Abstract
Habitat loss affects the nature of biotic interactions in all ecosystems and at all levels of the food web. Nevertheless, little attention has been given to soil nematodes in tropical habitats despite their important role in ecosystem functioning worldwide. Here, we analyzed the influence of anthropogenic habitat disturbance on the absolute and relative density and composition (i.e., trophic guilds) of soil nematode communities associated with the rhizosphere of the tropical herb Heliconia collinsiana in continuous mature forests and human-induced secondary forests. We compared nematode densities based on the following feeding guilds: bacterivores, fungivores, herbivores and predators. Thereafter, we classified herbivorous nematodes into genera and described soil properties in both habitat types including pH, electrical conductivity, and organic matter content. Herbivores were significantly the most abundant feeding guild for both habitats represented by Criconemella spp., Helicotylenchus spp., and Meloidogyne spp., which showed no significant differences in density between habitats. Relative but not absolute nematode density differed between habitats, with fungivore nematodes being significantly lower in secondary forests. No significant differences in soil properties were detected. Overall, our results suggest that forest disturbance affects the nematode community associated with the rhizosphere of H. collinsiana which may affect forest succession and the dynamics of the soil biota. Our study contributes to the understanding of biotic interactions in conserved and disturbed tropical habitats.
1. Introduction
Deforestation, habitat loss and fragmentation influence the nature of biological interactions by changing the environmental conditions in which native species live, including drastic alterations in soil properties and their associated biota [,,]. The impact of habitat loss on biotic interactions (i.e., antagonistic, commensalism and mutualistic) depends on the taxonomic and functional groups involved [,]. Nevertheless, most studies have been focused on mutualistic plant–animal interactions, such as seed dispersal and pollination, leaving aside antagonistic interactions such as pest and pathogen attack to plants as well as commensal symbiotic interactions [,,,,]. Mutualistic interactions have generally shown to be negatively affected by human disturbance [], while the opposite has been observed for antagonistic interactions, such as those that plants have with their natural enemies [,,,].
Drastic changes in environmental conditions across human-disturbed habitats affect species richness, diversity, and composition of several invertebrate taxa and that of their host plants [,]. Some of the biotic interactions that have been little explored in the context of habitat disturbance are those between parasitic nematodes and their host plants [,,]. Recently, nematodes have been recognized as the most species-rich animal taxon worldwide [,]. Terrestrial nematodes fill all trophic guilds in the soil food web, having various functional roles including the following: bacterivores, fungivores, herbivores, omnivores, and predators [,,,,]. Broadly speaking and based on feeding guilds, various plant–nematode interactions could be inferred, including the following: antagonistic interactions when feeding on their host plants; mutualistic interactions when feeding on pathogens infecting plant tissue such as fungi, bacteria, and pathogenic nematodes; and commensal interactions, in which nematode species benefit while the plant receives neither benefit nor damage (some omnivorous nematodes). Nematodes are considered as important bioindicators of soil health and are part of key ecological processes in the soil, such as nutrient cycling and decomposition [,,]. Nevertheless, besides tropical agricultural systems, we still know very little about the impact of habitat loss on plant–soil–nematode interactions in the wet tropics [].
Altered microclimatic conditions, after anthropogenic disturbance and the novel plant and animal communities that establish thereafter, may favor certain invertebrate groups as well as pioneer plant species that may become potential hosts. Exotic and/or invasive species of invertebrates accidentally introduced through various human activities may become common in human-modified landscapes [,]. Nematodes could be accidentally spread by terrestrial wild and domestic animals or by people when moving plants, soil material and even themselves across natural and agricultural habitats. Despite their importance for ecosystem functioning, few quantitative and qualitative studies of the active belowground nematode communities under natural conditions in the tropics currently exist [,]. To the best of our knowledge, most research on nematode communities has been carried out in agroecosystems, because of their importance as pathogens for various crops and/or cultivars worldwide [,,,].
