A Review of Human-Elephant Ecological Relations in the Malay Peninsula: Adaptations for Coexistence
Abstract
:1. Introduction
2. Methodology
2.1. Study Site
2.2. Study Species
2.3. Literature Review
2.4. Field Observations
2.5. Analysis
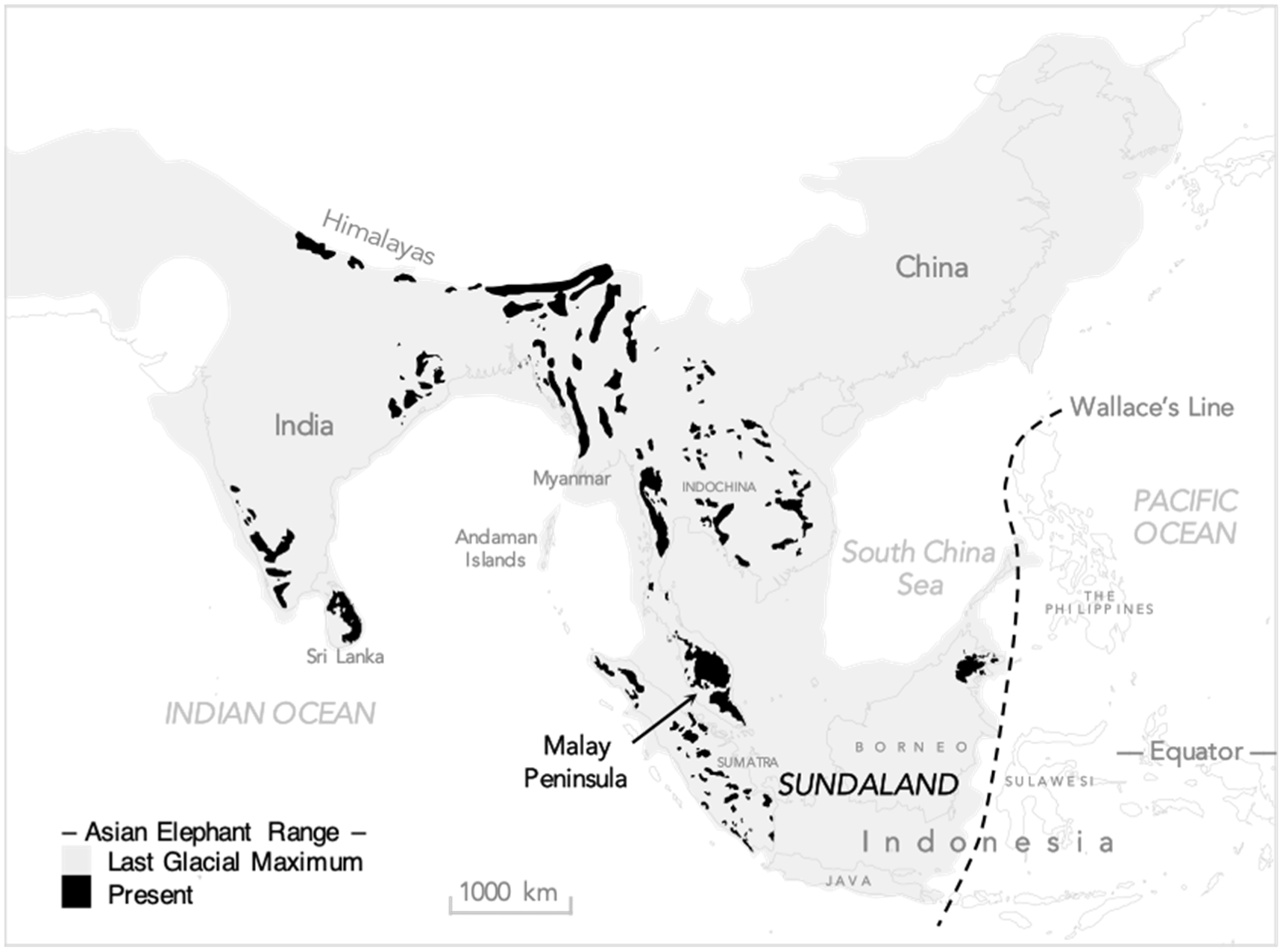
3. Overview of the Human–Elephant Ecological Overlap
4. Spatio-Temporal Niche Overall and Partitioning: The Elephant and the Person in the Room
4.1. Eurytopic Sympatry: From Coast to Mountain Crest
4.2. Shared Pathways: Elephant Forest Trails
4.3. Facultative Arborealism
4.4. Temporal Niche Partitioning: Diurnal & Nocturnal Activity Patterns
5. Trophic Niche: One Bite at a Time
5.1. Finding Food in the Rainforest
5.2. Dessert in the Green Desert: Carbohydrates from Palms
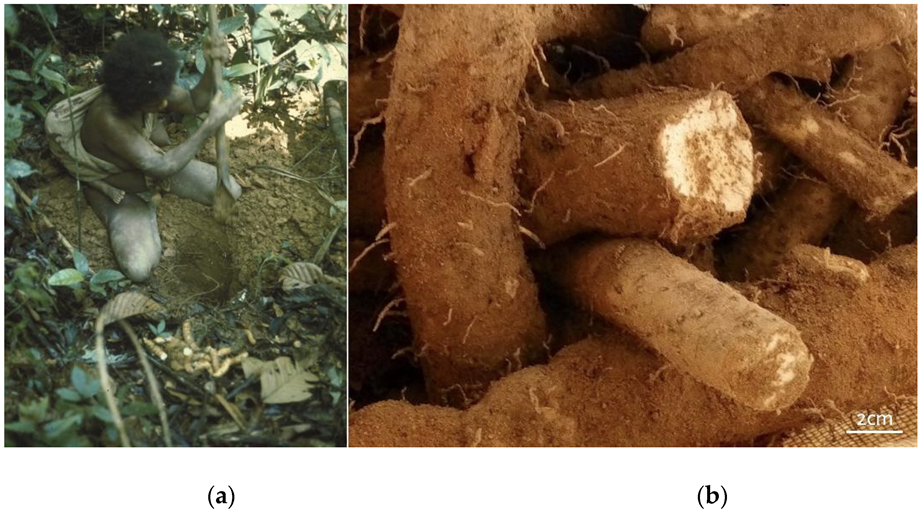
5.3. A Proboscidean Perspective on the Wild Yam Problem
5.4. Honey Hunters
5.5. Fruit Gardeners
5.6. Swidden Farming: Elephants in the Fallow Field
5.7. Predation: Calories from Animal Protein
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Shoshani, J.; Tassy, P. Advances in Proboscidean Taxonomy & Classification, Anatomy & Physiology, and Ecology & Behavior. Quat. Int. 2005, 126, 5–20. [Google Scholar]
- Palkopoulou, E.; Lipson, M.; Mallick, S.; Nielsen, S.; Rohland, N.; Baleka, S.; Karpinski, E.; Ivancevic, A.M.; To, T.-H.; Kortschak, R.D. A Comprehensive Genomic History of Extinct and Living Elephants. Proc. Natl. Acad. Sci. USA 2018, 115, E2566–E2574. [Google Scholar] [CrossRef] [Green Version]
- Agam, A.; Barkai, R. Elephant and Mammoth Hunting during the Paleolithic: A Review of the Relevant Archaeological, Ethnographic and Ethno-Historical Records. Quaternary 2018, 1, 3–31. [Google Scholar] [CrossRef] [Green Version]
- Ben-Dor, M.; Gopher, A.; Hershkovitz, I.; Barkai, R. Man the Fat Hunter: The Demise of Homo erectus and the Emergence of a New Hominin Lineage in the Middle Pleistocene (ca. 400 Kyr) Levant. PLoS ONE 2011, 6, e28689. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.D.; Zeder, M.A. The Onset of the Anthropocene. Anthropocene 2013, 4, 8–13. [Google Scholar] [CrossRef]
- Malhi, Y.; Doughty, C.E.; Galetti, M.; Smith, F.A.; Svenning, J.-C.; Terborgh, J.W. Megafauna and Ecosystem Function from the Pleistocene to the Anthropocene. Proc. Natl. Acad. Sci. USA 2016, 113, 838–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brittain, S.; Bata, M.N.; Ornellas, P.D.; Milner-Gulland, E.J.; Rowcliffe, M. Combining Local Knowledge and Occupancy Analysis for a Rapid Assessment of the Forest Elephant Loxodonta cyclotis in Cameroon’s Timber Production Forests. Oryx 2020, 54, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, A.; Lahiri Choudhury, D.K.; Desai, A.; Duckworth, J.W.; Easa, P.S.; Johnsingh, A.J.T.; Fernando, P.; Hedges, S.; Gunawardena, M.; Kurt, F.; et al. Elephas maximus. The IUCN Red List of Threatened Species 2008. Available online: https://doi.org/10.2305/IUCN.UK.2008.RLTS.T7140A12828813.en (accessed on 31 October 2020).
- Chase, M.J.; Schlossberg, S.; Griffin, C.R.; Bouché, P.J.C.; Djene, S.W.; Elkan, P.W.; Ferreira, S.; Grossman, F.; Kohi, E.M.; Landen, K.; et al. Continent-Wide Survey Reveals Massive Decline in African Savannah Elephants. PeerJ 2016, 4, e2354. [Google Scholar] [CrossRef]
- Blake, S.; Hedges, S. Sinking the Flagship: The Case of Forest Elephants in Asia and Africa. Conserv. Biol. 2004, 18, 1191–1202. [Google Scholar] [CrossRef]
- Lima-Ribeiro, M.S.; Nogués-Bravo, D.; Terribile, L.C.; Batra, P.; Diniz-Filho, J.A.F. Climate and Humans Set the Place and Time of Proboscidean Extinction in Late Quaternary of South America. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 392, 546–556. [Google Scholar] [CrossRef]
