Abstract
The zooplankton community composition in shallow lakes is influenced by numerous factors, such as environmental factors and the land use patterns around the lake. To investigate the interaction between the spatial differences in the zooplankton community structure, aquatic parameters, and land use patterns in the Lake Chen Yao complex (Lake Chen Yao and Lake Feng Sha), we assessed them in four seasons from October 2020 to August 2021. The results showed that the zooplankton density and biomass of Lake Chen Yao were higher than the latter. The results of Pearson correlation and RDA analysis revealed that electrical conductivity (EC), Chlorophyll a (Chl.a), dissolved oxygen (DO), and pH were the main environmental factors affecting the zooplankton community structure in the two lakes. The nutrient content of nitrogen (N) and phosphorus (P) were significantly higher in Lake Chen Yao, and there was a considerable relationship with the distribution of land use patterns around the two lakes. The land use patterns were the main reason for the difference in water quality and thus the spatial variation in the characteristics of the zooplankton communities in the two lakes.
1. Introduction
Changes in the zooplankton community structure in shallow lakes are influenced by various environmental factors, such as aquatic parameters and land use patterns resulting from human activities around the lake (e.g., the distribution of areas such as mountains, forests, farmland, and towns). Many authors have studied the effects of aquatic parameters (e.g., nutrients, pH, water temperature, and transparency) on local zooplankton [1,2]. For example, water temperature was thought to be a vitally important environmental factor affecting zooplankton community structure [3]. Helland et al. [4] found that temperature was the key factor driving changes in the zooplankton community composition in Lake Stechlin (Germany). DO is a critical variable in evaluating water quality in lakes, reservoirs, or any freshwater system. Low DO concentrations of water bodies directly affect the abundance of zooplankton, thus altering the ecological balance [5]. Transparency was also an important environmental factor affecting zooplankton communities [6,7]. It was also reported that increased zooplankton grazing had contributed to improvements in transparency [8]. In addition, anthropogenic land use changes in the watershed and the resulting decline in water quality are also considered to be important drivers affecting the distribution of the zooplankton community structure. It has been demonstrated that agricultural land is the main source of nutrients and organic matter in lakes and reservoirs, and when the levels of these nutrients and organic matter exceed certain thresholds, the water quality is affected to some extent [9,10], resulting in ecological changes such as eutrophication, which can cause the deterioration of water quality. The presence of mountain forest vegetation also plays an important role in improving the water quality of lakes: it can improve the ecological environment around a lake and thus indirectly influence the water quality of the lake itself [11]. Zooplankton, an important factor in the ecosystem cycle of lakes [12], participate in the energy flow and material cycle of the lake ecosystem [13]. As primary consumers of aquatic ecosystems, they can control the growth and reproduction of phytoplankton through grazing and are important food sources for many fish and shrimp as well as other swimming animals in the water column, performing a key role in the ecological processes of material cycling, energy flow, and information transfer in aquatic ecosystems [14,15,16]. Hence, it is crucial to understand the effects of various environmental drivers on the zooplankton communities to assess their impact on lake ecosystems [17,18].
Lake Chen Yao and Lake Feng Sha are located in Zongyang County, Anhui Province, on the north bank of the middle and lower reaches of the Yangtze River. Before the 1950s, these two lakes were one intact lake joined by a water channel. Their low-lying terrain and the open area enabled good flood storage and drought prevention with diverse types of wetland habitats, and they were an important part of the shallow lakes in Anhui Province, rich in aquatic species diversity that occupied an important position in the ecological zone of the middle and lower reaches of the Yangtze River [19]. Since the middle of the last century, the area of the lakes has been drastically reduced due to the large area that has been reclaimed for farmland. The two lakes are separated by a dam, forming two relatively independent lakes, thus creating a spatial difference between the two lakes. In recent years, due to the development of agriculture and the rise of aquaculture, the water quality of Lake Chen Yao has started deteriorating and its biodiversity is decreasing. Zhang et al. found that the number and species of birds in the Chen Yao Lake wetland were affected to varying degrees due to the intervention of human activities and environmental changes [20]. Meanwhile, in this study, except for the river estuary, no aquatic vegetation was found on the surface, which was contrary to previous studies [21]. Before the summer of 2020, the surface was rich in aquatic vegetation with lotus and gorgonians as the dominant species. These were widely distributed on the surface of Lake Chen Yao, but after mowing carried out by the local government and the impact of extreme floods in the summer of 2020, the aquatic vegetation on the surface disappeared, gradually followed by the submerged plants. The decay and disappearance of aquatic vegetation makes the bottom mud more abundant and higher in nutrient salts such as nitrogen and phosphorus.
Lake Feng Sha is not covered with aquatic vegetation all year round, while the surrounding mountains and forests are rich in vegetation, which is a natural habitat. Human activities have less influence on it. There are significant habitat differences in the spatial distribution of the two lakes in terms of land use patterns; therefore, it is scientifically important to study the distribution of the zooplankton community structure and their interactions with land use patterns and aquatic parameters in the two lakes based on the resulting habitat differences.
To explore the driving effects of the land use patterns and aquatic parameters on the changing characteristics of the zooplankton community composition, samples were collected and analyzed from shallow lakes in the Lake Chen Yao Complex. This study aimed to (1) explore the characteristics of the zooplankton community structure’s changes in the two lakes in different seasons, and (2) assess the direct and indirect effects of the aquatic parameters and land use patterns in the water column on the zooplankton community structure of the two lakes. This study is a comparative study of two lakes, based on the effect of land use patterns and physicochemical factors on the spatial differences of the zooplankton community structure. This study is important for understanding the effects of environmental changes on zooplankton and providing a scientific basis for water quality evaluation and pollution control in shallow lakes.
2. Materials and Methods
2.1. Sampling Point Setting and Sampling Time
According to the topography and water area of the study area, combined with local hydrological characteristics and human activities, and referring to previous research experience [22], we established a total of ten sampling points in the two lakes (Figure 1). These points were used to collect water samples and zooplankton samples and measure water quality indicators on-site. Sampling was conducted in October 2020 (autumn), March 2021 (winter), April (spring), June (summer), and August (summer).
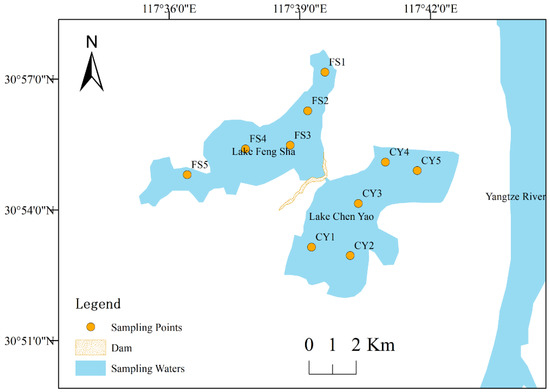
Figure 1.
Distribution of sampling points in Lake Chen Yao and Lake Feng Sha study area.
