Effect of Different Types of Continuous Cropping on Microbial Communities and Physicochemical Properties of Black Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Soil Physicochemical Analysis
2.3. DNA Extraction, PCR, and Sequencing
2.4. Bioinformatics and Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
3.2. Diversity of Microbial Communities
3.3. Bacterial and Fungal Populations in Soil Composition
3.4. Network of Soil Bacterial and Soil Fungal Communities That Co-Occur
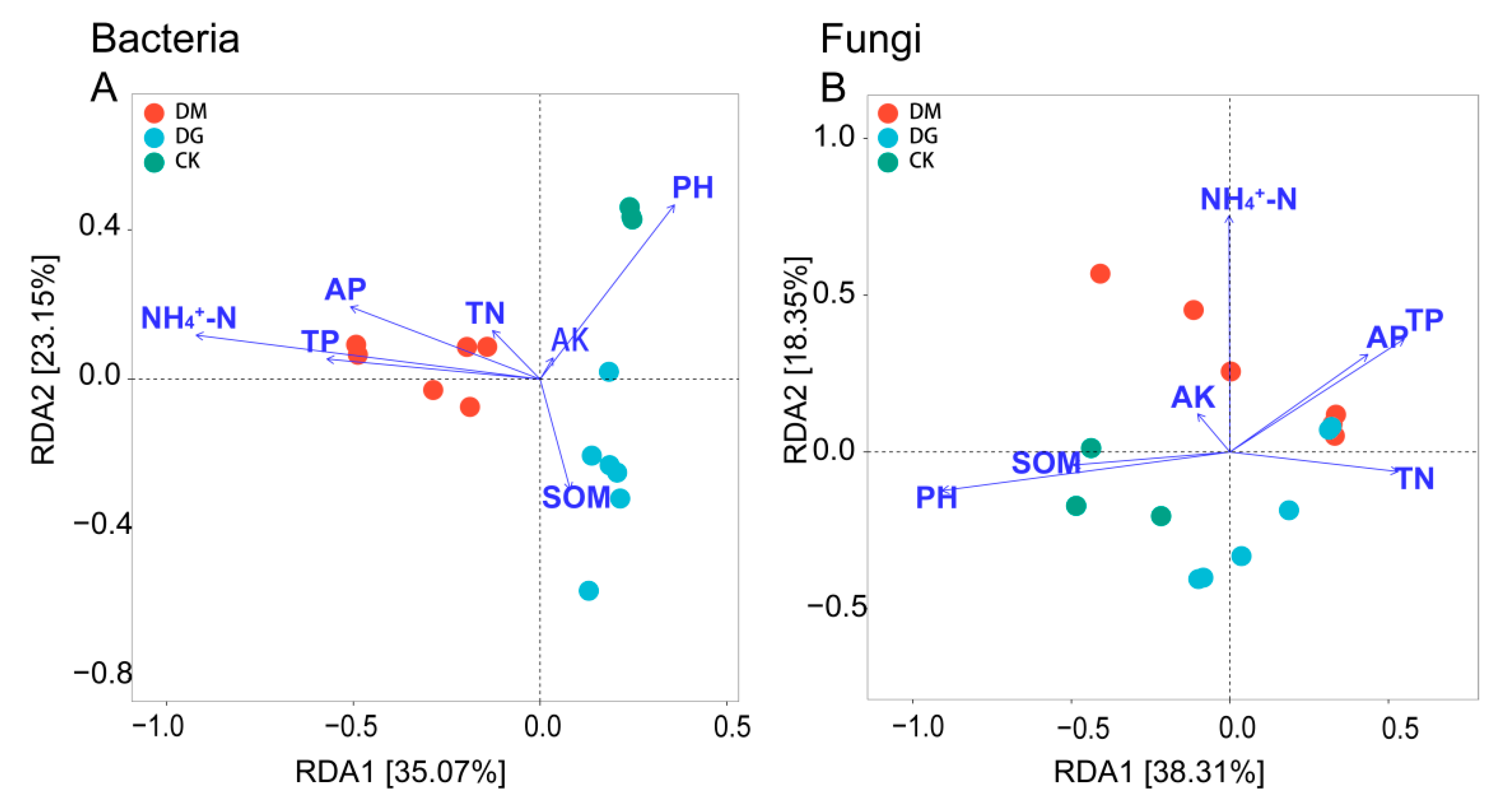
3.5. Relationships between Soil Physicochemical Characteristics and Microbial Populations
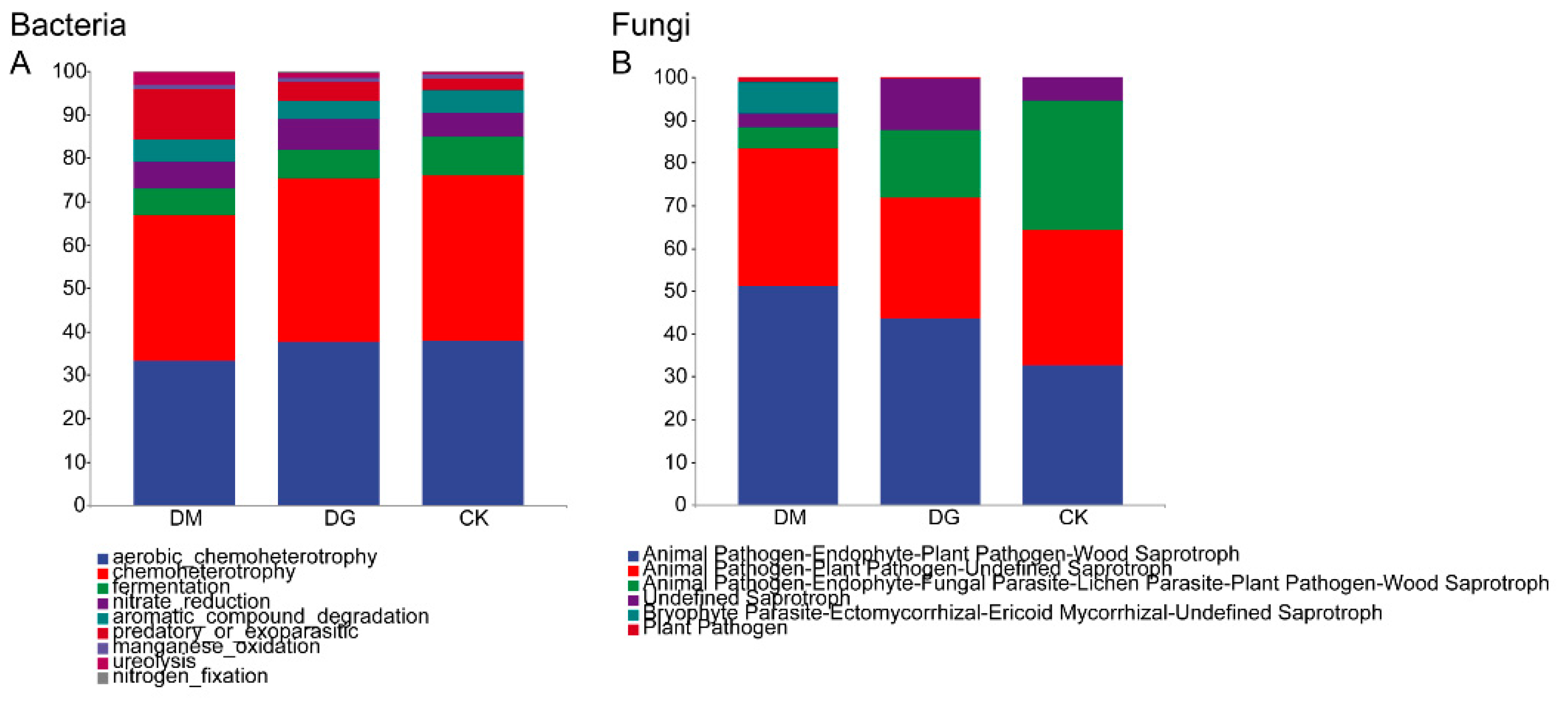
3.6. Prediction of Microbial Community Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, H.; Wang, L.S.; Guo, S.H.; Gong, S.L. Prospects of Sustainable Grain Production in Northeast of China. In Proceedings of the 2012 World Automation Congress (Wac), Puerto Vallarta, Mexico, 24–28 June 2012. [Google Scholar]
- Liu, X.B.; Zhang, X.Y.; Wang, Y.X.; Sui, Y.Y.; Zhang, S.L.; Herbert, S.J.; Ding, G. Soil degradation: A problem threatening the sustainable development of agriculture in Northeast China. Plant Soil Environ. 2010, 56, 87–97. [Google Scholar] [CrossRef]
- Wang, X.Y. Study on Continuous Cropping Obstacle and Control Strategy of Medicinal Plants. Adv. Soc. Sci. Educ. Hum. 2017, 119, 839–842. [Google Scholar]
- Shi, Q.L.; Lin, Y.Z.; Zhang, E.P.; Yan, H.M.; Zhan, J.Y. Impacts of Cultivated Land Reclamation on the Climate and Grain Production in Northeast China in the Future 30 Years. Adv. Meteorol. 2013, 2013, 853098. [Google Scholar] [CrossRef]
- Yang, X.M.; Zhang, X.P.; Deng, W.; Fang, H.J. Black soil degradation by rainfall erosion in Jilin, China. Land Degrad. Dev. 2003, 14, 409–420. [Google Scholar] [CrossRef]
- Yu, G.R.; Fang, H.J.; Gao, L.P.; Zhang, W.J. Soil organic carbon budget and fertility variation of black soils in Northeast China. Ecol. Res. 2006, 21, 855–867. [Google Scholar] [CrossRef]
- Waldrop, M.P.; Balser, T.C.; Firestone, M.K. Linking microbial community composition to function in a tropical soil. Soil Biol. Biochem. 2000, 32, 1837–1846. [Google Scholar] [CrossRef]