- Stuart, A.J. The Extinction of Woolly Mammoth (Mammuthus primigenius) and Straight-Tusked Elephant (Palaeoloxodon antiquus) in Europe. Quat. Int. 2005, 126–128, 171–177. [Google Scholar] [CrossRef]
- Lobban, R.A., Jr.; De Liedekerke, V. Elephants in Ancient Egypt and Nubia. Anthrozoös 2000, 13, 232–244. [Google Scholar] [CrossRef]
- Elvin, M. The Retreat of the Elephants: An Environmental History of China, Illustrated ed.; Yale University Press: New Haven, CT, USA, 2001; ISBN 978-0-300-11993-0. [Google Scholar]
- Parker, I.S.C.; Graham, A.D. Men, Elephants and Competition. Symp. Zool. Soc. Lond. 1989, 61, 241–252. [Google Scholar]
- Barnes, R.F.W. The Conflict between Humans and Elephants in the Central African Forests. Mammal Rev. 1996, 26, 67–80. [Google Scholar] [CrossRef]
- Sukumar, R. A Brief Review of the Status, Distribution and Biology of Wild Asian Elephants, Elephas maximus. Int. Zoo Yearb. 2006, 40, 1–8. [Google Scholar] [CrossRef]
- Laden, G.T. Ethnoarchaeology and Land Use Ecology of the Efe (Pygmies) of the Ituri Rain Forest, Zaire. PhD Thesis, Harvard University, Cambridge, MA, USA, 1992. [Google Scholar]
- Endicott, K. Malaysia’s Original People: Past, Present and Future of the Orang Asli; NUS Press: Singapore, 2016; ISBN 978-9971-69-861-4. [Google Scholar]
- Whitmore, T.C. Tropical Rain Forests of the Far East; Clarendon Press: Oxford, UK, 1975. [Google Scholar]
- Kealhofer, L. Looking into the Gap: Land Use and the Tropical Forests of Southern Thailand. Asian Perspect. 2003, 42, 72–95. [Google Scholar] [CrossRef] [Green Version]
- Ray, N.; Adams, J. A GIS-Based Vegetation Map of the World at the Last Glacial Maximum (25,000-15,000 BP). Internet Archaeol. 2001, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Haynes, G. Mammoths, Mastodonts, and Elephants: Biology, Behavior and the Fossil Record; Cambridge University Press: Cambridge, UK, 1993; ISBN 978-0-521-45691-3. [Google Scholar]
- Cerling, T.E.; Harris, J.M.; Leakey, M.G. Browsing and Grazing in Elephants: The Isotope Record of Modern and Fossil Proboscideans. Oecologia 1999, 120, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Todd, N.E. New Phylogenetic Analysis of the Family Elephantidae Based on Cranial-Dental Morphology. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2010, 293, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Puspaningrum, M.R.; van den Bergh, G.D.; Chivas, A.R.; Setiabudi, E.; Kurniawan, I. Isotopic Reconstruction of Proboscidean Habitats and Diets on Java since the Early Pleistocene: Implications for Adaptation and Extinction. Quat. Sci. Rev. 2020, 228, 106007. [Google Scholar] [CrossRef]
- Vidya, T.N.C.; Sukumar, R.; Melnick, D.J. Range-Wide MtDNA Phylogeography Yields Insights into the Origins of Asian Elephants. Proc. R. Soc. B Biol. Sci. 2009, 278, 798. [Google Scholar] [CrossRef] [Green Version]
- van der Made, J. The Evolution of the Elephants and Their Relatives in the Context of Changing Climate and Geography. In Elefantentreich—Eine Fossilwelt in Europa; Verlag Beier & Beran: Langenweißbach, Germany, 2010; pp. 340–360. ISBN 978-3-939414-48-3. [Google Scholar]
- Wen, H.; Jian, Y.; He, Y.; Gao, Y. Initial research on wild elephants in China during the historical period. In The Change of the Plant and Animal in China During Different Historical Period; Wen, R., Wen, H., Eds.; Chongqing Press: Chongqing, China, 1995; pp. 185–201. [Google Scholar]
- Détroit, F.; Mijares, A.S.; Corny, J.; Daver, G.; Zanolli, C.; Dizon, E.; Robles, E.; Grün, R.; Piper, P.J. A New Species of Homo from the Late Pleistocene of the Philippines. Nature 2019, 568, 181–186. [Google Scholar] [CrossRef] [PubMed]
