Phenotypic Diversity Analysis of the Progeny Variation of a ‘Mosaic Leaf’ Loropetalum chinense var. rubrum Based on Flower Organ Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Measurement of Phenotypic Traits of Flower Organs
2.3. Determination of L*, a* and b* Parameters of CIELab Chromaticity System
2.4. Determination of Name of Flower Color Based on ISCC-NBS Method
2.5. Distribution and Content Determination of Anthocyanins in Flowers
2.6. Statistical Analysis
3. Results
3.1. Phenotypic Analysis of Flower Organs in L. chinense var. rubrum
3.1.1. Analysis of Genetic Diversity of Phenotypic Traits in Floral Organs
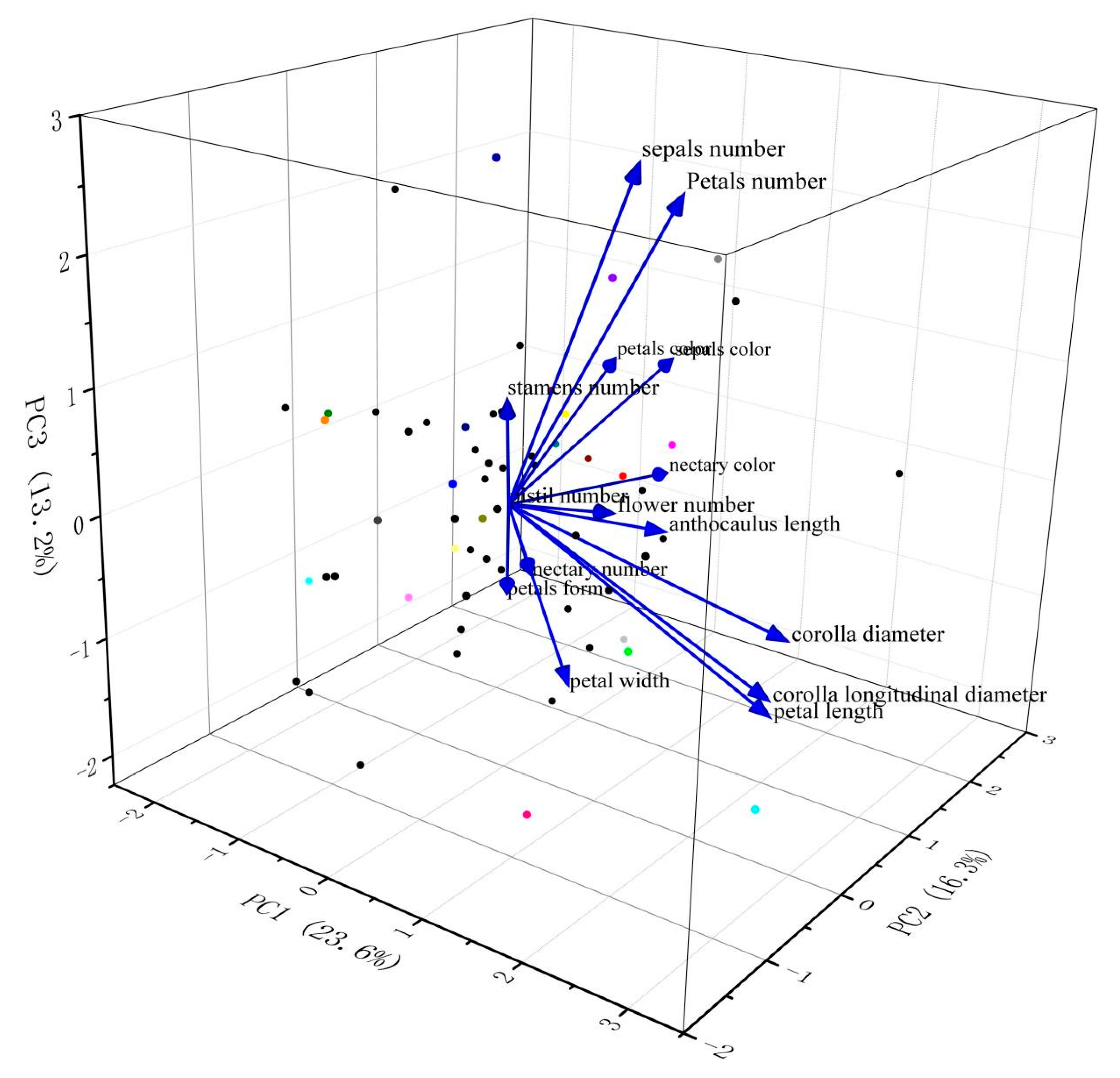
3.1.2. Principal Component Analysis Based on Phenotypic Traits
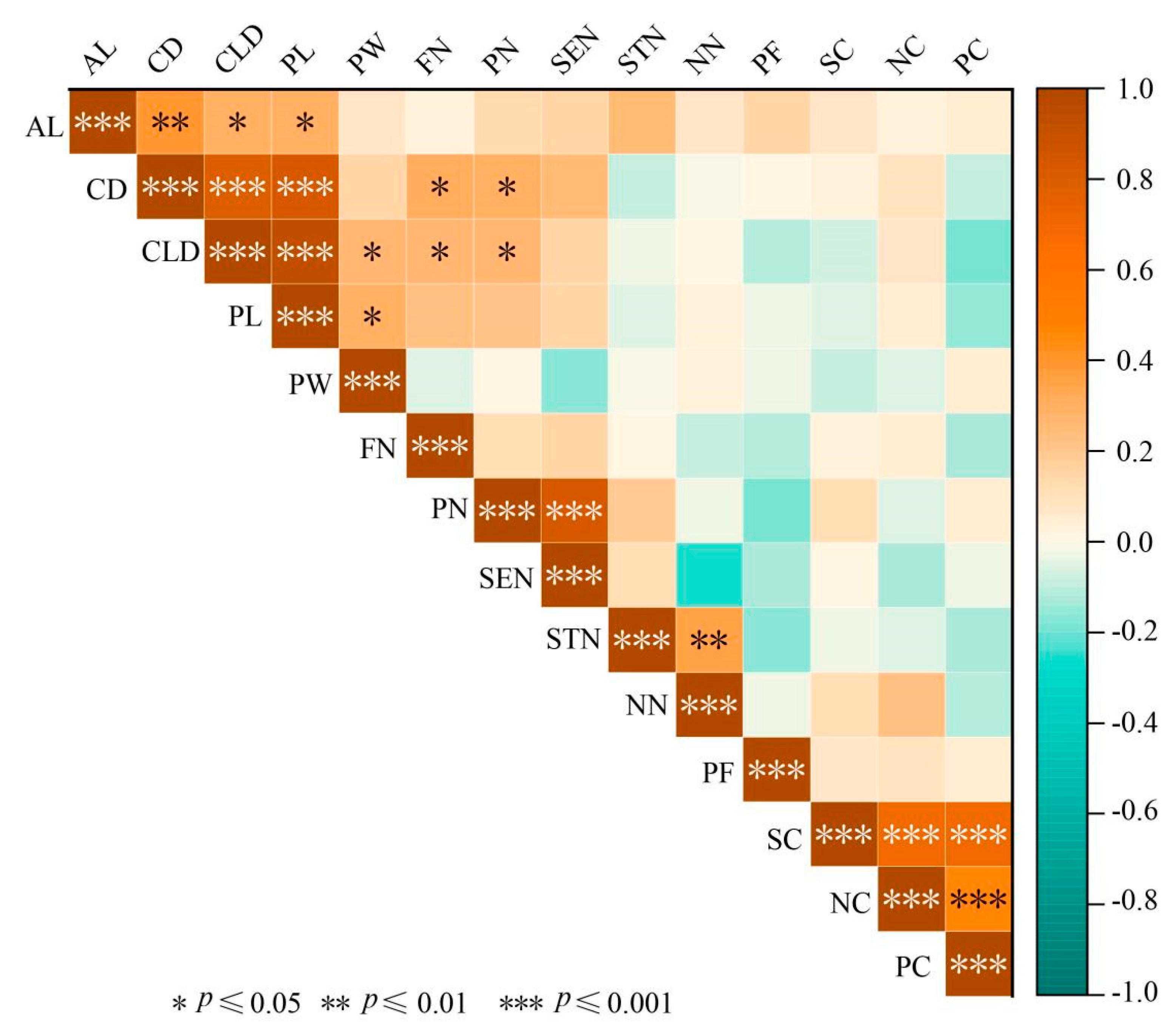
3.1.3. Correlation Analysis of Phenotypic Traits in Flower Organs
3.2. Classification of Flower Color Phenotypes of L. chinense var. rubrum
3.2.1. Flower Color Naming and Classification Based on ISCC-NBS Method
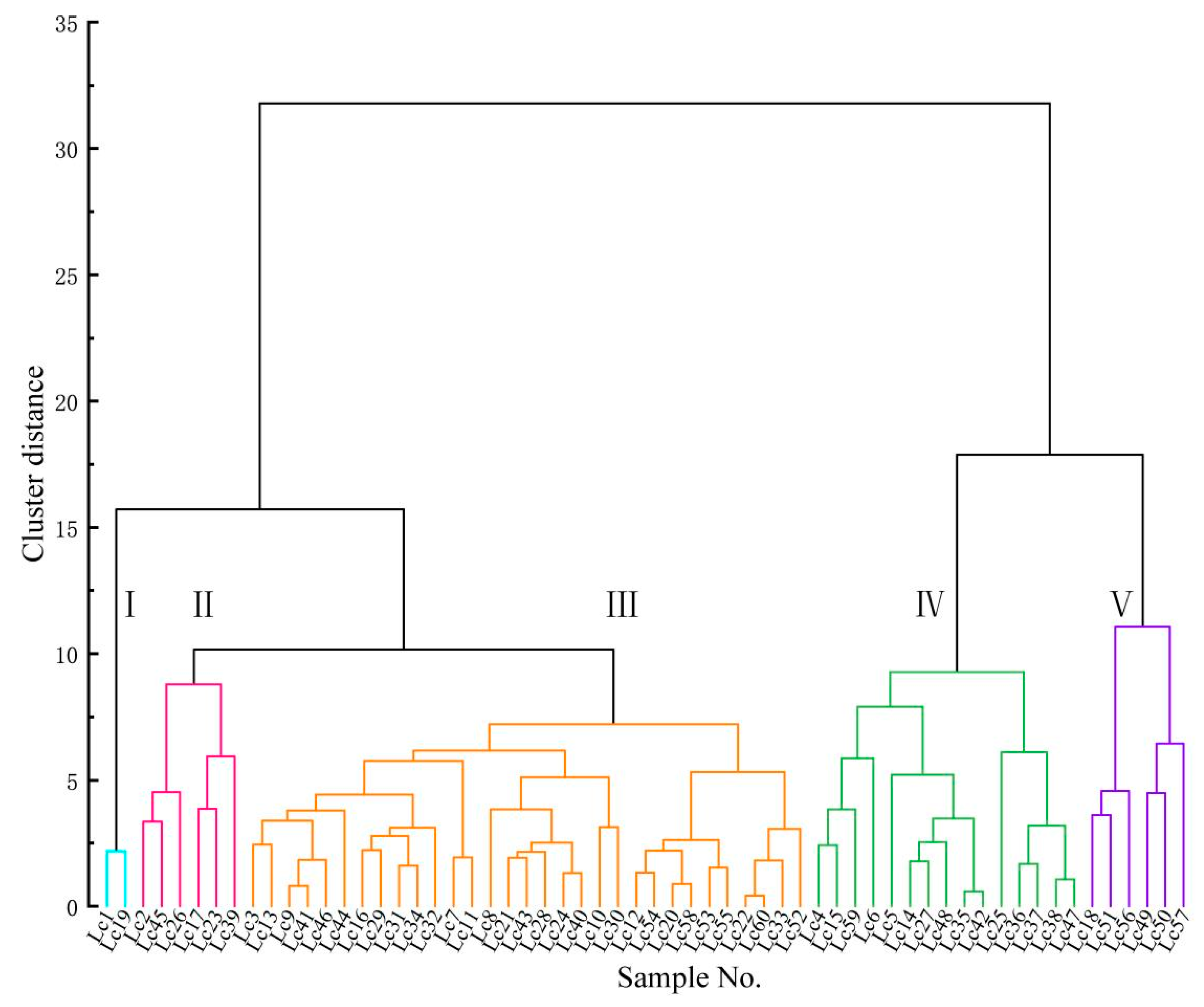
3.2.2. Flower Color Classification Based on CIELab Data Clustering Analysis
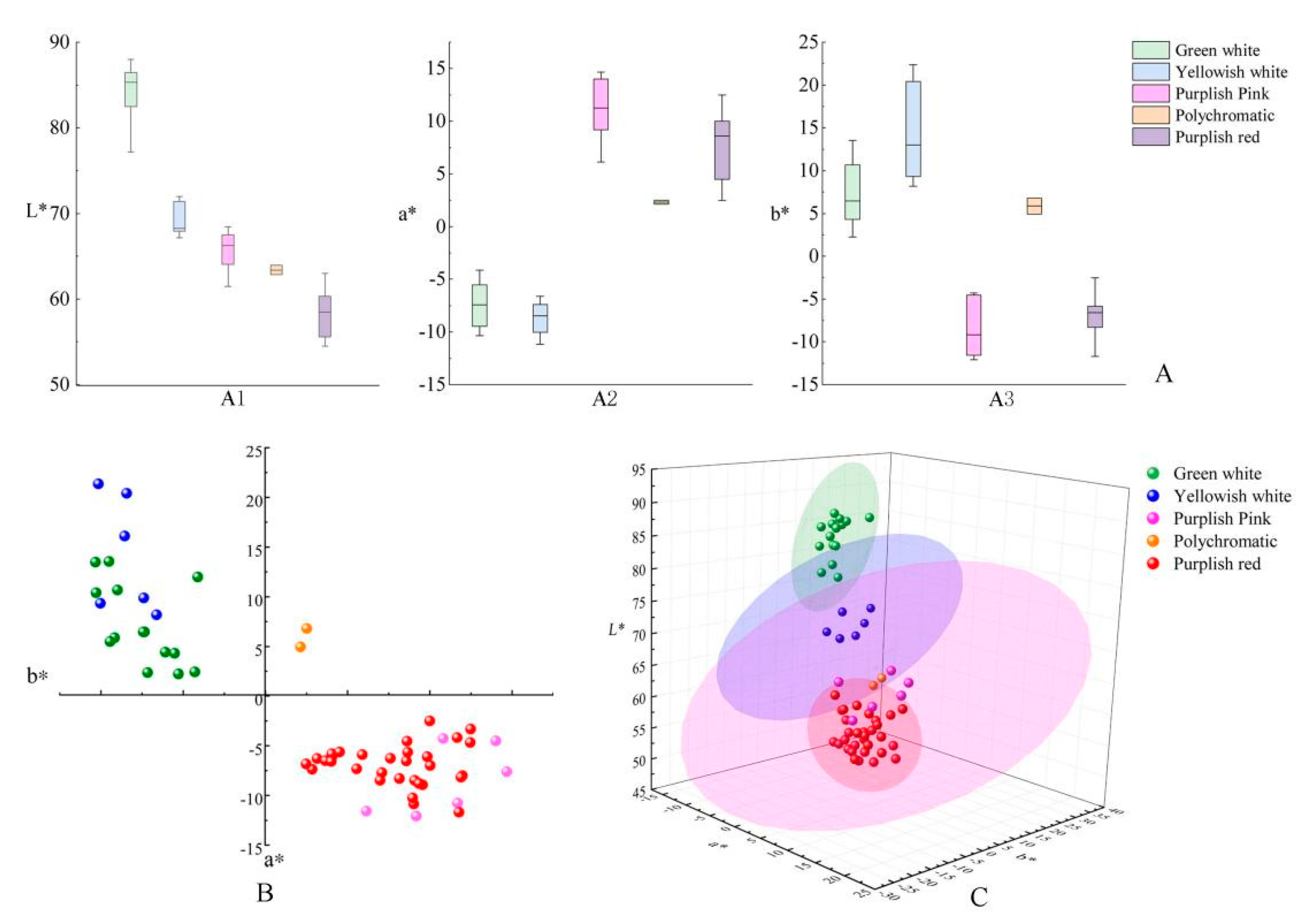
3.2.3. Flower Color Classification Based on L*, a* and b* Parameter Feature Analysis
3.2.4. Observation of the Distribution of Anthocyanins in Petals
3.2.5. Observation of the Distribution of Anthocyanins in Calyx and Nectary
4. Discussion
4.1. Diversity Analysis of Flower Organ Phenotypic Traits of L. chinense var. rubrum
4.2. Taxonomic Analysis of Flower Color Phenotype of L. chinense var. rubrum
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Plant Code | CIELab Color Coordinate | Munsell Color System | Color Group According to CIELab | |||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | C* | H | V | C | ||
| Lc1 | 62.85 | 2.14 | 4.95 | 5.39 | 7.45YR | 6.18 | 0.87 | Polychromatic |
| Lc19 | 63.95 | 2.52 | 6.79 | 7.24 | 8.08YR | 6.29 | 1.15 | Polychromatic |
| Lc4 | 78.30 | −9.16 | 5.87 | 10.88 | 1.60G | 7.77 | 1.5 | Green-white |
| Lc14 | 86.46 | −4.28 | 2.43 | 4.92 | 2.44G | 8.61 | 0.64 | Green-white |
| Lc27 | 85.00 | −5.28 | 2.23 | 5.73 | 4.59G | 8.46 | 0.79 | Green-white |
| Lc35 | 83.34 | −5.51 | 4.31 | 6.99 | 0.39G | 8.29 | 0.89 | Green-white |
| Lc42 | 83.36 | −6.07 | 4.43 | 7.52 | 0.83G | 8.29 | 0.97 | Green-white |
| Lc48 | 86.15 | −7.15 | 2.37 | 7.53 | 6.40G | 8.58 | 1.07 | Green-white |
| Lc59 | 82.51 | −9.44 | 5.49 | 10.92 | 2.25G | 8.2 | 1.48 | Green-white |
| Lc5 | 88.00 | −7.35 | 6.47 | 9.79 | 9.70GY | 8.77 | 1.19 | Yellowish white |
| Lc6 | 77.19 | −8.99 | 10.66 | 13.94 | 7.55GY | 7.65 | 1.82 | Yellowish white |
| Lc15 | 79.87 | −7.42 | 6.45 | 9.83 | 9.66GY | 7.93 | 1.28 | Yellowish white |
| Lc25 | 87.53 | −4.11 | 11.98 | 12.67 | 1.24GY | 8.72 | 1.41 | Yellowish white |
| Lc36 | 86.65 | −8.99 | 10.66 | 13.94 | 7.74GY | 8.63 | 1.68 | Yellowish white |
| Lc37 | 85.62 | −10.29 | 10.41 | 14.64 | 8.79GY | 8.52 | 1.83 | Yellowish white |
| Lc38 | 85.30 | −10.33 | 13.47 | 16.98 | 7.18GY | 8.49 | 2.09 | Yellowish white |