The species of the tropical genus Heliconia (Heliconiaceae) provide an excellent model system for studying multitrophic interactions in conserved and human-disturbed tropical habitats; this is because of their prevalence in conserved and human-impacted landscapes as well as their well-known taxonomy and their relatively well-known pollinator and pest and disease-causing taxa [,,,]. For instance, the abundance and richness of arthropod communities within various trophic guilds living in heliconias have shown different responses to habitat disturbance [,,,]. Several species of Heliconia are grown and commercialized around the world [,,,]; therefore, certain pests, pathogens and benefic organisms are relatively well known for several commercially important species in the genus, but information on their enemies under natural conditions is still scarce. The large number of recorded pathogenic microorganisms associated with specific structures of heliconias (i.e., bracts, flowers, roots, leaves, and stems) include viruses, bacteria, nematodes, algae, and fungi [,,,,]. Based on this context, we aimed to describe, for the first time, the nematode community associated with the rhizosphere of wild populations of Heliconia collinsiana Griggs var. collinsiana, in natural forest gaps within mature continuous forests and in human-induced secondary forests. Because human disturbance drastically changes soil physical, chemical, and biological traits and increases plant susceptibility to diseases, we expected that nematode communities would differ in terms of density and composition between tropical mature and secondary forests. We expected higher density (absolute or relative) of plant–parasitic nematodes in the rhizosphere of H. collinsiana individuals growing in secondary forests [,,,,]. Plant–parasitic nematodes cause significant economic losses in tropical crops through yield reduction, but there is no information regarding their impact on plant populations and communities under natural conditions; thus, it is necessary to generate information on this topic to generate management strategies and conservation practices. Ideally, the impact of human activities on the diversity of soil nematode communities should be analyzed in four dimensions: ecologically, taxonomically, functionally, and genetically. Gathering information of these four diversity dimensions must allow for the improvement of biodiversity assessments and an increased understanding of anthropogenic impacts in natural communities. There is no information, however, on the prevalence and intensity of nematode infestation during seasons, not between years, nor on the consequences for H. collinsiana host populations. Nevertheless, in this study, we were able to describe, for the first time, the prevalence and abundance of four recognized functional nematode groups, i.e., feeding guilds, indicative of shifts in the structure and functioning of nematode communities in a human-modified ecosystem.
2. Materials and Methods
2.1. Study Site
The study was conducted within the Montes Azules Biosphere Reserve (MABR), Chiapas, southeastern Mexico (120 masl; 16°06′ N, 90°56′ W). The MABR is within the Selva Lacandona region that comprises part of Guatemala and Mexico []. Human activities have dramatically reduced the original forested area (500,000 ha) by one-third in 40 years. The MABR contains most of the remaining tropical forest of Mesoamerica (3310 km2) and constitutes the main component of the region biodiversity hotspot []. The primary vegetation type is lowland tropical rainforest, with some trees reaching up to 40 m in height in alluvial terraces along main rivers. There are roughly 4000 species of vascular plants []. Maximum and minimum annual temperatures are 32 °C (April–May), and 18 °C (January–February), respectively. Annual precipitation averages 3000 mm. Currently, the landscape is composed of a mosaic of land-uses including forest fragments, secondary vegetation of various ages, human settlements, croplands, pastures, and paved and unpaved roads.
Two habitat-types corresponding to areas where the focal species is commonly found were considered for the study, including the following (for further information see []): (a) naturally opened forest gaps in mature continuous forests (>100 m2); (b) secondary vegetation regrowth after human disturbance along paved roads. Microclimatic conditions differed between habitats; secondary forests hold significantly higher air temperature, higher light income and lower air relative humidity compared with forest gaps []. The number of H. collinsiana shoots is ten-fold greater in secondary vegetation (44.4 ± 12 clumps/314 m2) than in forest gaps (4.0 ± 2.1 clumps/314 m2) []. When compared with individuals growing in the continuous forest, individuals of H. collinsiana in secondary forests present shorter but more shoots in their clumps, greater leaf toughness and greater levels of leaf fungal damage []. Secondary vegetation patches were 3–6 km away from the nearest primary continuous forest and were dominated by early successional shrubs and herbs, including other species of heliconia such as H. latispatha and H. bourgaeana [,].
2.2. Study Species
The study species, Heliconia collinsiana, is naturally distributed from southern Mexico to central Nicaragua and is a widely cultivated species [,,]. Individuals of H. collinsiana present a musoid growth type with a white waxy coating on the shoot, leaves, lower blade and bracts. The inflorescence is pendant with 6–14 cm with dark red to orange red bracts; flowers are yellow to orange yellow or gold. In the study area, flowers are visited by the stripe throated hermit hummingbird species Phaethornis striingularis (Castillo-Muñoz, pers. comm.). The species needs 50% light to proliferate and is mostly found in large canopy gaps, in secondary forests and along riparian vegetation, while rarely found in the understory of mature forests [].
2.3. Nematodes Associated with the Genus Heliconia
The nematodes we are dealing with are microscopic roundworms that inhabit the soil and feed on various plant structures, microbes, and animals, including other nematodes. Of special economic importance are plant–parasitic nematodes that cause nutritional deficiencies in the plant and serve as vectors of various diseases including leaf rot, root or rhizome rot, flower or bulb rot, and seed damage. Nematode genera and species that have been found infesting heliconia cultivars include the following: Aorolaimus, Criconemella, Helicotylenchus spp., Helicotylenchus erythrinae, Helicotylenchus crenacauda, Helicotylenchus dihystera, Hemicycliophora sp., Meloidogyne sp., Meloidogyne incognita, Mesocriconema sp., Pratylenchus zeae, Rotylenchulus reniformis, Trichodorus sp., Xiphinema sp., among others [,,]. Other infectious agents that have been described attacking heliconias include fungi (e.g., Alternaria solani, Fusarium oxysporum, Rhizoctonia solani, Colletotrichum gloeosporioides), viruses (Banana streak virus, Cucumber mosaic virus), bacteria (e.g., Pseudomonas solanacearum) and algae [].
