1. Introduction
Different land uses modify natural environments and negatively impact terrestrial and aquatic ecosystems, along with the associated biodiversity [
1,
2,
3]. Among the different land uses, the conversion of forests into farmland and pastures has increased the degradation of aquatic ecosystems and water quality due to the removal of vegetation from the surrounding water bodies, silting of channels, and water pollution [
3,
4,
5,
6]. These impacts modify environments and can affect insect assemblages, thus, causing highly negative effects on aquatic ecosystems and their biodiversity [
3,
6,
7].
Agriculture is one of the leading economic activities in Brazil. Additionally, this activity causes the highest rate of conversion of natural landscapes [
3]. According to MapBiomas [
8], 30.97% of Brazilian territory is used for agriculture, especially in the Pampas, Cerrado, and Atlantic Forest domains, where large areas have been converted to grow crops and graze livestock. In Atlantic Forest fragments, present in 17 Brazilian states, intensive exploitation has led to drastic reductions and modifications of native areas [
3,
9], and therefore, these Atlantic Forest fragments have been identified among the priority regions for conservation. The Atlantic Forest houses vast biological diversity and is highly susceptible to human intervention [
10,
11].
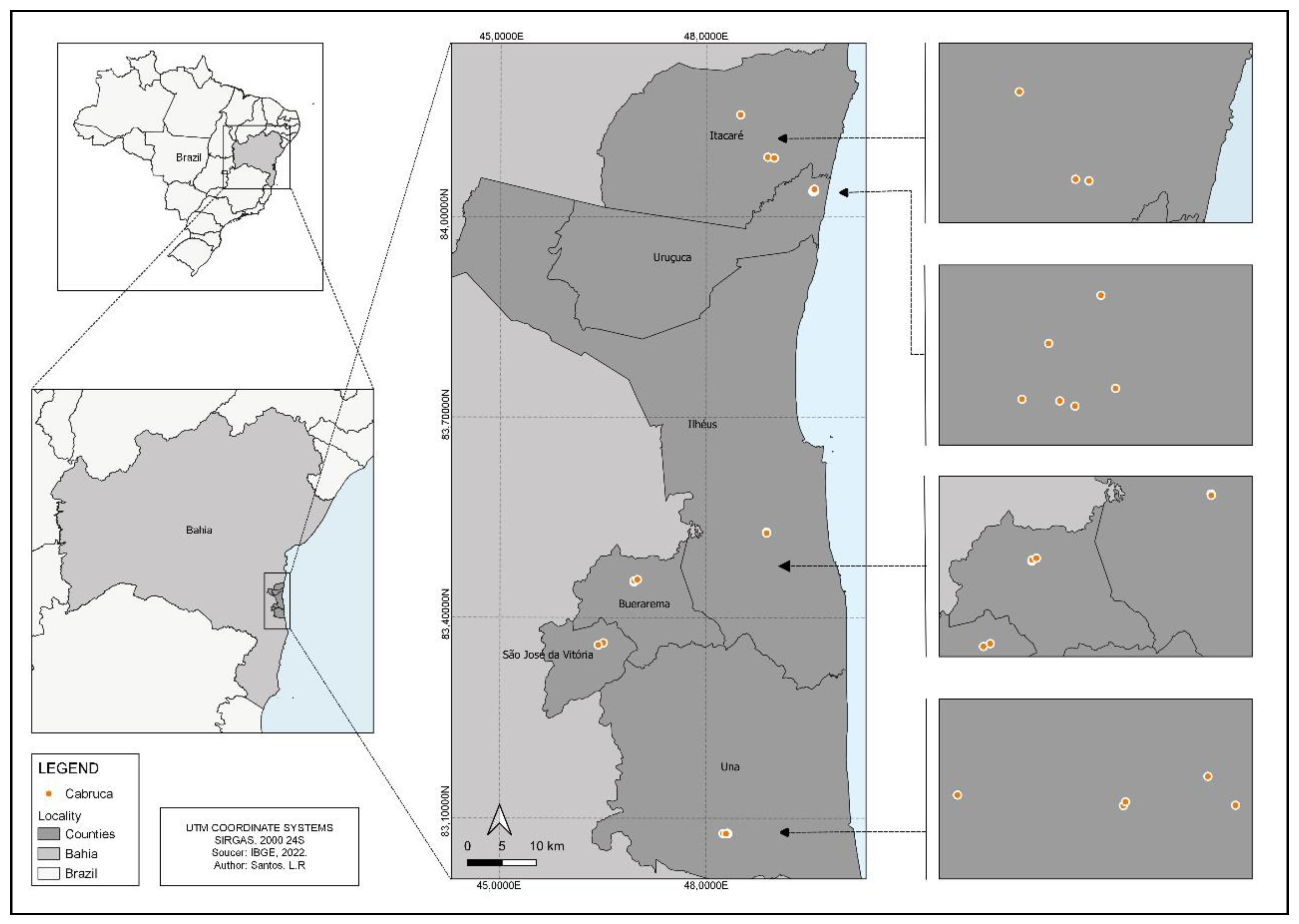
In this regard, the southern region of the state of Bahia can be highlighted, due to the cultivation of cocoa (
Theobroma cacao L. 1753) since the 18th century. This type of cultivation has had an important economic impact on the region. Studies in cocoa growing areas have demonstrated that these areas have been able to maintain part of the region’s flora and fauna, helping to conserve biodiversity [
12,
13,
14]. In the agroforestry system used to cultivate cocoa, plants grow under the shade of the native trees in the forest, locally known as cabruca or cacao-cabruca areas [
13,
14,
15]. This agroforestry system is considered to be favorable for the conservation of natural resources and local biodiversity, since it maintains part of the native forest structure and, consequently, protects terrestrial ecosystems, sustains some ecological services, and maintains the species diversity of fauna and flora [
12,
13,
14,
15].