- Liu, H.; Pan, F.J.; Han, X.Z.; Song, F.B.; Zhang, Z.M.; Yan, J.; Xu, Y.L. Response of Soil Fungal Community Structure to Long-Term Continuous Soybean Cropping. Front. Microbiol. 2019, 9, 03316. [Google Scholar] [CrossRef]
- Tang, H.; Xiao, C.H.; Ma, J.Z.; Yu, M.; Li, Y.M.; Wang, G.L.; Zhang, L.P. Prokaryotic diversity in continuous cropping and rotational cropping soybean soil. FEMS Microbiol. Lett. 2009, 298, 267–273. [Google Scholar] [CrossRef]
- Chen, S.L.; Zhou, B.L.; Lin, S.S.; Li, X.; Ye, X.L. Accumulation of cinnamic acid and vanillin in eggplant root exudates and the relationship with continuous cropping obstacle. Afr. J. Biotechnol. 2011, 10, 2659–2665. [Google Scholar]
- Huang, L.F.; Song, L.X.; Xia, X.J.; Mao, W.H.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Plant-Soil Feedbacks and Soil Sickness: From Mechanisms to Application in Agriculture. J. Chem. Ecol. 2013, 39, 232–242. [Google Scholar] [CrossRef]
- Hu, Q.L.; Tan, L.; Yang, X.X.; Deng, Y.; Gu, S.S.; Chen, J.R.; Cai, L.; Zhou, Q.; Hu, X.X.; Qin, Y.Z.; et al. The Divergence of Bacterial Communities among Continuous Cropping, Rotational Cropping and New Planting Potato Soils. Int. J. Agric. Biol. 2020, 23, 721–729. [Google Scholar] [CrossRef]
- Wu, L.K.; Yang, B.; Li, M.L.; Chen, J.; Xiao, Z.G.; Wu, H.M.; Tong, Q.Y.; Luo, X.M.; Lin, W.X. Modification of Rhizosphere Bacterial Community Structure and Functional Potentials to Control Pseudostellaria heterophylla Replant Disease. Plant Dis. 2020, 104, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.Y.; Han, M.K.; Hu, Y.Y.; Li, Z.Q.; Liu, C.F.; Wang, X.; Tian, Q.; Jiao, W.J.; Hu, J.M.; Liu, L.F.; et al. Effects of Continuous Cropping of Sweet Potato on the Fungal Community Structure in Rhizospheric Soil. Front. Microbiol. 2019, 10, 2269. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.C.; Rodibaugh, K.J.; Hahn, D.; Nowlin, W.H. Bacterial community composition and carbon metabolism in a subtropical riverscape. Hydrobiologia 2017, 792, 209–226. [Google Scholar] [CrossRef]
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Hu, Y.T.; Zhang, S.S.; Noll, L.; Bockle, T.; Dietrich, M.; Herbold, C.W.; Eichorst, S.A.; Woebken, D.; Richter, A.; et al. Soil multifunctionality is affected by the soil environment and by microbial Choo community composition and diversity. Soil Biol. Biochem. 2019, 136, 107521. [Google Scholar] [CrossRef]