2.2. Sample Collection and Processing
Water samples were conducted seasonally in the two lakes. During sampling, water column parameters were measured, and zooplankton samples were collected. The field measurements included water temperature (WT), dissolved oxygen (DO), electrical conductivity (EC), pH, and turbidity (Turb). Measurements were taken by the SX751 portable water quality analyzer and the Hach 2100Q portable turbidimeter. Water depth (WD) and Secchi depth (SD) were determined by the Secchi disk. Water samples from each lake were also taken to measure chlorophyll a levels -[23] and the nutrient concentrations in the laboratory, including total phosphorus (TP) [24], total nitrogen (TN) [25], ammonia nitrogen (NH4+-N) [26], and nitrate nitrogen (N03−-N) [27]. The collection of zooplankton included rotifers, cladocerans, and copepods, and the samples were divided into qualitative and quantitative sets. Qualitative samples of rotifers were collected with a 25# (pore size = 64 μm) plankton net in the surface water that was dragged in the shape of “∞” for 3~5 min, filtered and concentrated into 50 mL quantitative bottles, and then fixed by adding 1 mL of Lugol iodine solution on-site. The quantitative samples were collected in 1 L sample bottles, fixed by adding 10 mL of Lugol iodine solution on-site before being brought back to the laboratory for precipitation and concentration to 30 mL. Qualitative samples of cladocerans and copepods were dragged through a 13# (pore size = 112 μm) plankton net in the surface water in the shape of “∞” for 3–5 min. The samples were concentrated into 50 mL quantification bottles, and then fixed by adding 4% formaldehyde solution 1 mL on-site. The quantified samples were collected in 10 L of water with a Plexiglas water collector, filtered through a 25# plankton net, concentrated into a 50 mL quantified bottle, fixed by adding 4% formaldehyde solution 1 mL on-site, and labeled. The plankton nets were washed three times [28].
The species composition of zooplankton is extremely complex. Identifying the species of zooplankton requires specialized knowledge and training. Generally, the classification and identification of zooplankton are based on the morphological characteristics and size of zooplankton; for more information, please refer to “Chinese Freshwater Rotifers” and “Chinese Fauna Crustacea”. Species identification was performed under light and dissecting microscopes.
3. Data Analyses
The Shannon–Wiener index (H′), Pielou index (J), and Margalef index (D) were used to represent zooplankton biodiversity [29,30], while comprehensive “surface water environmental quality standards” (GB3838-2002) were used to evaluate the lake’s water quality standards and nutritional status. The standard for evaluating water pollution status by the H′ index is as follows: 0 ≤ H′ ≤ 1, heavy pollution; 1 < H′ ≤ 2, α-moderate pollution; 2 < H′ ≤ 3, β-moderate pollution; H′ > 3, light pollution or no pollution [31,32]
The formula for calculating the diversity index of zooplankton is as follows:
In the formula, N is the total number of zooplankton individuals, S is the total number of zooplankton species, and Pi is the ratio of the number of individuals of the i-th species to the total number in the sample (Ni/N). The calculation of the water body nutrient state parameters employs the mean and standard deviation; selects the SD, TN, TP, and Chl.a 4 indicators; and evaluates them with comprehensive nutrient state parameters: TLI(∑) < 30, indicates poor nutrition; 30 ≤ TLI(∑) ≤ 50, signifies moderate nutrition; 50 < TLI(∑) ≤ 60, indicates mild eutrophication; 60 < TLI(∑) ≤ 70, means moderate eutrophication; TLI(∑) > 70, signals severe eutrophication [33,34,35]. The calculation formula is as follows:
In the formula, TLI(∑) represents the comprehensive nutritional state parameter; Wj is the relative weight of the nutritional state parameter of the j-th parameter; rij is the j-th parameter and the reference parameter; m is the number of evaluation parameters; TLI(j) is the nutritional state parameters representing the first parameter. They are calculated by the following formulas:
In the formula, Chl.a is the concentration of chlorophyll a (ug/L), TP is the concentration of total phosphorus in the water (mg/L), TN is the concentration of total nitrogen in the water (mg/L), and SD is the transparency of the lake water (m).
The distribution map of the sampling points was drawn using ArcGIS10.2 software. The distribution of the land use types was cropped utilizing Envi5.3 software applied to remote-sensing images of the distribution area. The area within the 1000 m buffer zone near the mountain ridgeline was used as the cutting area because Lake Feng Sha is surrounded by mountains to the west and is close to the mountains; Lake Chen Yao is surrounded by farmland and is a buffer plain near the Yangtze River. The area of the 1000 m buffer zone around the lake is used as the area for cropping classification, followed by supervised classification of land use types such as farmland, mountain forest vegetation, and towns, and the proportion of area distribution of land use types around the two lakes is also classified and calculated by employing ArcGIS software (Version 10.2, ESRI http://www.esri.com). We completed the Pearson’s correlation analysis of zooplankton species, density, biomass, and environmental factors with IBM SPSS Statistics (Version 19.0, http://www.ibm.com) Due to the large degree of variability in the raw data for zooplankton and environmental factors, the raw data were transformed by lg(x + 1) to meet the normality required for ANOVA. We employed the kriging method to process the data for the base map of the study area and mapped the spatial distribution of the species diversity indices with ArcGIS 10.2 software. We performed an RDA utilizing Canoco 5 to explain the relationship between dominant zooplankton communities and aquatic parameters. We used the Monte Carlo permutation test to screen the aquatic parameters, and then plotted their relationship with dominant species.
4. Results
4.1. Distribution of Land Use Types
Employing Envi5.3 software to crop and supervise the classification of remote-sensing images of Lake Chen Yao and Lake Feng Sha, respectively, and ArcGIS10.2 software to rasterize the cropped and supervised classified images and calculate the proportion of the classified area, we concluded that there are significant differences in the distribution of land use types between the two lakes. Lake Feng Sha is surrounded by mountains in the west, and its main land use types are mountain forest vegetation; the distribution of its mountainous vegetation, agricultural land, and towns is 54.53%, 26.93%, and 18.53%, respectively. In contrast, Lake Chen Yao is dominated by the distribution of farmland and towns, and its four sides are mainly surrounded by farmland with wide coverage, accounting for 79.59%, followed by towns, accounting for 20.41% (Figure 2).
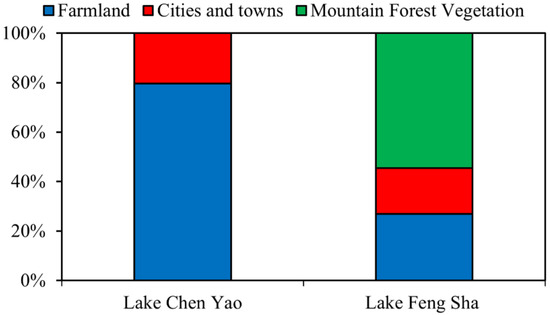
Figure 2.
Distribution of land use types around Lake Chen Yao and Lake Feng Sha.
