Branch Growth, Leaf Canopies and Photosynthetic Responses of Zizyphus jujube cv. “Huizao” to Nutrient Addition in the Arid Areas of Northwest China †
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of the Test Site
2.2. Test Materials and Design
2.3. Determination Method
2.3.1. Determination of Growth Indicators
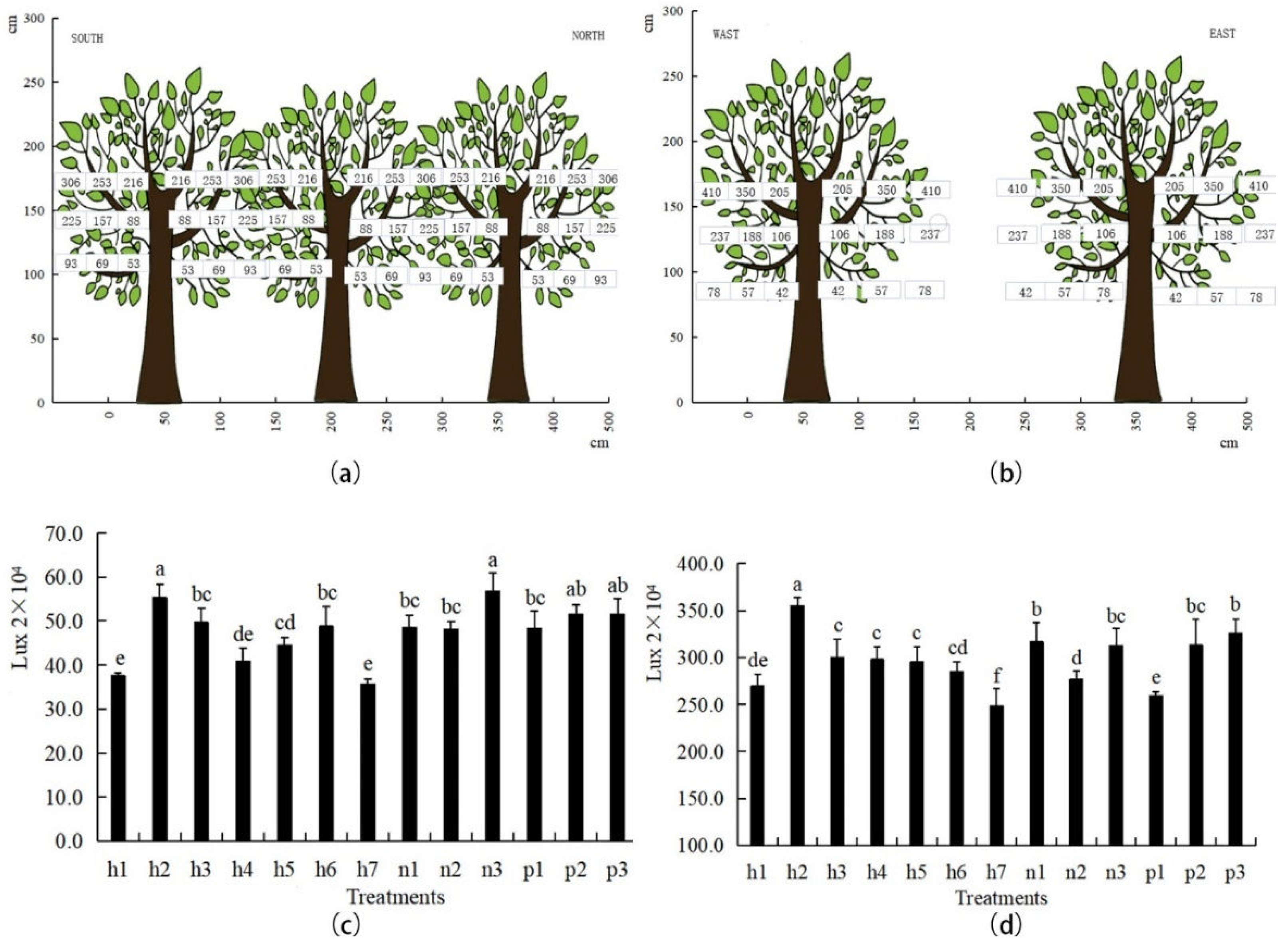
2.3.2. Determination of the Tree Canopy and Light Intensity
2.3.3. Determination of Photosynthetic Traits
2.3.4. Determination of the Soil and Plant Analyzer Development (SPAD) Value of the Leaves
2.4. Data Processing
3. Results
3.1. Effects of Different Fertilization Treatments on the Growth Indices of the Fruit-Bearing Shoot in Ziziphus jujuba
3.2. Effects of Different Fertilization Treatments on the Light Interception Ability of Jujube Trees
3.3. Light Distribution Characteristics
3.4. Effect of Different Fertilization Treatments on the SPAD Value of the Jujube Leaves
3.5. Effects of Different Fertilization Treatments on the Jujube Leaf’s Photosynthetic Traits
3.6. Correlation Analysis of the Physiological Growth Indices of the Jujube Leaves
3.7. Principal Component Analysis of the Physiological Growth Indexes of Jujube Trees
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LG | length growth |
| DG | diameter growth |
| Leaves | leaf number |
| LAI | leaf area index |
| MLA | mean leaf angle |
| TC | transmission coefficient |
| ICLI | jujube tree interior canopy light intensity |
| ECLI | jujube tree external canopy light intensity |
| SPAD | soil and plant analyzer development value |
| Pn | net photosynthetic rate |
| Gs | stomatal conductance |
| Ci | intercellular CO2 concentration |
| Tr | transpiration rate |
| WUE | water use efficiency |
References
- Guo, Y.X.; Dan, G.H. Chinese Jujube; Shanghai Science and Technology Press: Shanghai, China, 2010. [Google Scholar]
- Liu, M.J.; Zhao, Z.H. Germplasm resources and production of jujube in China. Int. Jujube Symp. 2008, 840, 25–32. [Google Scholar] [CrossRef]
- Wang, X.; Shen, L.; Liu, T. Microclimate, yield, and income of a jujube–cotton agroforestry system in Xinjiang, China. Ind. Crops Prod. 2022, 182, 114941. [Google Scholar] [CrossRef]
- Kuang, L.X.; Nie, J.Y.; Li, Z.X.; Guan, D.K.; Wu, Y.L.; Yan, Z.; Cheng, Y. Factor analysis and cluster analysis of mineral elements contents in different apple varieties. Sci. Agric. Sin. 2017, 50, 2807–2815. [Google Scholar]
