Bloom of Prorocentrum cordatum in Paracas Bay, Peru
Abstract
1. Introduction
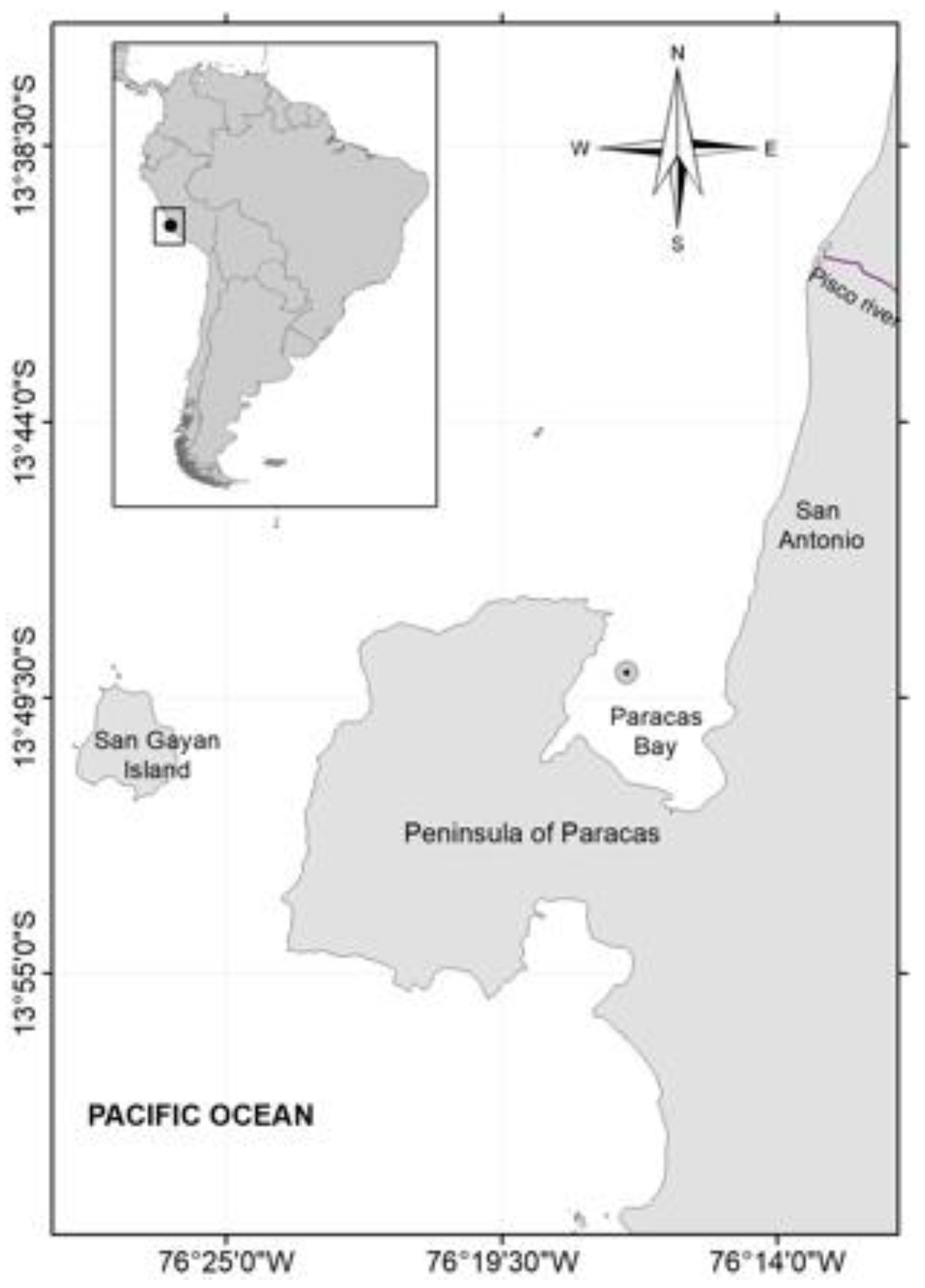
2. Materials and Methods
2.1. Environmental Parameters
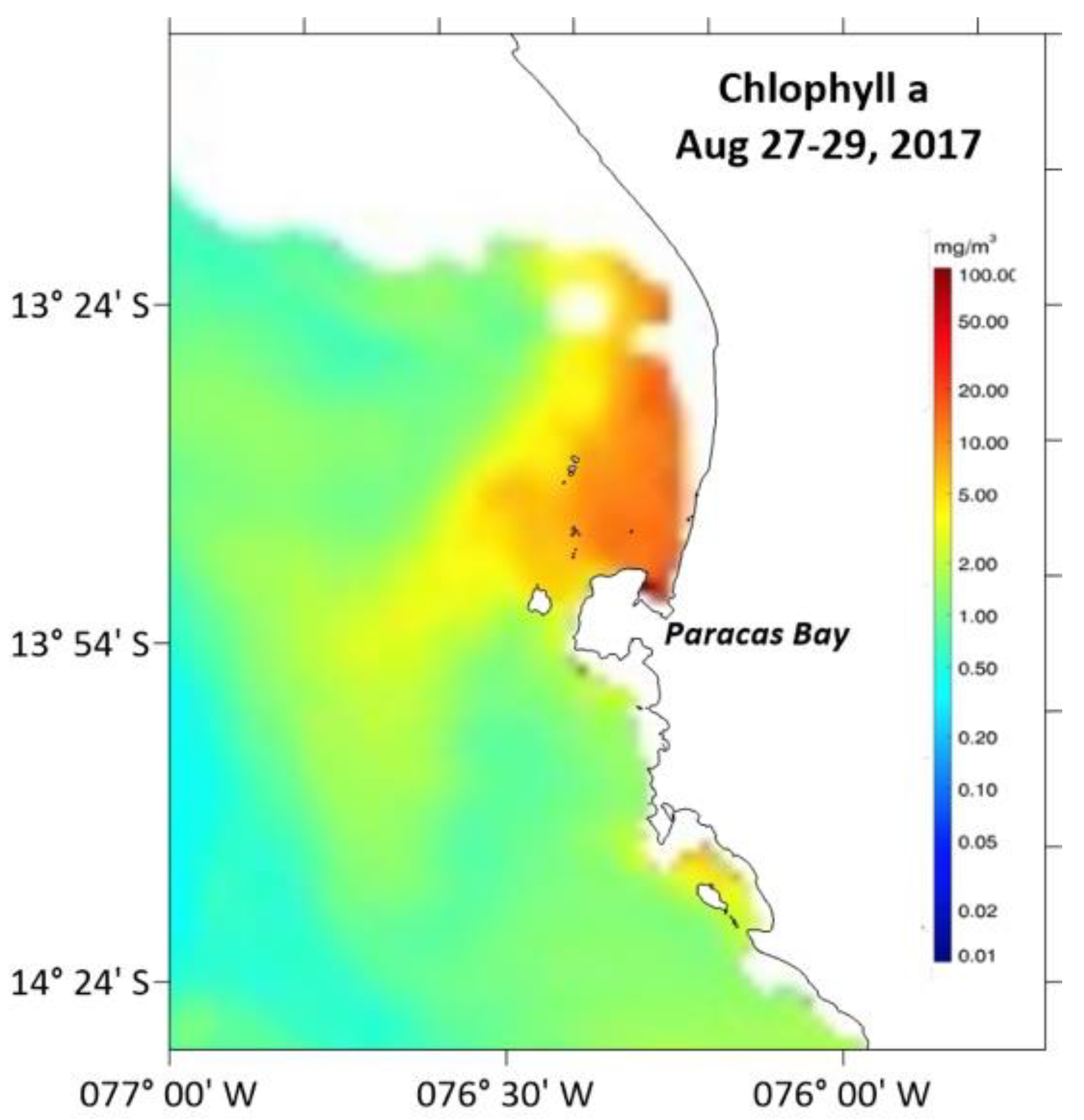
2.1.1. Satellite Imagery of Chlorophyll-a
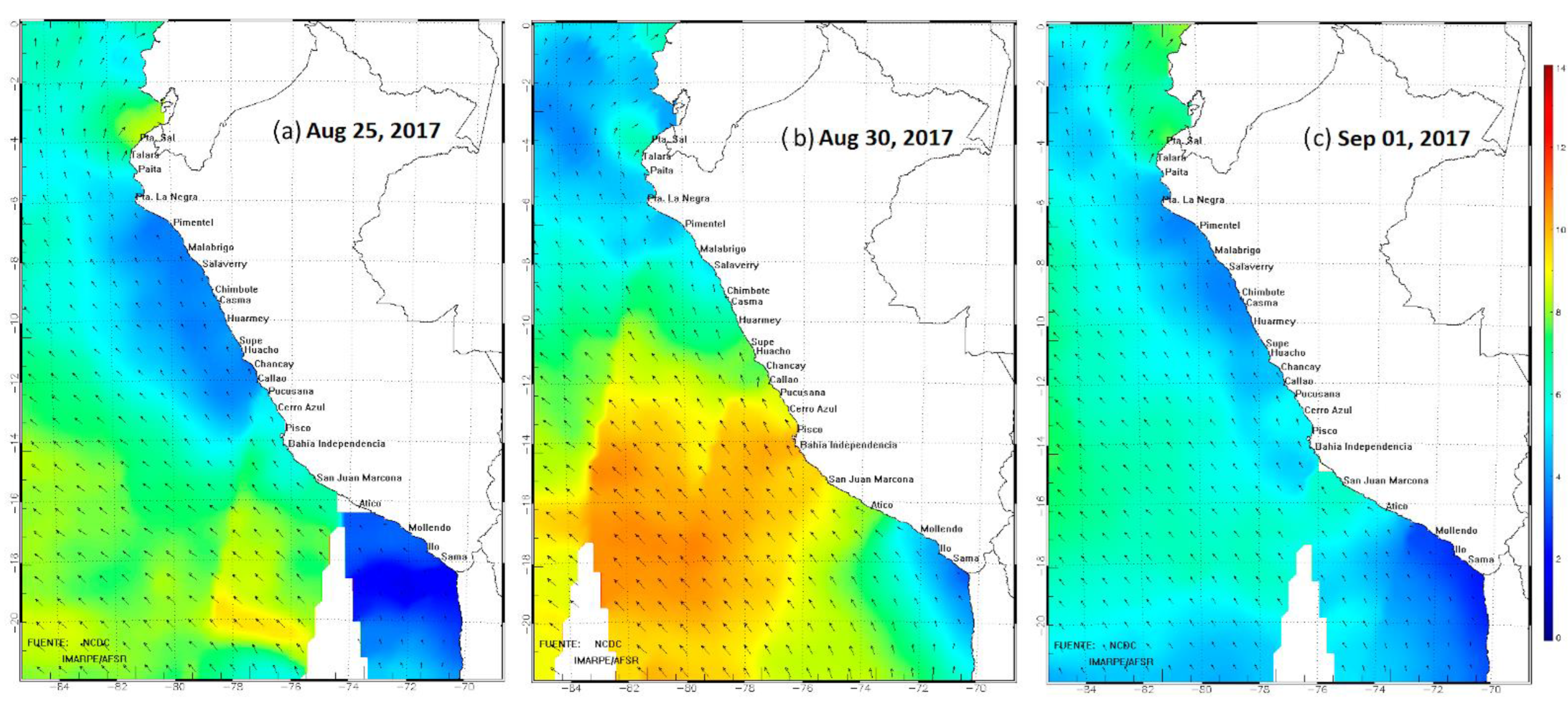
2.1.2. Satellite Wind Imagery
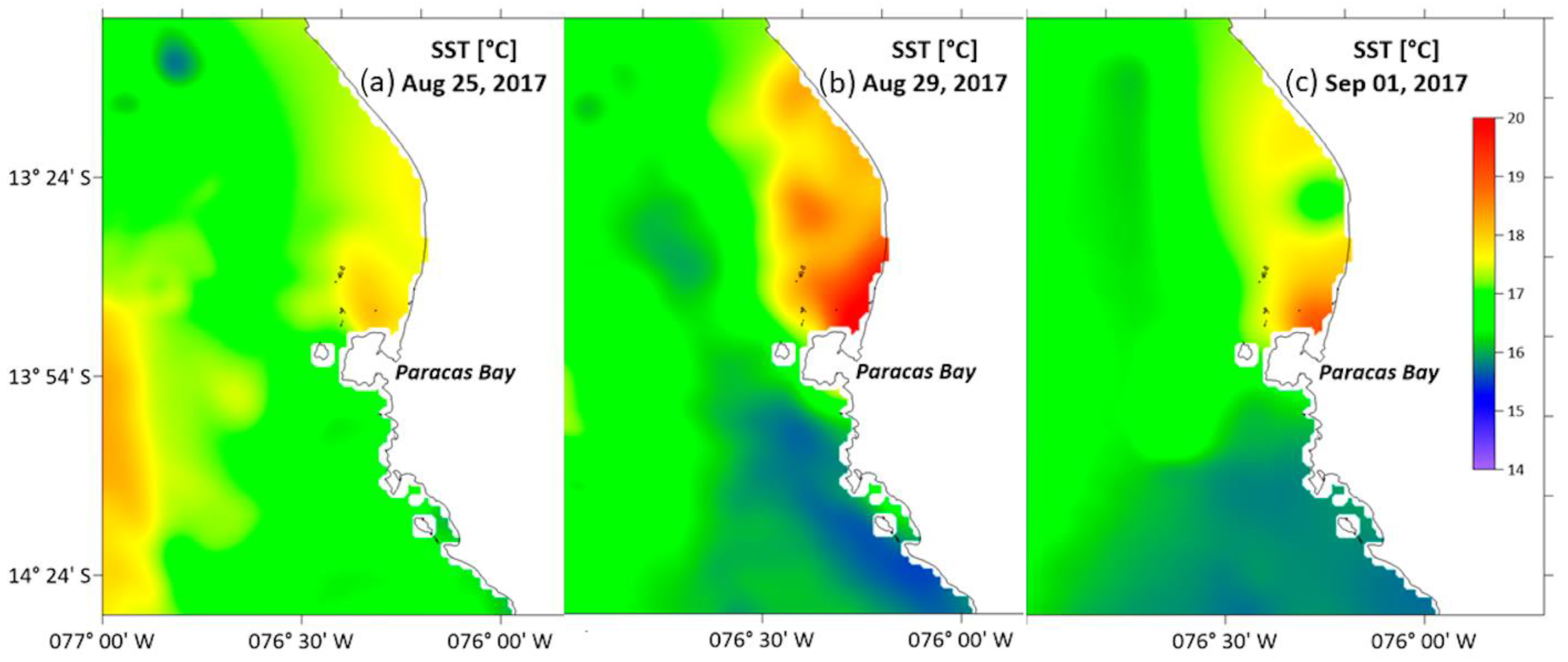
2.1.3. Satellite Sea Surface Temperature
2.2. Strain Obtention and Culture Conditions
2.3. Morphological Description
2.4. Molecular and Phylogenetic Analysis
2.4.1. DNA and PCR Extraction
2.4.2. Bioinformational Analysis or Phylogenetic Analysis
2.5. Toxin Analysis
3. Results
3.1. Environmental Parameters
3.1.1. Satellite Chlorophyll-a
3.1.2. Satellite Winds
3.1.3. Sea Surface Temperature (SST)
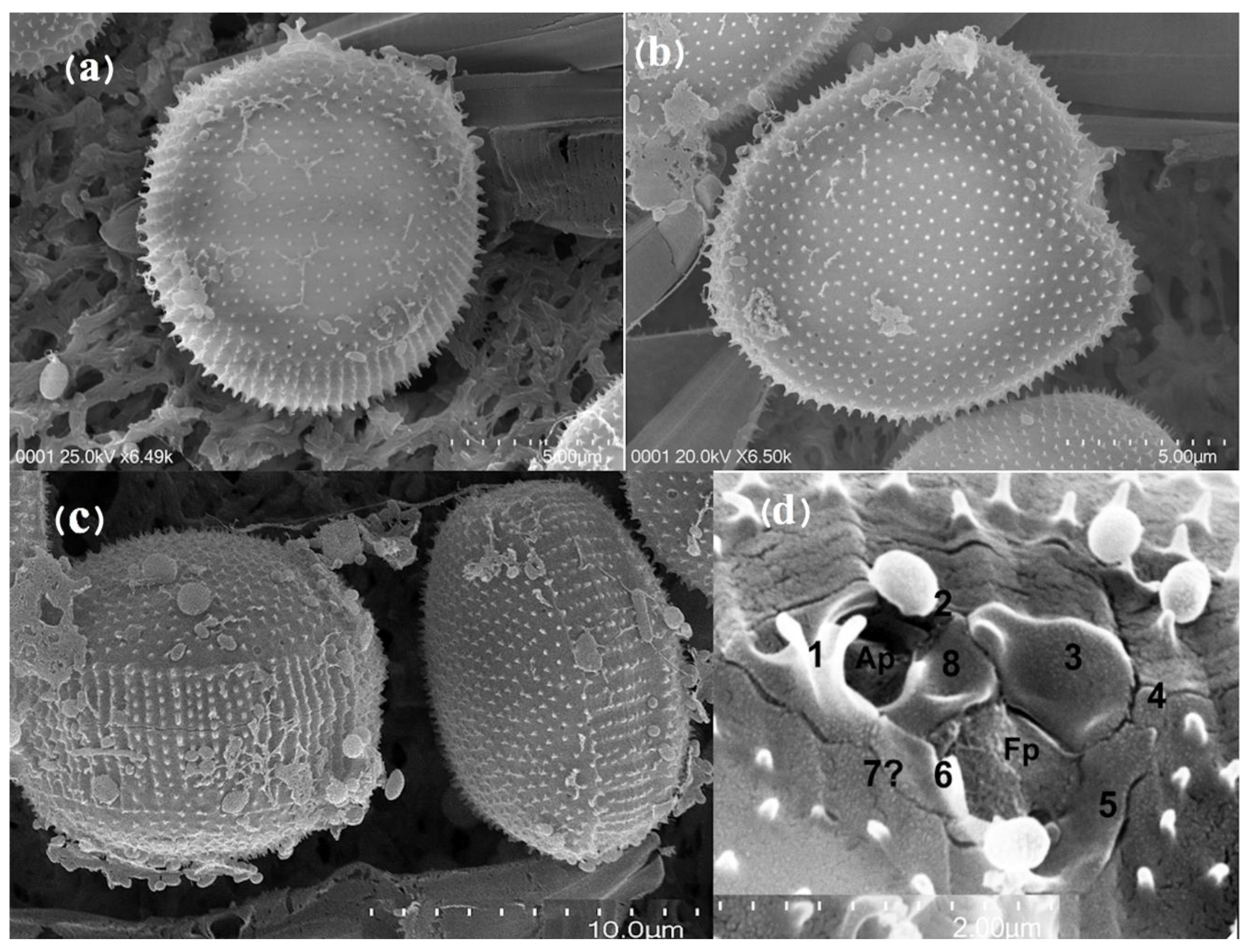
3.2. Morphology
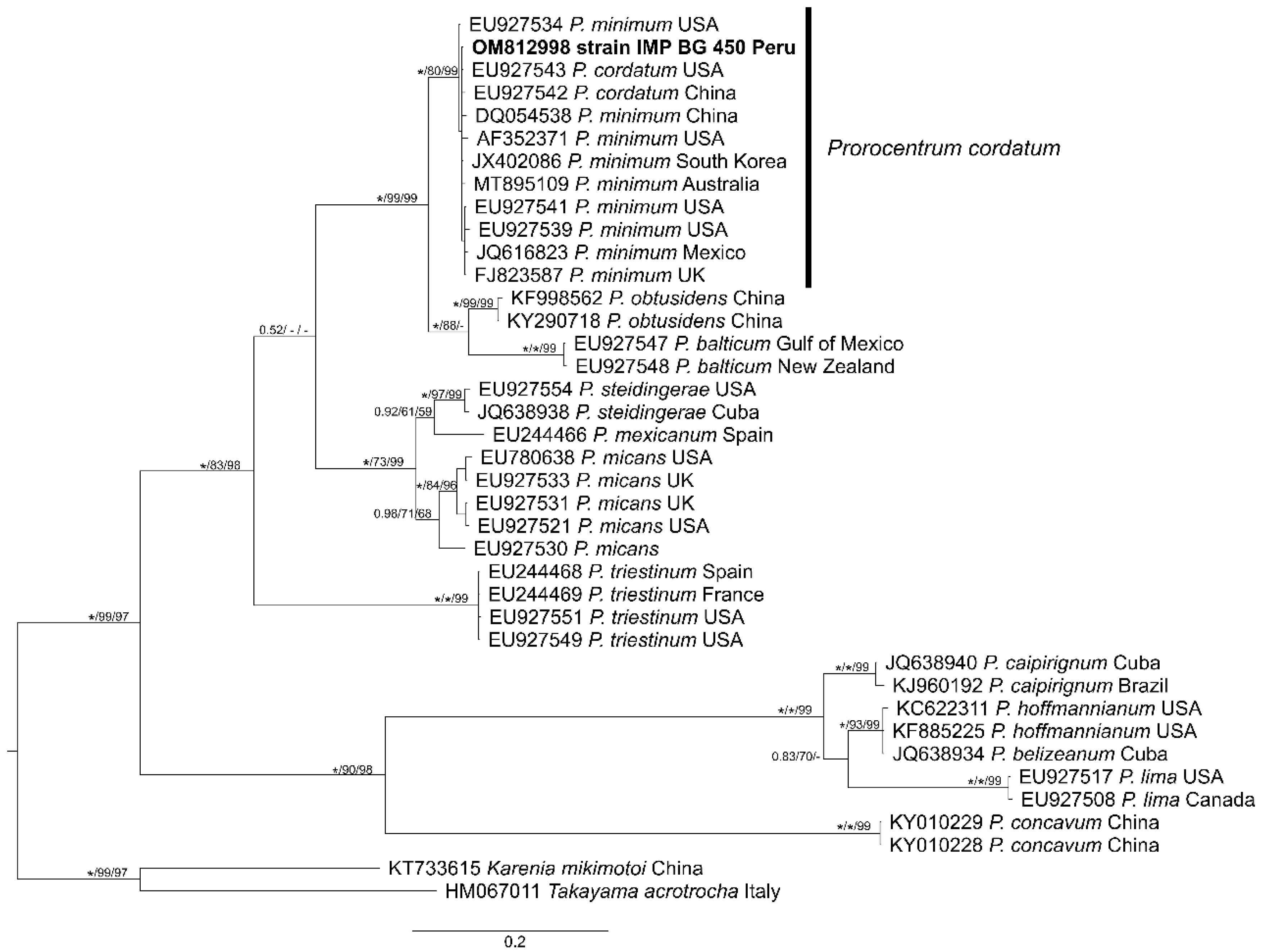
3.3. Phylogeny
3.4. Toxin Analysis
4. Discussion
4.1. Environmental Parameters
4.2. Morphology
4.3. Phylogeny
4.4. Toxins Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Glibert, P.M. Harmful algae at the complex nexus of eutrophication and climate change. Harmful Algae 2020, 91, 101583. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef]
- Glibert, P.M.; Burkholder, J.M. Harmful Algal Species Fact Sheet: Prorocentrum. In Harmful Algal Blooms: A Compendium Desk Reference; Shumway, S.E., Burkholder, J.M., Morton, S.L., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2018; pp. 625–628. [Google Scholar]
- Heisler, J.; Glibert, P.; Burkholder, J.; Anderson, D.; Cochlan, W.; Dennison, W.; Gobler, C.; Dortch, Q.; Heil, C.; Humphries, E.; et al. Eutrophication and Harmful Algal Blooms: A Scientific Consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M. Toxic algal bloom and red tides: A global perspective. In Red Tides: Biology, Environmental Science and Technology; Okaichi, T., Anderson, D.M., Nemoto, T., Eds.; Elsevier: New York, NY, USA, 1989; pp. 11–16. [Google Scholar]
- Hallegraeff, G.M. Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge 1. J. Phycol. 2010, 46, 220–235. [Google Scholar] [CrossRef]
