Abstract
Dense aggregations of serpulid worms were encountered in the Daymaniyat Islands (Gulf of Oman) from 10 to 20 m depth, over the period January–March, 2021. The species responsible for these aggregations belongs to the Filograna/Salmacina-complex (Annelida: Serpulidae). This species has been present in the area and observed along the Oman coastline, but high-density aggregates like this have not been reported before. The most probable cause of the aggregations, supported by field observations and Aqua-MODIS satellite data, was natural eutrophication with a subsequent algal bloom linked to the local winter monsoon. This observation emphasises the importance of documenting biodiversity and dynamics of reef communities along the Oman coastline.
Keywords:
algal blooms; outbreak; bioindicator; coral reefs; eutrophication; infestation; Serpulidae; monsoon; Daymaniyat Islands As sedentary filter-feeders in coastal waters, tube-dwelling polychaetes of the families Sabellidae and Serpulidae are often considered bioindicators owing to potential increases in their abundance in relation to eutrophication [1,2,3]. Some serpulids occur in clusters and are considered habitat formers, especially as fouling organisms on manmade substrates [4,5]. Furthermore, serpulid worms account for 15% of the alien polychaetes species recognized worldwide [6,7,8].
Dense aggregations and outbreaks of Serpulidae can be opportunistic responses to changes in environmental conditions [9], especially to nutrient pollution [10]. These worms may thrive in conditions that are unfavourable to many other marine fauna [11,12]. The aggregations often develop in sheltered areas, sometimes at salinity levels outside the normal oceanic range [9,13], and with limited water movement facilitating larval settlement [14].
In January–March, 2021, dense aggregations of serpulid worms were observed in reef communities of Jabal Al Kabir Island (also known as D3 Island) in the Daymaniyat Islands Nature Reserve, north of Oman (Figure 1). The worms were mainly overgrowing hard substrates in the sheltered bays and seaward cliffs, forming fragile, branching clusters up to 20 cm in diameter from 10 to 20 m depth (Figure 2). The aggregations occurred during a phytoplankton bloom and were relatively short-lived. By the end of April, the density of the worms had decreased, and only remnants of the tube clusters remained. They were no longer evident in February 2022 when we revisited the area.
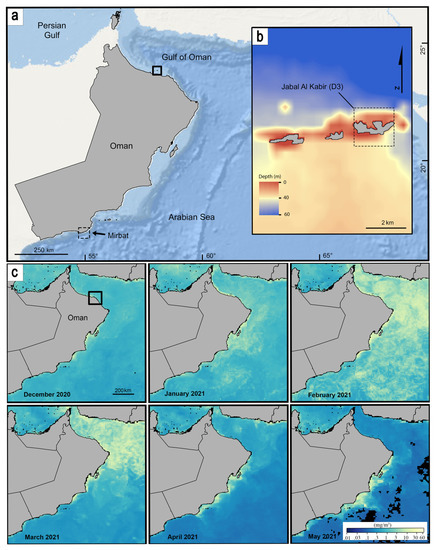
Figure 1.
(a) Coastline of Oman with the Daymaniyat Islands in the north (inset). (b) The inset shows Jabal Al Kabir (D3) bathymetric data around the islands. Daymaniyat Islands consist of nine uninhabited islands (also called aD1–D9 islands), composed of Miocene limestones uplifted by Pliocene folding [15]. The northern shores have small cliffs and narrow embayments, whereas sandy beaches line most of the southern side of the islands. (c) Image series of monthly average concentration of Chlorophyll data from the Aqua/MODIS satellite. Notice the high concentration in February-March, 2021, representing algal blooms.

Figure 2.
Serpulid aggregations of Filograna/Salmacina-complex. (a,b) Pseudo-colonies forming fragile constructions, Jabal Al Kabir (D3). (c) Individual tubes covered by algae. Green arrows: inflated tips of the radioles. Pinnately branched radioles resemble octocoral polyps with eight tentacles and pinnules, Bandar Al Khiran. (d) Aggregations over hard surfaces and crustose coralline algae, Daymaniyat islands, D3 (photos (a,b,d), J. Al Asfoor; (c), M. Claereboudt).
These serpulids, although uncommon, have been present in the area and observed along the Oman coastline as individuals or in small clusters. However, high density aggregates and outbreaks of these worms have not been reported until now and we do not have field observations to confirm the formation of high-density aggregations in other locations.
