Physiological and Ecological Correlates of the Cellular and Humoral Innate Immune Responses in an Insular Desert Bat: The Fish-Eating Myotis (Myotis vivesi)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Parasite Load
2.3. Immune Challenge
2.4. Bacterial Killing Ability
2.5. Steroid Hormone Analyses
2.6. Data Analysis
3. Results
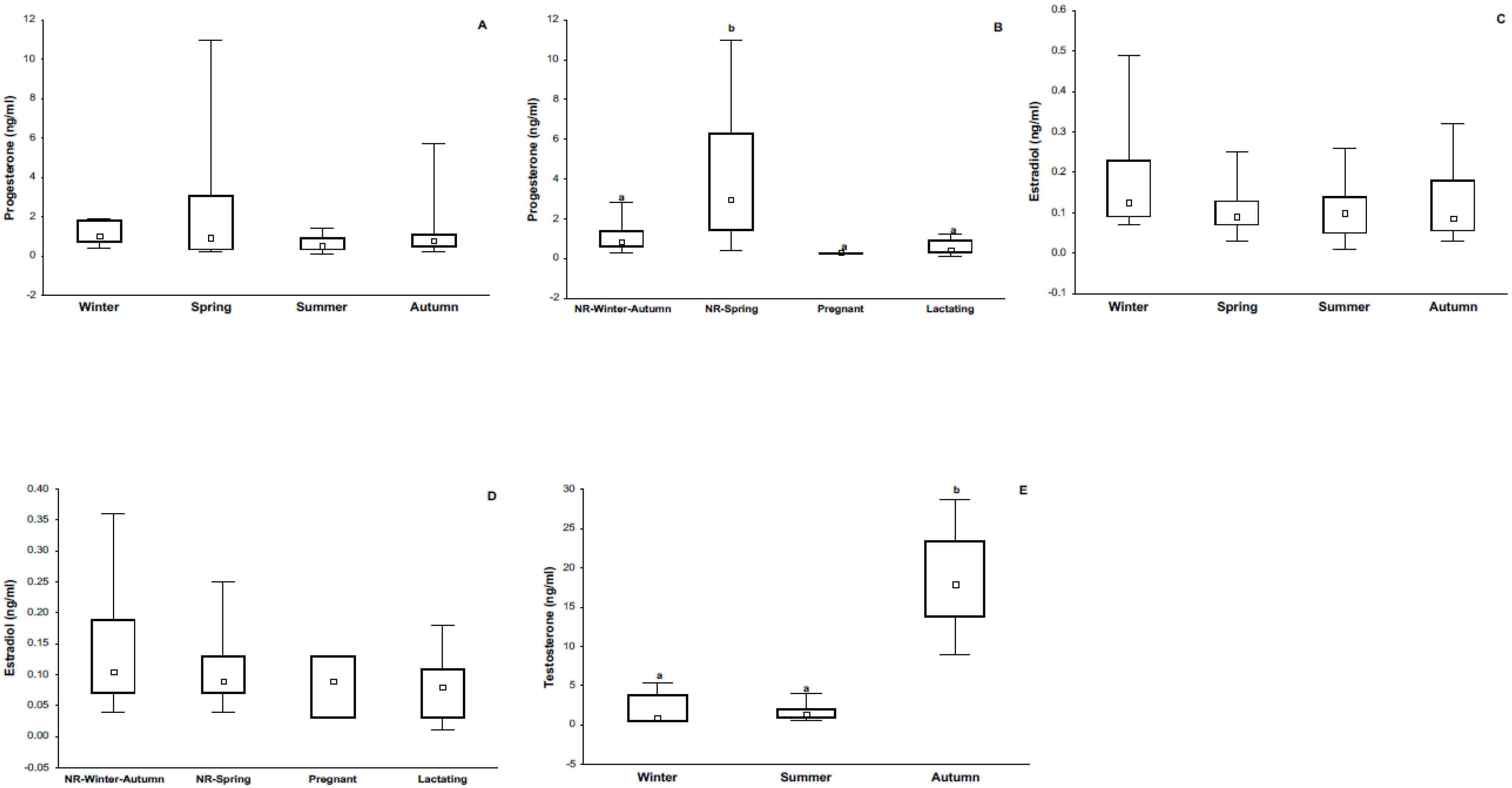
3.1. Body Mass, Reproductive Condition, and Sexual Hormones
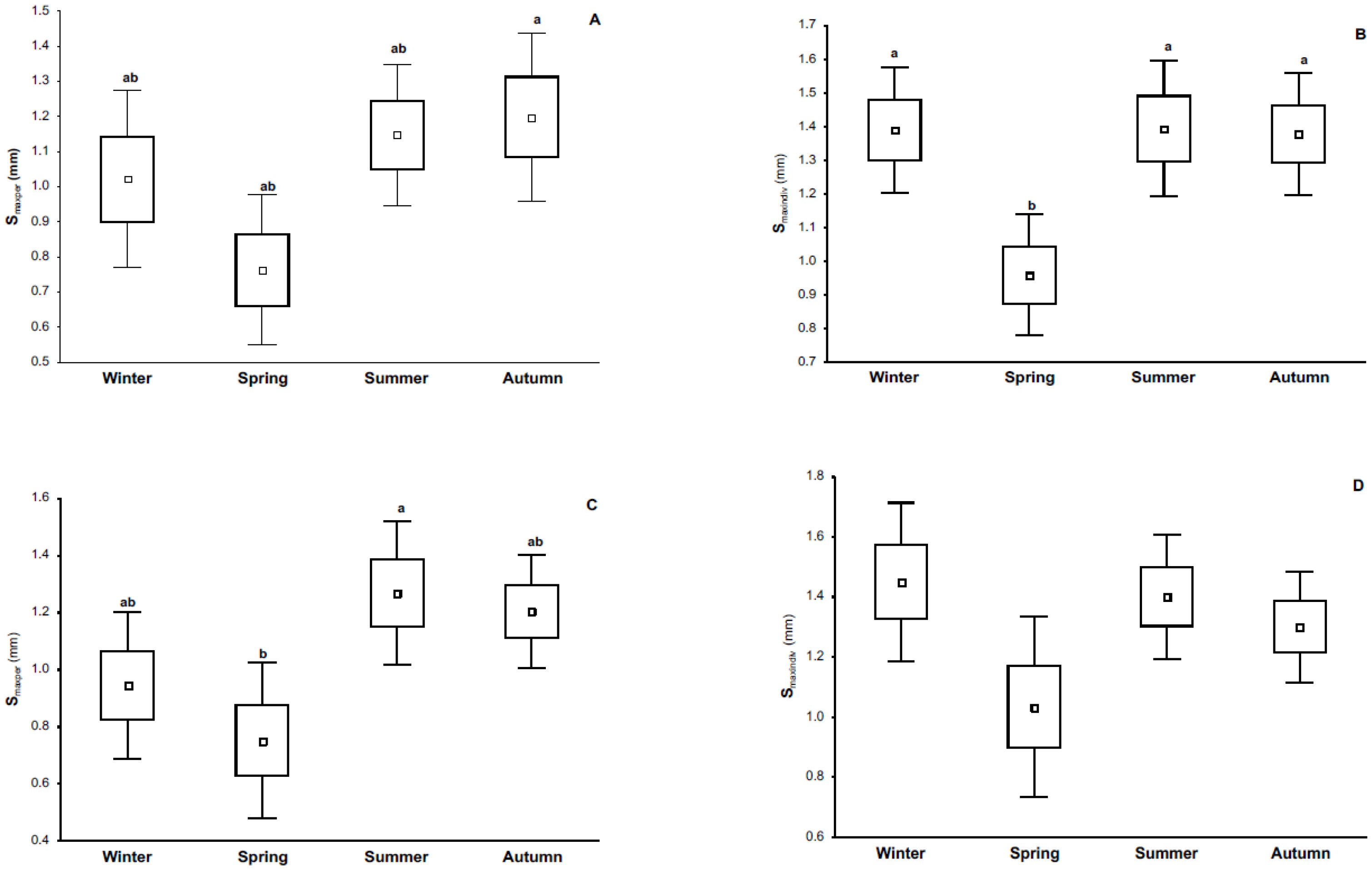
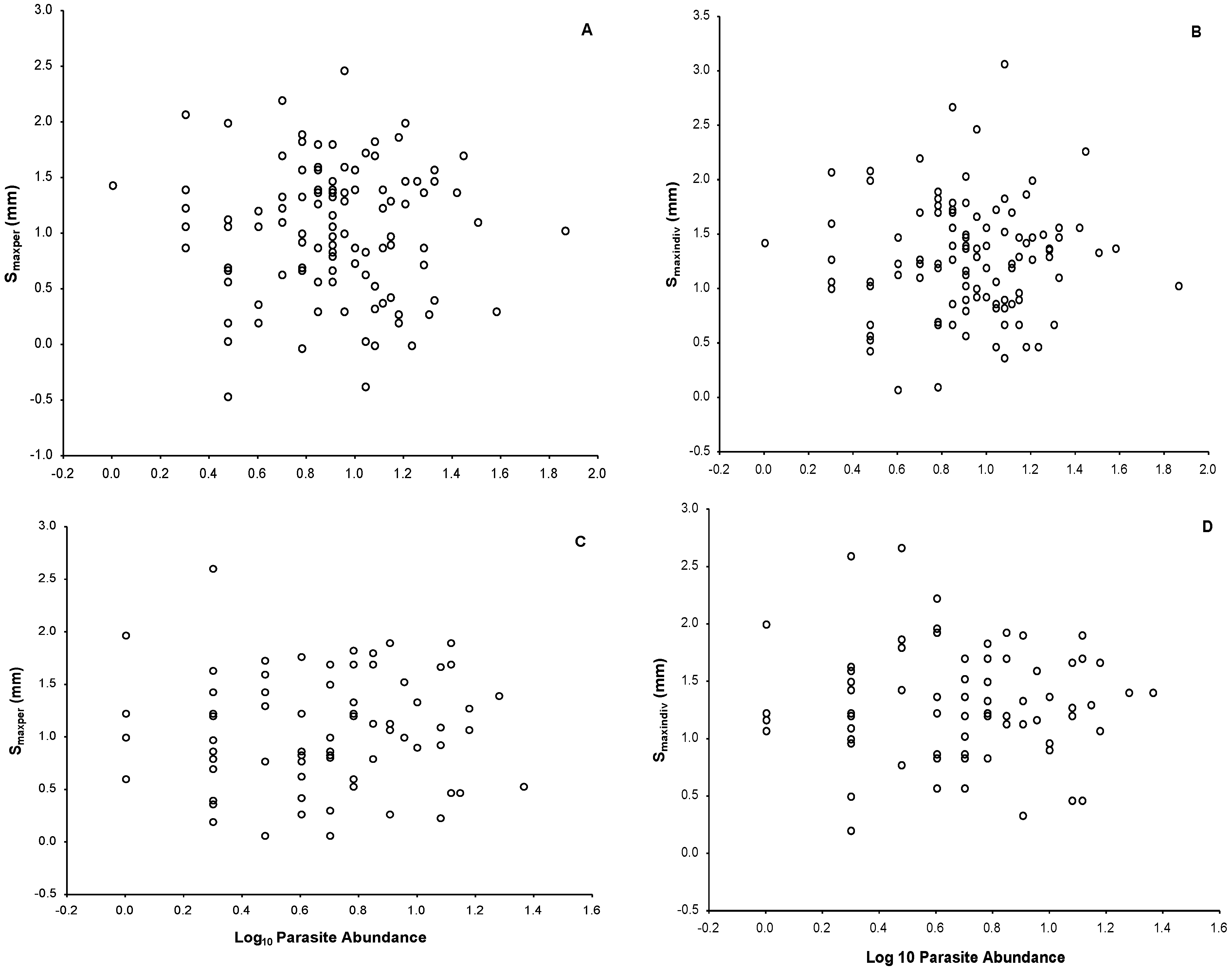
3.2. Phytohemagglutinin (PHA) Measurements
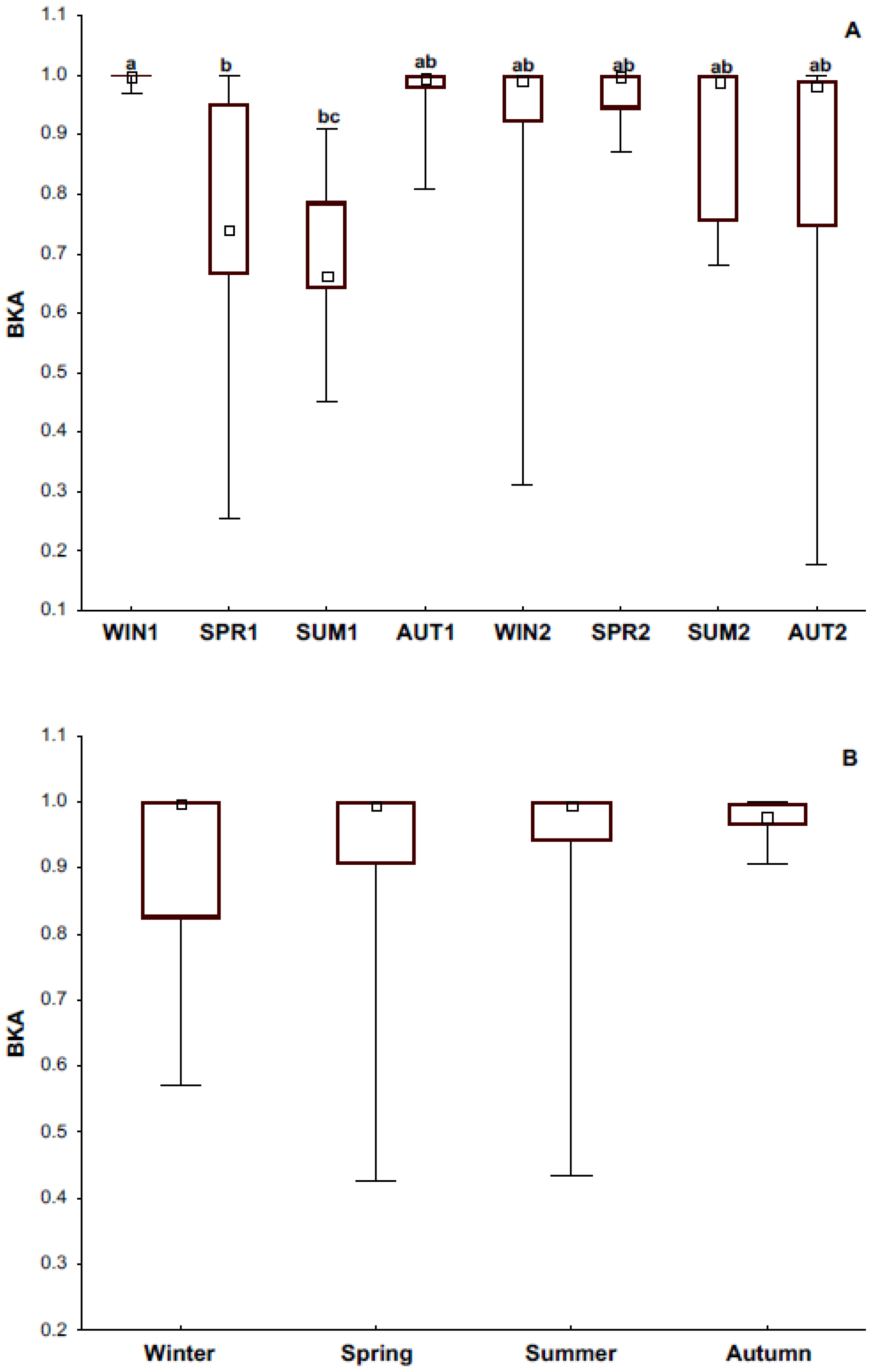
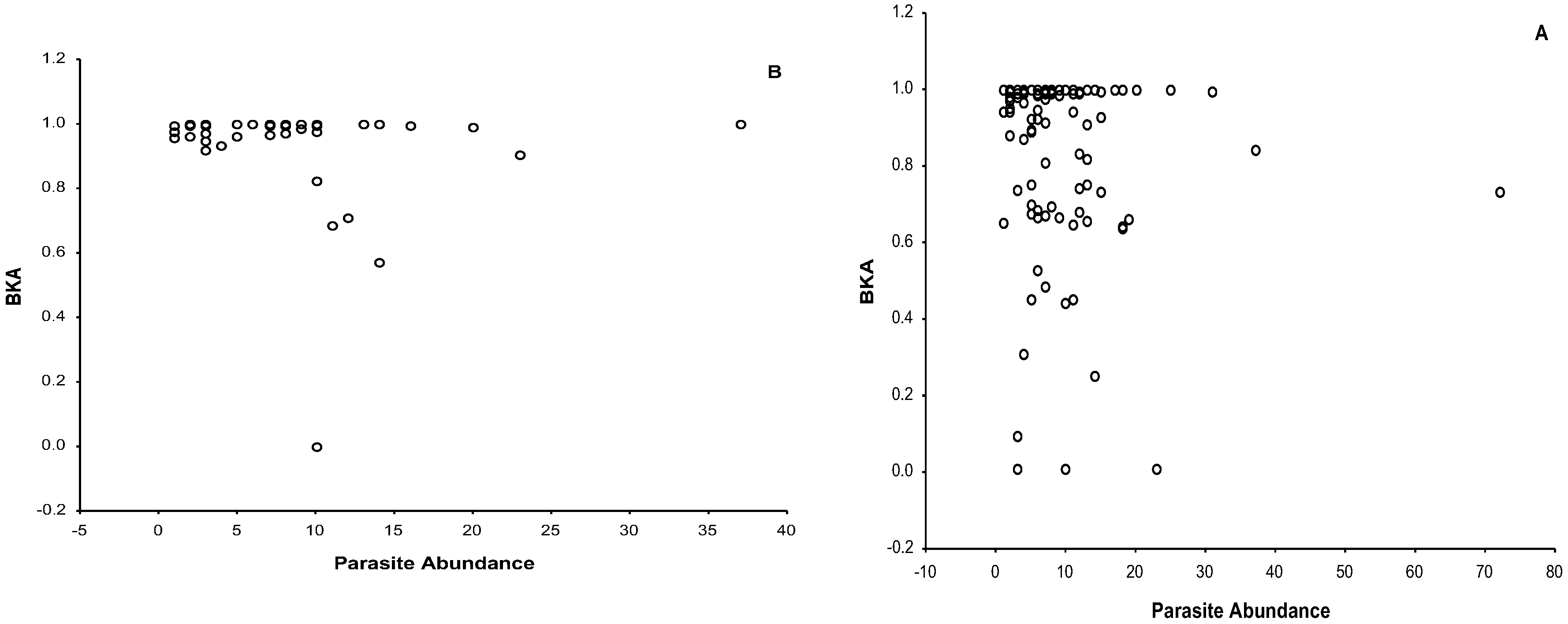
3.3. Bacteria Killing Ability
3.4. Ectoparasite Load
4. Discussion
4.1. Immune Response and Its Relationship with Season and Reproductive Condition
4.2. Immune Response and Its Relationship with Parasite Load and Body Mass
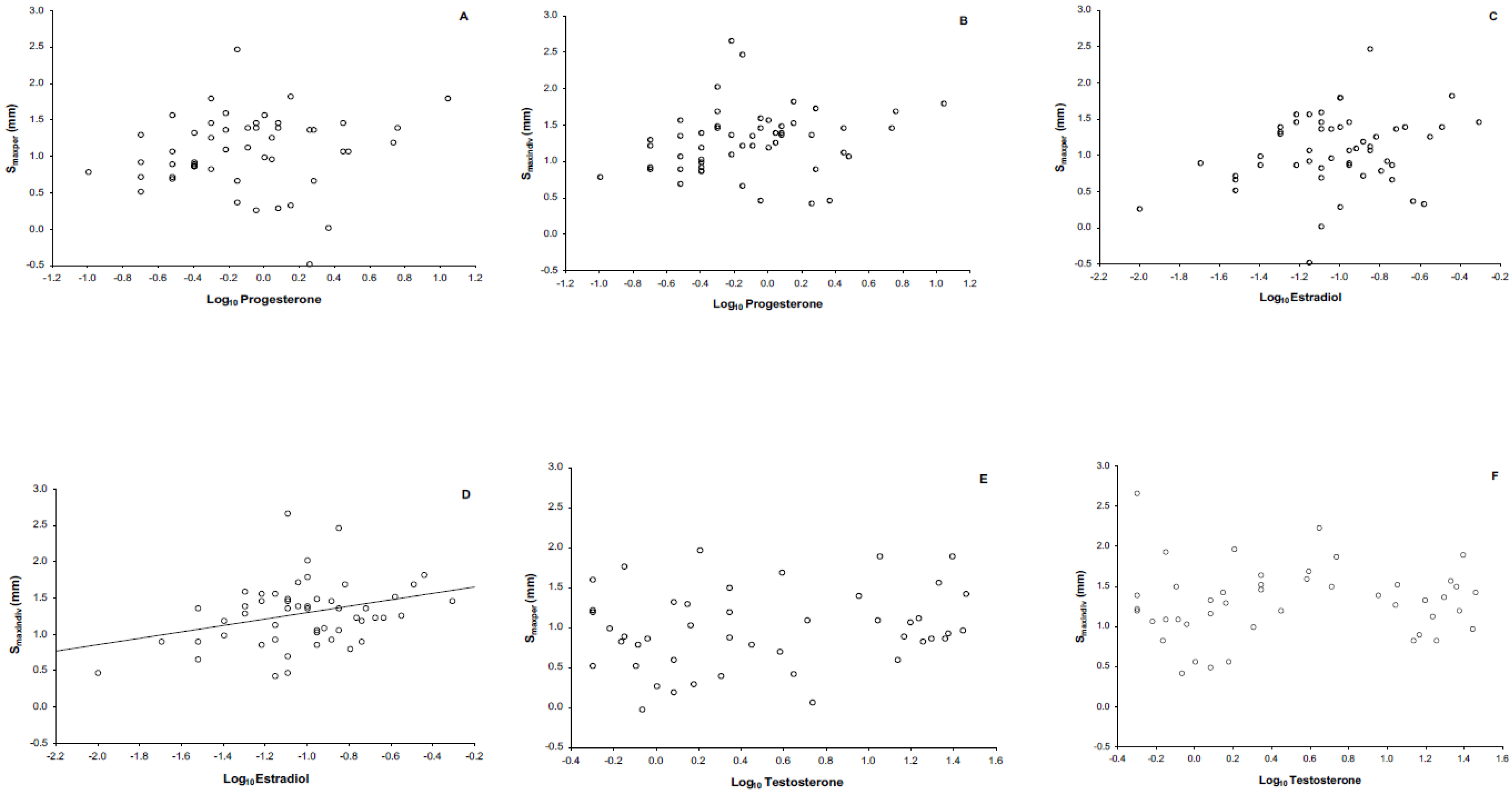
4.3. Immune Response and Its Relationship with Steroid Sex Hormones