Environmental changes caused by different land uses are directly related to the structuring and maintenance of biodiversity associated with aquatic ecosystems. Since any modification to the physical conditions of surrounding water bodies alters these environments and the physicochemical parameters of the water, local assemblages are also drastically modified [
16,
17,
18,
19]. These changes in the abiotic parameters of the water, caused by the reduced riparian vegetation, increase the entry of allochthonous material and sunlight [
20,
21,
22]. Therefore, it is critical to understand the relationships of environmental and spatial variables among cacao-cabruca areas and the diversity of associated species, and how this type of cultivation affects biodiversity. This understanding can help to identify and to quantify the impacts (negative and positive) of the management and use of land, thus, supporting more effective decision making to protect species and their habitats [
19,
21].
Among aquatic invertebrate species, dragonflies have been widely used in studies to assess the effect of changes in environmental variables on their biodiversity in areas with different land uses [
20,
23,
24,
25,
26]. This is due to the fact that there are species that can, more or less, tolerate changes in the natural environment, and therefore, are able to reflect the local conditions, and therefore, have been widely used as bioindicators of water quality and the impacts caused to the surroundings of aquatic ecosystems [
27,
28,
29,
30]. Dragonflies are organisms that are extremely dependent on aquatic ecosystems, using these environments for oviposition and development of larvae, in addition to depending on the surrounding terrestrial ecosystems, since adults use these environments to feed, to defend their territories for reproduction, and to perform important physiological functions such as thermoregulation [
30,
31,
32].
These ecological and behavioral characteristics of the species allow them to reflect the characteristics and integrity of the ecosystems they live in [
27,
30,
31,
32], which makes the different species ideal as forest and open-area specialists and habitat generalists [
33]. In this regard, the relationships among the different local and spatial environmental variables and the different Odonata species in cacao-cabruca areas should be evaluated to understand the effects of this type of cultivation on Odonata biodiversity. Moreover, the group can be used as a “surrogate” for other groups of aquatic insects [
34].
Accordingly, in the present study, we evaluated the diversity of Odonata in cocoa cultivation areas and the relationships with local and spatial environmental variables between adults and larvae at the sampling sites. The following two specific objectives were proposed:
- (I).
To assess the diversity (richness and abundance) of adult and larvae dragonflies in cocoa farming areas among sampling sites. Our prediction is that cocoa farming areas, which are considered to be agroforestry systems, can maintain a wide range of Odonata species by also maintaining groups of species considered to be forest specialists, habitat generalists, and open-area specialists [
14].
- (II).
To assess the influence of environmental physical variables, the physicochemical variables of water, and the spatial relationships in the structuring of dragonfly assemblages (adult and larvae individuals) in cocoa farms. In the present study, the local and physical variables such as canopy cover, channel width, bank structure, and amount of riparian vegetation sites, as well as abiotic water variables (dissolved oxygen, pH, and conductivity) and the distances between sampling sites were found to be important factors in the structuring of Odonata assemblages.
3. Results
A total of 689 adult Odonata individuals were captured, representing seven families and 50 species, 520 individuals distributed in 20 species of Zygoptera and 169 individuals and 30 species of Anisoptera (
Appendix A,
Table A2. Among the Zygoptera, the most representative species were
Argia chapadae Calvert, 1909;
Hetaerina rosea Selys, 1853; and
Heteragrion lencionii Vilela, Farias & Santos, 2021, with, respectively, 154, 87, and 87 individuals. Among the Anisoptera,
Erythrodiplax fusca Rambur, 1842, and
Perithemis thais Kirby, 1889 totaled 62 and 18 individuals, respectively.