- Zhang, H.; Zhao, Q.; Wang, Z.; Wang, L.; Li, X.; Fan, Z.; Zhang, Y.; Li, J.; Gao, X.; Shi, J.; et al. Effects of nitrogen fertilizer on photosynthetic characteristics, biomass, and yield of wheat under different shading conditions. Agronomy 2021, 11, 1989. [Google Scholar] [CrossRef]
- Zhang, X.W.; Bai, M.D.; Hao, G.W.; Fu, C.B.; Yang, S.; Wang, Y.P.; Gao, P.; Guo, H.P. Analysis on the accumulation of dry matter, nitrogen, phosphorus and potassium in the growth period of yuluxiang pear. Shanxi Agric. Sci. 2020, 48, 1758–1762. [Google Scholar]
- Lai, Y.; Tong, Y.N.; Chen, L.L.; Gao, Y.M.; Yang, J.F. Effect of fertilization on kiwifruit yield and quality. Northwest Sci-Tech Univ. Agric. For. (Nat. Sci. Ed.) 2011, 39, 171–176. [Google Scholar]
- Zhang, M.H.; Sun, D.Y.; Niu, Z.R.; Yan, J.X.; Zhou, X.L.; Kang, X. Effects of combined organic/inorganic fertilizer application on growth, photosynthetic characteristics, yield and fruit quality of Actinidia chinesis cv ‘Hongyang’. Global Ecol. Biogeogr. 2020, 22, e00997. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, S.; Mao, J. Effects of shading on the synthesis of volatile organic compounds in ‘Marselan’ grape berries (Vitis vinifera L.). J. Plant Growth Regul. 2021, 40, 679–693. [Google Scholar] [CrossRef]
- Newete, S.W.; Abutaleb, K.; Byrne, M.J. Mapping the distribution and tree canopy cover of jacaranda mimosifolia and platanus× acerifolia in johannesburg’s urban forest. Sci. Rep. 2022, 12, 5998. [Google Scholar] [CrossRef]
- Mirheidari, F.; Khadivi, A.; Saeidifar, A. Selection of superior genotypes of Indian jujube (Ziziphus mauritiana Lamk.) as revealed by fruit-related traits. Food Sci. Nutr. 2022, 10, 903–913. [Google Scholar] [CrossRef]
- Wang, C.; She, H.Z.; Liu, X.B. Effects of fertilization on leaf photosynthetic characteristics and grain yield in tartary buckwheat Yunqiao1. Photosynthetica 2017, 55, 77–84. [Google Scholar] [CrossRef]
- Ye, S.L.; Liu, T.C.; Niu, Y. Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi. Open Chem. 2020, 18, 537–545. [Google Scholar] [CrossRef]
- Erisman, J.W.; Bleeker, A.; Galloway, J. Reduced nitrogen in ecology and the environment. Environ. Pollut. 2007, 150, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Yuan, H.X.; Liu, Y.; Li, J.; Zheng, J.Y.; Sun, S.; Xing, G.M. Photosynthetic responses of tomato to different concentrations of CO2 enrichment in greenhouse. Fruit. Sci. 2018, 24, 1010–1018. [Google Scholar]