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, R.C.; Lymbery, A.J.; Smith, A. Parasites, emerging disease and wildlife conservation. Int. J. Parasitol. 2010, 40, 1163–1170. [Google Scholar] [CrossRef]
- Owen, J.P.; Nelson, A.C.; Clayton, D.H. Ecological immunology of bird-ectoparasite systems. Trends Parasitol. 2010, 26, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Carrillo, J.L.; Garcia, F.P.C.; Coronado OGGarcia, M.A.M.; Cordero, J.F.C. Physiology and pathology of innate immune response against pathogens. In Physiology and Pathology of Immunology; Rezaei, N., Ed.; IntechOpen Ltd.: London, UK, 2017; pp. 99–134. [Google Scholar]
- Lazzaro, B.P.; Little, T.J. Immunity in a variable world. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.B.; Weil, Z.M.; Nelson, R.J. Seasonal changes in vertebrate immune activity: Mediation by physiological trade-offs. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 321–339. [Google Scholar] [CrossRef] [PubMed]
- French, S.S.; DeNardo, D.F.; Moore, M.C. Trade-offs between the reproductive and immune systems: Facultative responses to resources or obligate responses to reproduction? Am. Nat. 2007, 170, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Taneja, V. Sex hormones determine immune response. Front. Immunol. 2018, 9, 1931. [Google Scholar] [CrossRef]
- Foo, Y.Z.; Nakagawa, S.; Rhodes, G.; Simmons, L.W. The effects of sex hormones on immune function: A meta-analysis. Biol. Rev. 2017, 92, 551–571. [Google Scholar] [CrossRef]
- Cattadori, I.; Boag, B.; Bjornstad, O.; Cornell, S.; Hudson, P. Peak shift and epidemiology in a seasonal host-nematode system. Proc. Biol. Sci. 2005, 272, 1163–1169. [Google Scholar] [CrossRef]
- Lourenço, S.; Palmeirim, J.M. Which factors regulate the reproduction of ectoparasites of temperate-zone cave-dwelling bats? Parasitol. Res. 2008, 104, 127–134. [Google Scholar] [CrossRef]
- Podmokła, E.; Dubiec, A.; Drobniak, S.M.; Arct, A.; Gustafsson, L.; MCichoń, M. Avian malaria is associated with increased reproductive investment in the blue tit. J. Avian Biol. 2014, 45, 219–224. [Google Scholar] [CrossRef]