The observed species can be attributed to the Filograna/Salmacina-complex. While nearly 800 polychaete species, including 48 serpulids, have been recorded from the waters around the Arabian Peninsula, only two nominal species have been reported in this complex: Filograna implexa Berkeley, 1835, and Salmacina dysteri Huxley, 1855 [16]. Filograna implexa is the only valid species in that genus [17], characterised by two spoon-shaped opercula. The genus Salmacina, which comes closest to our specimens, includes nine species and one uncertain attribution, all non-operculate, some with inflated radiole tips (Figure 2c). Poor descriptions of most species in this group, and lack of assessment of intra-specific variability, make it currently impossible to confidently identify the specimens.
Molecular studies have shown that some of the previously reported serpulids with wide, almost circumtropical distributions are actually a mix of several taxa, each with restricted regional distributions [18,19,20]. For example, the widespread taxa Spirobranchus kraussii and S. tetraceros (both recorded from Arabian Seas), appear each to consist of more than six species, all with geographically limited distributions [18,19]. The same is likely true for the taxa Filograna implexa and Salmacina dysteri, both originally described from the temperate coasts of south-eastern Great Britain and later reported from around the globe, including the Arabian Seas [16,17,21]. Although it is likely that the worms encountered in Oman represent a new species, any further identification requires genetic studies and a taxonomic revision to establish diversity and relationship within the Filograna/Salmacina complex [21,22].
Filograna/Salmacina species construct calcareous tubes attached to hard substrates. The individual adults are small, usually less than 350 µm in diameter, and a length of only a few millimetres [11,23]. They reproduce sexually, and asexually by scissiparity. Even if sexual reproduction can contribute to the growth of aggregates [23], the branching tube pattern is a consequence of asexual reproduction [24]. Although the worms and aggregates observed in the Daymaniyat Islands show signs of both sexual and asexual reproduction, the asexual reproduction is likely the main mode of “pseudo-colony” formation [23,24], followed by settlement of larvae on conspecific tubes [9,25]. The tube accretion rate depends on environmental parameters, such as water temperature, salinity, food availability, and can reach up to several millimetres per day in S. dysteri [23]. As a result, pseudo-colonies are formed from numerous joined branching tubes, protruding from the seabed [21,26]. Similar aggregations of tubes (Figure 2a,b) were illustrated by Dalyell [27] (for Salmacina dysteri, as Filipora filograna most probably from subtidal Scotland, North Sea), by Pernet [24] (for Salmacina amphidentata from intertidal and shallow subtidal zones of the Indian River Lagoon), by Bianchi [28] (for Filograna sp., Italy, probably Ligurian Sea), and by Enrichetti et al. [29] (for Filograna/Salmacina complex, at 30–160 m depth on a muddy–sandy seafloor of the Ligurian Sea). The fragile structures of this group often do not accrete to form reefs and are sensitive to physical disturbances [30,31], unlike some other serpulid species that can make aggregates larger than 1 m in diameter and make extensive bioherms [26,32,33,34,35]. All the aggregates encountered in our study site were also fragile and did not accrete to form reefs, but grew on the rocky surfaces (Figure 2, Figure 3 and Figure 4).

Figure 3.
Rocky walls in the seaward side of the Daymaniyat Islands, reaching about 30 m depth, covered with Filograna/Salmacina aggregations (a) Aggregations starting with tubes overgrowing surfaces, then joining up, and building thicker branches. White arrow: overgrowth on a sponge. (b) Aggregations on overhangs, in between gorgonians, such as Bebryce stellata (white arrow), and Astrogorgia sp. (square outline), slowly getting smothered by the overgrowing worms. (c) Worms growing over rocks, crustose coralline algae, and sponges. (d) View of the wall in upward direction, with worm pseudo-colonies up to 20 cm in diameter (photos J. Al Asfoor).

Figure 4.
Filograna/Salmacina aggregations south of Oman, Dhofar region. (a) Worm clusters overgrowing black coral stem. (b–e) Aggregations growing over rocks, crustose coralline algae, and sponges. (f) Square outline in figure e, showing the branching asexual pattern, as described by Pernet [24]) (photos M. Claereboudt, G. Paulay).
Nutrient levels in coastal waters of Oman are mostly linked to monsoonal cycles. A strong, moist, summer southwest monsoon, and a weaker, dry, winter northeast monsoon, both result in upwelling and advection of nutrients to the surface in coastal water [36,37]. These are reflected in the algal bloom patterns, with two annual peaks in January–April and August–September along the northern Oman coastline [38].