- Giles, J. Nitrogen study fertilizes fears of pollution. Nature 2005, 433, 791–792. [Google Scholar] [CrossRef]
- Oikawa, S.; Ainswort, E.A. Changes in leaf area, nitrogen content and canopy photosynthesis in soybean exposed to an ozone concentration gradient. Environ. Pollut. 2016, 215, 347–355. [Google Scholar] [CrossRef]
- Wei, T.B.; Chai, Q.; Wang, W.M.; Wang, J.Q. Effects of coupling of irrigation and nitrogen application as well as planting density on photosynthesis and dry matter accumulation characteristics of maize in oasis irrigated areas. Sci. Agric. Sin. 2019, 52, 428–444. [Google Scholar]
- Niklas, K.J.; Owens, T.; Reich, P.B.; Cobb, E.D. Nitrogen/phosphorus leaf stoichiometry and the scaling of plant growth. Ecol. Lett. 2005, 8, 636–642. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Cai, Z.Q.; Liu, G.Z. Effects of fertilization on the growth, photosynthesis, and biomass accumulation in juvenile plants of three coffee (Coffea arabica L.) cultivars. Photosynthetica 2017, 55, 134–143. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Wu, Z.B.; Shi, Y.J. Effects of different fertilization treatments on photosynthetic characteristics and yield of Jun jujube. Agric. Res. Arid. Areas 2015, 33, 83–87. [Google Scholar]
- Liu, Y.Z.; Zhu, J.H.; Su, C.W.; Liu, D.H.; Li, J.Z. Photosynthetic efficiency and its seasonal changes of peach trees with different growth types. Agric. Res. Appl. 2016, 36, 1836–1845. [Google Scholar]
- Shi, Y.; Bai, M.; Li, Y. Evaluation index of winter wheat water use efficiency analysis based on DSSAT simulation. Water Sav. Irrig. 2021, 09, 1–6,11. [Google Scholar] [CrossRef]
- Nielsen, G.H.; Nielsen, D.; Herbert, L.C. Response of apple to fertigation of N and K under conditions susceptible to the development of K deficiency. J. Am. Soc. Hortic. Sci. 2004, 129, 26–31. [Google Scholar] [CrossRef]
- Nasar, J.; Khan, W.; Khan, M.Z. Photosynthetic activities and photosynthetic nitrogen use efficiency of maize crop under different planting patterns and nitrogen fertilization. J. Soil Sci. Plant Nutr. 2021, 21, 2274–2284. [Google Scholar] [CrossRef]
- Kou, R.; Jia, S.H.; Bai, Y.H.; Zhao, X.; Ma, Y.; Cao, R.; Xu, C.J.; Zheng, Z.Q.; Gao, F.; Yang, S.R. Effects of different nitrogen fertilizer levels on jujube growth indexes and leaf spad values under drip irrigation. China Rural. Water Hydropower 2021, 08, 123–126. [Google Scholar]