- Dale, B.; Edwards, M.; Reid, P.C. Climate change and harmful algal blooms. In Ecology of Harmful Algae; Springer: Berlin/Heidelberg, Germany, 2006; pp. 367–378. [Google Scholar]
- Edwards, M.; Johns, D.G.; Leterme, S.C.; Svendsen, E.; Richardson, A.J. Regional climate change and harmful algal blooms in the northeast Atlantic. Limnol. Oceanogr. 2006, 51, 820–829. [Google Scholar] [CrossRef]
- Echevin, V.; Gévaudan, M.; Espinoza-Morriberón, D.; Tam, J.; Aumont, O.; Gutierrez, D.; Colas, F. Physical and biogeochemical impacts of RCP8. 5 scenario in the Peru upwelling system. Biogeosciences 2020, 17, 3317–3341. [Google Scholar] [CrossRef]
- Chavez, F.P.; Bertrand, A.; Guevara-Carrasco, R.; Soler, P.; Csirke, J. The northern Humboldt Current System: Brief history, present status and a view towards the future. Prog. Oceanogr. 2008, 79, 95–105. [Google Scholar] [CrossRef]
- Lomas, M.W.; Glibert, P.M. Interactions between NH+ 4 and NO− 3 uptake and assimilation: Comparison of diatoms and dinoflagellates at several growth temperatures. Mar. Biol. 1999, 3, 541–551. [Google Scholar] [CrossRef]
- Goldman, J.C. Potential role of large oceanic diatoms in new primary production. Deep Sea Res. Part I Oceanogr. Res. Pap. 1993, 40, 625–628. [Google Scholar] [CrossRef]
- Kudela, R.M.; Dugdale, R.C. Nutrient regulation of phytoplankton productivity in Monterey Bay, California. Deep-Sea Res. Part II 2000, 47, 1023–1053. [Google Scholar] [CrossRef]
- Wilkerson, F.P.; Dugdale, R.C.; Kudela, R.M.; Chavez, F.P. Biomass and productivity in Monterey Bay, California: Contribution of the large phytoplankton. Deep Sea Res. Part II Top. Stud. Oceanogr. 2000, 47, 1003–1022. [Google Scholar] [CrossRef]
- Heil, C.A.; Glibert, P.M.; Fan, C. Prorocentrum minimum (Pavillard) Schiller A review of a harmful algal bloom species of growing worldwide importance. Harmful Algae 2005, 4, 449–470. [Google Scholar] [CrossRef]
- Klanjšček, J.; Geček, S.; Klanjšček, T.; Legović, T. Nutrient quotas and carbon content variability of Prorocentrum minimum (Pavillard) Schiller, 1933. Harmful Algae 2016, 51, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Glibert, P.M.; Mayorga, E.; Seitzinger, S. Prorocentrum minimum tracks anthropogenic nitrogen and phosphorus inputs on a global basis: Application of spatially explicit nutrient export models. Harmful Algae 2008, 8, 33–38. [Google Scholar] [CrossRef]