- Ji, L.; Tian, L.; Nasir, F.; Chang, J.J.; Chang, C.L.; Zhang, J.F.; Li, X.J.; Tian, C.J. Impacts of replanting American ginseng on fungal assembly and abundance in response to disease outbreaks. Arch. Microbiol. 2021, 203, 2157–2170. [Google Scholar] [CrossRef]
- Luo, S.S.; Wang, S.J.; Tian, L.; Li, S.Q.; Li, X.J.; Shen, Y.F.; Tian, C.J. Long-term biochar application influences soil microbial community and its potential roles in semiarid farmland. Appl. Soil Ecol. 2017, 117, 10–15. [Google Scholar] [CrossRef]
- Shi, S.H.; Tian, L.; Nasir, F.; Bahadur, A.; Batool, A.; Luo, S.S.; Yang, F.; Wang, Z.C.; Tian, C.J. Response of microbial communities and enzyme activities to amendments in saline-alkaline soils. Appl. Soil Ecol. 2019, 135, 16–24. [Google Scholar] [CrossRef]
- Tian, L.; Shi, S.H.; Sun, Y.; Tran, L.S.P.; Tian, C.J. The compositions of rhizosphere microbiomes of wild and cultivated soybeans changed following the hybridization of their F1 and F2 generations. Eur. J. Soil Biol. 2020, 101, 103249. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Srivastava, D.S.; Duffy, J.E.; Wright, J.P.; Downing, A.L.; Sankaran, M.; Jouseau, C. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 2006, 443, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Enwall, K.; Nyberg, K.; Bertilsson, S.; Cederlund, H.; Stenstrom, J.; Hallin, S. Long-term impact of fertilization on activity and composition of bacterial communities and metabolic guilds in agricultural soil. Soil Biol. Biochem. 2007, 39, 106–115. [Google Scholar] [CrossRef]
- Janvier, C.; Villeneuve, F.; Alabouvette, C.; Edel-Hermann, V.; Mateille, T.; Steinberg, C. Soil health through soil disease suppression: Which strategy from descriptors to indicators? Soil Biol. Biochem. 2007, 39, 1–23. [Google Scholar] [CrossRef]
- Wang, R.Q.; Xiao, Y.P.; Lv, F.J.; Hu, L.Y.; Wei, L.G.; Yuan, Z.Q.; Lin, H.X. Bacterial community structure and functional potential of rhizosphere soils as influenced by nitrogen addition and bacterial wilt disease under continuous sesame cropping. Appl. Soil Ecol. 2018, 125, 117–127. [Google Scholar] [CrossRef]