In commercial cultivars, nematodes are known to affect the roots, which results in major disease symptoms such as brown, rotted roots, swollen roots or root knots, and root lesions. Nematode infestations of roots may occur alone but sometimes are accompanied by pathogenic fungi. When feeding, herbivorous nematodes puncture the root or even enter the root, which leaves an opening for other opportunistic pathogenic soil microorganisms. Some of the pathogens infecting heliconias are nonspecific; however, there are some pathogens that are specialized in the order Zingiberales. Heliconias growing under natural conditions and under cultivation may be affected by more than one disease-causing agent at a time. For instance, most leaves (70%) of H. collinsiana in the study area showed symptoms of foliar lesions (67% in forest gaps and 72% in secondary vegetation) characteristic of pathogenic fungi such as Bipolaris, Cylindrocladium, Cladosporium, Cercospora, and Puccinia-like damage [] (Benítez-Malvido, unpublished data). Our study however, consisted solely in counting the number of nematodes and their feeding guilds associated with H. collinsiana individuals present in mature (experimental control) and secondary forests within the study area.
2.4. Nematode Sampling and Processing
To assess if anthropogenic disturbance alters the local density of nematodes associated with H. collinsiana, in August 2019, ten individuals of H. collinsiana in secondary vegetation and 10 individuals in various forest gaps were randomly selected. In both habitats, sampled individuals were at least 50 m away from the nearest conspecific. We only considered the heliconias located in floodplains to keep soil type relatively constant.
One composite soil sample per individual of H. collinsiana was taken; the soil sample comprised 4 subsamples (~100 mL of soil each) taken on four equidistant corners of the plant using a soil core at a depth of 25 cm. A total of 80 subsamples were taken, 40 for primary forests and 40 for secondary forests. Samples were transported in an ice box until processed at the Agroecology Laboratory at the Research Institute for Ecosystems and Sustainability (IIES), National Autonomous University of Mexico (UNAM), Morelia, Michoacán, Mexico.
2.5. Nematode Extractions and Counts
A 100 mL aliquot of the composite soil sample taken around each heliconia plant was used to extract the nematodes. To collect the 100 mL aliquot, the soil sample was spread out on a bench, and subsamples were taken from all corners and the center of the sample using a spatula. Enough random sample was taken to fill a 100 mL beaker. Thereafter, the 100 mL aliquot was placed in two liters of water and stirred using a metal spatula for two minutes to disaggregate the sample. The suspension was allowed to settle for 30 s and then passed through a series of 60 and 500 mesh sieves (openings of 250 and 25 μm, respectively). The 60-mesh sieve fraction was discarded. Fraction collected in the 25 μm sieve was transferred to conical polypropylene centrifuge tubes (50 mL), spun at 600 g for 3 min, and the centrifuge was stopped using the brake. The supernatant was discarded, and a 45% sugar solution was added to the remaining pellet, which was resuspended by stirring and spun at 600 g for an additional two minutes before stopping using the break. The supernatant was passed through the 25 μm, and the suspension was collected in a beaker for further nematode characterization and counting.
Thereafter, two 15 mL aliquots of the nematode suspension were counted in an 85 mm gridded Petri plate. Nematodes were counted using a compound microscope at 40X magnification, and 108 (9 mm2 each) squares from the grid were counted. The number of individuals from the 108 counts was extrapolated to the total area of the counting plate, and nematode populations are given per 100 mL of soil. Based on their mouth morphology, nematodes were categorized into four feeding guilds including the following: bacterivores, fungivores, herbivores, and nematode predators []. It was only possible to identify plant feeder or herbivorous nematodes to the genus level.
2.6. Soil pH, Electrical Conductivity (EC), and Organic Matter Content
From each original composite sample, subsamples were taken to determine soil pH, EC, and the organic matter content. Soil pH and EC were determined in a 1:10 soil-to-water ratio using a LAQUA water quality analyzer pH/EC meter F-74, (HORIBA Scientific, Japan). The organic matter content (% of organic carbon) in the soil was determined following the method proposed by Walkley [] where chromic acid oxidizes the active forms of organic carbon in the soil, while leaving the inert forms unaltered.
2.7. Statistical Analysis
To test if habitat disturbance affected nematode density and feeding guild composition, we fitted two types of generalized linear mixed effect models, the first with absolute guild density as the response variable and the second with relative guild density (proportions). In both models, guilds, soil properties, habitat and their interaction were included as predictor variables. A Poisson distribution was assumed for absolute densities and a binomial for relative densities. In both cases, a random effect of the H. collinsiana individual plant was included to account for the non-independence of counts of different guilds from the same plant, as well as a random effect of each observation to account for overdispersion []. Models were fitted using the ‘glmer’ function in the lme4 library for R []. Post hoc tests with Bonferroni correction to assess differences across habitats within guilds were performed using the ‘pairs’ function in the emmeans package for R []. The analysis included a total of 10 rhizosphere soil samples per habitat type (N = 20). Significance was set at the p < 0.05 level.