Increase in nutrient concentrations create a cascade of effects: shifts in phytoplankton composition and biomass, increase in the abundance of phytoplankton grazers, followed by phytoplankton die-off, decomposition, and oxygen depletion [39,40] particularly below the thermocline, occasionally accompanied by mass mortalities of other organisms [41,42,43,44]. These natural cycles in productivity contribute to ocean acidification and specialized shallow-water communities [45].
Our field observations together with Chlorophyll–a data obtained from Aqua–MODIS satellite, confirmed an algal bloom during February–March, 2021, in the Daymaniyat Islands, with monthly averages of 11.17 mg/m3 and 4.65 mg/m3 [46] (23.8° N, 58.1° E; 0.1°–pixel), presumably driven by elevated nutrient levels in the water column. The temporal correlation between the high abundance of Filograna/Salmacina and the phytoplankton bloom, the rapid growth rate of these animals, and the tendency of serpulids to respond to elevated food levels, all suggest that the bloom could be partly responsible for the outbreak.
During field work in the Arabian Sea coast of Oman around Mirbat (Figure 1) in January 2022, we encountered smaller aggregations of what appeared to be the same species (Figure 4, vouchers deposited at Florida Museum of Natural History, UF Annelida 10242, 10255, 10456). This coast undergoes much more intense upwelling than the Gulf of Oman, and therefore these worms might regularly bloom in that area, lending support to phytoplankton productivity driving these population increases.
Although serpulid outbreaks could be a sign of environmental degradation, it seems that they responded indirectly here to a natural increase in planktonic productivity driven by upwelling-enhanced nutrient levels. It is unknown how these serpulids affected the benthic communities in the Daymaniyat Islands, but they could potentially increase water clarity through their suspension feeding [47] and affect their habitat by providing shelter, food, and substrate for epibiont organisms [9,48,49,50]. We did not observe any sign of smothering or overgrowth on corals, unlike serpulid infestations in the Persian Gulf [51] and the Gulf of Oman following the 2008–2009 Cochlodinium polykrikoides bloom [42], and high densities of Spirobranchus in the Caribbean [50].
These observations illustrate the need for a better taxonomic coverage of invertebrate biodiversity in the region and the importance of long-term monitoring of benthic communities.
Author Contributions
Conceptualization, K.S.-N. and B.W.H.; validation, K.S.-N., H.A.t.H., M.R.C., G.P. and B.W.H.; formal analysis, K.S.-N., H.A.t.H., G.P., M.R.C. and B.W.H.; investigation, K.S.-N., H.A.t.H., G.P., M.R.C. and B.W.H.; data curation, K.S.-N.; writing—original draft preparation, K.S.-N., H.A.t.H., G.P., M.R.C. and B.W.H.; writing—review and editing, K.S.-N., H.A.t.H., G.P., M.R.C. and B.W.H.; visualization, K.S.-N. All authors have read and agreed to the published version of the manuscript.
Funding
The research and partial fieldwork supported by The Richard Lounsbery Foundation, Alfred P. Sloan Foundation, the Census of Marine Life are gratefully acknowledged for the research grants to K.S.-N., and by NSF DEB-1457817 to G.P.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to J. Al Asfoor for the images and partial field work, and E. Looker (Five Oceans Environmental Services LLC) for support. J.H. Ausubel (Rockefeller University), L. Brown (Lounsbery Foundation), and L. C. Woodall (Oxford University) are greatly appreciated for their continued support and encouragement. Three reviewers are appreciated for their constructive comments and suggestions, which helped improve the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Yoana, D.-P.-R.; Jose Antonio, D.-O.-C.; Angel, L.-F.; Luis Miguel, F.-V.; Francisca, G.-C.; Jose Luis, S.-L. Assessment of soft-bottom Polychaeta assemblage affected by a spatial confluence of impacts: Sewage and brine discharges. Mar. Pollut. Bull. 2009, 58, 776–782. [Google Scholar] [CrossRef]