4.2. The Water Quality Characteristics of Lake Chen Yao and Lake Feng Sha
Each quarter, the physical and chemical components of Lake Chen Yao and Lake Feng Sha were tested (Figure 3). The results revealed that the water temperature fluctuated seasonally. Lake Chen Yao and Lake Feng Sha had the highest water temperatures in summer, at (27.84 ± 0.8) °C and (27.99 ± 0.24) °C, respectively, and the lowest water temperatures in winter, at (7.28 ± 0.56) °C and (7.3 ± 0.43) °C, respectively. The seasonal change in the dissolved oxygen (DO) level was more obvious in Lake Chen Yao and was significantly lower in summer than in autumn and winter. The peak value of the dissolved oxygen in Lake Chen Yao was (10.33 ± 0.64) mg/L in winter, while that in Lake Feng Sha was (11.57 ± 0.69) mg/L in autumn, and the lowest values appeared in summer (6.64 ± 1.80) mg/L and (9.57 ± 0.26) mg/L. This is significantly different from the research results of Shang (2022) and others regarding the relationship between zooplankton and environmental factors in Lake Sheng Jin. Their results depict an opposite relationship. The mean values of the comprehensive trophic state parameters (TLI) of the two lakes were 65.21 ± 12.27 and 61.64 ± 12.21. The water quality of the two lakes corresponded to moderately eutrophic water bodies.
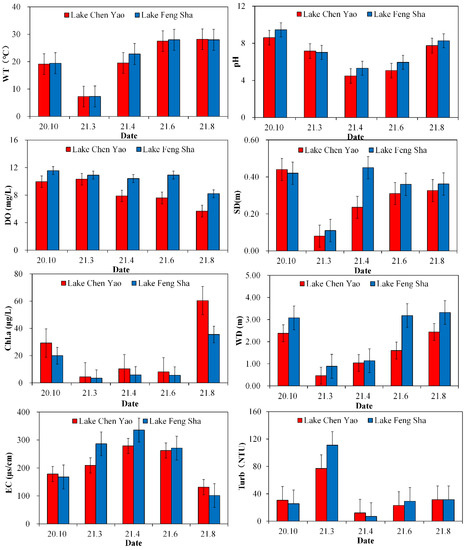
Figure 3.
Seasonal changes of physical and chemical factors in Lake Chen Yao and Lake Feng Sha.
The independent samples t-test demonstrated that, with respect to the physicochemical factors, there were significant spatial differences between the two lakes. During the survey period (Table 1), the water temperature, pH, turbidity, chlorophyll a concentration, TP content, TN content, and NO3-N concentration in the two lakes were (20.32 ± 0.56) °C, (6.61 ± 0.31), (32.61 ± 14.62) NTU, (22.5 ± 10.3) μg/L, (0.72 ± 0.02) mg/L, (4.69 ± 0.45) mg/L, and (0.12 ± 0.004) mg/L for Lake Chen Yao and (21.1 ± 0.35) °C, (7.2 ± 0.11), (40.86 ± 14.1) NTU, (14.07 ± 7.74) μg/L, (0.69 ± 0.01) mg/L, (4.07 ± 0.16) mg/L, and (0.08 ± 0.005) mg/L for Lake Feng Sha, showing highly significant correlations (p < 0.01). The data results indicated that the mean transparency value of Lake Chen Yao (0.28 m) was lower than that of Lake Feng Sha (0.34 m), but the contents of nutrients such as TN, TP, NH4+-N, and Chl.a were higher than that of Feng Sha Lake, especially the concentrations of NH4+-N. The Chl.a levels were significantly higher than that of Lake Feng Sha—nearly 2~3 times that of the latter.

Table 1.
Physical and chemical factors of Lake Chen Yao and Lake Feng Sha (mean ± standard deviation).
4.3. Species Composition of Zooplankton
During the whole survey period, a total of 49 species of zooplankton in 13 families and 29 genera were identified in Lake Chen Yao, including 6 families, 13 genera, and 28 species of rotifers, accounting for 57.14% of the total zooplankton; 5 families, 9 genera, and 13 species ofcladocera, accounting for 26.53% of the total zooplankton; and 2 families, 7 genera, and 8 species of copepods, accounting for 16.33% of the total zooplankton. A total of 48 species of zooplankton from 14 families and 29 genera were identified in Lake Feng Sha, including 27 species of rotifers from 7 families and 13 genera, accounting for 56.25% of the total; 10 species of cladocerafrom 5 families and 9 genera, accounting for 20.83% of the total; and 11 species of copepods from 2 families and 7 genera, accounting for 22.92% of the total (Figure 4). In terms of species composition, there were no obvious differences between the two lakes, with rotifers being the most widely distributed and making up the highest proportion, followed by branchiopods and copepods, but in general, except for copepods, the composition and proportion of zooplankton in Lake Chen Yao were higher than those in Lake Feng Sha, showing some spatial variability.
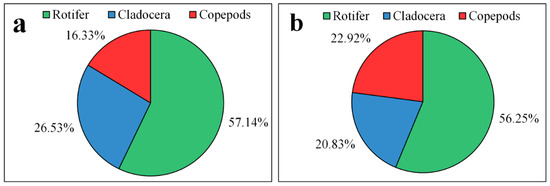
Figure 4.
Percentage species composition of the zooplankton community in Lake Chen Yao (a) and Lake Feng Sha (b).
4.4. Species Diversity
For the zooplankton in Lake Chen Yao, the H′ index was 2.4 (2~2.75), D was 2.47 (1.87~3.10), and J was 0.76 (0.51~1.44). The changing trends of the H′ and D indices are basically the same. Both reach their highest values in autumn, at 2.75 ± 0.57 and 3.10 ± 0.57, respectively, and their lowest values in winter, at 2.00 ± 0.21 and 2.34 ± 0.50; J is at its highest in the summer and does not vary significantly in the spring, autumn, or winter. For the zooplankton in Lake Feng Sha, the H′ index was 2.81 (2.58~3.22), D was 2.85 (2.13~4.11), and J was 0.71 (0.69~0.75). The changing trends of the H′ and D indices are basically the same. Both realize their highest values in autumn, at 3.22 ± 0.14 and 4.11 ± 0.49, respectively, and their lowest values in summer, at 2.64 ± 0.18 and 2.31 ± 0.35, respectively. The changes in the J indices in the four seasons are not obvious (Figure 5).
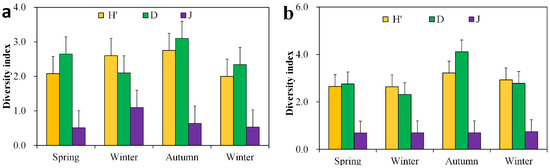
Figure 5.
Seasonal distribution of zooplankton diversity index in Lake Chen Yao (a) and Lake Feng Sha (b).
Spatially, the H′ index of Lake Chen Yao shows a general trend of gradually increasing from north to south, the D index shows a trend of gradually increasing from southwest to northeast, and the spatial distribution trend of the J index is basically consistent with the D index. For Lake Feng Sha, the H′ and D indices are consistent, both showing a decreasing trend from west to east, while the J index demonstrates a general decreasing trend from southwest to northeast (Figure 6).