3. Results
Overall, our results indicate that the interaction between H. collinsiana and its rhizosphere nematodes is altered in secondary forests originated by human activities. Particularly, our results show that guild composition (i.e., relative density) differs between habitats. It seems that some specific guilds are more sensitive to human disturbance, as shown below.
3.1. Nematode Density
All H. collinsiana individuals that were sampled presented nematodes in their rhizosphere, in both mature continuous forests and secondary forests. The density of nematodes in continuous forests varied from 112 to 373 nematodes per 100 mL of soil (262.8 ± 77.7 mean ± se), whereas in secondary forests, it varied from 81 to 684 nematodes per 100 mL of soil (356.4 ± 186.7 mean ± se). Although all nematode feeding guilds were present in both habitats, not all sampled individuals presented all four feeding guilds concurrently (Figure 1). Overall, the number of nematodes per 100 mL of soil differed between guilds (χ2 = 313.4, df = 3, p < 0.001), declining from herbivores to bacterivorous, to fungivores, to predators (Figure 1). In mature continuous forests, the density of feeding guilds declined from herbivores, to fungivores, to bacterivorous, to predators. For secondary forests, the number of nematodes declined from herbivores, to bacterivorous, to fungivores, to predators (Figure 1). The term that reflects the interaction of habitat and guild was marginally statistically significant for explaining variability in nematode density (χ2 = 7.03, df = 3, p < 0.071). Fixed predictors explained 87.2% of variation in density. Guild alone can explain 84.9% of the variance in relative density, the Habitat × Guild interaction, 1.1%, and the other fixed factors an additional 1.2%. Random factors (H. collinsiana ID and observation ID) explained 12.7% of the variation in density.
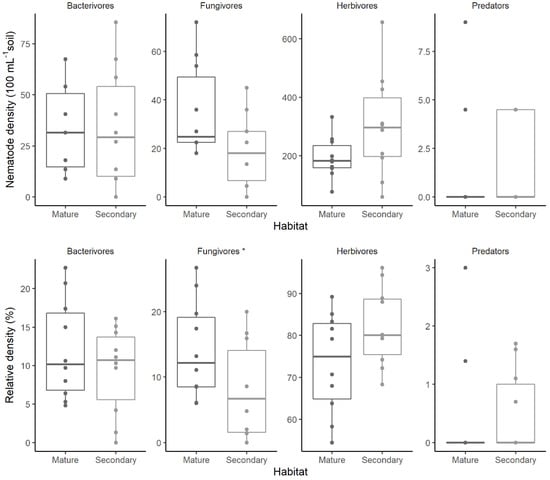
Figure 1.
Absolute (upper panels) and relative (lower panels) density of nematodes from different feeding guilds in the rhizosphere of the tropical herb Heliconia collinsiana inhabiting mature continuous forests and secondary forests in southern Mexico. Names with an asterisk indicate significant differences among habitats (p < 0.05).
For herbivorous nematodes, we identified three genera, Criconemella spp., Helicotylenchus spp., and Meloidogyne spp., but no significant differences in their density were detected within and between habitat types.
3.2. Nematode Guild Composition
The relative density of nematode guilds was significantly affected by the interaction between habitat and guild (χ2 = 9.48, df = 3, p = 0.024). Post hoc comparisons showed that relative density was significantly affected for fungivores (Z = 2.558, p = 0.011), but not for other guilds. Fungivore relative density was significantly higher in mature forests than in secondary forests (Figure 1). Fixed predictors explained 89.9% of variation in relative density. Guild alone is able to explain 88.0% of the variance in relative density, while the Habitat × Guild interaction explained an additional 1.9%. Random factors (H. collinsiana ID and observation ID) explained 10.2% of the variation in relative density.
3.3. Soil pH, Electrical Conductivity, and Organic Matter Content
Soil pH in secondary forests was slightly less acidic than in soils in the mature continuous forest, but these differences were not statistically significant. No significant differences in soil EC were found in the soils of both habitat types. Soils from the mature continuous forest had slightly more organic carbon when compared with soils from the secondary forest, although these differences were not statistically significant (Figure 2). None of the soil properties considered in our statistical model were related with nematode density
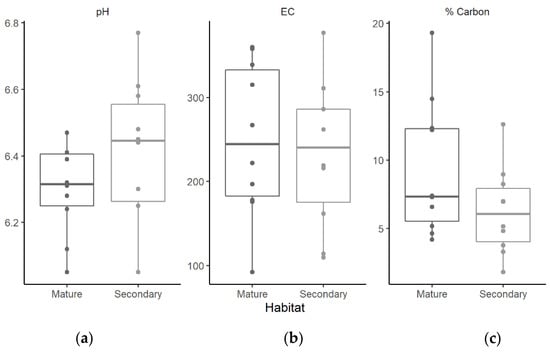
Figure 2.