In relation to larvae, 647 specimens were collected, comprising five families and 11 genera of Zygoptera and three families and 23 genera of Anisoptera. The most representative genera were
Homeoura and
Heteragrion with 43 and 76 individuals, respectively, (Zygoptera), along with
Epigomphus with 72 individuals (Anisoptera). Regarding the life stages (adult and larvae), 14 genera were collected, four genera of the suborder Zygoptera and 10 genera of the suborder Anisoptera. Sixteen genera were only found as adults and 20 genera were recorded only for the larvae (
Appendix A,
Table A3 and
Table A4).
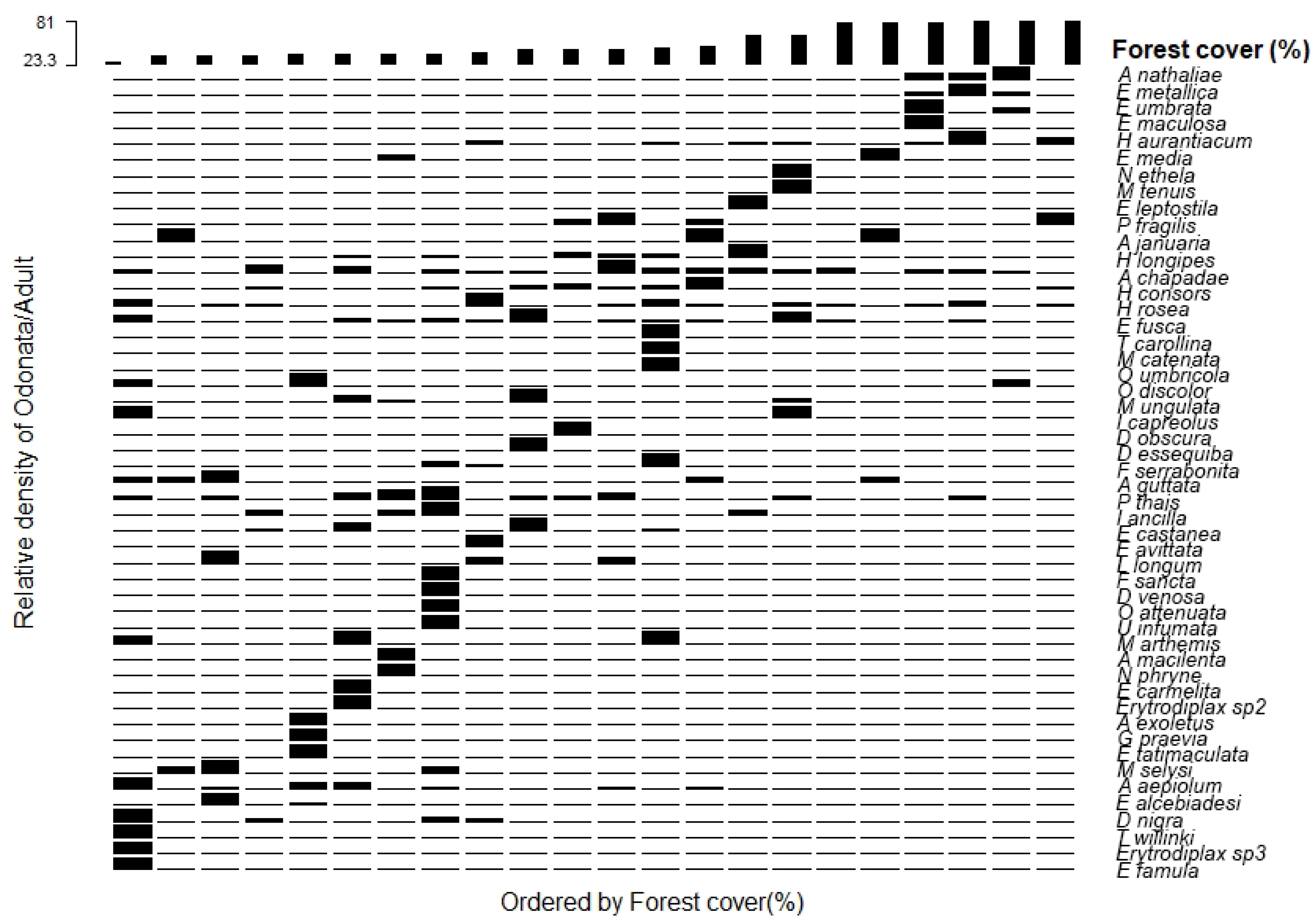
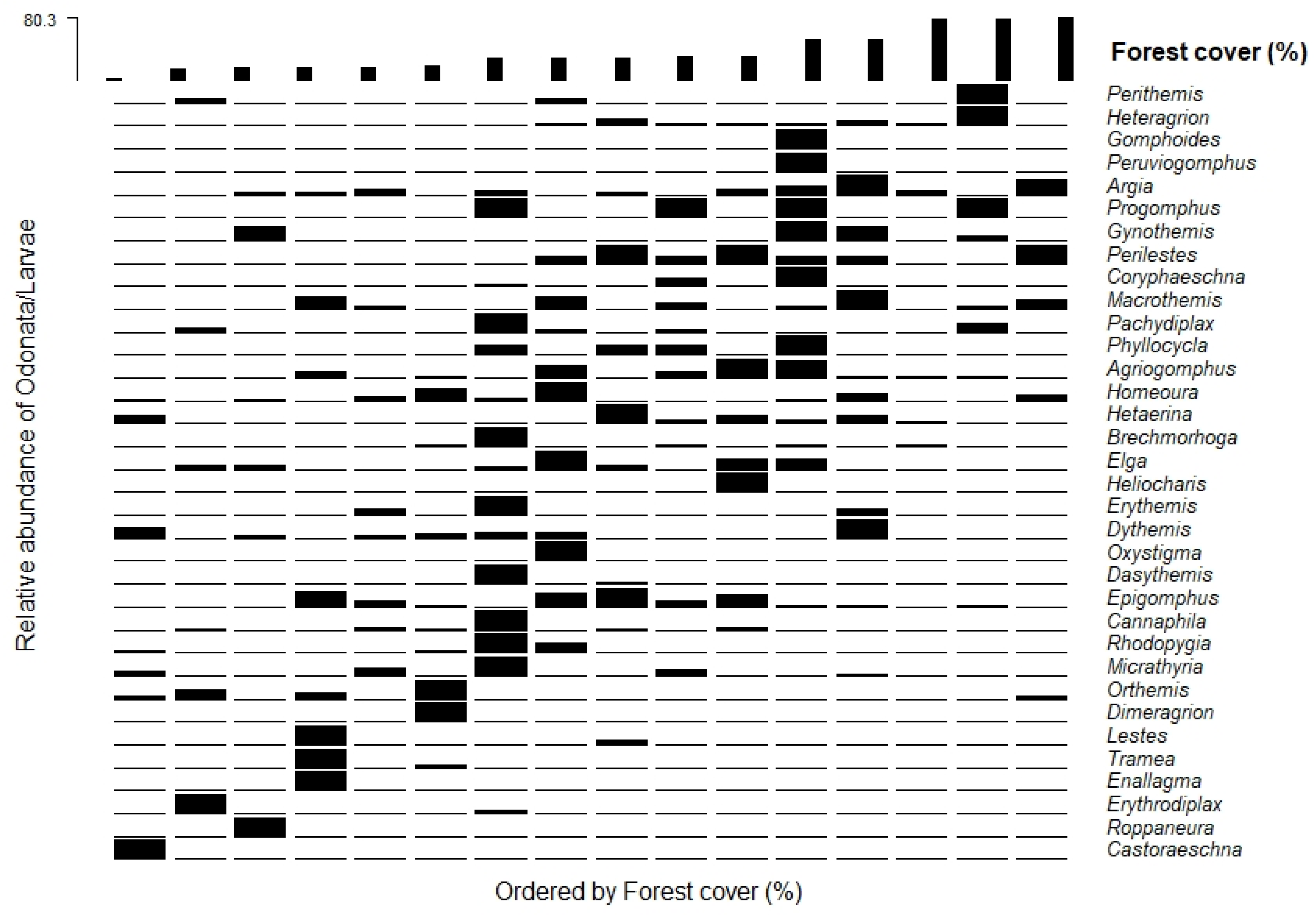
The relationship between the relative abundance with the amount of forest cover in the cocoa areas indicated alteration of some species as a function of the amount of forest cover for adult and larvae in the evaluated areas (
Figure 2 and
Figure 3). Some species and genera were related to areas with low amounts of forest cover in the sampled areas. The most open-area specialist genera and species targeted sites with a lower amount of forest cover, as in the case of the species
Telebasis willinki Fraser, 1948;
Acanthagrion aepiolum Tennessen, 2004;
Erythrodiplax famula Erichson in Schomburgk, 1848;
Elasmothemis alcebiadesi Santos, 1945; and
Diastatops nigra Montgomery, 1940; and the genera
Castoraeschna,
Roppaneura,
Erythrodiplax, Enallagma, Lestes, and
Tramea, located on the left side of both graphs (
Figure 2 and
Figure 3).
The species of adults and genera of larvae considered to be habitat generalists are represented in the central strips of the charts, which show that these groups may have greater tolerance for the loss of forest cover surrounding streams in the cocoa growing areas (
Figure 3 and
Figure 4). Finally, species and genera that were associated with cocoa growing areas with greater amounts of forest cover were
Aceratobasis nathaliae Lencioni, 2004;
Epipleoneura metallica Rácenis, 1955;
Erythrodiplax maculosa Hagen, 1861; and
Heteragrion aurantiacum Selys, 1862. The genera
Peruviogomphus,
Gomphoide, and
Heteragrion were more abundant in the cacao-cabruca areas with a higher level of amount forest cover (
Figure 4).