| Lc47 | 85.99 | −9.51 | 13.55 | 16.56 | 6.73GY | 8.56 | 2 | Yellowish white |
| Lc18 | 68.13 | −10.03 | 9.34 | 13.71 | 9.01GY | 6.72 | 1.93 | Yellowish white |
| Lc49 | 68.40 | −11.15 | 22.35 | 24.98 | 4.35GY | 6.74 | 3.35 | Yellowish white |
| Lc50 | 71.39 | −8.43 | 20.40 | 22.07 | 2.63GY | 7.05 | 2.86 | Yellowish white |
| Lc51 | 67.93 | −6.61 | 8.17 | 10.51 | 7.04GY | 6.70 | 1.40 | Yellowish white |
| Lc56 | 71.96 | −7.39 | 9.88 | 12.33 | 6.73GY | 7.11 | 1.62 | Yellowish white |
| Lc57 | 67.16 | −8.55 | 16.11 | 18.24 | 4.72GY | 6.62 | 2.40 | Yellowish white |
| Lc2 | 67.50 | 14.01 | −4.53 | 14.72 | 1.80RP | 6.66 | 3.89 | Purplish Pink |
| Lc17 | 61.46 | 9.18 | −12.08 | 15.17 | 3.31P | 6.05 | 3.84 | Purplish Pink |
| Lc23 | 64.07 | 11.69 | −10.76 | 15.89 | 5.93P | 6.31 | 4.06 | Purplish Pink |
| Lc26 | 66.14 | 14.68 | −7.61 | 16.53 | 9.49P | 6.52 | 4.27 | Purplish Pink |
| Lc39 | 66.39 | 6.14 | −11.58 | 13.11 | 0.94P | 6.55 | 3.46 | Purplish Pink |
| Lc45 | 68.46 | 10.81 | −4.28 | 11.63 | 0.86RP | 6.76 | 3.17 | Purplish Pink |
| Lc3 | 56.83 | 11.98 | −8.00 | 14.41 | 7.80P | 5.58 | 3.58 | Purplish red |
| Lc7 | 55.59 | 12.46 | −4.67 | 13.30 | 1.46RP | 5.46 | 3.32 | Purplish red |
| Lc8 | 62.60 | 7.08 | −7.70 | 10.46 | 5.02P | 6.16 | 2.68 | Purplish red |
| Lc9 | 58.10 | 9.08 | −8.47 | 12.42 | 5.83P | 5.71 | 3.1 | Purplish red |
| Lc10 | 63.00 | 12.49 | −3.32 | 12.92 | 2.60RP | 6.2 | 3.37 | Purplish red |
| Lc11 | 57.29 | 11.66 | −4.18 | 12.39 | 1.62RP | 5.63 | 3.13 | Purplish red |
| Lc12 | 56.20 | 4.08 | −6.03 | 7.28 | 2.48P | 5.52 | 1.84 | Purplish red |
| Lc13 | 59.27 | 11.89 | −8.17 | 14.43 | 7.57P | 5.83 | 3.65 | Purplish red |
| Lc16 | 55.36 | 9.03 | −10.86 | 14.12 | 3.96P | 5.44 | 3.44 | Purplish red |
| Lc20 | 55.30 | 3.15 | −6.28 | 7.03 | 0.31P | 5.43 | 1.8 | Purplish red |
| Lc21 | 61.60 | 8.58 | −6.54 | 10.79 | 6.92P | 6.06 | 2.77 | Purplish red |
| Lc22 | 60.77 | 3.66 | −6.52 | 7.48 | 1.17P | 5.97 | 1.98 | Purplish red |
| Lc24 | 58.96 | 8.66 | −5.66 | 10.35 | 7.88P | 5.79 | 2.61 | Purplish red |
| Lc28 | 60.32 | 8.62 | −4.55 | 9.75 | 9.40P | 5.93 | 2.49 | Purplish red |
| Lc29 | 56.35 | 9.56 | −8.93 | 13.08 | 5.80P | 5.53 | 3.23 | Purplish red |
| Lc30 | 61.29 | 9.99 | −2.51 | 10.30 | 2.77RP | 6.03 | 2.67 | Purplish red |
| Lc31 | 54.47 | 8.15 | −8.29 | 11.63 | 5.25P | 5.35 | 2.85 | Purplish red |
| Lc32 | 54.66 | 10.02 | −6.98 | 12.21 | 7.54P | 5.37 | 3.02 | Purplish red |
| Lc33 | 59.26 | 4.04 | −5.82 | 7.08 | 2.79P | 5.82 | 1.82 | Purplish red |
| Lc34 | 55.57 | 6.98 | −8.47 | 10.98 | 3.97P | 5.46 | 2.71 | Purplish red |
| Lc40 | 58.45 | 7.61 | −6.26 | 9.85 | 6.53P | 5.74 | 2.48 | Purplish red |
| Lc41 | 58.79 | 9.34 | −8.79 | 12.83 | 5.78P | 5.78 | 3.21 | Purplish red |
| Lc43 | 60.20 | 9.84 | −6.10 | 11.58 | 8.25P | 5.92 | 2.95 | Purplish red |
| Lc44 | 58.47 | 11.78 | −11.69 | 16.60 | 5.42P | 5.75 | 4.09 | Purplish red |
| Lc46 | 59.26 | 8.96 | −10.24 | 13.61 | 4.49P | 5.83 | 3.39 | Purplish red |
| Lc52 | 63.01 | 2.85 | −7.38 | 7.91 | 0.17P | 6.18 | 2.22 | Purplish red |