- van den Bergh, G.D.; de Vos, J.; Sondaar, P.Y. The Late Quaternary Palaeogeography of Mammal Evolution in the Indonesian Archipelago. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2001, 171, 385–408. [Google Scholar] [CrossRef]
- Louys, J.; Curnoe, D.; Tong, H. Characteristics of Pleistocene Megafauna Extinctions in Southeast Asia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 243, 152–173. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Janis, C.M.; Goodall, R.H.; Purnell, M.A. An Examination of Feeding Ecology in Pleistocene Proboscideans from Southern China (Sinomastodon, Stegodon, Elephas), by Means of Dental Microwear Texture Analysis. Quat. Int. 2017, 445, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Wang, Y.; Jin, C.; Hu, Y.; Bocherens, H. Ecological Flexibility and Differential Survival of Pleistocene Stegodon orientalis and Elephas maximus in Mainland Southeast Asia Revealed by Stable Isotope (C, O) Analysis. Quat. Sci. Rev. 2019, 212, 33–44. [Google Scholar] [CrossRef]
- Olivier, R.C. Ecology and behavior of living elephants: Bases for assumptions concerning the extinct woolly mammoths. In Paleoecol. of Beringia; Academic Press: New York, NY, USA, 1982; pp. 291–305. [Google Scholar]
- Olivier, R. Distribution and Status of the Asian Elephant. Oryx 1978, 14, 379–424. [Google Scholar] [CrossRef]
- Terborgh, J.; Davenport, L.C.; Ong, L.; Campos-Arceiz, A. Foraging Impacts of Asian Megafauna on Tropical Rain Forest Structure and Biodiversity. Biotropica 2018, 50, 84–89. [Google Scholar] [CrossRef]
- Olivier, R. On the Ecology of the Asian Elephant, Elephas maximus Linn, with Particular Reference to Malaya and Sri Lanka. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 1978. [Google Scholar]
- Roberts, P.; Louys, J.; Zech, J.; Shipton, C.; Kealy, S.; Carro, S.S.; Hawkins, S.; Boulanger, C.; Marzo, S.; Fiedler, B. Isotopic Evidence for Initial Coastal Colonization and Subsequent Diversification in the Human Occupation of Wallacea. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Louys, J.; Roberts, P. Environmental Drivers of Megafauna and Hominin Extinction in Southeast Asia. Nature 2020, 586, 402–406. [Google Scholar] [CrossRef]
- Rambo, A.T. Human Ecology of the Orang Asli: A Review of Research on the Environmental Relations of the Aborigines of Peninsular Malaysia. Fed. Mus. J. 1979, 24, 41–74. [Google Scholar]
- Lim, T. Malaysia: Illegalities in Forest Clearance for Large-Scale Commercial Plantations; Forest Trends Association: Washington, DC, USA, 2013. [Google Scholar]
- Hannigan, T. Beyond Control: Orientalist Tensions and the History of the “Upas Tree” Myth. J. Commonw. Lit. 2020, 55, 173–189. [Google Scholar] [CrossRef]
- Noor, F.A. The Discursive Construction of Southeast Asia in 19th Century Colonial-Capitalist Discourse; Amsterdam University Press: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Rappaport, R.A. Pigs for the Ancestors: Ritual in the Ecology of a New Guinea People; Waveland Press: Long Grove, USA, 2000. [Google Scholar]
- Reich, D. Who We Are and How We Got Here: Ancient DNA and the New Science of the Human Past; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Lev, M.; Barkai, R. Elephants Are People, People Are Elephants: Human–Proboscideans Similarities as a Case for Cross Cultural Animal Humanization in Recent and Paleolithic Times. Quat. Int. 2016, 406, 239–245. [Google Scholar] [CrossRef]
- Endicott, K. The Hunting Methods of the Batek Negritos of Malaysia. Canberra Anthropol. 1979, 2, 7–22. [Google Scholar] [CrossRef]
- Rambo, A.T. Why Are the Semang? Ecology and Ethnogenesis of Aboriginal Groups in Peninsular Malaysia. In Ethnic Diversity and the Control of Natural Resources in Southeast Asia; Rambo, A.T., Gillogly, K., Hutterer, K.L., Eds.; University of Michigan Press: Ann Arbor, MI, USA, 1988; pp. 19–35. [Google Scholar]