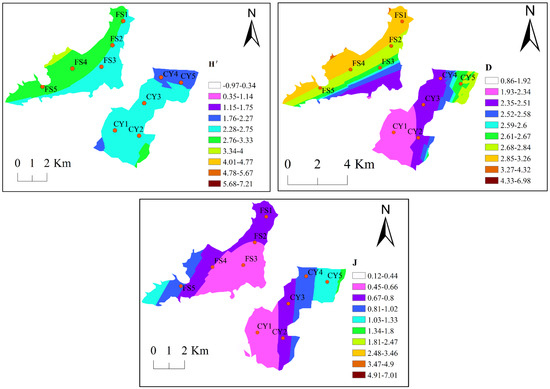
Figure 6.
Spatial distribution of zooplankton diversity indices in Lak Chen Yao and Lake Feng Sha.
4.5. Dominant Species of Zooplankton
According to the principle wherein a dominance of Y ≥ 0.02 represents the dominant species [29], we concluded that there were 14 dominant species in two lakes during the study period (Table 2 and Table 3). This dominance mainly consisted of rotifers, but there were significant spatial differences in their distribution in the two lakes. In Lake Chen Yao, the number of dominant species was the largest in spring and summer, with 10 species, and the least in autumn, with only 5 species. The species with the highest dominance was Polyarthra trigla, which had the highest dominance in winter with a value of 0.49. Brachionus budapestiensis and Keratella cochlearis were the dominant species throughout the year, and both had their highest dominance values in autumn, at 0.21 and 0.41, respectively. In Lake Feng Sha, the number of dominant species was the largest in summer, with 11 species, while the least were in spring, with only 2 species. There was little difference in the number of dominant species in fall and winter, with six and seven species, respectively. Keratella cochlearis is the dominant species throughout the year, and its dominance is not very different in the four seasons. The highest dominance in winter is 0.18, and the lowest in summer is 0.10.

Table 2.
Dominant species and dominance of zooplankton in Lake Chen Yao.

Table 3.
Dominant species and dominance of zooplankton in Lake Feng Sha.
4.6. Seasonal Distribution of Zooplankton Species
The analysis of the number of zooplankton species in Lake Chen Yao and Lake Feng Sha in the four seasons illustrated that there were obvious seasonal variations (Figure 7), and the trends of the seasonal distribution of zooplankton in the two lakes were as follows: Lake Chen Yao: summer (31 species) > autumn and winter (both 30 species) > spring (22 species); Lake Feng Sha: autumn (41 species) > spring (31 species) > winter (27 species) > summer (26 species). There were obvious seasonal differences in the two lakes.
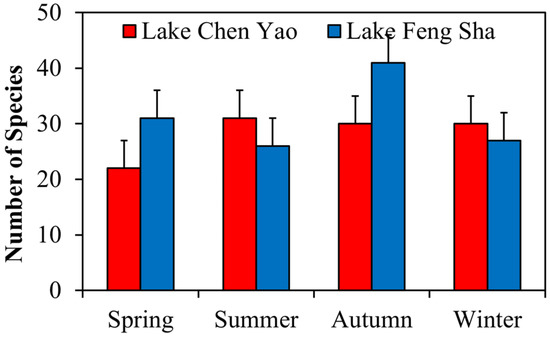
Figure 7.
Seasonal distribution of zooplankton species in Lake Chen Yao and Lake Feng Sha.
4.7. Zooplankton Density and Biomass
4.7.1. Time Distribution of Density and Biomass
In Lake Chen Yao and Lake Feng Sha, the yearly average zooplankton densities were (947.12 ± 106.78)/L and (260.44 ± 23.46)/L, respectively, while the annual average biomass was (17.73 ± 0.77) mg/L and (15.76 ± 0.41) mg/L. Regarding Lake Chen Yao, the average annual density of rotifers is the highest, accounting for 96.83% of the total density, and the biomass is also the highest, at 36.1 mg/L. The average annual density of cladocerans was the smallest, making up 0.53% of the total density, and the biomass was also the smallest, at 0.95 mg/L. Its annual mean density and biomass of copepods were (156.92 ± 28.24) and (12.99 ± 1.75) mg/L, respectively. The density and biomass of zooplankton in Lake Feng Sha were not significantly different from those in Lake Chen Yao.
Throughout the study period, the zooplankton density in Lake Chen Yao was highest in spring and lowest in summer and autumn, whereas the biomass was highest in winter and lowest in summer and autumn, and the overall density variation trend was spring > winter > autumn > summer. The seasonal variation trend of the overall biomass is winter > spring > autumn > summer. This is different from the situation in Lake Feng Sha, where the density and biomass of zooplankton are both highest in autumn and lowest in spring. The seasonal change trends regarding the overall density and biomass were as follows: autumn > summer > winter > spring (Figure 8).
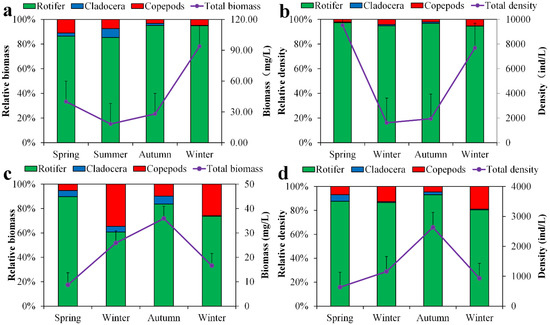
Figure 8.
Seasonal distribution of zooplankton biomass and density in Lake Chen Yao (a,b) and Lake Feng Sha (c,d).
4.7.2. Spatial Distribution of Density and Biomass
As exhibited in Figure 9, there were some differences in the distribution of the two lakes regarding the density and biomass of zooplankton between the sampling sites throughout the study period. With respect to Lake Chen Yao, the average density of zooplankton at sampling point CY5 is the highest (1781.23 ± 343.35)/L, and the average density at sampling point CY1 is (578.8 ± 64.88)/L. The overall spatial variation trend of the density is as follows: CY5 > CY4 > CY3 > CY2 > CY1. The overall performance is low in the west and high in the east, increasing from south to north. The trend fluctuation of biomass at each point is not large; it is high in the central area, and relatively low near the shore. The highest value was (39.25 ± 3.8) mg/L at sampling point CY3, and the lowest value was (23.05 ± 2.43) mg/L at sampling point CY1. Concerning Lake Feng Sha, compared with Lake Chen Yao, the density and biomass distribution of zooplankton are higher in the center of the lake and relatively lower on both sides. The highest density value (349.22 ± 30.31) was found in FS4, while the lowest value (209.66 ± 17.93) was detected in FS1. The maximum biomass value was found in FS3 (28.03 ± 2.33), while the lowest value was located in FS1 (12.31 ± 1.52). Overall, the density distribution of biomass in the core area is still higher than on either side. In the waters of Lake Chen Yao and Lake Feng Sha, rotifers had the largest relative density and biomass, followed by cladocerans and copepods.
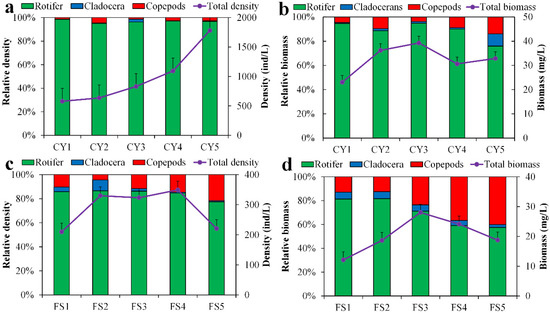
Figure 9.