| Lc53 | 55.87 | 5.89 | −5.91 | 8.34 | 5.41P | 5.48 | 2.08 | Purplish red |
| Lc54 | 57.39 | 4.51 | −5.62 | 7.20 | 3.92P | 5.64 | 1.81 | Purplish red |
| Lc55 | 55.37 | 5.55 | −7.32 | 9.19 | 3.31P | 5.43 | 2.28 | Purplish red |
| Lc58 | 55.52 | 2.48 | −6.82 | 7.26 | 1.45P | 5.43 | 1.97 | Purplish red |
| Lc60 | 61.00 | 4.01 | −6.58 | 7.71 | 1.80P | 6 | 2.02 | Purplish red |
References
- Creech, J.L. On the distribution of Loropetalum chinense. Am. Hortic. Mag. 1960, 39, 236. [Google Scholar]
- Tang, Q.R. Study on the Genetic Diversity among Loropetalum chinense var. rubrum Introductions and Changes of Physiology and Bioehemistry during Its Leaf Colour Transformation. Ph.D. Dissertation, Hunan Agricultural University, Changsha, China, 2001. [Google Scholar]
- Huang, R.K.; Yang, C.K.; He, Z.H.; LI, J.C.; Liu, Y.Q. Resources investigation of Loropetalum chinense var. rubrum. Hunan Agric. Sci. 1998, 4, 44–45. [Google Scholar]
- Shao, Z.X.; Hou, W.; Long, X.Z.; Yang, G.D.; Chen, C.H.; Zeng, X.J.; Hou, B.X.; Yu, G.F.; Wu, W.W. The formation and development of the geography symbol product Loropetalum chinense var.rubrum in Liuyang City. Hunan For. Sci. Technol. 2007, 2, 71–73. [Google Scholar]
- Liang, S.L.; Tian, H. “Panda in plants”-Loropetalum chinense var. rubrum. China Flower Hortic. 2003, 22, 40. [Google Scholar]
- Hou, B.X. The development and prospect of the characteristic flower industry of Loropetalum chinense var. rubrum. Technol. Mark. (Landsc. Eng.) 2006, 11, 40–43. [Google Scholar]
- Gawel, N.J.; Johnson, G.R.; Sauve, R. Identification of genetic diversity among Loropetalum chinense var. rubrum introductions. J. Environ. Hortic. 1996, 14, 38–41. [Google Scholar] [CrossRef]
- Wang, W.T. Preliminary study on annual cultivation and conservation of Loropetalum chinense var. rubrum. Hunan For. Sci. Technol. 2004, 31, 67–68. [Google Scholar]
- Hou, B.X.; Yu, G.F.; Song, Q.G.; Yi, A.Q. Cultivation and management techniques of Loropetalum chinense var. rubrum. Hunan For. Sci. Technol. 2003, 1, 34–35. [Google Scholar]
- Li, H.W.; Duan, Z.G.; Feng, J.T.; Liu, J.J. Characteristics and cultivation and management techniques of Loropetalum chinense var. rubrum. Agric. Dev. Equip. 2017, 8, 185. [Google Scholar]
- Tang, Q.R.; Chen, D.F.; Chen, Y.Y.; Zhang, H.Z.; Zhou, P.H. Changes of physiology and biochemistry during leaf color transformation in Loropetalum chinense var. rubrum. Sci. Silvae Sin. 2006, 2, 111–115. [Google Scholar]
- Li, Y.L. Studies on Genetic and Biological Characteristics of Flower Leaf Bud of Loropetalum chinense var. Rubrum. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2009. [Google Scholar]
- Chen, Q.R.; Cai, W.Q.; Zhang, X.; Zhang, D.M.; Li, W.D.; Xu, L.; Yu, X.Y. The comparative studies on phytochemicals of leaf coloration of Loropetalum chinense var. rubrum. Acta Hortic. Sin. 2021, 48, 1969–1982. [Google Scholar]
- Chen, X.L.; Chen, J.H.; Hou, B.X.; Chen, G. Comparative analysis on photosynthetic characteristics of Loropetalum chinense var. rubrum. J. Cent. South Univ. For. Technol. 2010, 30, 119–121. [Google Scholar]