- Eisenberg, J.F.; Seidensticker, J. Ungulates in Southern Asia: A Consideration of Biomass Estimates for Selected Habitats. Biol. Conserv. 1976, 10, 293–308. [Google Scholar] [CrossRef]
- Evans, I.H.N. The Negritos of Malaya; University Press: Cambridge, UK, 1937. [Google Scholar]
- Lye, T.-P. Changing Pathways: Forest Degradation and the Batek of Pahang, Malaysia; Lexington Books: Lanham, MD, USA, 2004. [Google Scholar]
- Endicott, K.; Bellwood, P. The Possibility of Independent Foraging in the Rain Forest of Peninsular Malaysia. Hum. Ecol. 1991, 19, 151–185. [Google Scholar] [CrossRef]
- Torre, J.A.; Lechner, A.M.; Wong, E.P.; Magintan, D.; Saaban, S.; Campos-Arceiz, A. Using Elephant Movements to Assess Landscape Connectivity under Peninsular Malaysia’s Central Forest Spine Land Use Policy. Conserv. Sci. Pract. 2019, 1. [Google Scholar] [CrossRef]
- Evans, L.J.; Asner, G.P.; Goossens, B. Protected Area Management Priorities Crucial for the Future of Bornean Elephants. Biol. Conserv. 2018, 221, 365–373. [Google Scholar] [CrossRef]
- Symington, C.F. Foresters’ Manual of Dipterocarps; Ashton, P.S., Appanah, S., Revs Barlow, H.S., Eds.; Forest Research Institute Malaysia: Kuala Lumpur, Malaysia, 2004. [Google Scholar]
- Owen-Smith, N. Pleistocene Extinctions: The Pivotal Role of Megaherbivores. Paleobiology 1987, 13, 351–362. [Google Scholar] [CrossRef]
- Haynes, G. Mammoth Landscapes: Good Country for Hunter-Gatherers. Quat. Int. 2006, 142–143, 20–29. [Google Scholar] [CrossRef]
- Wadey, J.; Beyer, H.L.; Saaban, S.; Othman, N.; Leimgruber, P.; Campos-Arceiz, A. Why Did the Elephant Cross the Road? The Complex Response of Wild Elephants to a Major Road in Peninsular Malaysia. Biol. Conserv. 2018, 218, 91–98. [Google Scholar] [CrossRef]
- Wall, J.; Douglas-Hamilton, I.; Vollrath, F. Elephants Avoid Costly Mountaineering. Curr. Biol. 2006, 16, R527–R529. [Google Scholar] [CrossRef] [Green Version]
- Blake, S.; Inkamba-Nkulu, C. Fruit, Minerals, and Forest Elephant Trails: Do All Roads Lead to Rome? Biotropica 2004, 36, 392–401. [Google Scholar] [CrossRef]
- Kingdon, J. Mammalian evolution in Africa. In Mammals of Africa; Kingdon, J., Happold, D., Butynski, T., Hoffmann, M., Happold, M., Kalina, J., Eds.; Bloomsbury: London, UK, 2013; Volume 1, pp. 75–100. ISBN 978-1-4081-8996-2. [Google Scholar]
- Keil, P.G. Elephant-Human Dandi: How Humans and Elephants Move through the Fringes of Forest and Village. Confl. Negot. Coexistence Rethink. Hum.-Elephant Relat. South Asia 2016, 242–271. [Google Scholar] [CrossRef]
- Lim, T. Human-Elephant Relations in Peninsular Malaysia. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2019. [Google Scholar]
- Kromann-Clausen, A. How Mineral Deposits Impact Behaviour of Megafauna and Shape the Structure of a Malaysian Forest. Master’s Thesis, University of Copenhagen, København, Denmark, 2015. [Google Scholar]
- Campos-Arceiz, A.; Blake, S. Megagardeners of the Forest–the Role of Elephants in Seed Dispersal. Acta Oecologica 2011, 37, 542–553. [Google Scholar] [CrossRef]
- Ong, L.; McConkey, K.; Solana-Mena, A.; Campos-Arceiz, A. Elephant Frugivory and Wild Boar Seed Predation of Irvingia malayana, a Large-Fruited Tree, in a Rainforest of Peninsular Malaysia. Raffles Bull. Zool. 2019, 67, 160170. [Google Scholar] [CrossRef]
- Remis, M.J. The Gorilla Paradox. In Primate Locomotion: Recent Advances; Strasser, E., Fleagle, J.G., Rosenberger, A.L., McHenry, H.M., Eds.; Springer: Boston, MA, USA, 1998; pp. 95–106. ISBN 978-1-4899-0092-0. [Google Scholar]
- Goodall, J.M. Nest Building Behavior in the Free Ranging Chimpanzee. Ann. N. Y. Acad. Sci. 1962, 102, 455–467. [Google Scholar] [CrossRef]