- Elías, R.; Méndez, N.; Muniz, P.; Cabanillas, R.; Gutiérrez-Rojas, C.; Rozbaczylo, N.; Londoño-Mesa, M.H.; Gárate Contreras, P.J.; Cárdenas-Calle, M.; Villamar, F.; et al. Los poliquetos como indicadores biológicos en Latinoamérica y el Caribe. Mar. Fish. Sci. 2020, 34, 37–107. [Google Scholar] [CrossRef]
- Van der Schoot, R.J.; Hoeksema, B.W. Abundance of coral-associated fauna in relation to depth and eutrophication along the leeward side of Curaçao, southern Caribbean. Mar. Environ. Res. 2022, 181, 105738. [Google Scholar] [CrossRef]
- Tovar-Hernández, M.A.; Méndez, N.; Villalobos-Guerrero, T.F. Fouling polychaete worms from the Southern Gulf of California: Sabellidae and Serpulidae. Syst. Biodivers. 2009, 7, 319–336. [Google Scholar] [CrossRef]
- Musco, L.; Licciano, M.; Giangrande, A. Diversity and distribution of Sabellida (Annelida) under protection regimes. Water 2021, 13, 1491. [Google Scholar] [CrossRef]
- Ben-Eliahu, M.N.; ten Hove, H.A. Serpulidae (Annelida: Polychaeta) from the Suez Canal—From a Lessepsian migration perspective (a Monograph). Zootaxa 2011, 2848, 1. [Google Scholar] [CrossRef]
- Grosse, M.; Pérez, R.; Juan-Amengual, M.; Pons, J.; Capa, M. The elephant in the room: First record of invasive gregarious species of serpulids (calcareous tube annelids) in Majorca (western Mediterranean). Sci. Mar. 2021, 85, 15–28. [Google Scholar] [CrossRef]
- Çinar, M.E. Alien polychaete species worldwide: Current status and their impacts. J. Mar. Biol. Assoc. UK 2013, 93, 1257–1278. [Google Scholar] [CrossRef]
- Ten Hove, H.A.; van den Hurk, P. A review of Recent and fossil serpulid “reefs”; actuopalaeontology and “upper Malm” serpulid limestones in NW Germany. Geol. Mijnb. 1993, 72, 23–67. [Google Scholar]
- Ben-Eliahu, M.N.; Dafni, J. A new reef-building serpulid genus and species from the Gulf of Eilat and the Red Sea, with notes on other gregarious tubeworms from Israeli waters. Isr. J. Ecol. Evol. 1979, 28, 199–208. [Google Scholar]
- Ten Hove, H.A. Different causes of mass occurrence in serpulids. In Biology and Systematics of Colonial Organisms; Larwood, G.P., Rosen, B.R., Eds.; Academic Press: London, UK; New York, NY, USA, 1979; pp. 281–298. [Google Scholar]
- Ferrero, L.; Obenat, S.; Zárate, M.A. Mid-Holocene serpulid build-ups in an estuarine environment (Buenos Aires Province, Argentina). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 222, 259–271. [Google Scholar] [CrossRef]
- Glumac, B.; Berrios, L.; Greer, L.; Curran, H.A. Holocene tufa-coated serpulid mounds from the Dominican Republic: Depositional and diagenetic history, with comparison to modern serpulid aggregates from Baffin Bay. In Proceedings of the 11th Symposium on the Geology of the Bahamas and Other Carbonate Regions, San Salvadore, Bahamas; Gerace Research Center: San Salvadore, Bahamas, 2004; pp. 49–65. [Google Scholar]
- Moore, C.G.; Saunders, G.R.; Harries, D.B. The status and ecology of reefs of Serpula vermicularis L. (Polychaeta: Serpulidae) in Scotland. Aquat. Conserv. 1998, 8, 645–656. [Google Scholar] [CrossRef]
- Searle, M. Geology of the Oman Mountains, Eastern Arabia; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Wehe, T.; Fiege, D. Annotated checklist of the polychaete species of the seas surrounding the Arabian Peninsula: Red Sea, Gulf of Aden, Arabian Sea, Gulf of Oman, Arabian Gulf. Fauna Arabia 2002, 19, 7–238. [Google Scholar]
- Read, G.; Fauchald, K. World Polychaeta Database. Available online: https://www.marinespecies.org/polychaeta (accessed on 28 August 2022).