Variation in soil pH (a), electrical conductivity (b), and % organic carbon (c) in mature continuous forests and secondary forests in southern Mexico.
4. Discussion
Overall, we found that habitat disturbance significantly affected the relative density of nematodes with the proportion of fungivorous nematodes declining from mature to secondary forests. Nevertheless, the selected soil characteristics in the rhizosphere of the native tropical plant Heliconia collinsiana, did not show significant differences between habitats. Compared to other trophic guilds, the density of herbivorous nematodes, known to infect roots and rhizomes of several crops, was the highest in both habitat types. The three genera of herbivorous nematodes, Criconemella spp., Helicotylenchus spp., and Meloidogyne spp., are commonly found infecting several plant species in tropical agroecosystems including heliconia cultivars []. The relative density of fungivores was significantly higher in mature forests compared with secondary forests. In contrast, bacterivorous and predatory nematodes remained relatively unchanged between habitats. The trends regarding density of feeding guilds found in our study area match those trends reported for tropical moist forests worldwide, with predatory nematodes being the less abundant and herbivorous nematodes the most abundant [,,]. Changes in nematode relative density between habitats could be the consequence of a complex interplay of factors that include local environmental conditions affecting nematode populations and those affecting host availability and susceptibility to pathogen attack. In this study, however, we do not know the relative importance of such factors on the observed trends.
4.1. Habitat Disturbance and Nematode Populations
The structure of soil nematode populations and communities and soil processes are affected by natural and anthropogenic disturbances. Along forest successional trajectories and in various types of agricultural management, nematode feeding guilds have shown different responses in their populations [,,]. Plant parasitic nematodes are commonly abundant in tropical soils as compared to other feeding guilds []. Compared to other studies, differences detected in nematode communities between habitat types, were primarily driven by fungivore nematodes. Decreased relative density of fungivore nematodes could have negative consequences for the health of the soil ecosystem as fungal pathogens reduce plant productivity, disrupt plant nutrient and water transfer, and decrease fruit and tuber quality and size []. Free living nematodes such as bacterivores, fungivores and predators have fundamental roles in processing organic matter, in controlling soil microorganism populations and in plant growth and are good indicators of soil health []. Nevertheless, whether habitat disturbance changed nematode species richness and composition remains unknown in our study [].
4.2. Habitat Disturbance and Nematode Infection
Disease agents, including nematodes, may only infect a host plant under the influence of other factors such as a favorable environment, a virulent pathogen, and a susceptible host []. The physical (e.g., light, temperature, pH, soil fertility and moisture) and biological environments (e.g., host density, host identity, soil microbiome) drive the prevalence and infection of plant pathogens []. The physical environment, however, is the most important factor determining the development of a disease, and therefore, shifts in a particular physical factor in a disturbed habitat predispose the host plant to pathogen attack [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,].
Species of the three genera of herbivorous nematodes found in the present study are known to infect the roots of several cultivated tropical plant species. Herbivorous nematode feeding sites may be root-hairs, epidermal, cortical, or vascular structures. Several species of the genus Helicotylenchus (e.g., Helicotylenchus erythrinae, H. crenacauda, H. dihystera) have been found infecting species of Heliconiaceae, whereas the genera Criconemella, and Meloidogyne (e.g., Meloidogyne incognita) are known to infect several plant species in agroecosystems [,,]. Depending on the host, species of the genus Helicotylenchus exhibit an ectoparasitic and semi-endoparasitic feeding habit, reducing crop production in the Musaceae. Herbivorous nematodes may be polyphagous, feeding on several host species, or show host specificity, such as in those that feed in the Heliconiaceae and Musaceae.
Apparently, secondary forests in the study area provided suitable conditions for the prevalence of herbivorous nematodes in the rhizosphere of H. collinsiana, as has been shown for leaf pathogenic fungi under several types of human disturbances [,,]. High conspecific densities and denser clumps of H. collinsiana in secondary forests may also provide larger and denser root systems for nematodes to infest. Density-dependent mortality factors, including plant predation and diseases, might operate more strongly therein [,,]. Individuals of H. collinsiana in different habitats might have different rates of root growth and decay as a physiological response to changes in environmental factors within secondary forests. Changes in the root system under secondary forest conditions may facilitate nematode–fungus relationships known to cause diseases in various tropical crops [].