- Prasetyo, D.; Ancrenaz, M.; Morrogh-Bernard, H.C.; Atmoko, S.S.U.; Wich, S.A.; van Schaik, C.P. Nest Building in Orangutans; Oxford University Press: Oxford, UK; ISBN 978-0-19-170756-8.
- Noble, A.G. Traditional Buildings: A Global Survey of Structural Forms and Cultural Functions; I.B. Tauris: London, UK, 2007; ISBN 978-1-84511-305-6. [Google Scholar]
- Baker, S.W. The Rifle and the Hound in Ceylon; Longman: London, UK, 1854. [Google Scholar]
- Santiapillai, C. Mitigation of Human-Elephant Conflicts in Sri Lanka. Gajah 1996, 15, 1–8. [Google Scholar]
- Henderson, P.; Mornement, A. Treehouses; Frances Lincoln: London, UK, 2005; ISBN 978-0-7112-2437-7. [Google Scholar]
- Kroeber, A.L. Peoples of the Philippines; American Museum Press: New York, NY, USA, 1919. [Google Scholar]
- Skeat, W.W. Pagan Races of the Malay Peninsula; Macmillan: London, UK, 1906; Volume 2. [Google Scholar]
- Cameron, J. Our Tropical Possessions in Malayan India: Being a Descriptive Account of Singapore, Penang, Province Wellesley, and Malacca; Their Peoples, Products, Commerce, and Government; Smith, Elder & Co.: London, UK, 1865. [Google Scholar]
- Kelsall, H.J. Account of a Trip up the Pahang, Tembeling, and Tahan Rivers, and an Attempt to Reach Gunong Tahan. J. Straits Br. R. Asiat. Soc. 1894, 25, 33–56. [Google Scholar]
- Hornaday, W.T. Account of a Naturalist’s Visit to the Territory of Selangor. J. Straits Br. R. Asiat. Soc. 1879, 3, 124–131. [Google Scholar]
- Dunn, F.L. Rain-Forest Collectors and Traders: A Study of Resource Utilization in Modern and Ancient Malaya; Malaysian Branch of the Royal Asiatic Society: Kuala Lumpur, Malaysia, 1975. [Google Scholar]
- Roseman, M. Singers of the Landscape: Song, History, and Property Rights in the Malaysian Rain Forest. Am. Anthropol. 1998, 100, 106–121. [Google Scholar] [CrossRef]
- Manickam, S.K. Taming the Wild: Aborigines and Racial Knowledge in Colonial Malaya; NUS Press: Singapore, 2015. [Google Scholar]
- Schebesta, P. Among the Forest Dwarfs of Malaya; Hutchinson: London, UK, 1929. [Google Scholar]
- Dentan, R.K. The Semai: A Nonviolent People of Malaya; Holt, Rinehart, and Winston: New York, NY, USA, 1968. [Google Scholar]
- Larramendi, A. Shoulder Height, Body Mass, and Shape of Proboscideans. Acta Palaeontol. Pol. 2015, 61, 537–574. [Google Scholar] [CrossRef] [Green Version]
- Hii, N. Asian Elephants’ Social Structure and Mineral Lick Usage in a Malaysian Rainforest Using Camera Traps. Master’s Thesis, University of Nottingham, Selangor, Malaysia, 2017. [Google Scholar]
- Campos-Arceiz, A.; Takatsuki, S.; Ekanayaka, S.K.K.; Hasegawa, T. The Human-Elephant Conflict in Southeastern Sri Lanka: Type of Damage, Seasonal Patterns, and Sexual Differences in the Raiding Behavior of Elephants. Gajah 2009, 31, 5–14. [Google Scholar]
- Wilson, S.; Davies, T.E.; Hazarika, N.; Zimmermann, A. Understanding Spatial and Temporal Patterns of Human–Elephant Conflict in Assam, India. Oryx 2015, 49, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Robarchek, C.A.; Robarchek, C.J. A comparative study of Waorani and Semai. In Aggression and Peace in Humans and Other Primates; Silverberg, J., Patrick Gray, J., Eds.; Oxford University Press: Oxford, UK, 1992; pp. 189–213. [Google Scholar]
- Headland, T.N. The Wild Yam Question: How Well Could Independent Hunter-Gatherers Live in a Tropical Rain Forest Ecosystem? Hum. Ecol. 1987, 15, 463–491. [Google Scholar] [CrossRef]
- Bailey, R.C.; Headland, T.N. The Tropical Rain Forest: Is It a Productive Environment for Human Foragers? Hum. Ecol. 1991, 19, 261–285. [Google Scholar] [CrossRef]
- Headland, T.N. Could ‘Pure’ Hunter-Gatherers Live in a Rain Forest? Available online: https://scholars.sil.org/thomas_n_headland/controversies/wild_yam (accessed on 27 October 2020).