- Zhang, Z.Z. Photosynthetic physiological characteristics of Loropetalum chinense var. rubrum and its application in landscape. Qinghai Sci. Technol. Agric. For. 2011, 2, 17–19. [Google Scholar]
- Liu, J.; Zhang, L.; Li, W.D.; Li, T.; Li, Y.L.; Yu, X.Y. Comparative analysis of photosynthetic physiological characteristics of several Loropetalum chinense lines with different flower colors. Non Wood For. Res. 2020, 38, 245–251. [Google Scholar]
- Wani, G.A.; Shah, M.A.; Tekeu, H.; Reshi, Z.A.; Atangana, A.R.; Khasa, D.P. Phenotypic variability and genetic diversity of phragmites australis in Quebec and Kashmir reveal contrasting population Structure. Plants 2020, 9, 1392. [Google Scholar] [CrossRef] [PubMed]
- Bigard, A.; Berhe, D.T.; Maoddi, E.; Sire, Y.; Boursiquot, J.M.; Ojeda, H.; Peros, J.P.; Doligez, A.; Romieu, C.; Torregrosa, L. Vitis vinifera L. fruit diversity to breed varieties anticipating climate changes. Front. Plant Sci. 2018, 9, 455. [Google Scholar] [CrossRef]
- Manco, R.; Basile, B.; Capuozzo, C.; Scognamiglio, P.; Forlani, M.; Rao, R.; Corrado, G. Molecular and phenotypic diversity of traditional european plum (Prunus domestica L.) germplasm of southern Italy. Sustainability 2019, 11, 4112. [Google Scholar] [CrossRef]
- Wei, G.M.; Wang, S.M.; Luo, L.S.; Leng, J.G.; Hou, Y.H. Phenotypic trait diversity of acorus calamus germplasm resources. Guizhou Agric. Sci. 2002, 50, 15–20. [Google Scholar]
- Zhang, B.B.; Cai, Z.X.; Shen, Z.J.; Yan, J.; Ma, R.J.; Yu, M.L. Diversity Analysis of phenotypic characters in germplasm resources of ornamental peaches. Sci. Agric. Sin. 2021, 54, 2406–2418. [Google Scholar]
- Duan, J.J.; Ling, Z.; Jia, M.L.; Song, Z.Q.; Zhang, C.; Cao, D.M. Phenotypic diversity analysis of wild resources of hemerocallis in Shanxi Province. J. Plant Resour. Environ. 2021, 30, 29–38. [Google Scholar]
- Chen, J.Y. Ornamental Plants (Diversity); Chen, J.Y., Ed.; Beijing Forestry University, Chinese Society of Horticulture: Beijing, China, Selected Essays by Professor Chen Junyu; China Agricultural Science and Technology Press: Beijing, China, 1997; pp. 73–77. [Google Scholar]
- Wang, J.; Xu, L.F.; Wang, L.; Qi, X.Y.; Song, M.; Cao, Y.W.; He, G.R.; Tang, Y.C.; Yang, P.P.; Ming, J. The numerical classification of flower color phenotype in lily. Acta Hortic. Sin. 2022, 49, 571–580. [Google Scholar]
- Liu, Y.P.; Wu, F.F.; He, D.; Zhuang, Y.; Kong, D.Z. Numerical classification of lotus cultivars based on flower color phenotype. J. Zhejiang Univ. (Agric. Life Sci.) 2020, 46, 319–326. [Google Scholar]
- Wang, C.; Rong, L.Q.; Feng, L.J.; Liu, H.Q. Preliminary study of mechanism on diversity of floral organ variants in Anemone rivularis var. flore-minore. Acta Hortic. Sin. 2017, 44, 89–96. [Google Scholar]
- Hou, B.X.; Lin, F.; Yi, A.Q.; Yu, G.F.; Wang, X.M.; Song, Q.A. Study of the dissected structure of Loropetalum chinense and Loropetalum chinense var. Rubrum. Chin. Wild Plant Resour. 2004, 23, 47–49. [Google Scholar]