- Guo, J.H.; Liu, X.J.; Zhang, Y. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Lu, Y.L.; Yu, X.L.; Li, R.L. Effects of different potassium fertilizer application periods on the yield and quality of Fuji apple. Chin. J. Appl. Ecol. 2015, 26, 1179–1185. [Google Scholar]
- Kang, L.Y.; Chang, G.Z.; Gao, N.N. Effects of different nitrogen and potassium fertilizing amount on nutrition absorption, nutrition distribution and yield of muskmelon. Sci. Agric. Sin. 2018, 51, 1758–1770. [Google Scholar]
- Salman, S.R.; Abou-Hussein, S.D.; Abdel-Mawgoud, A.M.R. Fruit yield and quality of watermelon as affected by hybrids and humic acid application. J. Appl. Sci. Res. 2005, 1, 51–58. [Google Scholar]
- Ma, Y.L.; Guo, S.J. The relationship between leaf microenvironment and branches and leaves growth and fruit yield of chestnut. J. Cent. S. Univ. For. Technol. 2021, 41, 47–57. [Google Scholar]
- Xu, D.Q.; Gao, W.; Ruan, J. Effects of light quality on plant growth and development. Acta Phytophysiol. Sin. 2015, 51, 1217–1234. [Google Scholar]
- Brooke, J.M.; Basinger, P.S.; Birckhead, J.L. Effects of fertilization and crown release on white oak (Quercus alba) masting and acorn quality. For. Ecol. Manag. 2019, 433, 305–312. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Peng, S.L. Effects of warming on the biomass allocation and allometric growth of the invasive shrub Lantana camara. Acta Ecol. Sin. 2018, 38, 6670–6676. [Google Scholar]
- Huang, Z.; Zhong, Q.P.; Cao, L.Q.; Guo, H.Y.; Yan, C.; Yuan, T.T.; Yuan, Y.Q.; Luo, S. Effects of drought stress on photosynthesis of adult Camellia oleifera forest. Nonwood For. Res. 2017, 35, 72–79. [Google Scholar]
- Niu, R.X.; Zhao, X.M.; Wang, C.B. Effects of canopy characteristics on fruit yield and quality with different training systems in nectarines. J. Fruit. Sci. 2019, 36, 1667–1674. [Google Scholar]
- Zhang, H.P.; Ma, J.; Wen, J.; Zhou, T.H. Effects of potassium application on the photosynthetic characteristics, yield, and fiber properties of different transgenic cotton varieties. Cott. Sci. 2012, 24, 548–553. [Google Scholar]
- Wu, C.Y.; Jiang, H.; Li, T.H.; Yu, J. Effect of soil potassium fertilization on leaf photosynthesis and fruit quality of jujube. China Soils Fert. 2018, 276, 105–112. [Google Scholar]