Spatial distribution of zooplankton biomass and density in Lake Chen Yao (a,b) and Lake Feng Sha (c,d).
As displayed in Figure 10, regarding the composition of zooplankton in terms of density and biomass in the two lakes, both had the highest percentage of rotifers, followed by copepods and Cladocera. However, the total zooplankton biomass and total density in Lake Chen Yao were higher than those in Lake Feng Sha, showing obvious spatial differences. In terms of density, both lakes have the widest distribution of rotifers and the lowest distribution of cladoceran species, with the density of rotifers in Lake Chen Yao being higher than that in Lake Feng Sha, and the density of cladoceran species and copepods being lower than that in Lake Feng Sha. In terms of biomass, the two lakes still had a high biomass of rotifers, with Lake Chen Yao having a higher biomass of rotifers, while the biomass of branchiopods and copepods was lower compared to Lake Feng Sha.
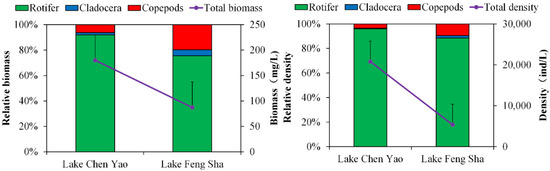
Figure 10.
Comparison of biomass and density distribution of zooplankton in Lake Chen Yao and Lake Feng Sha.
4.8. Relationship between Zooplankton Community Structure, and Environmental Factors
4.8.1. Correlation Analysis
The Pearson’s correlation analysis between the zooplankton density and biomass and the aquatic parameters in Lake Chen Yao and Lake Feng Sha(Table 4) revealed that rotifer density has a significant positive correlation with NH4+-N concentration (p < 0.01) and a significant negative correlation with water depth (p < 0.05); rotifer biomass showed a significant negative correlation with water temperature (p < 0.05); cladoceran density and biomass did not indicate a significant correlation with aquatic parameters; and copepod density also exhibited a significant negative correlation with chlorophyll concentration (p < 0.05). The biomass of rotifers was negatively correlated with water temperature (p < 0.05); the density and biomass of Cladocera were not significantly correlated with aquatic parameters; the density of copepods was negatively correlated with chlorophyll concentration (p < 0.05).

Table 4.
Pearson’s correlation analysis of zooplankton density and biomass with environmental factors in Lake Chen Yao and Lake Feng Sha zooplankton.
4.8.2. Redundancy Analysis
The biomass of nine dominant species of zooplankton in Lake Chen Yao and Lake Feng Sha were selected for a redundancy analysis with aquatic parameters such as the water temperature, DO levels, Chl.a concentration, water depth, transparency, pH, and total phosphorus concentration, which were closely related to the zooplankton community structure of the two lakes as determined by RDA pre-selection and Monte Carlo replacement test screening. The aquatic parameters that had the strongest influence on the zooplankton structure of the two lakes were mainly EC, Chl.a, DO, and pH, among which Filinia maior and Brachionus diversicornis were significantly and positively correlated with DO, and negatively correlated with temperature and TP; Brachionus angularis was considerably and positively correlated with Chl.a concentration and pH, and significantly and negatively correlated with EC and ammonia nitrogen concentration (Table 5 and Figure 11).

Table 5.
Redundancy analysis of zooplankton biomass and environmental factors in Lake Chen Yao and Lake Feng Sha.
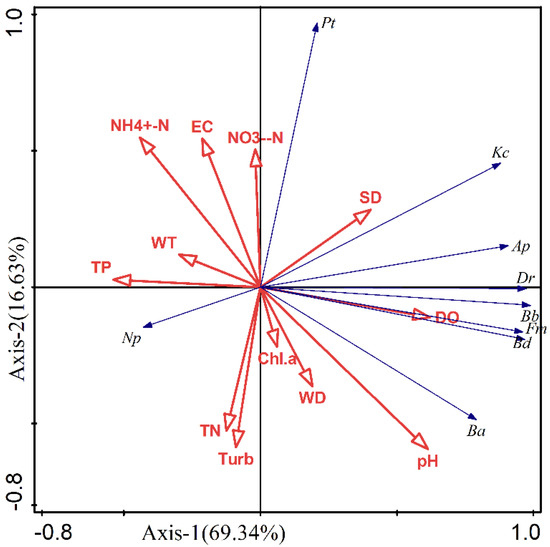
Figure 11.
RDA analysis ranking the biomass of dominant species of zooplankton and environmental factors in the surrounding area of Lake Chen Yao and Lake Feng Sha. (Bb: Brachionus budapestinensis; Kc: Keratella cochlearis; Dr: Diurella rousseoeti; Ap: Asplanchna priodonta; Pt: Polyarthra trigla; Fm: Filinia maior; Ba: Brachionus angularis; Np: Nauplius; Bd—Brachionus diversicornis).
5. Discussion
5.1. Effects of Water Quality and Land Use in Lake Chen Yao and Lake Feng Sha
According to the classification standard of lake eutrophication [33], the average total nitrogen concentrations in these two lakes were above 1.1 mg/L and 0.9 mg/L, the average total phosphorus (TP) concentration was above 0.6 mg/L, and the overall TLI index was above 60. This indicates that the water body is in a moderately eutrophic state. Many studies have shown that zooplankton are abundant in eutrophic lakes, and the dominant species are mostly pollution-tolerant species [36,37]. The co-dominant species are Polyarthra trigla, Brachionus budapestiensis, and Keratella cochlearis. All of these are indicators of eutrophic water bodies. The size of the diversity index is negatively correlated with the degree of water pollution; the greater the diversity index, the better the water quality [38,39]. According to the preliminary evaluation of the water quality with reference to the general standards, the D indices were 2.47 (1.87~3.10) and 2.85 (2.13~4.11), signaling that the water bodies were subject to moderate pollution. The H′ indices were 2.4 (2~2.75) and 2.81 (2.58~3.22), indicating that the water bodies were β-moderately polluted, and the J indices were 0.76 (0.51~1.44) and 0.71 (0.69~0.75), indicating that the water bodies were characterized by medium pollution. The comprehensive TLI index, dominant species characteristics, and biodiversity index revealed that the water bodies were in a state of moderate eutrophication, and the water quality was in a state of moderate pollution.
In this study, when analyzing the water quality of the two lakes, we concluded that the levels of organic matter and nutrient salts, such as TN, TP, NH4+-N, and NO3-N, as well as Chl.a, were notably higher in Lake Chen Yao than in Lake Feng Sha, which was significantly correlated with the habitat differences between the two lakes. The extensive coverage of farmland means that the use of pesticides, fertilizers, and herbicides is becoming more frequent. In addition to being partially absorbed by crops, a large portion of the pesticides, fertilizers, and herbicides that enter the surface enter Lake Chen Yao via the surface runoff, causing a marked increase in the organic matter content of N and P. Meanwhile, according to the summer survey in 2019, we discovered that gorgonians grew too densely in the waters of Lake Chen Yao, and 95% of the lake’s surface was covered by aquatic vegetation. The dominant species were gorgonians and lotus, with gorgonians reaching 90% of the total number of species [21]. The presence of aquatic vegetation exacerbates the effects of photosynthesis and promotes the uptake of nutrient salts such as N and P. However, there have been extreme floods since the summer of 2020, which have led to the presence of enriched nutrient salts in the bottom mud that has resulted in the process of gradual decay and the disappearance of submerged plants. This is one of the reasons for the higher concentrations of nutrient salts such as N and P in Lake Chen Yao.