The environmental variables in the PCA analysis for adults presented the first four axes and the observed values were greater than those estimated by the broken stick method. The four axes of the PCA accounted for 58.48% of the cumulative proportion. The PC1 axis explained 17.47%, PC2 15.11%, PC3 14.53%, and PC4 11.37% (the eigenvalues for the axes were PC1 = 4.541, PC2 = 3.927, PC3 = 3.777, and PC4 = 2.957). The variables that contributed the most on each axis were greater than 0.6. For the PC1 axis, the variables were conductivity (−0.684), salinity (−0.758), and margin structure (0.630). For the PC2 axis, it was stream width (0.665). In the formation of the PC3 axis, the variables were depth (−0.679), undercut margin (0.630), aquatic vegetation (0.614), and HII (0.727). For the PC4 axis, the variables were dissolved oxygen (0.785) and luminosity (0.686).
For the larvae, the first three axes showed the best response in the PCA analysis according to the broken stick method. These accounted for 50.64% of the cumulative PCA proportion. The PCA1 axis explained 20.32%, PCA2 15.67%, and PC3 14.65% (respectively, the eigenvalues for the axes were 5.283; 4.073, and 3.807). The environmental variables that most influenced the formation of axes with values greater than 0.6 included conductivity (−0.639), dissolved oxygen (−0.747), salinity (−0.678), and margin structure (0.669) for PCA1, width (−0.787), channel/sediment (0.603), and current and backwater, or meanders (−0.646) for PCA2, and riparian forest width (0.621) and habitat integrity index (0.608) for PCA3.
For the spatial variables, the PCNM axes 1, 2, and 5 were selected by the forward selection method, corresponding to the axes with better correlation with the data when related to adult individuals. The obtained results were PCNM2 with correlation factor R
2 = 0.199 (F = 4.97,
p = 0.006); PCNM1 with R
2 = 0.116 (F = 3.24,
p = 0.014), and PCNM5 with R
2 = 0.084 (F= 0.08,
p = 0.018). For the case of larvae, the selected axes were PCNM2 and -1. The obtained results were PCNM2 with R = 0.200 (F = 3.493,
p = 0.006) and PCNM1 with R
2 = 0.116 (F= 2.205,
p= 0.030) (
Table 1).
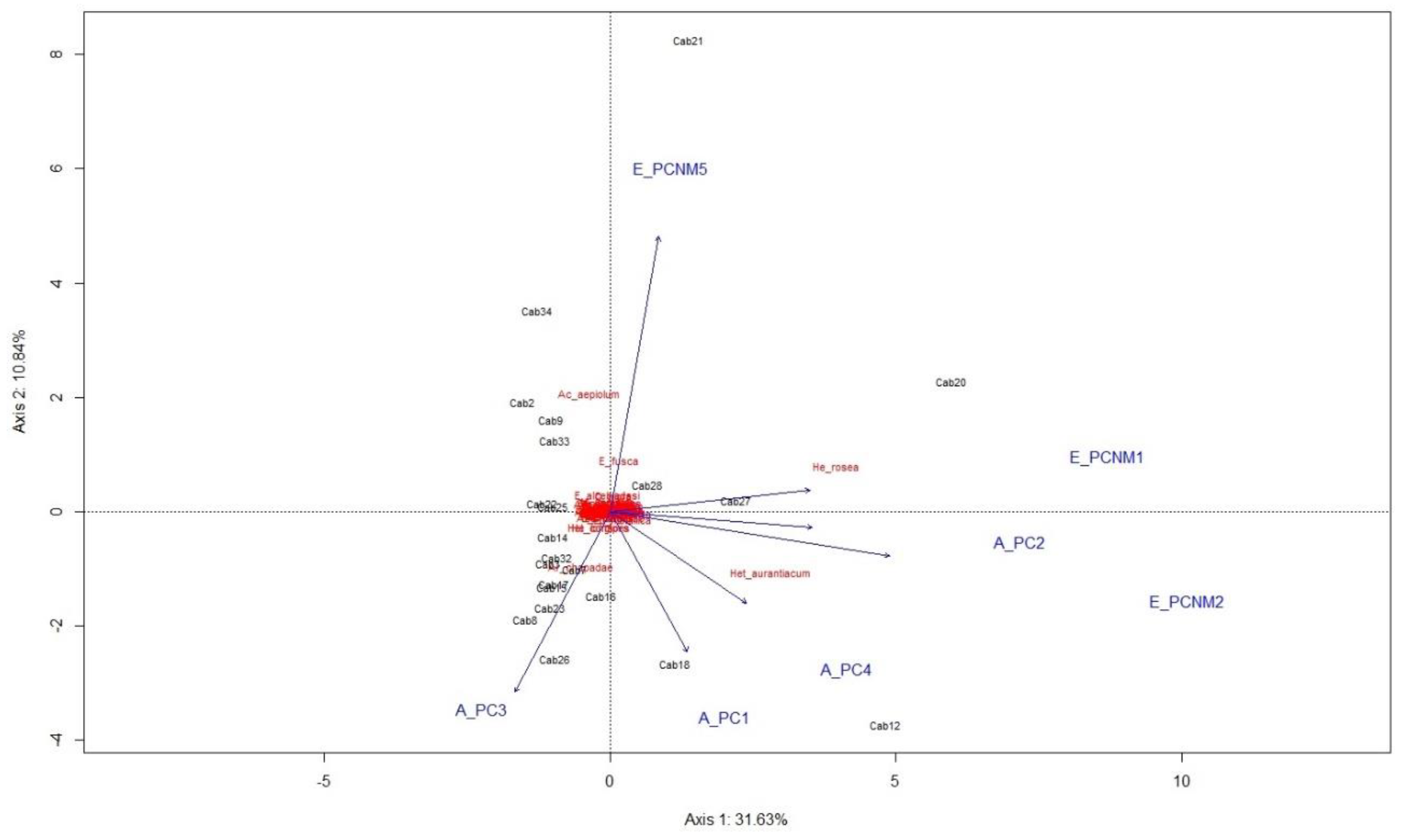
The result of the redundancy analysis (RDA) indicated that the combination of environmental and spatial variables accounted for 42.74% of the assemblage composition of adult individuals of Odonata in the cabruca areas. Axis 1 explained 31.63% and Axis 2 explained 10.84% of the data variation (
Figure 4). The ANOVA showed that the ordering analysis generated by the RDA was statistically significant (F = 2.67,