- Wang, Y. Research progress of Loropetalum chinense var. rubrum germplasm resources and their genetic characteristics. J. Hunan Univ. Arts Sci. (Nat. Sci. Ed.) 2007, 19, 66–68. [Google Scholar]
- Hou, B.X.; Lin, F.; Yu, G.F.; Yi, A.Q. Study of the number variation of outward characteristic of the Loropetalum chinense and Loropetalum chinense var. rubrum. J. Plant Genet. Resour. 2003, 4, 203–206. [Google Scholar]
- Hou, B.X.; Lin, F.; Li, W.P.; Wang, X.M.; Yu, G.F.; Song, Q.A. Classification system of Loropetalum chinense var. rubrum. For. Res. 2003, 16, 430–433. [Google Scholar]
- Gonnet, J.F. CLElab measurement, a precise communication in flower colour: An example with carnation (dianthus caryophyllus) cultivars. J. Hortic. Sci. 1993, 68, 499–510. [Google Scholar] [CrossRef]
- Sun, W.; Li, C.H.; Wang, L.S.; Dai, S.L. Study on the determination part of flower color of chrysanthemum tongue. J. Hortic. 2010, 37, 777–784. [Google Scholar]
- Wang, F.; Yang, S.H.; Liu, X.Y.; Cui, J.P.; Chang, Z.H.; Ge, H. Flower color diversity of rose germplasm resources and its relationship with anthocyanins. J. Hortic. 2017, 44, 1125–1134. [Google Scholar]
- Guo, X.; Cheng, F.Y.; Zhong, Y.; Chen, X.Y.; Tao, X.W. The quantitative classification of flower color phenotype in Paeonia rockii (flare tree peony). Acta Hortic. Sin. 2022, 49, 86–99. [Google Scholar]
- Yin, H.T.; Yin, J.M.; Liao, Y.; Lu, S.J.; Li, C.H. Phenotype classification based on flower color, pigment distribution and epidermal cell shape of Dendrobium hybrids. Acta Hortic. Sin. 2021, 48, 1907–1920. [Google Scholar]
- Hong, Y.; Bai, X.X.; Sun, W.; Jia, F.W.; Dai, S.L. Quantitative classification of flower color phenotypes of chrysanthemum cultivars. J. Hortic. 2012, 39, 1330–1340. [Google Scholar]
- Li, Y.Q.; Liu, X.Y.; Guo, X.Y.; Pan, J.S.; Tang, D.Q. Analyses of flower color phenotype and flower pigments of Petunia hybrida new germplasms. Subtrop. Plant Sci. 2021, 50, 378–387. [Google Scholar]
- Jiang, X.K. Studies on Test Guideline to New Vaarities on Distinctness Uniformity and Stability of Amorphophallus. Master’s Thesis, Southwest University, Chongqing, China, 2012. [Google Scholar]
- Ren, X.Y. Study on Mutation and Identification of Hibiscus Syriacus Seeds by Colchicine and EMS. Master’s Thesis, Central South University of Forestry & Technology, Changsha, China, 2019. [Google Scholar]
- Zhao, B.T.; Zhao, W.; Liu, L.W. Advances in the analytical methods of anthocyanin com pounds. J. Northwest A F Univ. (Nat. Sci. Ed.) 2014, 42, 180–188. [Google Scholar]
- Romero, I.; Sanchez-Ballesta, M.T.; Maldonado, R.; Isabel escribano, M.; Merodio, C. Anthocyanin, antioxidant activity and stress-induced gene expression in high CO2-treated table grapes stored at tow temperature. J. Plant Physiol. 2008, 165, 522–530. [Google Scholar] [CrossRef]
- Wrolstad, R.; Culbertson, J.; Cornwell, C.; Mattick, L. Detection of adulteration in blackberry juice concentrates and wines. Assoc. Off. Anal. Chem. 1982, 65, 1417–1423. [Google Scholar] [CrossRef]