- Hart, T.B.; Hart, J.A. The Ecological Basis of Hunter-Gatherer Subsistence in African Rain Forests: The Mbuti of Eastern Zaire. Hum. Ecol. 1986, 14, 29–55. [Google Scholar] [CrossRef]
- Dierenfeld, E.S. Nutrition. In Biology, Medicine, and Surgery of Elephants; Fowler, M., Mikota, S.K., Eds.; Blackwell Publishing: Ames, IA, USA, 2008; pp. 57–65. ISBN 978-0-470-34411-8. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; Institute of Medicine of the National Academies: Washington, DC, USA, 2002; ISBN 978-0-309-08525-0. [Google Scholar]
- Dentan, R.K. Potential Food Sources for Foragers in Malaysian Rainforest: Sago, Yams and Lots of Little Things. Bijdr. Tot Taal-Land-En Volkenkd. J. Humanit. Soc. Sci. Southeast Asia 1991, 147, 420–444. [Google Scholar] [CrossRef]
- Brosius, J.P. Foraging in Tropical Rain Forests: The Case of the Penan of Sarawak, East Malaysia (Borneo). Human Ecol. 1991, 19, 123–150. [Google Scholar] [CrossRef]
- Kitanishi, K. Variability in the Subsistence Activities and Distribution of Food among Different Aged Males of the Aka Hunter-Gatheres in Northeastern Congo. Afr. Study Monogr. 1996, 17, 35–57. [Google Scholar] [CrossRef]
- Endicott, K.M.; Endicott, K.L. The Headman Was a Woman: The Gender Egalitarian Batek of Malaysia; Waveland: Long Grove, IL, USA, 2008. [Google Scholar]
- Stanton, W.R. Perspective on, and Future Prospects for, the Sago Industry. Sago Palm 1993, 1, 2–7. [Google Scholar]
- Phillipps, Q. Phillipps’ Field Guide to the Mammals of Borneo and Their Ecology: Sabah, Sarawak, Brunei, and Kalimantan; Princeton University Press: Princeton, NJ, USA, 2016. [Google Scholar]
- Dounias, E. The Management of Wild Yam Tubers by the Baka Pygmies in Southern Cameroon. Afr. Study Monogr. Suppl. Issue 2001, 26, 135–156. [Google Scholar] [CrossRef]
- Turner, I.M. A Catalogue of the Vascular Plants of Malaya. Gardens’ Bul. 1994, 47, 19980602199. [Google Scholar]
- Burkill, I.H. A Dictionary of the Economic Products of the Malay Peninsula. Ministry of Agriculture: Kuala Lumpur, Malaysia, 1935 (2002 Reprint). Available online: https://books.google.com.my/books?id=a4AKAQAAIAAJ (accessed on 13 January 2021).