The land use type of Lake Feng Sha is mainly mountain forest vegetation. Its west side is covered by mountain forests from north to south. The water source is mainly water from the mountain, and the surface is not covered by aquatic vegetation for the entire year, so the content of organic matter and chlorophyll is lower compared with that of Lake Chen Yao. Therefore, land use can directly affect the water quality of shallow river lakes and can thus indirectly affect the distribution of zooplankton communities.
5.2. Effects of Physical and Chemical Factors on the Community Structure of Zooplankton
In this study, the results of the Pearson’s correlation analysis and RDA redundancy analysis on the density and biomass of zooplankton and aquatic parameters in these two lakes showed that the EC, Chl.a, DO, and pH were closely related to the community structure of zooplankton.
Not all water is suitable for the survival of different species of zooplankton because these species require different levels of water alkalinity [2]. Related studies have shown that in acidic waters, rotifers are distributed in more species and fewer numbers, while in alkaline waters the situation happens to be the opposite [40]. In this study, Lake Chen Yao was acidic in spring and summer (pH 4.48 and 6.41, respectively) and alkaline in autumn and winter (pH 8.61 and 7.17, respectively), and the rotifer density distribution was higher in spring and winter than in summer and autumn, with the highest value of 9269.5 ind/L in spring and the lowest value of 1882.5 ind/L in autumn, which shows opposite results compared to the previous studies. In Lake Feng Sha, the water bodies were alkaline in all seasons except spring (pH 5.31). The number of rotifer species were the highest in spring and autumn, with 31 and 41 species, respectively, and the rotifer density dropped to the lowest value in spring, with 555 ind/L (Figure 5 and Figure 6). The distribution of rotifers showed more species but fewer numbers, which was consistent with previous studies and presented the spatial distribution of rotifer density and species in the two lakes. This reinforces the results of previous studies and illustrates the difference in the spatial distribution of the rotifers’ density and species between the two lakes.
Oxygen is required for zooplankton’s respiration; they mainly obtain oxygen for respiration from dissolved oxygen in the water column. In addition, the dissolved oxygen content also indirectly responds to the water quality condition, and it is generally lower in water bodies with poor water quality [41,42]. The zooplankton community structure depends on the water quality conditions; so, the change in the dissolved oxygen content in the water column will also cause the zooplankton community structure to evolve. Therefore, it is also an important factor affecting the zooplankton community structure. In this study, Keratella cochlearis and Asplanchna priodonta showed a significant positive correlation with the dissolved oxygen concentration.
5.3. Effect of Land Use on Zooplankton Community Structure
Land use is an important driver of the zooplankton community composition and can influence physicochemical parameters such as the water quality in lakes, where land types such as agricultural land have been shown to be a major source of nutrient inputs to freshwater lakes [43,44]. Regarding the lakes in this study, Lake Chen Yao is surrounded by farmland and covers a wide area, while Lake Feng Sha is near the mountainous area and the distribution of land use types is dominated by mountain and forest vegetation. The survey and analysis results show that the content of nutrient salts in Lake Chen Yao is appreciably higher than that in Lake Feng Sha (Table 1), which is consistent with previous studies. The land use type contributes considerably to the composition and distribution of zooplankton communities. This can be seen in the difference between the breeding and growth of the zooplankton communities in the two lakes in the different growing seasons.
In this study, the number of copepod species in Lake Chen Yao was lower than that in Lake Feng Sha, presumably because copepods are larger and more easily predated by fish [45]; previous studies have shown that fish predation and the distribution of aquatic vegetation as well as the stability of water levels are important factors influencing changes in the zooplankton community structure [46,47]. Under the same conditions, filter-feeding fish will prefer zooplankton with weaker escape ability and larger individuals [48], and the larger crustacean post-larvae are at a competitive disadvantage compared to rotifers, leading to the miniaturization of the post-larvae [49]. Due to the wide coverage of agricultural fields and the frequent agricultural activities, Lake Chen Yao is covered with aquatic vegetation all year round, so the nutrient content of N and P and the concentrations of Chl.a are higher, which promotes the growth of some fish. On the other hand, compared with Lake Chen Yao, the water level of Lake Feng Sha is stable and less volatile throughout the year. These conditions encourage the growth of large zooplankton. The water level of Lake Chen Yao fluctuated greatly during the investigation period because of the extreme floods that started in the summer of 2020 and continued through the end of 2020. The perennial water level was lower than that of Lake Feng Sha, especially in the background of a low water level in winter and spring, which was still lower than that of Lake Feng Sha (Figure 3). Such low water levels are not conducive to the growth of zooplankton. The increase in the nutrient content of N, P, and other nutrients, as well as the increase in the Chl.a concentration, promoted the reproduction and growth of the phytoplankton community and thus also favored the growth and reproduction of the zooplankton community. As a result, the density and biomass of the zooplankton community in Lake Chen Yao were higher than those in Lake Feng Sha (Figure 8). This phenomenon was closely related to the difference in the distribution of the land use types around the two lakes.
6. Conclusions
This study focused on the seasonal dynamics of zooplankton communities in Lake Chen Yao and Lake Feng Sha and their relationships with aquatic parameters and land use. The land use types around the two lakes are mainly farmland, mountain and forest vegetation, and towns, among which the distribution around Lake Chen Yao is mainly farmland and towns. The wide distribution of farmland had a notable impact on the change in its water quality. The difference in the water quality of the two lakes, due to the distribution of farmland and forest vegetation, results in different zooplankton community characteristics. The zooplankton in the two lakes were dominated by small zooplankton rotifers, and the zooplankton community structure did not differ much among the sample points, but the seasonal differences were considerable. The seasonal variation trend of the overall density of zooplankton in Lake Chen Yao followed the order of spring > winter > autumn > summer, while the seasonal variation trend of the overall biomass followed the order of winter > spring > autumn > summer. The seasonal trends of the overall density and biomass of zooplankton in Lake Feng Sha were in the following order: autumn > summer > winter > spring. Both the zooplankton density and biomass of Lake Chen Yao were higher than those of Lake Feng Sha.
The Pearson’s correlation analysis and redundancy analysis of the zooplankton and aquatic parameters revealed that the dissolved oxygen concentration, ammonia nitrogen concentration, Chl.a concentration, and the water temperature, depth, pH, and conductivity were closely related to the community structure of the zooplankton in the two lakes, wherein conductivity, pH, DO, and Chl.a concentration were the main factors affecting the community structure of the zooplankton in the two lakes. Through comprehensive physical and chemical indicators, the zooplankton diversity index, the comprehensive nutritional status index, the indicator species of the dominant zooplankton species, etc., it has been concluded that from October 2020 to August 2021, the water quality of the two lakes generally corresponded to the β-moderate pollution type and the nutrient levels were all at moderate eutrophication levels.