- Velikova, V.; Larsen, J. The Prorocentrum cordatum/Prorocentrum minimum taxonomic problem. Grana 1999, 38, 108–112. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication; National University of Ireland. Galway. Available online: https://www.algaebase.org/search/species/detail/?species_id=71381 (accessed on 8 July 2022).
- Moestrup, O.; Akselmann-Cardella, R.; Churro, C.F.S.; Hoppenrath, M.; Iwataki, M.; Larsen, J.; Lundholm, N.; Zingone, A. IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae. Prorocentrum cordatum (Ostenfeld) J.D.Dodge. 1976. Available online: http://www.marinespecies.org/hab/aphia.php?p=taxdetails&id=232376 (accessed on 8 July 2022).
- Khanaychenko, A.N.; Telesh, I.V.; Skarlato, S.O. Bloom-forming potentially toxic dinoflagellates Prorocentrum cordatum in marine plankton food webs. Protistology 2019, 13, 95–125. [Google Scholar] [CrossRef]
- Glibert, P.M.; Burkholder, J.M.; Kana, T.M. Recent insights about relationships between nutrient availability, forms, and stoichiometry, and the distribution, ecophysiology, and food web effects of pelagic and benthic Prorocentrum species. Harmful Algae 2012, 14, 231–259. [Google Scholar] [CrossRef]
- Sierra-Beltrán, A.; Cortés-Altamirano, R.; Cortés-Lara, M.d.C. Occurrences of Prorocentrum minimum (Pavillard) in México. Harmful Algae 2005, 4, 507–517. [Google Scholar] [CrossRef]
- Matantseva, O.; Skarlato, S.; Vogts, A.; Pozdnyakov, I.; Liskow, I.; Schubert, H.; Voss, M. Superposition of individual activities: Urea-mediated suppression of nitrate uptake in the dinoflagellate Prorocentrum minimum revealed at the population and single-cell levels. Front. Microbiol. 2016, 7, 1310. [Google Scholar] [CrossRef]
- Telesh, I.V.; Schubert, H.; Skarlato, S.O. Ecological niche partitioning of the invasive dinoflagellate Prorocentrum minimum and its native congeners in the Baltic Sea. Harmful Algae 2016, 59, 100–111. [Google Scholar] [CrossRef]