5. Conclusions
Based on our results, we might expect lower populations of fungivore nematodes to develop in secondary tropical forests, which may lead to increased susceptibility of host plants to pathogenic fungi [,,]. Furthermore, nematodes from a specific feeding guild could be used as indicators of soil health and hence develop alternatives to aid host plants to withstand various pathogens in disturbed habitats. For instance, some soil-borne plant diseases and pests, including nematodes, are frequently suppressed with organic mulch []. High organic matter content should increase microbial biodiversity in the rhizosphere, improving soil health. Soil physical, chemical, and biological attributes may differ between mature and secondary forests in tropical and temperate ecosystems []. Nevertheless, the dynamics of soil microbes and flora in conserved and disturbed tropical habitats are still poorly understood despite the relevance that pests and pathogens have on structuring plant communities and in the maintenance of their high biodiversity [,,].
Author Contributions
Conceptualization, J.B.-M.; methodology, J.B.-M. and P.F.J.-L.; investigation, J.B.-M., P.F.J.-L., J.M.L.-G., H.H.S.-C. and R.L.; resources J.B.-M., P.F.J.-L. and J.M.L.-G.; formal analysis, J.B.-M., P.F.J.-L., J.M.L.-G., F.M.-A. and H.H.S.-C.; writing—original draft preparation, J.B.-M.; writing—review and editing, J.B.-M., P.F.J.-L., J.M.L.-G., H.H.S.-C., F.M.-A. and R.L.; supervision, J.B.-M. and P.F.J.-L.; project administration, J.B.-M.; funding acquisition, J.B.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad Nacional Autónoma de México (Projects PAPIIT IN-214014, IN-202117 and IN201620 to J.B.-M.).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are openly available in Dryad at: https://datadryad.org/stash/share/ve00texezgounKgVxJBpBHjw0_ynsol3YakmDDxdN0s. Data were accessed on 10 September 2022.
Acknowledgments
We appreciate the logistic support from Universidad Nacional Autónoma de México.
Conflicts of Interest
The corresponding author confirms, on behalf of all authors, that there are no conflicting interests or involvements that might raise the question of bias in the work reported here or in the conclusions, implications or opinions stated.
References
- Ayala-Orozco, B.; Gavito, M.E.; Mora, F.; Siddique, I.; Balvanera, P.; Jaramillo, V.J.; Cotler, H.; Romero-Duque, L.P.; Martínez-Meyer, E. Resilience of soil properties to land-use change in a tropical dry forest ecosystem. Land Degrad. Dev. 2018, 29, 315–325. [Google Scholar] [CrossRef]
- Carrillo-Saucedo, S.M.; Gavito, M.E. Resilience of soil aggregation and exocellular enzymatic functions associated with arbuscular mycorrhizal fungal communities along a successional gradient in a tropical dry forest. Mycorrhiza 2020, 30, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Allek, A.; Crouzeilles, R. Soil dynamics in forest restoration: A data set for temperate and tropical regions. Ecology 2021, 102, e03207. [Google Scholar] [CrossRef]
- Berkelmans, R.; Ferris, H.; Tenuta, M.; van Bruggen, A.H.C. Effects of long-term crop management on nematode trophic levels other than plant feeders disappear after 1 year of disruptive soil management. Appl. Soil Ecol. 2003, 23, 223–235. [Google Scholar] [CrossRef]
- Benítez-Malvido, J.; Giménez, A.; Graciá, E.; Rodríguez-Caro, R.C.; De Ybáñez, R.R.; Siliceo-Cantero, H.H.; Traveset, A. Impact of habitat loss on the diversity and structure of ecological networks between oxyurid nematodes and spur-thighed tortoises (Testudo graeca L.). PeerJ 2019, 7, e8076. [Google Scholar] [CrossRef]
- Benítez-Malvido, J.; Martínez-Falcón, A.P.; Dáttilo, W.; del Val, E. Diversity and network structure of invertebrate communities associated to Heliconia species in natural and human disturbed tropical rain forests. Glob. Ecol. Conserv. 2014, 2, 107–117. [Google Scholar] [CrossRef]
- Benítez-Malvido, J.; Dáttilo, W.; Martínez-Falcón, A.P.; Durán-Barrón, C.; Valenzuela, J.; López, S.; Lombera, R. The multiple impacts of tropical forest fragmentation on arthropod biodiversity and on their patterns of interactions with host plants. PLoS ONE 2016, 11, e0146461. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Malvido, J.; Dáttilo, W. Interaction intimacy of pathogens and herbivores with their host plants influences the topological structure of ecological networks in different ways. Am. J. Bot. 2015, 102, 512–519. [Google Scholar] [CrossRef]