- Croce, R.; Amerongen, V.H. Natural strategies for photosynthetic light harvesting. Nat. Chem. Biol. 2014, 10, 492–501. [Google Scholar] [CrossRef]
- Nava Pour, S.; Morris, K.; Allen, R. Expression of senescence-enhanced genes in response to oxidative stress. J. Exp. Bot. 2003, 54, 2285–2292. [Google Scholar] [CrossRef]
- Li, J.; Ma, J.; Fan, S.; Mi, S.; Zhang, Y. Comparison of the nutritional and taste characteristics of 5 edible fungus powders based on the composition of hydrolyzed amino acids and free amino acids. J. Food Qual. 2022, 2022, 3618002. [Google Scholar] [CrossRef]
- Wang, Q.; Li, X.; Chen, H. Mapping combined with principal component analysis identifies excellent lines with increased rice quality. Sci. Rep. 2022, 12, 5969. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Shan, Y.; Li, P. Nondestructive characterization of citrus fruit by near-infrared diffuse reflectance spectroscopy (NIRDRS) with principal component analysis (PCA) and fisher linear discriminant analysis (FLDA). Anal. Lett. 2022, 55, 2554–2563. [Google Scholar] [CrossRef]


| Treatment (Code) | Group of Single Fertilization Amount (g·Tree) | |||
|---|---|---|---|---|
| CH4N2O | P2O5 | K2O | ||
| 1 | h1 | 564.12 | 326.08 | 588.23 |
| 2 | h2 | 460.77 | 489.13 | 588.23 |
| 3 | h3 | 396.97 | 652.17 | 588.23 |
| 4 | h4 | 524.57 | 326.08 | 588.23 |
| 5 | h5 | 524.57 | 326.08 | 1029.41 |
| 6 | h6 | 524.57 | 326.08 | 1176.46 |
| 7 | h7 | 524.57 | 326.08 | 441.17 |
| 8 | n1 | 326.08 | ||
| 9 | n2 | 652.17 | ||
| 10 | n3 | 978.26 | ||
| 11 | p1 | 326.08 | ||
| 12 | p2 | 652.17 | ||
| 13 | p3 | 978.26 | ||
| 14 | k1 | 294.12 | ||
| 15 | k2 | 588.23 | ||
| 16 | k3 | 882.35 | ||
| Treatments | Length Growth (LG) (cm) | Diameter Growth (DG) (cm) | Leaf Number (Leaves) (Leaf) |
|---|---|---|---|
| h1 | 2.96 ± 0.38 bc | 0.49 ± 0.01 bc | 14.07 ± 1.03 a |
| h2 | 2.24 ± 0.55 bcd | 0.22 ± 0.04 e | 13.47 ± 1.17 ab |
| h3 | 1.86 ± 0.31 d | 0.36 ± 0.09 d | 13.60 ± 1.06 ab |
| h4 | 1.90 ± 0.67 cd | 0.49 ± 0.14 bc | 12.80 ± 0.92 abc |
| h5 | 1.91 ± 0.77 cd | 0.46 ± 0.07 c | 12.73 ± 2.04 abc |
| h6 | 1.64 ± 0.44 d | 0.37 ± 0.08 d | 10.45 ± 0.73 c |
| h7 | 2.65 ± 1.06 bcd | 0.45 ± 0.03 c | 13.07 ± 2.34 ab |
| n1 | 3.47 ± 0.92 a | 0.51 ± 0.05 abc | 12.20 ± 0.8 abc |
| n2 | 2.68 ± 0.49 bcd | 0.63 ± 0.14 ab | 13.20 ± 1.25 ab |
| n3 | 1.97 ± 0.12 cd | 0.46 ± 0.04 c | 12.30 ± 1.51 abc |