- Su, Q.; Yang, Y.H.; Tian, M.; Zhang, J.Z.; Mao, L.Y.; Tang, Y.W. Bu, Z.Y.; Lu, Z.S. Phenotypic diversity analysis and comprehensive evaluation of 49 waterlily resources. Southwest China J. Agric. Sci. 2019, 32, 2670–2681. [Google Scholar]
- Petruccelli, R.; Ganino, T.; Ciaccheri, L.; Maselli, F.; Mariotti, P. Phenotypic diversity of traditional cherry Accessions present in the tuscan region. Sci. Hortic. 2013, 150, 334–347. [Google Scholar] [CrossRef]
- Lv, W.; Han, J.N.; Wen, F.; Ren, G.X.; Wang, R.P.; Liu, W.P. Phenotypic Diversity analysis of sesame germplasm resources. J. Plant Genet. Resour. 2020, 21, 234–242, 251. [Google Scholar]
- Wang, L.F.; Xu, J.J.; Huang, X.X.; Li, S.B.; Cheng, X.M. Phenotypic Traits and comprehensive evaluation of 57 modern Chinese rose germplasm resources. J. Southwest For. Univ. 2022, 42, 83–90. [Google Scholar]
- Yuan, X. Study on Genetic Diversity of Phenotype Traits of Lotus Germplasm Resources and Classification Based on Flower Color Phenotype. Master’s Thesis, Henan Agricultural University, Changsha, China, 2021. [Google Scholar]
- Li, Y.; Zhang, S.H.; Guo, Y.; Zhang, X.F.; Wang, G.P. Catkin phenotypic diversity and cluster analysis of 211 Chinese chestnut germplasms. Sci. Agric. Sin. 2020, 53, 4667–4682. [Google Scholar]
- Rui, W.J.; Wang, X.M.; Zhang, Q.N.; Hu, X.Y.; Hu, X.H.; Fu, X.J.; Gao, Y.M.; Li, J.S. Genetic diversity analysis of 353 tomato germplasm resources by phenotypic traits. Acta Hortic. Sin. 2018, 45, 561–570. [Google Scholar]
- Tucker, A.O.; Maciarello, M.J. A survey of colored harts for biological description. Taxon 1991, 40, 201–214. [Google Scholar] [CrossRef]
- Bao, Z.Y.; Zhang, H.; Bao, J.S.; Huang, Z. Genetic diversity of Loropetalum chinense var. rubrum was analyzed by RAPD. J. Zhejiang Univ. (Agric. Life Sci.) 2003, 29, 665–670. [Google Scholar]
- Bi, M.M.; Cao, Y.W.; Song, M.; Tang, Y.C.; He, G.R.; Yang, Y.; Yang, P.P.; Xu, L.F.; Ming, J. Advances in flower color of Lilium. Acta Hortic. Sin. 2021, 48, 2073–2086. [Google Scholar]
- Yu, P.C.; Tan, P.Y.; Gao, L.; Jia, G.X. Analysis of the development of flower color based on hybridization breeding process in Lilium OT hybrids. Acta Hortic. Sin. 2021, 48, 1885–1894. [Google Scholar]
- Zou, H.Z.; Zhou, L.; Han, L.L.; Lv, J.H.; Wang, Y. Changes of carotenoid components and expression of the related genes during petal coloring of Paeonia delavayi. Acta Hortic. Sin. 2021, 48, 1934–1944. [Google Scholar]


| Traits | Description of Phenotypic Traits |
|---|---|
| length (AL) | The distance from the flower to the stem |
| corolla diameter (CD) | Distance between petal apex at upper part of corolla |
| corolla longitudinal diameter (CLD) | Vertical distance from base of corolla to corolla spreading plane |
| petal length (PL) | Distance from base to tip of petals |
| petal width (PW) | Distance from widest point of petals |
| flower number (FN) | The number of flowers in an inflorescence |
| petal number (anthocaulus PN) | The number of petals per flower |
| sepal number (SEN) | The number of sepals per flower |