- Simon, C.A.; Helene van Niekerk, H.; Burghardt, I.; ten Hove, H.A.; Kupriyanova, E.K. Not out of Africa: Spirobranchus kraussii (Baird, 1865) is not a global fouling and invasive serpulid of Indo-Pacific origin. Aquat. Invasions 2019, 14, 221–249. [Google Scholar] [CrossRef]
- Pazoki, S.; Rahimian, H.; Struck, T.H.; Katouzian, A.R.; Kupriyanova, E.K. A New Species of the Spirobranchus kraussii-Complex (Annelida, Serpulidae) from the Persian Gulf and Gulf of Oman. Zootaxa 2020, 4748, 401–430. [Google Scholar] [CrossRef]
- Kupriyanova, E.K.; Flaxman, B.; Burghardt, I. A puzzle no more: The identity of Spirobranchus tetraceros (Schmarda, 1861), Annelida, Serpulidae, is resolved. Rec. Aust. Mus. 2022; in press. [Google Scholar]
- Ten Hove, H.A.; Kupriyanova, E.K. Taxonomy of Serpulidae (Annelida, Polychaeta): The state of affairs. Zootaxa 2009, 2036, 1–126. [Google Scholar] [CrossRef]
- Miguel De Matos Nogueira, J.; ten Hove, H.A. On a new species of Salmacina Claparède, 1870 (Polychaeta: Serpulidae) from São Paulo State, Brazil. Beaufortia 2000, 50, 151–161. [Google Scholar]
- Nishi, E.; Nishihira, M. Multi-clonal pseudo-colony formation in the calcareous tube worm Salmacina dysteri (Huxley) (Serpulidae, Polychaeta). Nat. Hist. Res. 1997, 4, 93–100. [Google Scholar]
- Pernet, B. Escape hatches for the clonal offspring of serpulid polychaetes. Biol. Bull. 2016, 200, 107–117. [Google Scholar] [CrossRef]
- Hanson, J. Formation and breakdown of serpulid tubes. Nature 1948, 161, 610–611. [Google Scholar] [CrossRef]
- Ingrosso, G.; Abbiati, M.; Badalamenti, F.; Bavestrello, G.; Belmonte, G.; Cannas, R.; Benedetti-Cecchi, L.; Bertolino, M.; Bevilacqua, S.; Bianchi, C.N.; et al. Mediterranean bioconstructions along the Italian coast. Adv. Mar. Biol. 2018, 79, 61–136. [Google Scholar] [PubMed]
- Dalyell, J.G. The Powers of the Creator Displayed in the Creation; or, Observations on Life Amidst the Various Forms of the Humbler Tribes of Animated Nature; Volume I-III, J. van Voorst: London, UK, 1853. [Google Scholar]
- Bianchi, C.N. Policheti Serpuloidei. Guide per il Riconoscimento delle Specie Animali delle Acque Lagunari e Costiere Italiane. 5. Collana del Progetto Finalizzato “Promozione della Qualità Dell’ambiente”, Serie AQ/1/96; CNR: Roma, Italy, 1981; pp. 1–187. [Google Scholar]
- Enrichetti, F.; Dominguez-Carrio’, C.; Toma, M.; Bavestrello, G.; Betti, F.; Canese, S.; Bo, M. Megabenthic communities of the Ligurian deep continental shelf and shelf break (NW Mediterranean Sea). PLoS ONE 2019, 14, e0223949. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C. Serpuloidea (Annelida: Polychaeta) from Milos, an island in the Aegean Sea with submarine hydrothermalism. J. Mar. Biol. Assoc. UK 2000, 80, 259–269. [Google Scholar] [CrossRef]
- Chan, B. Climate Change Impacts on the Serpulid Tubeworm Hydroides elegans: A Biomineralization Perspective. Ph.D. Thesis, University of Hong Kong, Pokfulam, Hong Kong, China, 2013. [Google Scholar] [CrossRef]
- Aliani, S.; Bianchi, C.N.; de Asmundis, C.; Meloni, R. Scanning electron microscope observations on the tube of the reef-forming serpulid Ficopomatus enigmaticus (Fauvel) (Annelida, Polychaeta). Boll. Zool. 1995, 62, 363–367. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Aliani, S.; Morri, C. Present-day serpulid reefs, with reference to an on-going research project on Ficopomatus enigmaticus. Publ. Serv. Géol. Luxemb. 1995, 29, 61–65. [Google Scholar]
- Hoeksema, B.W.; ten Hove, H.A. Aggregation of the reef-building tube worm Filogranella elatensis at Semporna, eastern Sabah, Malaysia. Coral Reefs 2011, 30, 839. [Google Scholar] [CrossRef]