- English, M.; Ancrenaz, M.; Gillespie, G.; Goossens, B.; Nathan, S.; Linklater, W. Foraging Site Recursion by Forest Elephants Elephas maximus borneensis. Curr. Zool. 2014, 60, 551–559. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto-Ebina, S.; Saaban, S.; Campos-Arceiz, A.; Takatsuki, S. Food Habits of Asian Elephants Elephas maximus in a Rainforest of Northern Peninsular Malaysia. Mammal Study 2016, 41, 155–161. [Google Scholar] [CrossRef]
- Kuchikura, Y. Wild Yams in the Tropical Rain Forest: Abundance and Dependence among the Semaq Beri in Peninsular Malaysia. Man Cult. Ocean. 1993, 9, 102. [Google Scholar]
- Barton, H.; Denham, T. Prehistoric Vegeculture and Social Life in Island Southeast Asia and Melanesia. In Why cultivate? Anthropological and Archaeological Approaches to Foraging-Farming Transitions in Southeast Asia; Barker, G., Janowski, M., Eds.; McDonald Institute for Anthropological Research: Cambridge, UK, 2011; pp. 17–25. [Google Scholar]
- Yasuoka, H. Concentrated Distribution of Wild Yam Patches: Historical Ecology and the Subsistence of African Rainforest Hunter-Gatherers. Hum. Ecol. 2009, 37, 577–587. [Google Scholar] [CrossRef]
- Knight, J. Half-man, half-elephant: Shapeshifting among the Baka. In Natural Enemies: People-wildlife Conflicts in Anthropological Perspective; Routledge: Abingdon, UK, 2000; pp. 50–77. ISBN 978-0-415-22440-6. [Google Scholar]
- Sukumar, R. The Living Elephants: Evolutionary Ecology, Behavior, and Conservation; Oxford University Press: New York, NY, USA, 2003; ISBN 978-0-19-510778-4. [Google Scholar]
- Suba, R.B.; Beveridge, N.G.P.; Kustiawan, W.; De Snoo, G.R.; De Iongh, H.H. Foraging Ecology and Diet of Bornean Elephants (Elephas maximus borneensis) in the Sebuku Forest Area, North Kalimantan Province of Indonesia: Do the Choices Matter? Integr. Zool. 2018, 13, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Kurt, F.; Hartl, G.B.; Tiedemann, R. Tuskless Bulls in Asian Elephant Elephas maximus. History and Poulation Genetics of a Man-Made Phenomenon. Acta Theriol. 1995, 40, 125–144. [Google Scholar] [CrossRef]
- Zaya, D.N.; Howe, H.F. The Anomalous Kentucky Coffeetree: Megafaunal Fruit Sinking to Extinction? Oecologia 2009, 161, 221–226. [Google Scholar] [CrossRef]
- Wilson, M.J. Clay Mineralogical and Related Characteristics of Geophagic Materials. J. Chem. Ecol. 2003, 29, 1525–1547. [Google Scholar] [CrossRef]
- Lundquist, C.A.; Varnedoe Jr, W.W. Salt Ingestion Caves. Int. J. Speleol. 2006, 35, 2. [Google Scholar] [CrossRef]
- Elyana, F.N.; Al-Mekhlafi, H.M.; Ithoi, I.; Abdulsalam, A.M.; Dawaki, S.; Nasr, N.A.; Atroosh, W.M.; Abd-Basher, M.H.; Al-Areeqi, M.A.; Sady, H. A Tale of Two Communities: Intestinal Polyparasitism among Orang Asli and Malay Communities in Rural Terengganu, Malaysia. Parasit. Vectors 2016, 9, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadkarni, N.M. Diversity of Species and Interactions in the Upper Tree Canopy of Forest Ecosystems. Am. Zool. 1994, 34, 70–78. [Google Scholar] [CrossRef]
- Latinis, D.K. The Development of Subsistence System Models for Island Southeast Asia and Near Oceania: The Nature and Role of Arboriculture and Arboreal-Based Economies. World Archaeol. 2000, 32, 41–67. [Google Scholar] [CrossRef]
- Kraft, T.S.; Venkataraman, V.V.; Dominy, N.J. A Natural History of Human Tree Climbing. J. Hum. Evol. 2014, 71, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Seeley, T.D.; Seeley, R.H.; Akratanakul, P. Colony Defense Strategies of the Honeybees in Thailand. Ecol. Monogr. 1982, 52, 43–63. [Google Scholar] [CrossRef]
- Bulbeck, F.D. The Guar Kepah human remains. In The Perak Man and Other Prehistoric Skeletons of Malaysia; Penerbit Universiti Sains Malaysia: Penang, Malaysia, 2005; pp. 383–423. ISBN 978-983-3391-12-7. [Google Scholar]
- Estienne, V.; Boesch, C. Underground Honey Extraction by Chimpanzees, Honey Badgers and Forest Elephants in Loango National Park, Gabon. Folia Primatol. 2015, 86, 276–277. [Google Scholar]
- King, L.; Pardo, M.; Weerathunga, S.; Kumara, T.V.; Jayasena, N.; Soltis, J.; de Silva, S. Wild Sri Lankan Elephants Retreat from the Sound of Disturbed Asian Honey Bees. Curr. Biol. 2018, 28, R64–R65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridley, H.N. On the Dispersal of Seeds by Mammals. J. Straits Br. R. Asiat. Soc. 1894, 25, 11–32. [Google Scholar]
- Maisels, F.; Blake, S.; Turkalo, A. Wild Forest Elephants Shake down Fruit and Leaves from Trees. Pachyderm 2002, 33, 88–90. [Google Scholar]
- Rutten, M. Over Olifantshoopen [On Elephant Dung]. Trop. Nat. 1939, 28, 19. [Google Scholar]
- Campos-Arceiz, A.; Traeholt, C.; Jaffar, R.; Santamaria, L.; Corlett, R.T. Asian Tapirs Are No Elephants When It Comes To Seed Dispersal. Biotropica 2012, 44, 220–227. [Google Scholar] [CrossRef]