- Telesh, I.; Schubert, H.; Skarlato, S. Abiotic stability promotes dinoflagellate blooms in marine coastal ecosystems. Estuar. Coast. Shelf Sci. 2021, 251, 107239. [Google Scholar] [CrossRef]
- Skarlato, S.O.; Telesh, I.V.; Matantseva, O.V.; Pozdnyakov, I.A.; Berdieva, M.A.; Schubert, H.; Filatova, N.A.; Knyazev, N.A.; Pechkovskaya, S.A. Studies of bloom-forming dinoflagellates Prorocentrum minimum in fluctuating environment: Contribution to aquatic ecology, cell biology and invasion theory. Protistology 2018, 12, 113–157. [Google Scholar] [CrossRef]
- Vlamis, A.; Katikou, P.; Rodriguez, I.; Rey, V.; Alfonso, A.; Papazachariou, A.; Zacharaki, T.; Botana, A.M.; Botana, L.M. First detection of tetrodotoxin in Greek shellfish by UPLC-MS/MS potentially linked to the presence of the dinoflagellate Prorocentrum minimum. Toxins 2015, 7, 1779–1807. [Google Scholar] [CrossRef]
- Bordin, P.; Dall’Ara, S.; Tartaglione, L.; Antonelli, P.; Calfapietra, A.; Varriale, F.; Guiatti, D.; Milandri, A.; Dell’Aversano, C.; Arcangeli, G.; et al. First occurrence of tetrodotoxins in bivalve mollusks from Northern Adriatic Sea (Italy). Food Control 2021, 120, 107510. [Google Scholar] [CrossRef]
- Turner, A.D.; Dhanji-Rapkova, M.; Coates, L.; Bickerstaff, L.; Milligan, S.; O’Neill, A.; Faulkner, D.; McEneny, H.; Baker-Austin, C.; Lees, D.N.; et al. Detection of Tetrodotoxin Shellfish Poisoning (TSP) Toxins and Causative Factors in Bivalve Molluscs from the UK. Mar. Drugs 2017, 15, 277. [Google Scholar] [CrossRef]
- Gerssen, A.; Bovee, T.; Klijnstra, M.; Poelman, M.; Portier, L.; Hoogenboom, R. First Report on the Occurrence of Tetrodotoxins in Bivalve Mollusks in The Netherlands. Toxins 2018, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Narahashi, T.; Moore, J.W.; Scott, W.R. Tetrodotoxin blockage of sodium conductance increase in lobster giant axons. J. Gen. Physiol. 1964, 47, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Narahashi, T. Pharmacology of tetrodotoxin. J. Toxicol. Toxin Rev. 2001, 20, 67–84. [Google Scholar] [CrossRef]