| stamen number (STN) | Number of stamens per flower |
| nectary number (NN) | Number of nectaries per flower |
| pistil number (PIN) | The number of pistils per flower |
| petal color (PC) | Red Purple = 1, purplish pink = 2, purplish red = 3, White = 4, polychromatic = 5 |
| petal form (PF) | Roll type petals = 1, petals slightly rolled = 2, straight petals = 3 |
| sepal color (SC) | Purplish Red = 1, purplish pink = 2, yellow green = 3, Chimera color = 4 |
| nectary color (NC) | Purplish red = 1, purplish pink = 2, yellow green = 3, white = 4, Chimera color = 5 |
| Trait | Mean | SD | Maximum | Minimum | CV (%) | Genetic Diversity Index (H’) |
|---|---|---|---|---|---|---|
| anthocaulus length (AL) | 26.35 | 8.58 | 64.59 | 9.19 | 32.55 | 1.82 |
| corolla diameter (CD) | 35.83 | 4.73 | 51.01 | 24.45 | 13.19 | 1.9 |
| corolla longitudinal diameter (CLD) | 22.68 | 3.66 | 32.57 | 15.5 | 16.15 | 1.97 |
| petal length (PL) | 19.85 | 3.66 | 30.57 | 11.17 | 18.46 | 1.97 |
| petal width (PW) | 1.96 | 0.37 | 3.04 | 1.29 | 18.99 | 1.99 |
| flower number (FN) | 5.73 | 1.05 | 9 | 4 | 18.25 | 2 |
| petal number (PN) | 4.32 | 0.23 | 5 | 3 | 5.24 | 1.75 |
| sepal number (SEN) | 4.34 | 0.25 | 6 | 3 | 5.7 | 1.79 |
| stamen number (STN) | 4.45 | 0.56 | 8 | 3 | 12.55 | 1.38 |
| nectary number (NN) | 5.11 | 0.85 | 8 | 4 | 16.63 | 1.86 |
| pistil number (PIN) | 2 | 0 | 2 | 2 | 0 | 0 |
| Trait | Frequency Distribution (%) | H’ | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| petal color (PC) | 33.34 | 20.00 | 8.33 | 35 | 3.33 | 1.39 |
| petal form (PF) | 20.00 | 51.67 | 28.33 | 1.02 | ||
| sepal color (SC) | 26.67 | 30.00 | 35.00 | 8.33 | 1.29 | |
| nectary color (NC) | 28.33 | 18.33 | 20.00 | 11.67 | 21.67 | 1.57 |
| Trait | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 |
|---|---|---|---|---|---|---|
| anthocaulus length (AL) | 0.24 | 0.12 | −0.02 | 0.24 | 0.56 | −0.08 |
| corolla diameter (CD) | 0.49 | 0.09 | −0.13 | −0.08 | 0.04 | −0.10 |
| corolla longitudinal diameter (CLD) | 0.49 | 0.02 | −0.20 | −0.02 | −0.10 | 0.03 |
| petal length (PL) | 0.49 | 0.03 | −0.24 | −0.04 | −0.03 | 0.03 |
| petal width (PW) | 0.14 | −0.02 | −0.28 | 0.04 | −0.01 | 0.71 |
| flower number (FN) | 0.21 | 0.00 | 0.04 | −0.10 | −0.39 | −0.51 |
| petal number (PN) | 0.28 | 0.05 | 0.55 | 0.05 | 0.03 | 0.18 |
| sepal number (SEN) | 0.24 | −0.03 | 0.60 | −0.11 | 0.12 | 0.01 |
| stamen number (STN) | 0.03 | −0.06 | 0.20 | 0.66 | 0.06 | −0.03 |
| nectary number (NN) | −0.01 | 0.10 | −0.16 | 0.65 | −0.14 | −0.07 |
| pistil number (PIN) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| petal form (PF) | −0.07 | 0.10 | −0.21 | −0.14 | 0.66 | −0.29 |
| sepal color (SC) | −0.04 | 0.61 | 0.10 | 0.00 | −0.08 | −0.03 |
| nectary color (NC) | −0.01 | 0.55 | −0.10 | 0.08 | −0.17 | −0.14 |
| petal color (PC) | −0.11 | 0.52 | 0.11 | −0.15 | 0.05 | 0.28 |
| eigenvalue | 3.31 | 2.28 | 1.85 | 1.50 | 1.13 | 1.06 |
| percentage of variance (%) | 23.64 | 16.30 | 13.19 | 10.74 | 8.06 | 7.61 |
| cumulative (%) | 23.64 | 39.95 | 53.14 | 63.88 | 71.94 | 79.55 |
