; Sun, Z.; Song, Y.; Liu, X.; Yang, J.; Wu, J.; Ci, X.; Li, J.; Lin, L.; Cao, M.; et al. Rare tree species have narrow environmental but not functional niches. Funct. Ecol. 2020, 35, 511–520. [Google Scholar] [CrossRef]
- Baker, T.P.; Jordan, G.J.; Fountain-Jones, N.M.; Balmer, J.; Dalton, P.J.; Baker, S.C. Distance, environmental and substrate factors impacting recovery of bryophyte communities after harvesting. Appl. Veg. Sci. 2018, 21, 64–75. [Google Scholar] [CrossRef]
- Hou, H.Y. Vegetation of China with reference to its geographical distribution. Ann. Mo. Bot. Gard. 1983, 70, 509–549. [Google Scholar] [CrossRef]
- Luan, J.; Liu, S.; Wang, J.; Zhu, X.; Shi, Z. Rhizospheric and heterotrophic respiration of a warm-temperate oak chronosequence in China. Soil Biol. Biochem. 2011, 43, 503–512. [Google Scholar] [CrossRef]
- Zhao, X.G.; Ma, C.H.; Xiao, L. The vegetation history of qinling mountains, China. Quat. Int. 2014, 325, 55–62. [Google Scholar] [CrossRef]
- Peng, J.; Li, J.; Wang, T.; Huo, J.; Yang, L. Effect of altitude on climate–growth relationships of Chinese white pine (Pinus armandii) in the northern Funiu Mountain, central China. Clim. Chang. 2019, 154, 273–288. [Google Scholar] [CrossRef]
- Zhu, W.; Li, S. The dynamic response of forest vegetation to hydrothermal conditions in the Funiu Mountains of western Henan Province. J. Geogr. Sci. 2017, 27, 565–578. [Google Scholar] [CrossRef][Green Version]
- Fan, Y.; Liu, H.; Hu, N.; Ding, S. Photosynthetic characteristics of plant functional groups in forest ecosystem at the national natural reserve of FuNiu Mountain. Acta Ecol. Sin. 2016, 36, 4609–4616. [Google Scholar]
- Blüthgen, N.; Menzel, F.; Hovestadt, T.; Fiala, B.; Blüthgen, N. Specialization, constraints, and conflicting interests in mutualistic networks. Curr. Biol. 2007, 17, 341–346. [Google Scholar] [CrossRef]
- Dormann, C.F.; Fründ, J.; Blüthgen, N.; Gruber, B. Indices, graphs and null models: Analyzing bipartite ecological networks. Open Ecol. J. 2009, 2, 7–24. [Google Scholar] [CrossRef]
- Toju, H.; Guimaraes, P.R.; Olesen, J.M.; Thompson, J.N. Assembly of complex plant–fungus networks. Nat. Commun. 2014, 5, 5273. [Google Scholar] [CrossRef]
- Dicks, L.V.; Corbet, S.A.; Pywell, R.F. Compartmentalization in plant–insect flower visitor webs. J. Anim. Ecol. 2002, 71, 32–43. [Google Scholar] [CrossRef]
- Bartomeus, I.; Vilà, M.; Santamaría, L. Contrasting effects of invasive plants in plant–pollinator networks. Oecologia 2008, 155, 761–770. [Google Scholar] [CrossRef]
- Nowak, A.; Plášek, V.; Nobis, M.; Nowak, S. Epiphytic Communities of Open Habitats in the Western Tian-Shan Mts (Middle Asia: Kyrgyzstan). Cryptogam. Bryol. 2016, 37, 415–433. [Google Scholar] [CrossRef]
- Studlar, S.M. Host specificity of epiphytic bryophytes near Mountain Lake, Virginia. Bryologist 1982, 85, 37–50. [Google Scholar] [CrossRef]
- Kuusinen, M. Epiphyte flora and diversity on basal trunks of six old-growth forest tree species in southern and middle boreal Finland. Lichenologist 1996, 28, 443–463. [Google Scholar] [CrossRef]
- Kovářová, M.; Pyszko, P.; Plášek, V. How Does the pH of Tree Bark Change with the Presence of the Epiphytic Bryophytes from the Family Orthotrichaceae in the Interaction with Trunk Inclination? Plants 2021, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Medina, N.G.; Albertos, B.; Lara, F.; Mazimpaka, V.; Garilleti, R.; Draper, D.; Hortal, J. Species richness of epiphytic bryophytes: Drivers across scales on the edge of the Mediterranean. Ecography 2014, 37, 80–93. [Google Scholar] [CrossRef]
- Mills, S.E.; Macdonald, S.E. Factors influencing bryophyte assemblage at different scales in the western Canadian boreal forest. Bryologist 2005, 108, 86–100. [Google Scholar] [CrossRef]
- Andersen, R.; Poulin, M.; Borcard, D.; Laiho, R.; Laine, J.; Vasander, H.; Tuittila, E.T. Environmental control and spatial structures in peatland vegetation. J. Veg. Sci. 2011, 22, 878–890. [Google Scholar] [CrossRef]
- Ódor, P.; Van Hees, A.F. Preferences of dead wood inhabiting bryophytes for decay stage, log size and habitat types in Hungarian beech forests. J. Bryol. 2004, 26, 79–95. [Google Scholar] [CrossRef]
- Evans, S.A.; Halpern, C.B.; McKenzie, D. The contributions of forest structure and substrate to bryophyte diversity and abundance in mature coniferous forests of the Pacific Northwest. Bryologist 2012, 115, 278–294. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, S.; Liu, W.; Liu, F.; Shao, Y.; Wang, J.; Yuan, Z. Associations between Epiphytic Bryophyte and Woody Plant Species in a Temperate Deciduous Broad-Leaved Forest. Diversity 2022, 14, 979. https://doi.org/10.3390/d14110979
Chen Y, Wang S, Liu W, Liu F, Shao Y, Wang J, Yuan Z. Associations between Epiphytic Bryophyte and Woody Plant Species in a Temperate Deciduous Broad-Leaved Forest. Diversity. 2022; 14(11):979. https://doi.org/10.3390/d14110979
Chicago/Turabian StyleChen, Yun, Senlin Wang, Wenxin Liu, Fengqin Liu, Yizhen Shao, Jing Wang, and Zhiliang Yuan. 2022. "Associations between Epiphytic Bryophyte and Woody Plant Species in a Temperate Deciduous Broad-Leaved Forest" Diversity 14, no. 11: 979. https://doi.org/10.3390/d14110979
APA StyleChen, Y., Wang, S., Liu, W., Liu, F., Shao, Y., Wang, J., & Yuan, Z. (2022). Associations between Epiphytic Bryophyte and Woody Plant Species in a Temperate Deciduous Broad-Leaved Forest. Diversity, 14(11), 979. https://doi.org/10.3390/d14110979