p = 0.001). The points most influenced by the evaluated axes were Sites 18 by Axis 1 of the PCA, Sites 8, 14, 15, 16, 17, 23, 26, and 32 by Axis 3 of the PCA, Sites 20, 27, and 28 by Axis 1 of the spatial analysis, and Sites 2, 9, 21, 33, and 34 by Axis 5 of the spatial analysis. The species associated with environmental and spatial variables were
Acanthagrion aepiolum and
Erythrodiplax fusca for the spatial axis (PCNM5) and
Hetearina rosea for the spatial axis (PCNM1). The species
Heteragrion aurantiacum was associated with the PC4 axis of the environmental variables and the species
Argia chapadae with the PC3 axis. The partition analysis of the environmental and spatial variables indicated that the environmental variables accounted for 32.75%, the spatial variable for 40.06%, and the two together in the model explained 57.25% of the data variation (
Table 2).
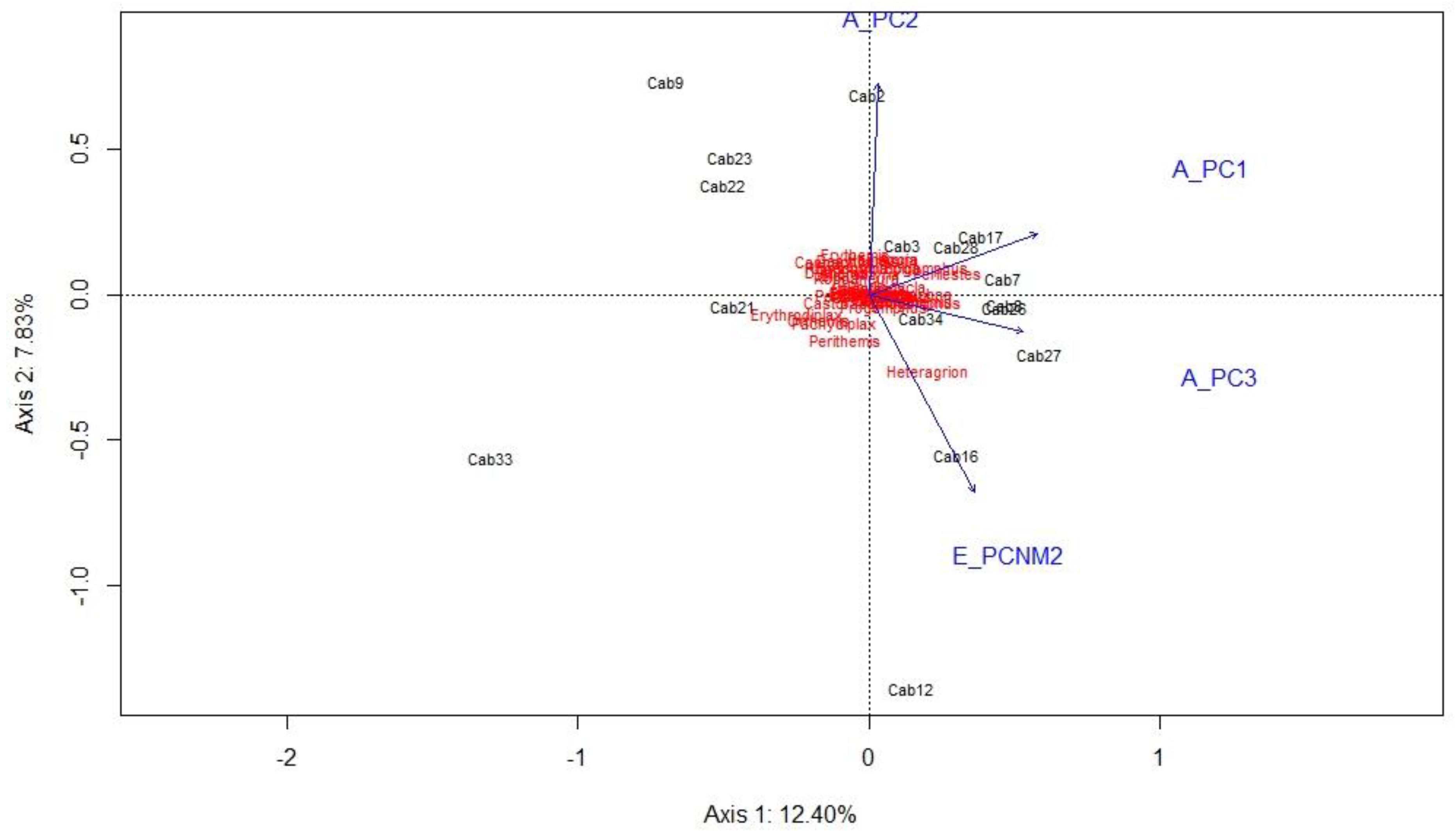
The RDA performed with the assemblages of larvae of Odonata and the environmental and spatial variables resulted in 31.58% of the explanation obtained in the first four axes that contributed the most (Axis 1, 12.40% and Axis 2, 7.83%). The ranking generated by ANOVA did not show significant values (F = 1.269,
p = 0.059) (
Figure 5). However, from the graph, it was observed that the sites associated with environmental and spatial variables were Sites 7, 17 and 28 for the PC1 axis, Site 2 for the PC2 axis, Sites 27 and 34 for the axis PC3, and Site 16 for the PCNM2 axis. The partition analysis indicated that the environmental variable was responsible for 27%, the spatial variable for 19%, and both explained 36%; the variables are presented in
Table 3.
4. Discussion
The results indicated that the cabruca areas maintain a vast diversity and richness of Odonata species (adults and larvae). Studies with the group in the Atlantic Forest that have compared different land uses have highlighted the importance of cocoa cultivation areas for the diversity of species in the region [
14]. Notably, these areas contain species that are considered to be specialists of forests areas, such as
Epipleoneura metallica Rácenis, 1955;
Aceratobasis nathaliae Lencioni, 2004;
Heteragrion aurantiacum Selys, 1862;
Forcepsioneura serrabonita Pinto & Kompier, 2018; and