| p1 | 2.65 ± 0.41 bcd | 0.66 ± 0.07 a | 11.50 ± 0.95 bc |
| p2 | 2.38 ± 0.23 bcd | 0.59 ± 0.18 abc | 12.50 ± 1.48 abc |
| p3 | 2.19 ± 0.45 bcd | 0.55 ± 0.09 abc | 13.07 ± 0.91 ab |
| k1 | 3.08 ± 0.36 b | 0.57 ± 0.13 abc | 11.73 ± 1.01 bc |
| k2 | 2.08 ± 0.24 bcd | 0.48 ± 0.14 bc | 13.53 ± 0.76 ab |
| k3 | 1.78 ± 0.38 d | 0.47 ± 0.05 c | 11.53 ± 1.91 bc |
| Treatments | Leaf Area Index (LAI) | Mean Leaf Angle (MLA) | Transmission Coefficient (TC) |
|---|---|---|---|
| h1 | 1.28 ± 0.14 a | 27.53 ± 6.55 a | 0.33 ± 0.05 bc |
| h2 | 0.94 ± 0.12 d | 33.32 ± 6.08 a | 0.40 ± 0.05 ab |
| h3 | 1.04 ± 0.16 bcd | 30.08 ± 8.18 a | 0.36 ± 0.07 abc |
| h4 | 1.23 ± 0.21 abc | 29.51 ± 0.65 a | 0.36 ± 0.01 abc |
| h5 | 1.12 ± 0.05 abcd | 28.62 ± 1.91 a | 0.35 ± 0.02 bc |
| h6 | 1.01 ± 0.19 cd | 30.55 ± 5.99 a | 0.37 ± 0.05 abc |
| h7 | 1.26 ± 0.04 ab | 25.63 ± 0.12 a | 0.31 ± 0.01 c |
| n1 | 1.06 ± 0.03 abcd | 29.87 ± 1.64 a | 0.36 ± 0.02 abc |
| n2 | 1.12 ± 0.01 abcd | 28.42 ± 0.52 a | 0.33 ± 0.01 c |
| n3 | 0.97 ± 0.025 d | 35.22 ± 2.37 a | 0.42 ± 0.02 a |
| p1 | 1.03 ± 0.09 cd | 31.37 ± 4.83 a | 0.37 ± 0.04 abc |
| p2 | 0.97 ± 0.12 d | 31.25 ± 6.14 a | 0.38 ± 0.05 abc |
| p3 | 0.93 ± 0.13 d | 35.23 ± 0.26 a | 0.43 ± 0.01 a |
| Treatment | Pn (μmol·m−2·s−1) | Gs (mmol·m−2·s−1) | Ci (μmol·mol−1) | Tr (mmol·m−2·s−1) | WUE (μmol·mmol−1) |
|---|---|---|---|---|---|
| h1 | 9.06 ± 0.02 e | 96.8 ± 0.11 de | 292.01 ± 2.01 c | 3.90 ± 0.23 bc | 2.28 ± 0.13 cd |
| h2 | 10.06 ± 0.83 d | 115 ± 1.88 c | 232.52 ± 3.47 d | 4.20 ± 0.34 ab | 2.35 ± 0.32 cd |
| h3 | 9.56 ± 0.62 de | 132.6 ± 1.05 bc | 301.82 ± 14.26 bc | 4.62 ± 0.33 a | 2.07 ± 0.06 e |
| h4 | 8.07 ± 0.28 f | 57.7 ± 0.53 f | 313.89 ± 9.55 b | 3.81 ± 0.12 cd | 2.15 ± 0.07 de |
| h5 | 8.62 ± 0.51 ef | 52.3 ± 0.37 fg | 242.58 ± 3.52 d | 3.76 ± 0.33 cd | 2.29 ± 0.13 cd |
| h6 | 7.34 ± 0.29 j | 56.1 ± 0.28 f | 400.22 ± 6.03 a | 3.46 ± 0.47 de | 2.15 ± 0.28 de |
| h7 | 10.50 ± 0.06 cd | 83.4 ± 0.29 e | 196.82 ± 5.68 de | 4.06 ± 0.25 bc | 2.59 ± 0.16 c |
| n1 | 11.54 ± 0.72 b | 153.4 ± 0.02 b | 189.18 ± 0.17 e | 4.06 ± 0.14 bc | 2.85 ± 0.28 ab |
| n2 | 12.76 ± 0.14 a | 186.4 ± 0.01 a | 182.27 ± 0.37 e | 4.27 ± 0.15 ab | 2.98 ± 0.08 a |
| n3 | 11.10 ± 0.49 bc | 93.6 ± 0.01 d | 218.24 ± 2.97 de | 4.01 ± 0.11 cd | 2.77 ± 0.16 ab |
| p1 | 9.47 ± 0.37 e | 32.5 ± 0.28 h | 140.46 ± 2.22 f | 3.63 ± 0.29 de | 2.61 ± 0.11 bc |