- Walters, K.E.; Martiny, J.B.H. Alpha-, beta-, and gamma-diversity of bacteria varies across habitats. PLoS ONE 2020, 15, e0233872. [Google Scholar] [CrossRef]
- Willis, A.D. Rarefaction, Alpha Diversity, and Statistics. Front. Microbiol. 2019, 10, 2407. [Google Scholar] [CrossRef]
- Ji, L.; Nasir, F.; Tian, L.; Chang, J.J.; Sun, Y.; Zhang, J.F.; Li, X.J.; Tian, C.J. Outbreaks of Root Rot Disease in Different Aged American Ginseng Plants Are Associated with Field Microbial Dynamics. Front. Microbiol. 2021, 12, 676880. [Google Scholar] [CrossRef]
- Bai, L.; Sun, H.B.; Zhang, X.Q.; Cai, B.Y. Next-generation sequencing of root fungal communities in continuous cropping soybean. Chil. J. Agric. Res. 2018, 78, 528–538. [Google Scholar] [CrossRef]
- Tan, Y.; Cui, Y.; Li, H.; Kuang, A.; Li, X.; Wei, Y.; Ji, X. Rhizospheric soil and root endogenous fungal diversity and composition in response to continuous Panax notoginseng cropping practices. Microbiol. Res. 2017, 194, 10–19. [Google Scholar] [CrossRef]
- Lu, L.H.; Yin, S.X.; Liu, X.; Zhang, W.M.; Gu, T.Y.; Shen, Q.R.; Qiu, H.Z. Fungal networks in yield-invigorating and -debilitating soils induced by prolonged potato monoculture. Soil Biol. Biochem. 2013, 65, 186–194. [Google Scholar] [CrossRef]
- Li, X.G.; Ding, C.F.; Zhang, T.L.; Wang, X.X. Fungal pathogen accumulation at the expense of plant-beneficial fungi as a consequence of consecutive peanut monoculturing. Soil Biol. Biochem. 2014, 72, 11–18. [Google Scholar] [CrossRef]
- Yang, Q.X.; Wang, R.F.; Xu, Y.Y.; Kang, C.X.; Miao, Y.; Li, M.J. Dynamic change of the rhizosphere microbial community in response to growth stages of consecutively monocultured Rehmanniae glutinosa. Biologia 2016, 71, 1320–1329. [Google Scholar] [CrossRef]
- Wu, J.P.; Jiao, Z.B.; Zhou, J.; Guo, F.L.; Ding, Z.L.; Qiu, Z.M. Analysis of bacterial communities in rhizosphere soil of continuously cropped healthy and diseased konjac. World J. Microb. Biot. 2017, 33, 134. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.H.; Yao, X.H.; An, L.K.; Bai, Y.X.; Xie, D.Q.; Wu, K.L. Rhizosphere Bacterial Community Response to Continuous Cropping of Tibetan Barley. Front. Microbiol. 2020, 11, 551444. [Google Scholar] [CrossRef]