There are some limitations in the overall study, which were due to the few previous studies on the joint effects of land use patterns and aquatic parameters on zooplankton and the lack of in-depth discussion on phytoplankton data.
Author Contributions
Conceptualization: Z.Z. and W.C.; Data curation: S.Z. and W.L.; Investigation: S.Z. and W.L.; Methodology: Z.Z.; Writing—original draft: S.Z.; Writing—review and editing: S.Z., Z.Z. and W.C.; Funding acquisition: Z.Z. and W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Aquatic Biological Resources Monitoring in Key Watershed of Anhui Province (No. ZF2021-18-0786), and the Anhui Provincial Natural Science Foundation (No. 1908085QC128).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from Siyong Zhang. E-mail: zhangsiyong2022@163.com.
Acknowledgments
We would like to thank the personnel of Lake Chen Yao and Lake Feng Sha Nature Reserve for their field support. We thank Mengmeng Zhou and Xinlin Rong for their help and support in the experiment. We would like to thank Marci Baun from the University of California, Los Angeles, for editing the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carrasco Navas-Parejo, J.C.; Corzo, A.; Papaspyrou, S. Seasonal cycles of phytoplankton biomass and primary production in a tropical temporarily open-closed estuarine lagoon—The effect of an extreme climatic event. Sci. Total Environ. 2020, 723, 138014. [Google Scholar] [CrossRef] [PubMed]
- Muhid, P.; Davis, T.W.; Bunn, S.E.; Burford, M.A. Effects of inorganic nutrients in recycled water on freshwater phytoplankton biomass and composition. Water Res. 2013, 47, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Jiang, F.Y.; Chen, H.; Dibar, D.T.; Wu, Q.L.; Zhou, Z.Z. Temporal and spatial variations in zooplankton communities in relation to environmental factors in four floodplain lakes located in the middle reach of the Yangtze River, China. Environ. Pollut. 2019, 251, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Helland, I.P.; Freyhof, J.; Kasprzak, P.; Mehner, T. Temperature sensitivity of vertical distributions of zooplankton and planktivorous fish in a stratified lake. Oecologia 2007, 151, 322–330. [Google Scholar] [CrossRef]
- Banerjee, A.; Chakrabay, M.; Rakshit, N.; Bhowmick, A.R.; Ray, S. Environmental factors as indicators of dissolved oxygen concentration and zooplankton abundance: Deep learning verus traditional regression approach. Ecol. Indic. 2018, 100, 99–117. [Google Scholar] [CrossRef]
- Wang, W.; Chen, F.; Gu, X.H. Community structures of zooplankton and its relation to environmental factors in five medium reservoirs in Nanjing city. J. Lake Sci. 2017, 29, 216–223. [Google Scholar]
- Mamun, A.A.; Didarul, A.M.; Aysha, A.; Xu, H.L.; Shafiqul, I.M.; Hena, M.K.A.; Muslem, U.M.; Wahidul, A.M. Annual pattern of zooplankton communities and their environmental response in a subtropical maritime channel system in the northern Bay of Bengal, Bangladesh. Acta Ecol. Sin. 2018, 37, 65–73. [Google Scholar]
- Auer, M.T.; Storey, M.L.; Effler, S.W.; Auer, N.A.; Sze, P. Zooplankton impacts on chlorophyll and transparency in Onondaga Lake, New York, USA. Hydrobiologia 1990, 200, 603–617. [Google Scholar] [CrossRef]
- Liberoff, A.L.; Flaherty, S.; Hualde, P.; García Asorey, M.I.; Fogel, M.L.; Pascual, M.A. Assessing land use and land cover influence on surface water quality using a parametric weighted distance function. Limnologica 2019, 74, 28–37. [Google Scholar] [CrossRef]
- Pratt, B.; Chang, H. Effects of land cover, topography, and built structure on seasonal water quality at multiple spatial scales. J. Hazard. Mater. 2012, 209–210, 48–58. [Google Scholar] [CrossRef]
- Mello, K.D.; Valente, R.A.; Randhir, T.O.; Alves dos Santos, A.C.; Vettorazzi, C.A. Effects of land use and land cover on water quality of low-order streams in Southeastern Brazil: Watershed versus riparian zone. Catena 2018, 167, 130–138. [Google Scholar] [CrossRef]
- Dhanasekaran, M.; Bhavam, P.S.; Manickam, N.; Kalpana, R. Physico-chemical characteristics and zooplankton diversity in a perennial lake at Dharmapuri (Tamil Nadu, India). J. Entomol. Zool. Stud. 2017, 5, 285–292. [Google Scholar]
- Sun, G.; Lang, Y.; Fang, Y. Characteristics of the post-zooplankton community in the aquatic ecosystem of South Lake Changchun. J. Jilin Univ. Sci. 2006, 44, 663–667. [Google Scholar]
- Xie, P.; Yang, Y. Long-term changes of Copepoda community (1957–1996) in a subtropical Chinese lake stocked densely with planktivorous filter-feeding silver and bighead carp. J. Plankton Res. 2000, 22, 1757–1778. [Google Scholar] [CrossRef][Green Version]
- Jeppesen, E.; Jensen, J.P.; Sondergard, M.; Lauridsen, T.; Pedersen, L.J.; Jensen, T. Top-down control in freshwater lakes: The role of nutrient state, submerged macrophytes and water depth. Hydrobiologia 1997, 342–343, 151–164. [Google Scholar] [CrossRef]
- Turner, J.T. Zooplankton community grazing impact on a bloom of Alexandrium fundyense in the Gulf of Maine. Harmful Algae 2010, 9, 578–589. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Reichwaldt, E.S.; Ghadouani, A. Effects of rainfall patterns on toxic cyanobacterial blooms in a changing climate: Between simplistic scenarios and complex dynamics. Water Res. 2012, 46, 1372–1393. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.B. Estimation of return period of flood in Chenyao Lake Basin of Anhui Province in 2016. Harnessing Huaihe River 2016, 12, 57. [Google Scholar]
- Zhang, H. Practice and discussion of wetland restoration technology in Lake Chen Yao. Anhui Agron. Bull. 2021, 27, 166–168. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Zhao, W.Q.; Guo, W.L.; Zhou, Z.Z. Effects of zonal mowing experiment of gorgonians on the community structure of post-zooplankton in Chen Yao Lake. J. Ecol. 2022, 42, 400–409. [Google Scholar]