- Rodriguez, I.; Alfonso, A.; Alonso, E.; Rubiolo, J.A.; Roel, M.; Vlamis, A.; Katikou, P.; Jackson, S.A.; Menon, M.L.; Dobson, A.; et al. The association of bacterial C9-based TTX-like compounds with Prorocentrum minimum opens new uncertainties about shellfish seafood safety. Sci. Rep. 2017, 7, 40880. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, S.; Bernales, A.; Delgado, E.; Chang, F.; Jacobo, N.; Quispe, J. Variability and Biogeographical Distribution of Harmful Algal Blooms in Bays of High Productivity off Peruvian Coast (2012–2015). J. Environ. Anal. Toxicol. 2017, 7, 2161-0525.1000530. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Eppley, R.W.; de Mendiola, B.R. Poblaciones de fitoplancton, nutrientes y fotosíntesis en aguas costeras peruanas. Bol. Instit. Mar. Perú 1969, 2, 4–45. [Google Scholar]
- Muck, P.E.T.E.R.; Rojas de Mendiola, B.; Antonietti, E.M.I.R.A. Comparative studies on feeding in larval anchoveta (Engraulis ringens) and sardine (Sardinops sagax). In The Peruvian Upwelling Ecosystem: Dynamics and Interactions; ICLARM: Manila, Philippines, 1989; Volume 18, pp. 86–96. [Google Scholar]
- Solano Cornejo, D.; Mendoza Díaz, V.; CONAM, PNUMA. Informe Sobre el Estado del Ambiente: GEO Bahía Paracas-Pisco; Lima, Peru, 2007; pp. 1–163. [Google Scholar]
- Sánchez, S.; Jacobo, N.; Bernales, A.; Franco, A.; Quispe, J.; Flores, G. Seasonal variability in the distribution of phytoplankton in Paracas Bay/Peru, as a response to environmental conditions. J. Environ. Sci. Eng. B 2018, 371–380. [Google Scholar] [CrossRef]
- Andersen, R.A.; Kawachi, M. Tradiotinal Microalgae Isolation Techniques. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 578, pp. 83–100. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR protocols: A Guide to Methods and Applications; Innis, M., Gelfand, D., Sninsky, J., White, T., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Silvestro, D.; Michalak, I. raxmlGUI: A graphical front-end for RAxML. Org. Divers. Evol. 2012, 12, 335–337. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut, A.; Suchard, M.A.; Xie, D.; Drummond, A.J. Tracer v1. 6. Available online: http://tree.bio.ed.ac.uk/software/tracer (accessed on 8 July 2022).