| p2 | 9.68 ± 0.17 de | 65.4 ± 0.50 f | 240.77 ± 13.82 d | 3.38 ± 0.48 e | 2.92 ± 0.48 ab |
| p3 | 10.42 ± 0.33 cd | 102.7 ± 0.05 cd | 268.51 ± 9.82 cd | 3.78 ± 0.23 cd | 2.76 ± 0.13 ab |
| Total Variance Explained | ||||||
|---|---|---|---|---|---|---|
| Component | Initial Eigenvalue | Extract the Load Sum of Squares | ||||
| Total | Variance (%) | Accumulation (%) | Total | Variance (%) | Accumulation (%) | |
| 1 | 4.597 | 35.360 | 35.360 | 4.597 | 35.360 | 35.360 |
| 2 | 3.865 | 29.727 | 65.087 | 3.865 | 29.727 | 65.087 |
| 3 | 2.224 | 17.105 | 82.192 | 2.224 | 17.105 | 82.192 |
| 4 | 0.791 | 6.084 | 88.275 | |||
| Treatment | PC1 | PC2 | PC3 | F | Ranking |
|---|---|---|---|---|---|
| h1 | 0.23 | 0.74 | −2.87 | −0.19 | 7 |
| h2 | 2.26 | −1.09 | 2.53 | 0.91 | 1 |
| h3 | 2.78 | −0.72 | −0.32 | 0.72 | 3 |
| h4 | 0.23 | −1.02 | −1.03 | −0.39 | 9 |
| h5 | −0.55 | −0.78 | −1.56 | −0.69 | 10 |
| h6 | 0.31 | −3.43 | −1.49 | −1.16 | 13 |
| h7 | −0.25 | 1.17 | −3.06 | −0.26 | 8 |
| n1 | 0.19 | 1.39 | 1.38 | 0.71 | 4 |
| n2 | 0.90 | 1.44 | 0.54 | 0.84 | 2 |
| n3 | −0.55 | 0.18 | 3.58 | 0.47 | 5 |
| p1 | −2.72 | −0.06 | −0.13 | −1.00 | 12 |
| p2 | −1.99 | −0.92 | 0.91 | −0.82 | 11 |
| p3 | −0.86 | −0.89 | 2.53 | −0.14 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, J.; Li, J.; Wang, G.; Tang, Z.; Zhi, J. Branch Growth, Leaf Canopies and Photosynthetic Responses of Zizyphus jujube cv. “Huizao” to Nutrient Addition in the Arid Areas of Northwest China. Diversity 2022, 14, 914. https://doi.org/10.3390/d14110914
Bao J, Li J, Wang G, Tang Z, Zhi J. Branch Growth, Leaf Canopies and Photosynthetic Responses of Zizyphus jujube cv. “Huizao” to Nutrient Addition in the Arid Areas of Northwest China. Diversity. 2022; 14(11):914. https://doi.org/10.3390/d14110914
Chicago/Turabian StyleBao, Jianping, Jiaxin Li, Guanli Wang, Zhihui Tang, and Jinhu Zhi. 2022. "Branch Growth, Leaf Canopies and Photosynthetic Responses of Zizyphus jujube cv. “Huizao” to Nutrient Addition in the Arid Areas of Northwest China" Diversity 14, no. 11: 914. https://doi.org/10.3390/d14110914
APA StyleBao, J., Li, J., Wang, G., Tang, Z., & Zhi, J. (2022). Branch Growth, Leaf Canopies and Photosynthetic Responses of Zizyphus jujube cv. “Huizao” to Nutrient Addition in the Arid Areas of Northwest China. Diversity, 14(11), 914. https://doi.org/10.3390/d14110914