- Wu, A.L.; Jiao, X.Y.; Fan, F.F.; Wang, J.S.; Guo, J.; Dong, E.W.; Wang, L.G.; Shen, X.M. Effect of continuous sorghum cropping on the rhizosphere microbial community and the role of Bacillus amyloliquefaciens in altering the microbial composition. Plant Growth Regul. 2019, 89, 299–308. [Google Scholar] [CrossRef]
- Mazzola, M. Assessment and management of soil microbial community structure for disease suppression. Annu. Rev. Phytopathol. 2004, 42, 35–59. [Google Scholar] [CrossRef]
- Alami, M.M.; Xue, J.Q.; Ma, Y.T.; Zhu, D.Y.; Abbas, A.; Gong, Z.D.; Wang, X.K. Structure, Function, Diversity, and Composition of Fungal Communities in Rhizospheric Soil of Coptis chinensis Franch under a Successive Cropping System. Plants 2020, 9, 244. [Google Scholar] [CrossRef]
- Gao, J.X.; Pei, H.X.; Xie, H. Synergistic effects of organic fertilizer and corn straw on microorganisms of pepper continuous cropping soil in China. Bioengineered 2020, 11, 1258–1268. [Google Scholar] [CrossRef]
- Li, C.G.; Li, X.M.; Kong, W.D.; Wu, Y.; Wang, J.G. Effect of monoculture soybean on soil microbial community in the Northeast China. Plant Soil 2010, 330, 423–433. [Google Scholar] [CrossRef]
- Liu, J.J.; Yu, Z.H.; Yao, Q.; Hu, X.J.; Zhang, W.; Mi, G.; Chen, X.L.; Wang, G.H. Distinct soil bacterial communities in response to the cropping system in a Mollisol of northeast China. Appl. Soil Ecol. 2017, 119, 407–416. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, D.; Yang, Y.Q.; Pan, Y.; Zhao, D.M.; Zhu, J.H.; Zhang, L.K.; Yang, Z.H. Dissecting the effect of continuous cropping of potato on soil bacterial communities as revealed by high-throughput sequencing. PLoS ONE 2020, 15, e0233356. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ning, P.; Li, J.Y.; Wei, X.M.; Ge, T.D.; Cui, Y.X.; Deng, X.P.; Jiang, Y.L.; Shen, W.J. Responses of soil microbial community composition and enzyme activities to long-term organic amendments in a continuous tobacco cropping system. Appl. Soil Ecol. 2022, 169, 104210. [Google Scholar] [CrossRef]