- Boundy, M.J.; Selwood, A.I.; Harwood, D.T.; McNabb, P.S.; Turner, A.D. Development of a sensitive and selective liquid chromatography-mass spectrometry method for high throughput analysis of paralytic shellfish toxins using graphitised carbon solid phase extraction. J. Chromatogr. A 2015, 1387, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hoppenrath, M.; Chomérat, N.; Horiguchi, T.; Schweikert, M.; Nagahama, Y.; Murray, S. Taxonomy and phylogeny of the benthic Prorocentrum species (Dinophyceae)—A proposal and review. Harmful Algae 2013, 27, 1–28. [Google Scholar] [CrossRef]
- Bakun, A.N.D.R.E.W.; Mendelssohn, R. Alongshore wind stress, 1953–1984: Correction, reconciliation and update through 1986. In The Peruvian Upwelling Ecosystem: Dynamics and Interactions, Proceedings of the ICLARM Conference Procceedings; ICLARM: Manila, Philippines, 1989; pp. 77–81. [Google Scholar]
- Gutiérrez, D.; Bouloubassi, I.; Sifeddine, A.; Purca, S.; Goubanova, K.; Graco, M.; Field, D.; Méjanelle, L.; Velazco, F.; Lorre, A.; et al. Coastal cooling and increased productivity in the main upwelling zone off Peru since the mid-twentieth century. Geophys. Res. Lett. 2011, 38, 1–6. [Google Scholar] [CrossRef]
- Calienes, R.; Guillén, O.; Lostaunau, N. Variabilidad espacio-temporal de clorofila, producción primaria y nutrientes frente a la costa peruana. Bol. Inst. Mar. Perú 1985, 10, 1–44. [Google Scholar]
- Sukhanova, I.N.; Flint, M.V.; Hibaum, G.; Karamfilov, V.; Kopylov, A.I.; Matveeva, E.; Rat’kova, T.N.; Sazhin, A.F. Exuviaella cordata red tide in Bulgarian coastal waters (May to June 1986). Mar. Biol. 1988, 99, 1–8. [Google Scholar] [CrossRef]
- Jeong, H.J.; Du Yoo, Y.; Kim, J.S.; Seong, K.A.; Kang, N.S.; Kim, T.H. Growth, feeding and ecological roles of the mixotrophic and heterotrophic dinoflagellates in marine planktonic food webs. Ocean. Sci. J. 2010, 45, 65–91. [Google Scholar] [CrossRef]
- SANIPES. Fisheries Health Organization of Perú- Organismo Nacional de Sanidad Pesquera. Available online: https://www.sanipes.gob.pe/web/index.php/es/fitoplancton (accessed on 8 July 2022).