- Shang, N.X.; Zhang, K.; Yuan, S.Q.; Meng, S.; Zhou, Z.Z. Community structure and environmental impact factors of post-zooplankton in Shengjin Lake after the removal of seine nets. J. Water Ecol. 2022, 43, 86–94. [Google Scholar] [CrossRef]
- Li, X.R. Study on Influencing Factors of determination of chlorophyll a in water by acetone extraction spectrophotometry. Chin. Soc. Environ. Sci. 2015, 1, 1300–1307. [Google Scholar]
- Wu, S.H.; Wu, C.H.; Xue, W.C. Determination of total phosphorus by ammonium molybdate spectrophotometry. Guangzhou Chem. Ind. 2020, 48, 96–97. [Google Scholar]
- Gu, D.J.; Liu, Q. Determination of total nitrogen in water by Ultraviolet Spectrophotometry with alkaline potassium persulfate digestion. Shandong Chem. Ind. 2020, 49, 103–105. [Google Scholar]
- Chen, Z.Q. Study on the determination of ammonia nitrogen in water samples by spectrophotometric method with nano reagent. Sci. Technol. Innov. 2020, 11, 34–35. [Google Scholar]
- Dong, Z.H.; Zhang, J. Interference and rapid removal of iron ions from nitrate nitrogen determined by UV spectrophotometry. Chem. Anal. Metrol. 2021, 30, 17–20. [Google Scholar]
- Chen, S.Z. Collection and counting methods of freshwater plankton. Bull. Biol. 1955, 6, 52–55. [Google Scholar]
- Wu, C.; Zhang, Q.G. Progress of freshwater plankton diversity and quantitative analysis methods. Anhui Agric. Bull. 2009, 15, 41–42. [Google Scholar]
- Zhao, F.; Yu, H.X.; Ma, C.X.; Junlin, S. Assessment of water quality in Zhalong Nature Reserve Zone by zooplankton communities. Chin. J. Fish. 2013, 26, 41–45. [Google Scholar]
- Chen, Q.; Cheng, G.P.; Li, Y.H.; Ji, Y.J.; Sang, M.Y.; Zuo, T.; Zhao, T.L. Characteristics of plankton population structure in cage culture area of Yongjiang River in Nanning. Oceanol. Limnol. Sin. 2013, 44, 49–55. [Google Scholar]
- Li, Z.F.; Xie, J.; Zhang, X.K.; Wang, G.J.; Lian, Y.X.; Yu, D.G.; Wang, C.C.; Yu, E.M.; Zhang, K. Community characteristics of zooplankton and assessment of water quality in high yield aquaculture ponds of the Pearl River Delta Acta Hydrobiol. Sin. 2017, 41, 1071–1079. [Google Scholar]
- Jin, C.; Zhang, W.T.; Sun, Z. Discussion on evaluation method and grading standard of lake and reservoir eutrophication. Guizhou Water Power 2011, 25, 4–6. [Google Scholar]
- Wang, M.C.; Liu, X.Q.; Zhang, J.H. Evaluation method and classification standard of lake eutrophication. Environ. Monit. China 2002, 5, 47–49. [Google Scholar]
- Wang, T.T.; Yi, Q.T.; Hu, Y.B.; Yan, J.P.; Yu, H.J.; Dong, X.L. Simulation experiment on eutrophication and nitrogen and phosphorus limitation of water body in coal mining subsidence area of Huaibei and Huaihe River. J. Lake Sci. 2013, 25, 916–926. [Google Scholar]
- Chu, Y.F.; Zhao, S.S.; Li, G.G.; Jin, T.X.; Ma, J.M. Plankton Community Structure and Evaluation of Water Quality in Chenqiao East Lake Wetland J. Yangtze River Sci. Res. Inst. 2019, 36, 23–29. [Google Scholar]
- He, M. Study on the Effect of Climate Warming and Water Eutrophication on Zooplankton Community Structure in Shallow Lakes. Master’s Thesis, Zhongnan University for Nationalities, Wuhan, China, 2016. [Google Scholar]
- Lv, Z.W.; Chen, Y.Q.; Peng, J.H.; Wu, Y.M.; Tang, Y.J.; Chen, X.P.; Su, H.Y.; Zeng, L.R. Evaluation of zooplankton diversity and water quality biology in Nansha wetland, Guangzhou. Green Technol. 2021, 23, 31–35+40. [Google Scholar] [CrossRef]
- García-Chicote, J.; Armengol, X.; Rojo, C. Zooplankton species as indicators of trophic state in reservoirs from Mediterranean river basins. Inland Waters 2019, 9, 113–123. [Google Scholar] [CrossRef]
- Bai, H.F.; Zhao, N.X.; Yin, X.W.; Lu, Y.Y.; Wu, W.; Xu, Z.X. Community structure of zooplankton in the Weihe River Basin and its relationship with environmental factors. J. Dalian Ocean Univ. 2014, 29, 260–266. [Google Scholar]
- Leira, M.; Cantonati, M. Effects of water-level fluctuations on lakes: An annotated bibliography. Hydrobiologia 2008, 613, 171–184. [Google Scholar] [CrossRef]
- Sanchez, E.; Colmenarejo, M.F.; Vicente, J.; Rubio, A.; Gancia, M.G.; Tranvieso, L.; Borja, R. Use of the water quality index and dissolved oxygen deficit as simple indicators of watersheds pollution. Ecol. Indic. 2007, 7, 315–328. [Google Scholar] [CrossRef]
- Katsiapi, M.; Mazaris, A.D.; Charalampous, E.; Mouataka-Gouni, M. Watershed land use types as drivers of freshwater phytoplankton structure. Hydrobiologia 2012, 698, 121–131. [Google Scholar] [CrossRef]
- Liao, J.Q.; Zhao, L.; Cao, X.F.; Sun, J.H.; Gao, Z.; Wang, J.; Jiang, D.; Fan, H.; Huang, Y. Cyanobacteria in lakes on Yungui Plateau, China are assembled via niche processes driven by water physicochemical property, lake morphology and watershed land-use. Sci. Rep. 2016, 6, 36357. [Google Scholar] [CrossRef]
- Špoljar, M.; Tomljanović, T.; Dražina, T.; Lajtner, J.; Štulec, H.; Matulić, D.; Fressl, J. Zooplankton structure in two interconnected ponds: Similarities and differences. Croat. J. Fish. 2016, 74, 6–13. [Google Scholar] [CrossRef]
- Lin, Z.; Wan, Y.; Xu, M.; Wang, C.C.; Wu, Q.L.; Zhou, Z.Z. Community structure of lacustrine post-larval zooplankton and its influencing factors in lakes in the coal mining subsidence area of Huainan Digou. Lake Sci. 2018, 30, 171–182. [Google Scholar]
- Akhurst, D.J.; Jones, G.B.; Clark, M.; Reichelt-Brushett, A. Effects of fish and macrophytes on phytoplankton and zooplankton community structure in a subtropical freshwater reservoir. Limnologica 2017, 62, 5–18. [Google Scholar] [CrossRef]
- Xu, M.; Wu, F.Y.; Liu, L.L.; Wang, B.; Sun, Q.Y.; Zhou, Z.Z. Seasonal dynamics of zooplankton crustacean community structure in Jiao Gang Lake. J. Ecol. 2016, 35, 1254–1262. [Google Scholar]
- Yang, L.J.; Lv, G.H.; Zhu, J.Q.; Xu, Z.; Jin, C.H. Structural characteristics of the post-zooplankton community and water quality evaluation in Heng Shan Reservoir. J. Aquat. Biol. 2014, 38, 720–728. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).