| Color Group | No. | Color Name | Sample Number |
|---|---|---|---|
| Green-white | 1 | Light Greenish Gray | 3 |
| 2 | Dark Olive Green | 3 | |
| 3 | Very Light Green | 2 | |
| Yellowish white | 4 | Moderate Yellowish Green | 1 |
| 5 | Deep Yellowish Green | 1 | |
| 6 | Very Deep Yellowish Green | 3 | |
| 7 | Deep Yellow Green | 4 | |
| 8 | Pale Yellowish Green | 3 | |
| 9 | Grayish Yellowish Green | 1 | |
| Light pink | 10 | Light Yellowish Brown | 1 |
| 11 | Brownish Pink | 1 | |
| 12 | Grayish Purplish Pink | 4 | |
| Reddish Purple | 13 | Pale Reddish Purple | 2 |
| Purple | 14 | Pale Purple | 5 |
| 15 | Very Pale Purple | 12 | |
| 16 | Vivid Violet | 2 | |
| 17 | Light Violet | 2 | |
| 18 | Pale Violet | 2 | |
| 19 | Light Purple | 2 | |
| 20 | Grayish Purple | 3 | |
| 21 | Very Light Purple | 1 | |
| 22 | Very Light Violet | 2 |
| Color Group | Number | Percentage(%) | L* | a* | b* | C* |
|---|---|---|---|---|---|---|
| Polychromatic | 2 | 3.33 | 62.85–63.95 | 2.14–2.52 | 4.95–6.79 | 5.39–7.24 |
| Green-white | 8 | 13.33 | 78.30–86.65 | −10.33–−4.28 | 2.23–13.47 | 4.92–16.98 |
| Yellowish white | 13 | 21.67 | 67.16–88.00 | −11.15–−4.11 | 4.31–22.35 | 6.99–24.98 |
| Light pink | 4 | 6.67 | 57.29–67.50 | 9.99–14.01 | −4.53–−2.51 | 10.30–14.72 |
| Reddish Purple | 2 | 3.33 | 55.59–68.46 | 10.81–12.46 | −4.67–−4.28 | 11.63–13.30 |
| Purple | 31 | 51.67 | 54.47–66.39 | 2.48–14.68 | −12.0–−4.55 | 7.03–16.60 |
| Color Group | Number of Samples | Proportion of Samples (%) | Anthocyanin Content Threshold (mg/100 g) |
|---|---|---|---|
| Green-white | 7 | 11.67 | 15.31–23.52 |
| Yellowish white | 14 | 23.33 | 15.52–33.88 |
| Polychromatic | 2 | 3.33 | 37.71–41.61 |
| Purplish pink | 6 | 10.00 | 99.43–264.96 |
| Purplish red | 31 | 51.67 | 97.06–334.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Yu, X.; Zhang, X.; Zhang, D.; Li, W.; Xiang, L.; Yang, Y.; Li, Y.; Xu, L. Phenotypic Diversity Analysis of the Progeny Variation of a ‘Mosaic Leaf’ Loropetalum chinense var. rubrum Based on Flower Organ Characteristics. Diversity 2022, 14, 913. https://doi.org/10.3390/d14110913
Zhang L, Yu X, Zhang X, Zhang D, Li W, Xiang L, Yang Y, Li Y, Xu L. Phenotypic Diversity Analysis of the Progeny Variation of a ‘Mosaic Leaf’ Loropetalum chinense var. rubrum Based on Flower Organ Characteristics. Diversity. 2022; 14(11):913. https://doi.org/10.3390/d14110913
Chicago/Turabian StyleZhang, Li, Xiaoying Yu, Xia Zhang, Damao Zhang, Weidong Li, Lili Xiang, Yujie Yang, Yanlin Li, and Lu Xu. 2022. "Phenotypic Diversity Analysis of the Progeny Variation of a ‘Mosaic Leaf’ Loropetalum chinense var. rubrum Based on Flower Organ Characteristics" Diversity 14, no. 11: 913. https://doi.org/10.3390/d14110913
APA StyleZhang, L., Yu, X., Zhang, X., Zhang, D., Li, W., Xiang, L., Yang, Y., Li, Y., & Xu, L. (2022). Phenotypic Diversity Analysis of the Progeny Variation of a ‘Mosaic Leaf’ Loropetalum chinense var. rubrum Based on Flower Organ Characteristics. Diversity, 14(11), 913. https://doi.org/10.3390/d14110913