- Aguilar, R.; Ashworth, L.; Galetto, L.; Aizen, M.A. Plant reproductive susceptibility to habitat fragmentation: Review and synthesis through a meta-analysis. Ecol. Lett. 2006, 9, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Malvido, J. Fungal diseases in Neotropical forests disturbed by humans. In Conservation Medicine: Applied Cases of Ecological Health; Aguirre, A., Daszak, P., Ostfeld, R.S., Eds.; Oxford University Press: London, UK, 2012; pp. 302–311. [Google Scholar]
- Santos, B.A.; Benítez-Malvido, J. Insect herbivory and leaf disease in natural and human disturbed habitats: Lessons from early-successional Heliconia herbs. Biotropica 2012, 44, 53–62. [Google Scholar] [CrossRef]
- Benítez-Malvido, J.; Lázaro, A.; Ferraz, I.D. Effect of distance to edge and edge interaction on seedling regeneration and biotic damage in tropical rainforest fragments: A long-term experiment. J. Ecol. 2018, 106, 2204–2217. [Google Scholar] [CrossRef]
- Krishnadas, M.; Kumar, A.N.; Comita, L.S. Edge effects reduce a-diversity but not b-diversity during community assembly in a human-modified tropical forest. Ecol. Appl. 2019, 29, e01996. [Google Scholar] [CrossRef]
- Boag, B.; Yeates, G.W. Soil nematode biodiversity in terrestrial ecosystems. Biodivers. Conserv. 1998, 7, 617–630. [Google Scholar] [CrossRef]
- Neher, D.A. Role of nematodes in soil health and their use as indicators. J. Nematol. 2001, 33, 161. [Google Scholar] [PubMed]
- van den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; de Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S.; et al. Soil nematode abundance and functional group composition at a global scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef] [PubMed]
- van den Hoogen, J.; Geisen, S.; Wall, D.H.; Wardle, D.A.; Traunspurger, W.; de Goede, R.G.; Crowther, T.W.; Adams, B.J.; Ahmad, W.; Ferris, H.; et al. A global database of soil nematode abundance and functional group composition. Sci. Data 2020, 7, 1–8. [Google Scholar] [CrossRef]
- Ingham, R.E.; Trofymow, J.A.; Ingham, E.R.; Coleman, D.C. Interactions of bacteria, fungi, and their nematode grazers: Effects on nutrient cycling and plant growth. Ecol. Monogr. 1985, 55, 119–140. [Google Scholar] [CrossRef]
- Procter, D.L. Global overview of the functional roles of soil-living nematodes in terrestrial communities and ecosystems. J. Nematol. 1990, 22, 1–7. [Google Scholar]
- Crowther, T.W.; Boddy, L.; Jones, T.H. Species-specific effects of soil fauna on fungal foraging and decomposition. Oecologia 2011, 167, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, S.; Talavera, M. Nematodes as environmental indicators in agroecosystems. Ecosistemas 2013, 22, 50–55. [Google Scholar]
- Sewake, K.T.; Uchida, J.Y. Diseases of Heliconia in Hawaii; Research Extension 159; Agriculture and Human Resources: Honolulu, HI, USA, 1995. [Google Scholar]
- López-Cardona, N.; Castaño-Zapata, J. Characterization of Phytopathogenic Fungi, Bacteria, Nematodes and Viruses in Four Commercial Varieties of Heliconia (Heliconia sp.). Rev. Fac. Nac. Agron. Medellín 2012, 65, 6697–6710. [Google Scholar]
- Mattos-Sobrinho, C.C.; Silveira, A.J.; César, F.B.C.; Oliveira, C.M.G.; Bittencourt, M.A.L. Phytonematodes associated with Heliconia spp. in commercial crops in the South coast of Bahia, Brazil. Nematropica 2012, 42, 351–355. [Google Scholar]
- Aristizábal, L.F.; Ospina, K.A.; Vallejo, U.A.; Henao, E.R.; Salgado, M.; Arthurs, S.P. Entomofauna Associated with Heliconia spp. (Zingiberales: Heliconiaceae) Grown in the Central Area of Colombia. Fla. Entomol. 2013, 96, 112–119. [Google Scholar] [CrossRef]
- Hadley, A.S.; Frey, S.J.K.; Robinson, W.D.; Betts, M.G. Forest fragmentation and loss reduce richness, availability, and specialization in tropical hummingbird communities. Biotropica 2018, 50, 74–83. [Google Scholar] [CrossRef]
- Berry, F.; Kress, W.J. Heliconia: An Identification Guide (No. 635.93421); Smithsonian Institution Press: Washington, DC, USA, 1991. [Google Scholar]
- Assis, S.M.P.; Mariano, R.L.R.; Gondim, M.G.C., Jr.; Menezes, M.; Rosa, R.C.T. Disease and Pests of Heliconias/Doenças e Pragas das Helicônias; Editora da UFRPE: Recife, Brazil, 2002. [Google Scholar]
- Lugo-Cruz, E.; del Rivero-Bautista, N.; Sánchez-Soto, S.; Osorio-Osorio, R.; Romero-Nápoles, J. Insectos fitófagos asociados a cultivos de heliconias (Heliconia spp.) en Tabasco, México. Agroproductividad 2020, 13, 31–36. [Google Scholar]