- Klein, S.L. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004, 26, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R. The physiological costs of reproduction in small mammals. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 375–398. [Google Scholar] [CrossRef] [PubMed]
- Nordling, D.; Andersson, M.; Zohari, S.; Gustafsson, L. Reproductive effort reduces specific immune response and parasite resistance. Proc. Biol. Sci. 1998, 265, 1291–1298. [Google Scholar] [CrossRef]
- Knowles, S.C.L.; Nakagawa, S.; Sheldon, B.C. Elevated reproductive effort increases blood parasitaemia and decreases immune function in birds: A meta-regression approach. Funct. Ecol. 2009, 23, 405–415. [Google Scholar] [CrossRef]
- Hayward, A.D.; Pilkington, J.G.; Wilson, K.; McNellly, T.N.; Watt, K.A. Reproductive effort influences intra-seasonal variation in parasite-specific antibody responses in wild Soay sheep. Funct. Ecol. 2019, 33, 1307–1320. [Google Scholar] [CrossRef]
- Albery, G.G.; Watt, K.A.; Keith, R.; Morris, S.; Morris, A.; Kenyon, F.; Nussey, D.H.; Pemberton, J.M. Reproduction has different costs for immunity and parasitism in a wild mammal. Funct. Ecol. 2020, 34, 229–239. [Google Scholar] [CrossRef]
- Huyghe, K.; Van Oystaeyen, A.; Pasmans, F.; Tadić, Z.; Vanhooydonck, B.; Van Damme, R. Seasonal changes in parasite load and a cellular immune response in a colour polymorphic lizard. Oecologia 2010, 163, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Veiga, J.P.; Salvador, A.; Merino, S.; Puerta, M. Reproductive effort affects immune response and parasite infection in a lizard: A phenotypic manipulation using testosterone. Oikos 1998, 82, 313–318. [Google Scholar] [CrossRef]
- Hughes, V.L.; Randolph, S.E. Testosterone depresses innate and acquired resistance to ticks in natural rodent hosts: A force for aggregated distributions of parasites. J. Parasitol. 2001, 87, 49–54. [Google Scholar] [CrossRef]
- Simmons, N.B.; Cirranello, A.L. Bat Species of the World: A Taxonomic and Geographic Database. 2021. Available online: https://batnames.org/ (accessed on 1 August 2022).