- Cha, J.Y.; Han, S.; Hong, H.J.; Cho, H.; Kim, D.; Kwon, Y.; Kwon, S.K.; Crusemann, M.; Lee, Y.B.; Kim, J.F.; et al. Microbial and biochemical basis of a Fusarium wilt-suppressive soil. ISME J. 2016, 10, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Delgado-Baquerizo, M.; Trivedi, C.; Hamonts, K.; Anderson, I.C.; Singh, B.K. Keystone microbial taxa regulate the invasion of a fungal pathogen in agro-ecosystems. Soil Biol. Biochem. 2017, 111, 10–14. [Google Scholar] [CrossRef]
- Long, S.R. Genes and signals in the Rhizobium-legume symbiosis. Plant Physiol. 2001, 125, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Logrieco, A.; Moretti, A.; Solfrizzo, M. Alternaria toxins and plant diseases: An overview of origin, occurrence and risks. World Mycotoxin J. 2009, 2, 129–140. [Google Scholar] [CrossRef]
- Pereira, A.S.; dos Santos, G.R.; Sarmento, R.A.; Galdino, T.V.D.; Lima, C.H.D.; Picanco, M.C. Key factors affecting watermelon yield loss in different growing seasons. Sci. Hortic. 2017, 218, 205–212. [Google Scholar] [CrossRef]
- Grube, M.; Furnkranz, M.; Zitzenbacher, S.; Huss, H.; Berg, G. Emerging multi-pathogen disease caused by Didymella bryoniae and pathogenic bacteria on Styrian oil pumpkin. Eur. J. Plant Pathol. 2011, 131, 539–548. [Google Scholar] [CrossRef]
- Chaisiri, C.; Liu, X.Y.; Yin, W.X.; Luo, C.X.; Lin, Y. Morphology Characterization, Molecular Phylogeny, and Pathogenicity of Diaporthe passifloricola on Citrus reticulata cv. Nanfengmiju in Jiangxi Province, China. Plants 2021, 10, 218. [Google Scholar] [CrossRef]
- Xiao, X.E.; Wang, W.; Crous, P.W.; Wang, H.K.; Jiao, C.; Huang, F.; Pu, Z.X.; Zhu, Z.R.; Li, H.Y. Species of Botryosphaeriaceae associated with citrus branch diseases in China. Persoonia 2021, 47, 106–135. [Google Scholar] [CrossRef]
- Huang, F.; Hou, X.; Dewdney, M.M.; Fu, Y.; Chen, G.Q.; Hyde, K.D.; Li, H.Y. Diaporthe species occurring on citrus in China. Fungal Divers. 2013, 61, 237–250. [Google Scholar] [CrossRef]