Perilestes fragilis Hagen in Selys, 1862. According to the data, the distribution of these species in cocoa farms was related to greater environmental integrity of the sampled areas, which directly reflected the local environmental variables. In other words, the environmental and spatial variables in the cabruca areas played important roles in structuring these Odonata assemblages. Most of the environmental variables were common to the two evaluated life stages.
Regarding the relative abundance of species with the amount of forest cover in cocoa areas, a similar pattern of occurrence was observed between adult species and larval genera. The cabruca areas with the lowest amount of forest cover values showed greater relative abundance of adult species and larval genera, formed by species considered to be specialists of open areas, associated with areas with greater canopy opening, greater solar incidence, more lentic environments, and the presence of macrophytes [
50,
51]. Some examples are
Erythrodiplax latimaculata Ris, 1911;
Telebasis willinki Fraser, 1948;
Diastatops nigra Montgomery, 1940; and
Elasmothemis alcebiadesi Santos, 1945 for the adults, and the genera
Erythrodiplax, Enallagma, and
Castoraeschna for the larvae.
A broad range of species had its distributions along the entire gradient of the forest cover, and therefore, can be considered to be habitat generalist species [
50,
52], as in the cases of
Hetaerina rosea Selys, 1853;
Argia chapadae Calvert, 1909; and
Micrathyria ungulata Förster, 1907; and the genera
Progomphus;
Argia, 1842; and
Hetaerina. The relative abundance of some adult species and genera of larvae was associated with areas with a higher amount of forest cover, as in the cases of the species
Aceratobasis nathaliae Lencioni, 2004;
Epipleoneura metallica Rácenis, 1955; and
Heteragrion aurantiacum Selys, 1862, usually considered to be forest specialist species, and the genera
Heteragrion, Gomphoide; and
Peruviogomphus, composed of more sensitive individuals that need greater environmental integrity and conditions more comparable to forest areas [
53,
54]. However, it is worth mentioning that some species considered to be specialists in forests that have already been recorded in the region in areas of native forest in the Atlantic Forest [
14] were not recorded in the cocoa cultivation areas sampled. For example,
Aceratobasis cornicauda Calvert, 1909;
Heliocharis amazona Selys, 1853;
Kiautagrion acutum Santos, 1961; and
Leptagrion macrurum Burmeister, 1839, the last two species being phytotelmata [
14]. Emphasizing that even crops that maintain a part of the native vegetation such as cabruca areas may not maintain some groups of species considered to be more sensitive or with more specialized habitats.