- Calisher, C.H.; Childs, J.E.; Field, H.E.; Holmes, K.V.; Schountz, T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006, 19, 531–545. [Google Scholar] [CrossRef]
- Mühldorfer, K. Bats and bacterial pathogens: A review. Zoonoses Public Health 2013, 60, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.L.; Schountzam, T.; Wang, L.F. Antiviral immune responses of bats: A review. Zoonoses Public Health 2013, 60, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Mandl, J.N.; Schneider, C.; Schneider, D.S.; Baker, M.L. Going to bat(s) for studies of disease tolerance. Front. Immunol. 2018, 9, 2112. [Google Scholar] [CrossRef]
- Banerjee, A.; Baker, M.L.; Kulcsar, K.; Misra, V.; Plowright, R.; Mossman, K. Novel insights into immune systems of bats. Front. Immunol. 2020, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Christe, P.; Arlettaz, R.; Vogel, P. Variation in intensity of a parasitic mite (Spinturnix myoti) in relation to the reproductive cycle and immunocompetence of its bat host (Myotis myotis). Ecol. Lett. 2000, 3, 207–212. [Google Scholar] [CrossRef]
- Lilley, T.M.; Stauffer, J.; Kanerva, M.; Eeva, T. Interspecific variation in redox status regulation and immune defence in five bat species: The role of ectoparasites. Oecologia 2014, 175, 811–823. [Google Scholar] [CrossRef]
- Schneeberger, K.; Courtiol, A.; Czirják, G.A.; Voigt, C.C. Immune profile predicts survival and reflects senescence in a small, long-lived mammal, the Greater Sac-Winged bat (Saccopteryx bilineata). PLoS ONE 2014, 9, e108268. [Google Scholar] [CrossRef]
- Warburton, E.M.; Pearl, C.A.; Vonhof, M.J. Relationships between host body condition and immunocompetence, not host sex, best predict parasite burden in a bat-helminth system. Parasitol. Res. 2016, 115, 2155–2164. [Google Scholar] [CrossRef]
- Ruoss, S.; Becker, N.I.; Otto, M.S.; Czirjá, G.Á.; Encarnação, J.A. Effect of sex and reproductive status on the immunity of the temperate bat Myotis daubentonii. Mammal. Biol. 2019, 94, 120–126. [Google Scholar] [CrossRef]
- Schneeberger, K.; Czirják, G.Á.; Voigt, C.C. Measures of the constitutive immune system are linked to diet and roosting habits of Neotropical bats. PLoS ONE 2013, 8, e54023. [Google Scholar] [CrossRef]
- Horrock, N.P.C.; Matson, K.D.; Tieleman, B.I. Pathogen pressure puts immune defense into perspective. Integr. Comp. Biol. 2011, 51, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Otálora-Ardila, A.; Herrera, M.L.G.; Flores-Martínez, J.J.; Voigt, C.C. Marine and terrestrial food sources in the diet of the fish-eating myotis (Myotis vivesi). J. Mammal. 2013, 94, 1102–1110. [Google Scholar] [CrossRef]
- Drinkwater, R.; Goodwin, A.; Cush, J.; Korstian, J.M.; Chumchal, M.M.; Herrera, M.L.G.; Valdez, M.; Otálora-Ardila, A.; Flores-Martinez, J.J.; Clare, E.L. Molecular diet analysis of the marine fish-eating bat (Myotis vivesi) and potential mercury exposure. Can. J. Zool. 2021, 99, 752–759. [Google Scholar] [CrossRef]
- Herrera, M.L.G.; Flores-Martínez, J.J.; Sánchez-Cordero, V. Geographical distribution and conservation status of an endemic insular mammal: The Vulnerable fish-eating bat Myotis vivesi. Oryx 2019, 53, 388–393. [Google Scholar] [CrossRef]
- Moyer, B.R.; Drown, D.M.; Clayton, D.H. Low humidity reduces ectoparasite pressure: Implications for host life history evolution. Oikos 2002, 97, 223–228. [Google Scholar] [CrossRef]
- Horrocks, N.P.; Hegemann, A.; Ostrowski, S.; Ndithia, H.; Shobrak, M.; Williams, J.B.; Matson, K.D.; Tieleman, B.I. Environmeonmental proxies of antigen exposure explain variation in immune investment better than indices of pace of life. Oecologia 2015, 177, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Matson, K.D.; Mauck, R.A.; Lynn, S.E.; Tieleman, B.I. Island life shapes the physiology and life history of Eastern Bluebirds (Sialia sialis). Physiol. Biochem. Zool. 2014, 87, 172–182. [Google Scholar] [CrossRef]
- Richman, A.D.; Herrera, M.L.G.; Ortega-García, S.; Flores-Martínez, J.J.; Arroyo-Cabrales, J.; Morales-Malacara, J.B. Class II DRB polymorphism and sequence diversity in two vesper bats in the genus Myotis. Int. J. Immunogenet. 2010, 37, 401–405. [Google Scholar] [CrossRef]
- Pilosof, S.; Korine, C.; Moore, M.S.; Krasnov, B.R. Effects of sewage-water contamination on the immune response of a desert bat. Mamm. Biol. 2014, 79, 183–188. [Google Scholar] [CrossRef]
- Korine, C.; Pilosof, S.; Gross, A.; Morales-Malacara, J.B.; Krasnov, B. The effect of water contamination and host-related factors on ectoparasite load in an insectivorous bat. Parasitol. Res. 2017, 116, 2517–2526. [Google Scholar] [CrossRef]
- Vinkler, M.; Bainová, H.; Albrecht, T. Functional analysis of the skin-swelling response to phytohaemagglutinin. Funct. Ecol. 2010, 24, 1081–1086. [Google Scholar] [CrossRef]
- Vinkler, M.; Svobodová, J.; Gabrielová, B.; Vainová, H.; Bryjová, A. Cytokine expression in phytohaemagglutinin-induced skin inflammation in a galliform bird. J. Avian Biol. 2014, 45, 43–50. [Google Scholar] [CrossRef]
- Millet, S.; Bennett, J.; Lee, K.A.; Hau, M.; Klasing, K.C. Quantifying and comparing constitutive immunity across avian species. Dev. Comp. Immunol. 2007, 31, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Greiner, S.; Stefanski, V.; Dehnhard, M.; Voigt, C.C. Plasma testosterone levels decrease after activation of skin immune system in a free-ranging mammal. Gen. Comp. Endocrin. 2010, 168, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Flores-Martínez, J.J.; Floyd, C.H.; Herrera, M.L.G.; May, B. Genetic variation and çpopulation size of the endangered fishing bat, Myotis vivesi in Isla Partida. In Contribuciones Mastozoológicas en Homenaje a Bernardo Villa; Sánchez Cordero, V., Medellín, R.A., Eds.; Universidad Nacional Autónoma de México, Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2005; pp. 187–192. [Google Scholar]
- Peig, J.; Green, A.J. New perspectives for estimating body condition from mass/length data: The scaled mass index as an alternative method. Oikos 2009, 118, 1883–1891. [Google Scholar] [CrossRef]
- Reiczigel, J.; Marozzi, M.; Fabian, I.; Rozsa, L. Biostatistics for parasitologists—A primer to Quantitative Parasitology. Trends Parasitol. 2019, 35, 277–281. [Google Scholar] [CrossRef]
- Smits, J.E.; Bortolotti, G.R.; Tella, J.L. Symplifying the phytohaemagglutinin skin-testing technique in studies of avian immunocompetence. Funct. Ecol. 1999, 13, 567–572. [Google Scholar] [CrossRef]
- Allen, L.C.; Turmelle, A.S.; Mendonça, M.T.; Navara, K.J.; Kunz, T.H.; McCracken, G.F. Roosting ecology and variation in adaptive and innate immune system function in the Brazilian free-tailed bat (Tadarida brasiliensis). J. Comp. Physiol. B 2009, 179, 315–323. [Google Scholar] [CrossRef]
- STATISTICA, version 10; Data Analysis Software System; StatSoft Inc.: Tulsa, OK, USA, 2011.