- Cheng, B.P.; Huang, Y.H.; Song, X.B.; Peng, A.T.; Ling, J.F.; Chen, X. First Report of Colletotrichum siamense Causing Leaf Drop and Fruit Spot of Citrus reticulata Blanco cv. Shiyue Ju in China. Plant Dis. 2013, 97, 1508. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Crowley, D.E.; Menge, J.A. 16S rDNA fingerprinting of rhizosphere bacterial communities associated with healthy and Phytophthora infected avocado roots. FEMS Microbiol. Ecol. 2001, 35, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, J.L.; Gu, T.Y.; Zhang, W.M.; Shen, Q.R.; Yin, S.X.; Qiu, H.Z. Microbial Community Diversities and Taxa Abundances in Soils along a Seven-Year Gradient of Potato Monoculture Using High Throughput Pyrosequencing Approach. PLoS ONE 2014, 9, e86610. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Li, Z.G.; Liu, H.J.; Xue, C.; Zhang, R.F.; Wu, H.S.; Li, R.; Shen, Q.R. The Effect of Long-Term Continuous Cropping of Black Pepper on Soil Bacterial Communities as Determined by 454 Pyrosequencing. PLoS ONE 2015, 10, e0136946. [Google Scholar] [CrossRef]
- Bartram, A.K.; Jiang, X.P.; Lynch, M.D.J.; Masella, A.P.; Nicol, G.W.; Dushoff, J.; Neufeld, J.D. Exploring links between pH and bacterial community composition in soils from the Craibstone Experimental Farm. FEMS Microbiol. Ecol. 2014, 87, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, S.D.; Palmer, A.S.; Winsley, T.; Lamb, E.; Bissett, A.; Brown, M.V.; van Dorst, J.; Ji, M.K.; Ferrari, B.C.; Grogan, P.; et al. Soil fertility is associated with fungal and bacterial richness, whereas pH is associated with community composition in polar soil microbial communities. Soil Biol. Biochem. 2014, 78, 10–20. [Google Scholar] [CrossRef]
- Zhalnina, K.; Dias, R.; de Quadros, P.D.; Davis-Richardson, A.; Camargo, F.A.O.; Clark, I.M.; McGrath, S.P.; Hirsch, P.R.; Triplett, E.W. Soil pH Determines Microbial Diversity and Composition in the Park Grass Experiment. Microb. Ecol. 2015, 69, 395–406. [Google Scholar] [CrossRef]
- Haichar, F.E.; Marol, C.; Berge, O.; Rangel-Castro, J.I.; Prosser, J.I.; Balesdent, J.; Heulin, T.; Achouak, W. Plant host habitat and root exudates shape soil bacterial community structure. ISME J. 2008, 2, 1221–1230. [Google Scholar] [CrossRef]
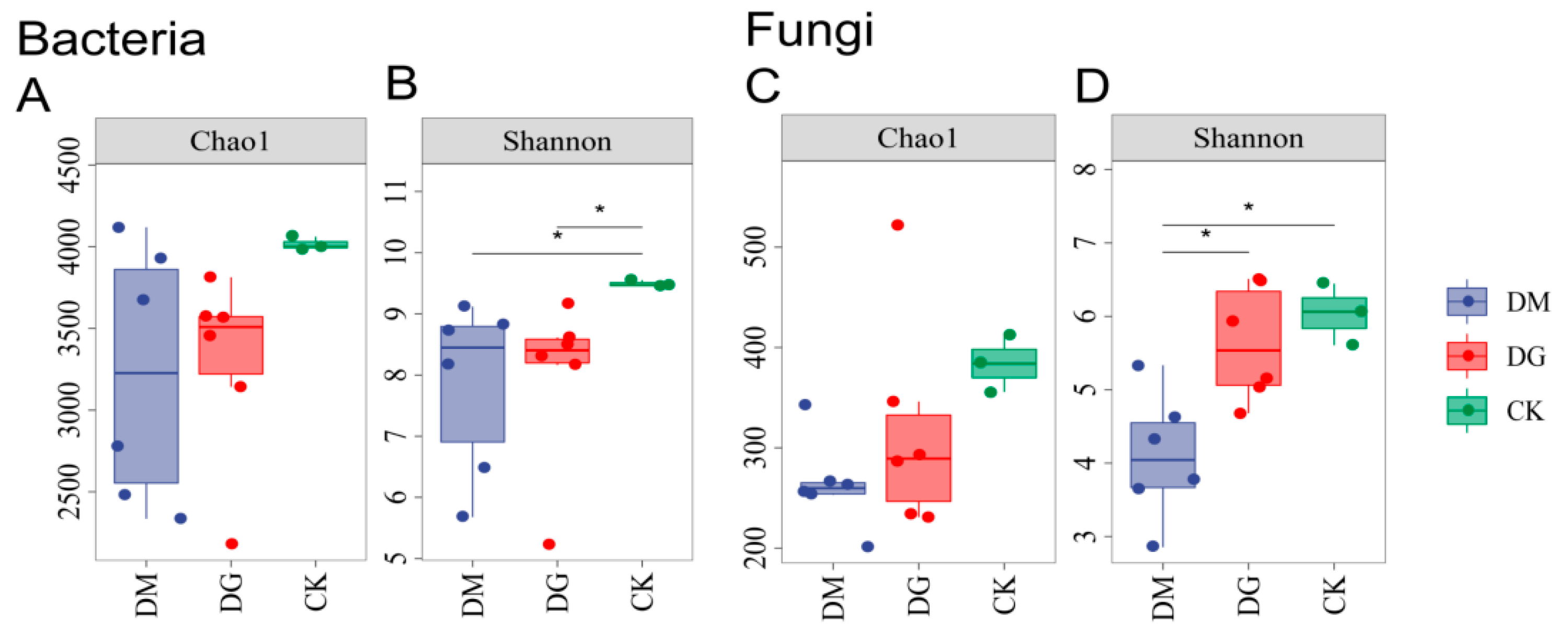
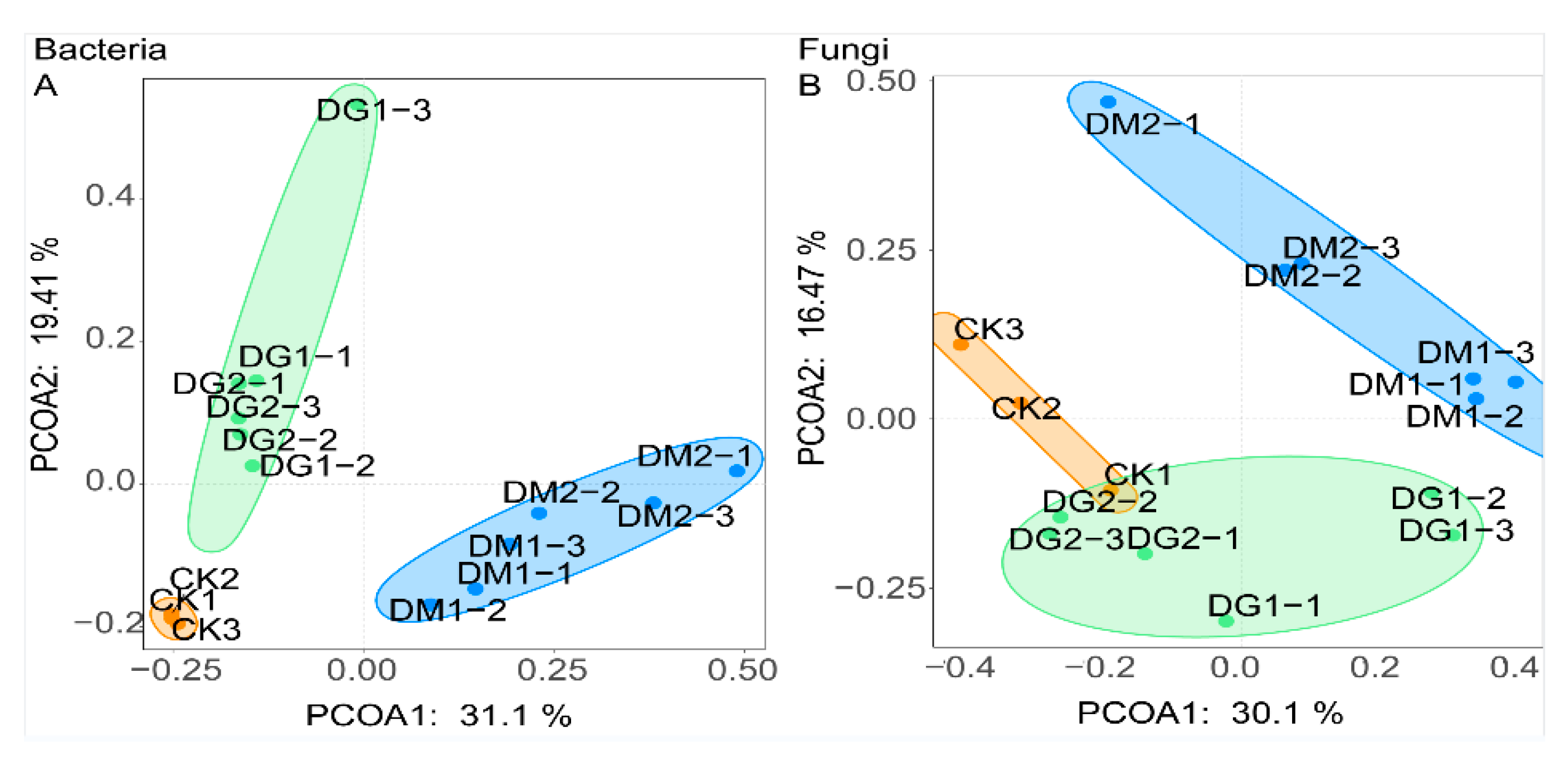

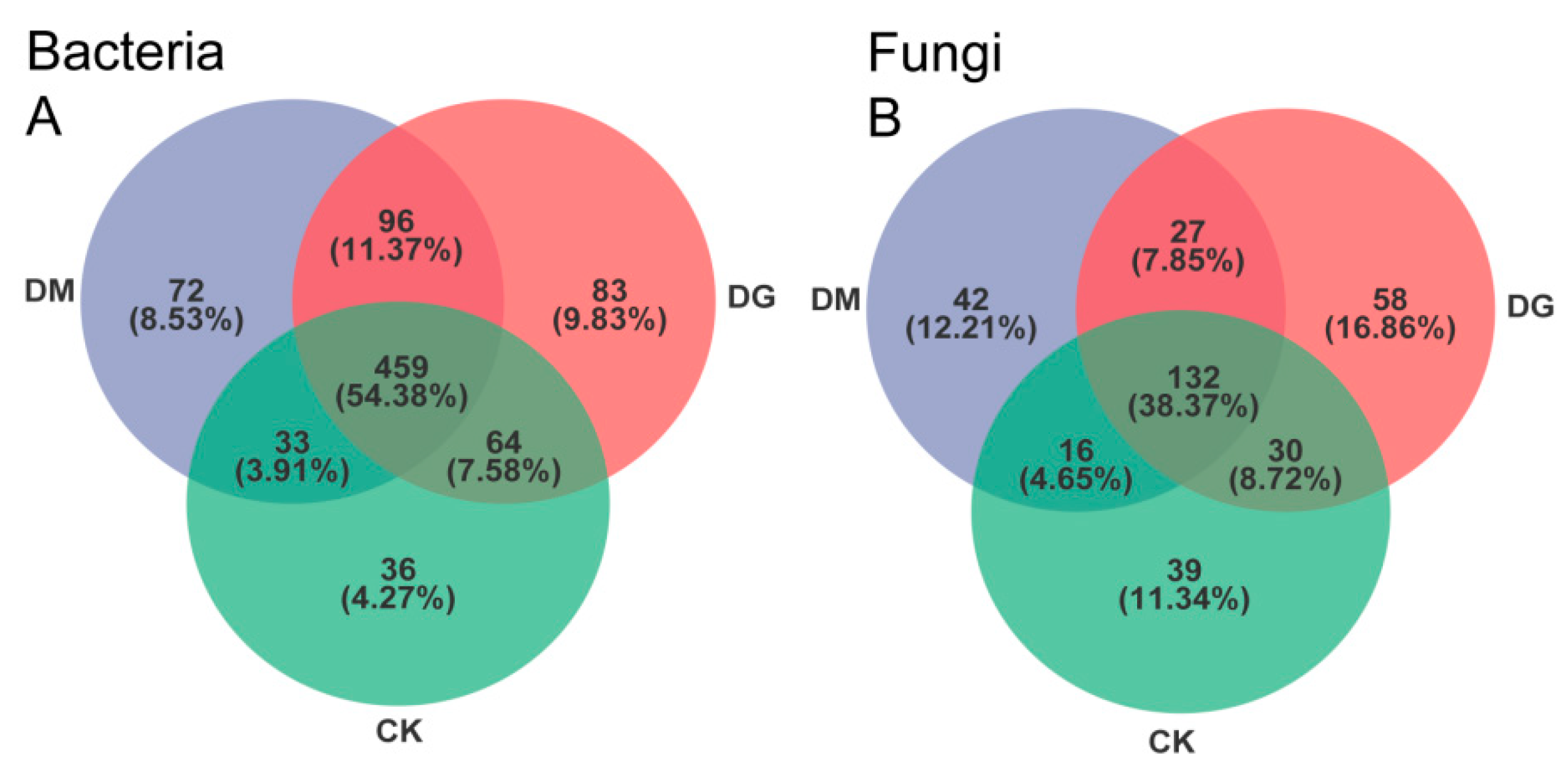
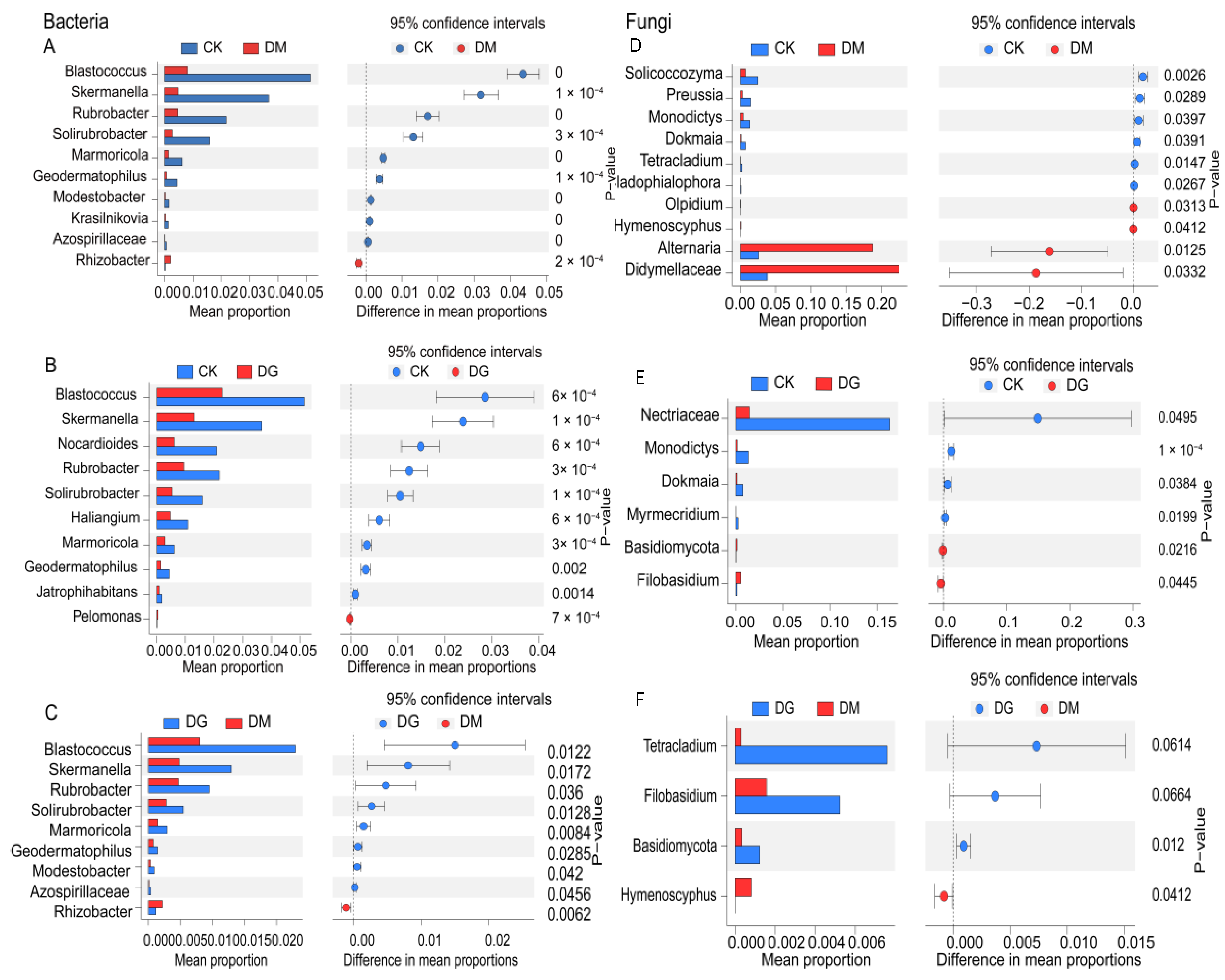


| Treat | CK | DG1 | DG2 | DM1 | DM2 |
|---|---|---|---|---|---|
| pH | 7.12 ± 0.01 a | 6.69 ± 0.02 c | 6.73 ± 0.01 c | 6.83 ± 0.02 b | 6.26 ± 0.03 d |