- Holt, T.J.; Rees, E.I.; Hawkins, S.J.; Seed, R. Biogenic Reefs, An Overview of Dynamic and Sensitivity Characteristics for Conservation Management of Marine SACs; Port Erin Marine Laboratory, University of Liverpool: Liverpool, UK, 1998. [Google Scholar]
- Tudhope, A.W.; Lea, D.W.; Shimmield, G.B.; Chilcott, C.P.; Head, S. Monsoon climate and Arabian Sea coastal upwelling recorded in massive corals from southern Oman. Palaios 1996, 11, 347–361. [Google Scholar] [CrossRef]
- Claereboudt, M.R. Oman. In World Seas: An Environmental Evaluation Volume II: The Indian Ocean to the Pacific; Sheppard, C.R.C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 25–47. [Google Scholar] [CrossRef]
- Al-Azri, A.; Piontkovski, S.; Al-Hashmi, K.; Al-Gheilani, H.; Al-Habsi, H.; Al-Khusaibi, S.; Al-Azri, N. The occurrence of algal blooms in Omani coastal waters. Aquat. Ecosyst. Health Manag. 2012, 15, 56–63. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, B.; Yao, Q.; Xue, L.; Sun, J.; Xin, M.; Yu, Z. Spatiotemporal variations in the summer hypoxia in the Bohai Sea (China) and controlling mechanisms. Mar. Pollut. Bull. 2019, 138, 125–134. [Google Scholar] [CrossRef]
- Xin, M.; Wang, B.; Xie, L.; Sun, X.; Wei, Q.; Liang, S.; Chen, K. Long-term changes in nutrient regimes and their ecological effects in the Bohai Sea, China. Mar. Pollut. Bull. 2019, 146, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Stachowitsch, M.; Riedel, B.; Zuschin, M.; Machan, R. Oxygen depletion and benthic mortalities: The first in situ experimental approach to documenting an elusive phenomenon. Limnol. Oceanogr. Methods 2007, 5, 344–352. [Google Scholar] [CrossRef]
- Richlen, M.L.; Morton, S.L.; Jamali, E.A.; Rajan, A.; Anderson, D.M. The catastrophic 2008–2009 red tide in the Arabian Gulf region, with observations on the identification and phylogeny of the fish-killing dinoflagellate Cochlodinium polykrikoides. Harmful Algae 2010, 9, 163–172. [Google Scholar] [CrossRef]
- Emery, N.C.; Ewanchuk, P.J.; Bertness, M.D. Competition and salt-marsh plant zonation: Stress tolerators may be dominant competitors. Ecology 2001, 82, 2471–2485. [Google Scholar] [CrossRef]
- Claereboudt, M.; Hermosa, G.; Mclean, E. Plausible cause of massive fish kills in the Gulf of Oman. In Proceedings of the First International Conference on Fisheries, Aquaculture and Environment in the Northern Indian Ocean, Muscat, Oman; 2001; pp. 123–132. [Google Scholar]
- Doney, S.C. The growing human footprint on coastal and open-ocean biogeochemistry. Science 2010, 328, 1512–1516. [Google Scholar] [CrossRef]
- Chlorophyll Concentration (1 Month–Aqua/MODIS) NASA. Available online: https://neo.gsfc.nasa.gov/view.php?datasetId=MY1DMM_CHLORA (accessed on 29 August 2022).
- Leung, J.Y.S.; Cheung, N.K.M. Feeding behaviour of a serpulid polychaete: Turning a nuisance species into a natural resource to counter algal blooms? Mar. Pollut. Bull. 2017, 115, 376–382. [Google Scholar] [CrossRef]
- Palmer, T.A.; Breaux, N.; Lebreton, B.; Guillou, G.; Beseres Pollack, J. Importance of serpulid reef to the functioning of a hypersaline estuary. Estuaries Coasts 2022, 45, 603–618. [Google Scholar] [CrossRef]
- Perry, O.; Sapir, Y.; Perry, G.; ten Hove, H.; Fine, M. Substrate selection of Christmas tree worms (Spirobranchus spp.) in the Gulf of Eilat, Red Sea. J. Mar. Biol. Assoc. UK 2018, 98, 791–799. [Google Scholar] [CrossRef]
- Hoeksema, B.W.; Wels, D.; van der Schoot, R.J.; ten Hove, H.A. Coral injuries caused by Spirobranchus opercula with and without epibiotic turf algae at Curaçao. Mar. Biol. 2019, 166, 60. [Google Scholar] [CrossRef]
- Samimi-Namin, K.; Risk, M.J.; Hoeksema, B.W.; Zohari, Z.; Rezai, H. Coral mortality and serpulid infestations associated with red tide, in the Persian Gulf. Coral Reefs 2010, 29, 509. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).