- Hernández-Arciga, U.; Herrera, M.L.G.; Ibáñez-Contreras, A.; Miranda-Labra, R.U.; Flores-Martínez, J.J.; Königsberg, M. Baseline and post-stress seasonal changes in immunocompetence and redox state maintenance in the fishing bat Myotis vivesi. PLoS ONE 2018, 13, e0190047. [Google Scholar] [CrossRef]
- Beguelini, M.R.; Santiago, C.S.; Guerra, L.H.A.; Santos, F.C.A.; Góes, R.M.; Morielle-Versute, E.; Taboga, S.R. The hormonal control of the uterus of the bat Myotis nigricans during its different reproductive phases: Emphasis on progesterone and estradiol. Cell Tissue Res. 2021, 384, 211–229. [Google Scholar] [CrossRef]
- Greiner, S.; Dehnhard, M.; Voigt, C.C. Differences in plasma testosterone levels related to social status are more pronounced during mating than nonmating season in the tropical bat Saccopteryx bilineata (greater sac-winged bat). Can. J. Zool. 2011, 89, 1157–1163. [Google Scholar] [CrossRef]
- Otálora-Ardila, A.; Herrera, M.L.G.; Flores-Martínez, J.J.; Welch, K.C., Jr. Metabolic cost of the activation of immune response in the fish- eating myotis (Myotis vivesi): The effects of inflammation and the acute phase response. PLoS ONE 2016, 11, e0164938. [Google Scholar] [CrossRef]
- Merlo, J.L.; Cutrera, A.P.; Luna, F.; Zenuto, R.R. PHA-induced inflammation is not energetically costly in the subterranean rodent Ctenomys talarum (tuco-tucos). Comp. Biochem. Physiol. Mol. Integr. Physiol. 2014, 175, 90–99. [Google Scholar] [CrossRef]
- O’Brien, K.A.; Waterman, J.M.; Anderson, W.G.; Bennett, N.C. Trade-offs between immunity and testosterone in male African ground squirrels. J. Exp. Biol. 2018, 22, jeb177683. [Google Scholar] [CrossRef]
- Li, D.; Hao, Y.; Liu, X.; Yao, Y.; Du, C.; Zhang, X.; Cui, S.; Wu, L.; Wu, Y. Changes in phytohemagglutinin skin-swelling responses during the breeding season in a multi-brooded species, the Eurasian Tree Sparrow: Do males with higher testosterone levels show stronger immune responses? J. Ornithol. 2015, 156, 133–141. [Google Scholar] [CrossRef]
- Oppliger AGiorgi, M.S.; Conelli, A.; Nembrini, N.; John-Alder, H.B. Effect of testosterone on immunocompetence, parasite load, and metabolism in the common wall lizard (Podarcis muralis). Can. J. Zool. 2004, 82, 1713–1719. [Google Scholar] [CrossRef]
- Casto, J.M.; Nolan, V., Jr.; Ketterson, E.D. Steroid hormones and immune function: Experimental studies in wild and captive dark-eyed juncos (Junco hyemalis). Am. Nat. 2001, 157, 408–420. [Google Scholar] [CrossRef]
- Duffy, D.L.; Bentley, G.E.; Drazen, D.L.; Ball, G.F. Effects of testosterone on cell-mediated and humoral immunity in non-breeding adult European starlings. Behav. Ecol. 2000, 11, 654–662. [Google Scholar] [CrossRef]
- Greenman, C.G.; Martin, L.B.; Hau, M. Reproductive state, but not testosterone, reduces immune function in male house sparrows (Passer domesticus). Physiol. Biochem. Zool. 2005, 78, 60–68. [Google Scholar] [CrossRef]
- Ezenwa, V.; Stefan, L.; Creel, S. Unravelling complex associations between testosterone and parasite infection in the wild. Funct. Ecol. 2012, 16, 123–133. [Google Scholar] [CrossRef]
- Merrill, L.; Stewart, T.E.; González-Gómez, P.L.; O’Loghlen, A.L.; Wingfield, J.C.; Ellis, V.A.; Rothstein, S.I. Epaulet size and current condition in red-winged blackbirds: Examining a semistatic signal, testosterone, immune function, and parasites. Physiol. Biochem. Zool. 2015, 88, 11–21. [Google Scholar] [CrossRef] [PubMed]
- LaVere, A.A.; Hamlin, H.J.; Lowers, R.R.H.; Parrott, B.B.; Ezenwa, V.O. Associations between testosterone and immune activity in alligators depend on bacteria species and temperature. Funct. Ecol. 2021, 35, 1018–1027. [Google Scholar] [CrossRef]
- Ádori, M.; Kiss, E.; Barad, Z.; Barabás, K.; Kiszely, E.; Schneider, A.; Kövesdi, D.; Sziksz, E.; Abrahám, I.M.; Matkó, J.; et al. Estrogen augments the T cell-dependent but not the T-independent immune response. Cell. Mol. Life Sci. 2010, 67, 1661–1674. [Google Scholar] [CrossRef] [PubMed]
- Carlsten, H.; Holmdahl, R.; Tarkowski, A.; Nilsson, L.A. Oestradiol- and testosterone-mediated effects on the immune system in normal and autoimmune mice are genetically linked and inherited as dominant traits. Immunology 1989, 68, 209–214. [Google Scholar]
- Li, L.; Zhang, S.; Tong, Z. In vivo effects of 17-β-estradiol on plasma immunoglobulin levels and leukocyte density in zebrafish Danio rerio. Chin. J. Ocean. Limnol. 2010, 28, 527–532. [Google Scholar] [CrossRef]