The environmental variables that most contributed to the formation of the PCA axes, for adults, included conductivity, salinity, dissolved oxygen, margin structure, stream width, channel undercut margin, depth, aquatic vegetation, and luminosity. For the larvae, the variables that most contributed to the formation of the PCA axes were almost the same as for the adults, except aquatic vegetation and undercut margin. These results emphasize the importance of the same set of environmental variables for dragonfly species (adults and larvae) in cocoa growing areas, and that the changes in these variables can modify the structuring of local assemblages in cabruca areas. These environmental variables have already been highlighted in other studies that have evaluated the structuring of Odonata assemblages in other types of land use or with different environmental modifications [
20,
24,
26,
29].
In cabruca areas, changes in the amount forest cover, riparian vegetation, canopy openings, erosion of stream banks, increase the number of vascular plants in the channels, and sediments in the stream, as well as the air and water temperature. These modifications may favor the abundance and permanence of species considered to be open-area specialists and habitat generalists [
33], as in the cases of
Perithemis thais Kirby, 1889;
Argia chapadae Calvert, 1909;
Telebasis willinki Fraser, 1948; and
Erythrodiplax latimaculata Ris, 1911, which have also been recorded in other studies of altered areas [
25,
26,
32,
55,
56].
Physicochemical variables of water also play important roles in structuring the assemblages of aquatic insects. Changes in water parameters and habitat affect most aquatic organisms, including Odonata [
26]. The conductivity, salinity, and dissolved oxygen variables stand out in the structuring of Odonata assemblages (adults and larvae) and can serve as parameters to define the specific oviposition sites selected by adults and are closely associated with development from larvae stages of many Odonata species [
42,
57]. This is especially true for more sensitive species that depend on more pristine environments and more particular habitat conditions for their development. The water in more preserved environments has higher dissolved oxygen concentrations [
58], which can help ensure the permanence of more sensitive species such as forest specialists. These forest specialists include
Heteragrion aurantiacum Selys, 1862;
Forcepsioneura serrabonita Pinto & Kompier, 2018; and
Perilestes fragilis Hagen in Selys, 1862; as well as species of the genera
Heteragrion,
Peruviogomphus, and
Agriogomphus [
26,
56,
59,
60,
61,
62].
Assemblage structuring between the sampling sites was also associated with the axes of spatial analysis. These results suggest that the spatial distance between the sampling points plays a critical role in the structuring of the assemblages in the cocoa cultivation sites. Areas of the same region have more homogeneous assemblages owing to the dispersal capacity of most of the sampled Odonata adults. The species
Acanthagrion aepiolum Tennessen, 2004;
Erythrodiplax fusca Rambur, 1842; and
Hetaerina rosea Selys, 1853, which are open-area specialists (the first two) and habitat generalists (the third species), have also been associated with spatial filters. More generalist or open-area specialist species or species with greater dispersal capabilities are usually associated with spatial filters [
26,
33,
51,
63,
64,
65].
In general, cabruca areas are favorable environments for the conservation of habitat structure and for the colonization of more sensitive species. Moreover, such areas are considered to have sustainable agricultural cultivation, as revealed in several studies with other groups such as mammals, birds, and insects (dragonflies) [
12,
14,
66]. However, few studies have related these areas to invertebrates in general and especially to aquatic invertebrates. In short, the results of this study emphasize that: (1) The cabruca areas manage to maintain a high diversity of Odonata and several species generally associated with more pristine areas. (2) The environmental and spatial variables are determinants in the structuring of Odonata assemblages in cabruca areas. More preserved areas or areas with higher amounts of forest cover maintain assemblages of species that are more sensitive to human impacts, whereas areas with lower amounts of forest cover have Odonata assemblages with more habitat generalist or open-area specialist species. (3) The relationship of the species with the amount of forest cover in the cabruca areas is reflected in the relative abundance of the species collected in the different sampled sites. From this perspective, the present study highlights that this cultivation system may be supporting the conservation of Odonata biodiversity in Atlantic Forest areas. Therefore, it is critical to ensure the integrity of these areas to maintain groups of more sensitive species. Notably, in Brazil, the recently approved Law 14,119 of 13 January 2021 establishes the National Policy on Payment for Environmental Services, which encourages recovery and recomposition through the planting of native species or by agroforestry systems. This law can further strengthen the relevance of cabruca areas as sites able to maintain large species diversity and as areas of economic, environmental, and social importance.