- Olenina, I.; Wasmund, N.; Hajdu, S.; Jurgensone, I.; Gromisz, S.; Kownacka, J.; Toming, K.; Vaiciūtė, D.; Olenin, S. Assessing impacts of invasive phytoplankton: The Baltic Sea case. Mar. Pollut. Bull. 2010, 60, 1691–1700. [Google Scholar] [CrossRef]
- Grzebyk, D.; Berland, B. Influences of temperature, salinity and irradiance on growth of Prorocentrum minimum (Dinophyceae) from the Mediterranean Sea. J. Plankton Res. 1996, 18, 1837–1849. [Google Scholar] [CrossRef]
- Tango, P.; Magnien, R.; Butler, W.; Luckett, C.; Luckenbach, M.; Lacouture, R.; Poukish, C. Impacts and potential effects due to Prorocentrum minimum blooms in Chesapeake Bay. Harmful Algae 2005, 4, 525–531. [Google Scholar] [CrossRef]
- Pertola, S. Diffusive and Ship-Mediated Spread of Dinoflagellates in the Baltic Sea with Prorocentrum Minimum as a Special Case; University of Helsinki: Helsinki, Finland, 2006. [Google Scholar]
- Hajdu, S.; Edler, L.; Olenina, I.; Witek, B. Spreading and establishment of the potentially toxic dinoflagellate Prorocentrum minimum in the Baltic Sea. Int. Rev. Hydrobiol. A J. Cover. All Asp. Limnol. Mar. Biol. 2000, 85, 561–575. [Google Scholar]
- Olenina, I.; Vaičiukynas, E.; Šulčius, S.; Paškauskas, R.; Verikas, A.; Gelžinis, A.; Bačauskienė, M.; Bertašiūtė, V.; Olenin, S. The dinoflagellate Prorocentrum cordatum at the edge of the salinity tolerance: The growth is slower but cells are larger. Estuar. Coast. Shelf Sci. 2016, 168, 71–79. [Google Scholar] [CrossRef]
- Knyazev, N.; Pechkovskaya, S.; Skarlato, S.; Telesh, I.; Filatova, N. The impact of temperature stress on DNA and RNA synthesis in potentially toxic dinoflagellates Prorocentrum minimum. J. Evol. Biochem. Physiol. 2018, 54, 383–389. [Google Scholar] [CrossRef]
- Pertola, S.; Kuosa, H.; Olsonen, R. Is the invasion of Prorocentrum minimum (Dinophyceae) related to the nitrogen enrichment of the Baltic Sea? Harmful Algae 2005, 4, 481–492. [Google Scholar] [CrossRef]
- Kimor, B.; Moigis, A.G.; Dohms, V.; Stienen, C. A case of mass occurrence of Prorocentrum minimum in the Kiel Fjord. Mar. Ecol. Prog. Ser. 1985, 27, 209–215. [Google Scholar] [CrossRef]
- Kahru, M.; Michell, B.G.; Diaz, A.; Miura, M. MODIS detects a devastating algal bloom in Paracas Bay, Peru. Eos Trans. Am. Geophys. Union 2004, 85, 465–472. [Google Scholar] [CrossRef]
- IMARPE. Instituto del Mar del Perú. Laboratorio Costero de Pisco. Bases Técnicas Para el Ordenamiento Pesquero y Acuícola de la Bahía Paracas. Available online: http://www2.produce.gob.pe/RepositorioAPS/3/jer/ACUISUBMENU4/estudios-bahia-paracas.pdf (accessed on 8 July 2022).
- Pitcher, G.C.; Bernales Jiménez, A.; Kudela, R.M.; Reguera, B. Harmful algal blooms in Eastern Boundary upwelling systems: A Geohab Core Research Project. Oceanography 2017, 30, 22. [Google Scholar] [CrossRef]
- Dyson, K.; Huppert, D.D. Regional economic impacts of razor clam beach closures due to harmful algal blooms (HABs) on the Pacific coast of Washington. Harmful Algae 2010, 9, 264–271. [Google Scholar] [CrossRef]
- Blanco-Pérez, J. Episodios nocivos por fitoplancton. In Los Moluscos Pectínidos de Iberoamérica: Ciencia y Acuicultura; Maeda-Martínez, A.N., Ed.; Limusa: Mexico City, Mexico, 2001; pp. 285–324. [Google Scholar]
- Borbor-Cordova, M.J.; Torres, G.; Mantilla-Saltos, G.; Casierra-Tomala, A.; Bermúdez, J.R.; Renteria, W.; Bayot, B. Oceanography of Harmful Algal Blooms on the Ecuadorian Coast (1997–2017): Integrating Remote Sensing and Biological Data. Front. Mar. Sci. 2019, 6, 13. [Google Scholar] [CrossRef]
- Méndez, S.; Ferrari, G. Floraciones algales nocivas en Uruguay: Antecedentes, proyectos en curso y revisión de resultados. In Floraciones Algales Nocivas en el Cono Sur Americano; Sar, E.A., Ferrario, M.E., Reguera, B., Eds.; Instituto Español de Oceanografia: Madrid, Spain, 2002; pp. 271–288. [Google Scholar]
- Ferrari, G.; Del Carmen Perez, M. Fitoplancton de la costa platense y Atlántica de Uruguay (1993–1994). Iheringia. Ser. Bot. 2002, 57, 263–278. [Google Scholar]
- Carreto, J.I.; Montoya, N.G.; Akselman, R.; Roja, P.M. Proyecto” Protección Ambiental del Río de la Plata y su Frente Marítimo. Prevención y Control de la Contaminación y Restauración de Hábitats” Proyecto PNUD/GEF RLA/99/G31. RLA 2004, 99, G31. [Google Scholar]
- Dodge, J. The Prorocentrales (Dinophyceae). II. Revision of the taxonomy within the genus Prorocentrum. Bot. J. Linn. Soc. 1975, 71, 103–125. [Google Scholar] [CrossRef]
- Monti-Birkenmeier, M.; Berden Zrimec, M.B.; Drinovec, L.; Beran, A.; Zrimec, A.; Cataletto, B.; Fonda Umani, S. Influence of salinity on growth and cell volume in three strains of Prorocentrum cordatum (Dinophyceae). Aquat. Biol. 2019, 28, 1–12. [Google Scholar] [CrossRef]
- Martin, G.W. Dinoflagellates from marine and brackish waters of New Jersey. In University of Lowa Studies in Natural History; 1929; Volume 12, pp. 3–32. [Google Scholar]
- Pertola, S.; Faust, M.A.; Kuosa, H.; Hallfors, G. Morphology of Prorocentrum minimum (Dinophyceae) in the Baltic Sea and in Chesapeake Bay: Comparison of Cell Shapes and Thecal Ornamentation. Bot. Mar. 2003, 46, 477–486. [Google Scholar] [CrossRef]