- Bilbo, S.D.; Nelson, R.J. Sex steroid hormones enhance immune function in male and female Siberian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R207–R213. [Google Scholar] [CrossRef]
- Ma, L.J.; Guzmán, E.A.; DeGuzman, A.; Muller, H.K.; Walker, A.M.; Owen, L.B. Local cytokine levels associated with delayed-type hypersensitivity responses: Modulation by gender, ovariectomy, and estrogen replacement. J. Endocrinol. 2007, 193, 291–297. [Google Scholar] [CrossRef]
- Khan, N. Possible protective role of 17β-estradiol against COVID-19. J. Allergy Infect. Dis. 2020, 1, 38–48. [Google Scholar]
- Klein, P.W.; Easterbrook, J.D.; Lalime, E.N.; Klein, S.L. Estrogen and progesterone affect responses to malaria infection in female C57BL/6 mice. Gend Med. 2008, 5, 423–433. [Google Scholar] [CrossRef]
- Wira, C.R.; Sullivan, D.A. Estradiol and progesterone regulation of immunoglobulin A and G and secretory component in cervicovaginal secretions of the rat. Biol. Reprod. 1985, 32, 90–95. [Google Scholar] [CrossRef]
- Rettew, J.A.; Huet, Y.M.; Marriott, I. Estrogens augment cell surface TLR4 expression on murine macrophages and regulate sepsis susceptibility in vivo. Endocrinology 2009, 150, 3877–3884. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.J.; Chumchal, M.M.; Bentz, A.B.; Platt, S.G.; Czirják, G.Á.; Rainwater, T.R.; Altizer, S.; Streicker, D.G. Predictors and immunological correlates of sublethal mercury exposure in vampire bats. R. Soc. Open Sci. 2017, 4, 170073. [Google Scholar] [CrossRef] [PubMed]
- Korine, C.; Adams, R.; Russo, D.; Fisher-Phelps, M.; Jacobs, D. Bats and water: Anthropogenic alterations threaten global bat populations. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Voigt, C.C., Kingston, T., Eds.; Springer: Cham, Switzerland, 2016; pp. 215–241. [Google Scholar]

| Bat sex | Season | BM (g) | BCI | n |
|---|---|---|---|---|
| Female | Winter | 28.53 ± 0.50 | 204.55 ± 3.05 | 31 |
| Spring | 28.25 ± 0.59 | 197.57 ± 3.62 | 2 | |
| Summer | 28.21 ± 0.46 | 200.69 ± 2.83 | 36 | |
| Autumn | 29.52 ± 0.56 | 210.92 ±3.40 | 25 | |
| Male | Winter | 25.83 ± 0.49 | 187.15 ± 3.44 | 18 |
| Spring | 24.80 ± 0.55 | 178.48 ± 3.90 | 14 | |
| Summer | 27.35 ± 0.41 | 196.80 ± 2.86 | 26 | |
| Autumn | 27.56 ± 0.48 | 199.20 ± 3.34 | 19 |
| Bat sex | Season | Prevalence (%) | Intensity | Abundance | n |
|---|---|---|---|---|---|
| Female | Winter | 96.8 (83.3–99.9) | 11.9 (8.3–20.0) | 11.5 (8.2–19.6) | 31 |
| Spring | 100.0 (90.5–100.0) | 5.9 (4.7–7.6) | 5.9 (4.7–7.6) | 37 | |
| Summer | 100.0 (90.3–100.0) | 11.1 (9.5–13.0) | 11.1 (9.5–12.9) | 36 | |
| Autumn | 100.0 (80.5–100.0) | 7.7. (5.7–10.6) | 7.7. (5.7–10.8) | 17 | |
| Male | Winter | 100.0 (85.1–100.0) | 5.72 (4.0–9.4) | 5.7 (4.0–9.4) | 18 |
| Spring | 100.0 (76.8–100.0) | 7.57 (5.1–10.1) | 7.57 (5.1–10.2) | 14 | |
| Summer | 100.0 (86.3–100.0) | 5.0 (3.9–6.3) | 5.0 (3.8–6.2) | 25 | |
| Autumn | 100.0 (78.2–100.0) | 7.6 (5.5–10.3) | 7.6 (5.4–10.2) | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otálora-Ardila, A.; Flores-Martínez, J.J.; Rosales, C.; Salame-Méndez, A.; Montalvo., L.G.H. Physiological and Ecological Correlates of the Cellular and Humoral Innate Immune Responses in an Insular Desert Bat: The Fish-Eating Myotis (Myotis vivesi). Diversity 2022, 14, 781. https://doi.org/10.3390/d14100781
Otálora-Ardila A, Flores-Martínez JJ, Rosales C, Salame-Méndez A, Montalvo. LGH. Physiological and Ecological Correlates of the Cellular and Humoral Innate Immune Responses in an Insular Desert Bat: The Fish-Eating Myotis (Myotis vivesi). Diversity. 2022; 14(10):781. https://doi.org/10.3390/d14100781
Chicago/Turabian StyleOtálora-Ardila, Aída, José Juan Flores-Martínez, Carlos Rosales, Arturo Salame-Méndez, and L. Gerardo Herrera Montalvo. 2022. "Physiological and Ecological Correlates of the Cellular and Humoral Innate Immune Responses in an Insular Desert Bat: The Fish-Eating Myotis (Myotis vivesi)" Diversity 14, no. 10: 781. https://doi.org/10.3390/d14100781
APA StyleOtálora-Ardila, A., Flores-Martínez, J. J., Rosales, C., Salame-Méndez, A., & Montalvo., L. G. H. (2022). Physiological and Ecological Correlates of the Cellular and Humoral Innate Immune Responses in an Insular Desert Bat: The Fish-Eating Myotis (Myotis vivesi). Diversity, 14(10), 781. https://doi.org/10.3390/d14100781