| AP (mg/kg) | 10.42 ± 0.10 bc | 6.02 ± 1.83 c | 7.57 ± 0.45 c | 13.06 ± 1.42 b | 30.08 ± 4.16 a |
| NH4+-N (mg/kg) | 32.16 ± 2.23 d | 18.43 ± 0.88 e | 44.21 ± 0.68 c | 94.71 ± 1.34 a | 73.81 ± 0.79 b |
| AK (mg/kg) | 176.52 ± 3.16 a | 174.77 ± 5.79 a | 170.03 ± 8.61 a | 177.34 ± 8.94 a | 164.51 ± 38.18 a |
| TP (g/kg) | 0.60 ± 0.01 d | 0.59 ± 0.02 e | 0.63 ± 0.01 c | 0.69 ± 0.02 b | 0.92 ± 0.01 a |
| TN (g/kg) | 1.93 ± 0.01 c | 1.60 ± 0.02 e | 1.95 ± 0.02 b | 2.95 ± 0.02 a | 1.66 ± 0.01 d |
| SOM (mg/kg) | 15.88 ± 0.20 b | 16.15 ± 0.29 b | 17.05 ± 0.14 a | 17.09 ± 0.56 a | 13.76 ± 0.11 c |
| Sample | Bacteria | Fungus | ||||||
|---|---|---|---|---|---|---|---|---|
| Nodes | Edges | Positive | Negative | Nodes | Edges | Positive | Negative | |
| DM | 97 | 360 | 81.11% | 18.89% | 19 | 21 | 57.14% | 42.86% |
| DG | 104 | 268 | 85.07% | 14.93% | 21 | 27 | 51.85% | 48.15% |
| CK | 113 | 1355 | 52.47% | 47.53% | 30 | 90 | 62.22% | 37.78% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Luo, S.; Yao, Z.; Zhang, J.; Chen, Y.; Sun, Y.; Wang, E.; Ji, L.; Li, Y.; Tian, L.; et al. Effect of Different Types of Continuous Cropping on Microbial Communities and Physicochemical Properties of Black Soils. Diversity 2022, 14, 954. https://doi.org/10.3390/d14110954
Zhang J, Luo S, Yao Z, Zhang J, Chen Y, Sun Y, Wang E, Ji L, Li Y, Tian L, et al. Effect of Different Types of Continuous Cropping on Microbial Communities and Physicochemical Properties of Black Soils. Diversity. 2022; 14(11):954. https://doi.org/10.3390/d14110954
Chicago/Turabian StyleZhang, Jianfeng, Shouyang Luo, Zongmu Yao, Jiafan Zhang, Yalin Chen, Yu Sun, Enze Wang, Li Ji, Yingxin Li, Lei Tian, and et al. 2022. "Effect of Different Types of Continuous Cropping on Microbial Communities and Physicochemical Properties of Black Soils" Diversity 14, no. 11: 954. https://doi.org/10.3390/d14110954
APA StyleZhang, J., Luo, S., Yao, Z., Zhang, J., Chen, Y., Sun, Y., Wang, E., Ji, L., Li, Y., Tian, L., & Tian, C. (2022). Effect of Different Types of Continuous Cropping on Microbial Communities and Physicochemical Properties of Black Soils. Diversity, 14(11), 954. https://doi.org/10.3390/d14110954