- Witek, B.; Plinski, M. The first recorded bloom of Prorocentrum minimum (Pavillard) Schiller in the coastal zone of the Gulf of Gdańsk. Oceanologia 2000, 42, 29–36. [Google Scholar]
- Marasović, I.; Pucher-Petković, T.; Petrova-Karadjova, V. Prorocentrum minimum (Dinophyceae) in the Adriatic and Black sea. J. Mar. Biolog. Assoc. UK 1990, 70, 473–476. [Google Scholar] [CrossRef]
- Muciño-Márquez, R.E.; Gárate-Lizárraga, I.; López-Cortés, D.J. Seasonal Variation of the Genus Prorocentrum (Dinophyceae) in Two Tuna Farms in the Bahía De La Paz, Mexico. Acta Biol. Colomb. 2014, 20, 195–206. [Google Scholar] [CrossRef]
- Petrova, D.; Gerdzhikov, D. Development of a traditional blooming phytoplankton species Prorocentrum cordatum Dodge, 1975 along the Bulgarian coast (2008–2010). In Proceedings of the Union of Scientists in Bulgaria-Varna, Series “Marine Science”, Bulgaria, Varna, 2013; pp. 12–17. [Google Scholar]
- Hajdu, S.; Pertola, S.; Kuosa, H. Prorocentrum minimum (Dinophyceae) in the Baltic Sea: Morphology, occurrence—A review. Harmful Algae 2005, 4, 471–480. [Google Scholar] [CrossRef]
- Monti, M.; Stoecker, D.K.; Cataletto, B.; Talarico, L. Morphology of the flagellar pore complex in Prorocentrum minimum (Dinophyceae) from the Adriatic and Baltic Seas. Bot. Mar. 2010, 53. [Google Scholar] [CrossRef]
- Loeblich, A.R., III. Dinoflagellate evolution: Speculation and evidence. J. Protozool. 1976, 23, 13–28. [Google Scholar] [CrossRef]
- Taylor, F. On dinoflagellate evolution. BioSystems 1980, 13, 65–108. [Google Scholar] [CrossRef]
- Taylor, F.; Fukuyo, Y.; Larsen, J.; Hallegraeff, G. Taxonomy of harmful dinoflagellates. In Harmful Marine Microalgae; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; UNESCO: Paris, France, 2003; pp. 389–482. [Google Scholar]
- Wayne Litaker, R.; Vandersea, M.W.; Kibler, S.R.; Reece, K.S.; Stokes, N.A.; Lutzoni, F.M.; Yonish, B.A.; West, M.A.; Black, M.N.D.; Tester, P.A. Recognizing Dinoflagellate species using ITS rDNA sequences. J. Phycol. 2007, 43, 344–355. [Google Scholar] [CrossRef]
- Stern, R.F.; Andersen, R.A.; Jameson, I.; Küpper, F.C.; Coffroth, M.A.; Vaulot, D.; Le Gall, F.; Véron, B.; Brand, J.J.; Skelton, H.; et al. Evaluating the ribosomal internal transcribed spacer (ITS) as a candidate dinoflagellate barcode marker. PLoS ONE 2012, 7, e42780. [Google Scholar] [CrossRef] [PubMed]
- McLennan, K.; Ruvindy, R.; Ostrowski, M.; Murray, S. Assessing the Use of Molecular Barcoding and qPCR for Investigating the Ecology of Prorocentrum minimum (Dinophyceae), a Harmful Algal Species. Microorganisms 2021, 9, 510. [Google Scholar] [CrossRef] [PubMed]
- Nakazima, M. Studies on the source of shellfish poison in Lake Hamana. III. Poisonous effects of shellfish feeding on Prorocentrum sp. Bull. Jpn. Soc. Sci. Fish 1965, 31, 281–285. [Google Scholar] [CrossRef]
- Al-Hashmi, K.A.; Smith, S.L.; Claereboudt, M.; Piontkovski, S.A.; Al-Azri, A. Dynamics of potentially harmful phytoplankton in a semi-enclosed bay in the Sea of Oman. Bull. Mar. Sci. 2015, 91, 141–166. [Google Scholar] [CrossRef]
- Denardou Queneherve, A.; Grzebyk, D.; Pouchus, Y.F.; Sauviat, M.P.; Alliot, E.; Biard, J.F.; Berland, B.; Verbist, J.F. Toxicity of French strains of the dinoflagellate Prorocentrum minimum experimental and natural contaminations of mussels. Toxicon 1999, 37, 1711–1719. [Google Scholar] [CrossRef]
- Wikfors, G.H.; Smolowitz, R.M. Experimental and histological studies of four life-history stages of the eastern oyster, Crassostrea virginica, exposed to a cultured strain of the dinoflagellate Prorocentrum minimum. Biol. Bull. 1995, 188, 313–328. [Google Scholar] [CrossRef]

| Compound | Transition | Collision Energy |
|---|---|---|
| 5,6,11-TrideoxyTTX | 272.1 > 162.1 | 40 |
| 5,6,11-TrideoxyTTX | 272.1 > 254.1 | 31 |
| 11-nor-TTX-6-ol | 290.1 > 162.1 | 40 |
| 11-nor-TTX-6-ol | 290.1 > 272.1 | 31 |
| 4,9-anhydroTTX | 302.1 > 162.1 | 40 |
| 4,9-anhydroTTX | 302.101 > 256.1 | 31 |
| 5-deoxyTTX, 11-deoxyTTX | 304.1 > 176.1 | 40 |
| 5-deoxyTTX, 11-deoxyTTX | 304.1 > 286.1 | 31 |
| TTX, 4-epiTTX | 320.1 > 162.1 | 40 |
| TTX, 4-epiTTX | 320.1 > 302.1 | 35 |
| C9-265 * | 265.1 > 179.1 | 35 |
| C9-265 * | 265.1 > 162.1 | 40 |
| C9-308 * | 308.1 > 180.1 | 40 |
| C9-308 * | 308.1 > 162.1 | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenorio, C.; Álvarez, G.; Perez-Alania, M.; Blanco, J.L.; Paulino, C.; Blanco, J.; Uribe, E. Bloom of Prorocentrum cordatum in Paracas Bay, Peru. Diversity 2022, 14, 844. https://doi.org/10.3390/d14100844
Tenorio C, Álvarez G, Perez-Alania M, Blanco JL, Paulino C, Blanco J, Uribe E. Bloom of Prorocentrum cordatum in Paracas Bay, Peru. Diversity. 2022; 14(10):844. https://doi.org/10.3390/d14100844
Chicago/Turabian StyleTenorio, Cecil, Gonzalo Álvarez, Melissa Perez-Alania, Jose Luis Blanco, Carlos Paulino, Juan Blanco, and Eduardo Uribe. 2022. "Bloom of Prorocentrum cordatum in Paracas Bay, Peru" Diversity 14, no. 10: 844. https://doi.org/10.3390/d14100844
APA StyleTenorio, C., Álvarez, G., Perez-Alania, M., Blanco, J. L., Paulino, C., Blanco, J., & Uribe, E. (2022). Bloom of Prorocentrum cordatum in Paracas Bay, Peru. Diversity, 14(10), 844. https://doi.org/10.3390/d14100844