- Alarcón-Restrepo, J.J. Enfermedades en la producción de heliconias en los departamentos de Caldas, Risaralda y Quindío. Agronomia 2007, 15, 45–61. [Google Scholar]
- Assis, T.C.; de Andrade, D.E.G.T. Fitonematoses em Zingiberales ornamentais no Estado de Pernambuco. An. Acad. Pernambucana Ciênc. Agron. 2007, 4, 185–198. [Google Scholar]
- Benítez-Malvido, J.; Lemus-Albor, A. The Seedling Community of Tropical Rain Forest Edges and Its Interaction with Herbivores and Pathogens. Biotropica 2005, 37, 301–313. [Google Scholar] [CrossRef]
- Medellín, R.A. Mammal diversity and conservation in the Selva Lacandona, Chiapas, Mexico. Conserv. Biol. 1994, 8, 780–799. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- López-Pérez, S.; Benítez-Malvido, J.; Lobato-García, J.M.; Siliceo-Cantero, H.H.; Santillán-Mendoza, R. A New State Record for Chelobasis bicolor Gray (Coleoptera: Chrysomelidae: Cassidinae: Arescini) and New Host Association with Heliconia bourgaeana Peterson (Heliconiaceae) in Mexico. Coleopt. Bull. 2020, 74, 572–575. [Google Scholar] [CrossRef]
- Lins, S.R.O.; Coelho, R.S.B. Ocorrência de Doenças em Plantas Ornamentais Tropicais no Estado de Pernambuco. Fitopatol. Bras. 2004, 29, 332–335. [Google Scholar] [CrossRef]
- Sardinha, D.H.S.; Rodrigues, A.A.C.; Diniz, N.B.; De Lemos, R.N.S.; Da Silva, G.S. Fungos e nematóides fitopatogênicos associados ao cultivo de flores tropicais em São Luís-MA. Summa Phytopathol. 2012, 38, 159–162. [Google Scholar] [CrossRef][Green Version]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera—An outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar]
- Walkley, A. A critical examination of a rapid method for determining organic carbon in soils-effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 1947, 63, 251–264. [Google Scholar] [CrossRef]
- Harrison, X. Using observation-level random effects to model overdispersion in count data in ecology and evolution. PeerJ 2014, 2, e616. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.V. emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version, 1.8.1-1. 2021. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 26 September 2022).
- Villegas-Urbano, N.P.; Restrepo-Alarcón, J.J.; Galindo, J.R. Enfermedades Limitantes de la Producción de Heliconias en los Departamentos de Caldas, Risaralda y Quindío; Instituto Colombiano Agropecuario: Bogotá, Colombia, 2006.
- Burdon, J.J. Fungal pathogens as selective forces in plant populations and communities. Austral. J. Ecol. 1991, 16, 423–432. [Google Scholar] [CrossRef]
- Yeates, G.W.; Coleman, D.C. Nematodes in decomposition. In Nematodes in Soil Ecosystems; Freckman, D.W., Ed.; University of Texas: Austin, TX, USA, 1982. [Google Scholar]
- Brussaard, L.; Behan-Pelletier, V.M.; Bignell, D.E.; Brown, V.K.; Didden, W.; Folgarait, P.; Fragoso, C.; Freckman, D.W.; Gupta, V.V.S.R.; Hattori, T.; et al. Biodiversity and ecosystem functioning in soil. Ambio 1997, 26, 563–570. [Google Scholar]
- Gruden, K.; Lidoy, J.; Petek, M.; Podpečan, V.; Flors, V.; Papadopoulou, K.K.; Pappas, M.L.; Martinez-Medina, A.; Bejarano, E.; Biere, A.; et al. Ménage à trois: Unraveling the mechanisms regulating plant–microbe–arthropod interactions. Trends Plant Sci. 2020, 25, 1215–1226. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant Pathology; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Escuer, M.; Lara, M.; Bello, A. Nematodos de la subfamilia Criconematinae (Nematoda: Criconematidae) en la España peninsular. Orsis 1997, 12, 39–63. Available online: www.raco.cat/index.php/Orsis/article/view/24394 (accessed on 10 September 2022).
- Jarosz, A.M.; Davelos, A.L. Effects of disease in wild plant populations and the evolution of pathogen aggressiveness. New Phytol. 1995, 129, 371–387. [Google Scholar] [CrossRef]
- Bagchi, R.; Gallery, R.E.; Gripenberg, S.; Gurr, S.J.; Narayan, L.; Addis, C.E.; Frecklenton, R.P.; Lewis, O.T. Pathogens and insect herbivores drive rainforest plant diversity and composition. Nature 2014, 506, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Connell, J.H. On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. Dyn. Popul. 1971, 298, 312. [Google Scholar]
- van Bruggen, A.H.C.; Semenov, A.M. In search of biological indicators for soil health and disease suppression. Appl. Soil Ecol. 2000, 15, 13–24. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).