- Plotnik, J.M.; Shaw, R.C.; Brubaker, D.L.; Tiller, L.N.; Clayton, N.S. Thinking with Their Trunks: Elephants Use Smell but Not Sound to Locate Food and Exclude Nonrewarding Alternatives. Anim. Behav. 2014, 88, 91–98. [Google Scholar] [CrossRef]
- Corlett, R.T. How to Be a Frugivore (in a Changing World). Acta Oecologica 2011, 37, 674–681. [Google Scholar] [CrossRef]
- Corner, E.J.H. The Durian Theory or the Origin of the Modern Tree on JSTOR. Ann. Bot. 1949, 13, 367–414. [Google Scholar] [CrossRef]
- Mayer, C. Trapping Wild Animals in Malay Jungles; Duffield: New York, NY, USA, 1922. [Google Scholar]
- Majid, A.; Kruspe, N. Hunter-Gatherer Olfaction Is Special. Curr. Biol. 2018, 28, 409–413. [Google Scholar] [CrossRef] [Green Version]
- Terrill, A.; Burenhult, N. Orientation as a Strategy of Spatial Reference. Stud. Lang. Int. J. Spons. Found. Found. Lang. 2008, 32, 93–136. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.H.; Sittimongkol, S.; Campos-Arceiz, A.; Sumpah, T.; Eichhorn, M.P. Fruit Gardens Enhance Mammal Diversity and Biomass in a Southeast Asian Rainforest. Biol. Conserv. 2016, 194, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Lim, T.; Loke, V.; Mena, A.; Pura, P.; Angah, R.; Tan, A.; Campos-Arceiz, A. Mapping the Distribution of People, Elephants, and Human-Elephant Conflict in Temengor Forest Complex, Peninsular Malaysia. Malay. Nat. J. 2017, 2017, 31–49. [Google Scholar]
- Wharton, C.H.; Komarek, E.V. Man, Fire and Wild Cattle in Southeast Asia. Proc. An. Tall Timbers Fire Ecol. Conf. 1968, 8, 107–167. [Google Scholar]
- Sukumar, R. The Asian Elephant: Ecology and Management; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Lorimer, J. Elephants as Companion Species: The Lively Biogeographies of Asian Elephant Conservation in Sri Lanka. Trans. Inst. Br. Geogr. 2010, 35, 491–506. [Google Scholar] [CrossRef]
- Fernando, P.; Wikramanayake, E.; Weerakoon, D.; Jayasinghe, L.K.A.; Gunawardene, M.; Janaka, H.K. Perceptions and Patterns of Human–Elephant Conflict in Old and New Settlements in Sri Lanka: Insights for Mitigation and Management. Biodivers. Conserv. 2005, 14, 2465–2481. [Google Scholar] [CrossRef]
- Speth, J.D.; Spielmann, K.A. Energy Source, Protein Metabolism, and Hunter-Gatherer Subsistence Strategies. J. Anthropol. Archaeol. 1983, 2, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Speth, J.D. The Paleoanthropology and Archaeology of Big-Game Hunting: Protein, Fat, or Politics? Springer: New York, NY, USA, 2010; pp. 149–161. [Google Scholar]
- Brown, C.; Warburton, K. Differences in Timidity and Escape Responses between Predator-Naive and Predator-Sympatric Rainbowfish Populations. Ethology 1999, 105, 491–502. [Google Scholar] [CrossRef]
- Hubback, T.B. The Malay Elephant. J. Bombay Nat. Hist. Soc. 1941, 42, 483–509. [Google Scholar]
- Tshen, L.T. Quaternary Elephas Fossils from Peninsular Malaysia: Historical Overview and New Material. Raffles Bull. Zool. 2013, 139–153. [Google Scholar]
- Muhammad, R.F.; Tshen, L.T.; Ibrahim, N.; Azmi Abdul Razak, M.; Mohd Razif, F.; Kem, Z.; Boon Tat, C. First discovery of Stegodon (Proboscidea) in Malaysia. War. Geol. 2020, 46, 196–198. [Google Scholar] [CrossRef]
- Loke, V.P.W.; Lim, T.; Campos-Arceiz, A. Hunting Practices of the Jahai Indigenous Community in Northern Peninsular Malaysia. Glob. Ecol. Conserv. 2020, 21, e00815. [Google Scholar] [CrossRef]
- Rambo, A.T. Bows, Blowpipes and Blunderbusses: Ecological Implications of Weapons Change among the Malaysian Negritos. Malays. Nat. J. 1978, 22, 209–216. [Google Scholar]
- Pfeffer, P. Fauna of humid tropical Asia. In Natural Resources of Tropical Asia; UNESCO: Paris, France, 1974; pp. 295–306. [Google Scholar]
- Stearman, A.M. Making a Living in the Tropical Forest: Yuqui Foragers in the Bolivian Amazon. Hum. Ecol. 1991, 19, 245–260. [Google Scholar] [CrossRef]
- Watson, P.J. Archaeology, Anthropology, and the Culture Concept. Am. Anthropol. 1995, 97, 683–694. [Google Scholar] [CrossRef]
- Gosselain, O.P. To Hell with Ethnoarchaeology! Archaeol. Dialogues 2016, 23, 215–228. [Google Scholar] [CrossRef]
- Barth, F. Ethnic Groups and Boundaries: The Social Organization of Culture Difference. In Results of a Symposium Held at the University of Bergen, 23rd to 26th February 1967; Universitetsforlage: Bergen, Norway, 1969. [Google Scholar]
- Roseman, M. Healing Sounds from the Malaysian Rainforest: Temiar Music and Medicine; University of California Press: Berkeley, CA, USA, 1991. [Google Scholar]
- Laundré, J.W.; Hernández, L.; Ripple, W.J. The Landscape of Fear: Ecological Implications of Being Afraid. Open Ecol. J. 2010, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Singh, J. Materialities of the Non-Human Animal and the Species Matrix of Postcolonial Remains in Selected Contemporary Writing. Interventions 2018, 20, 666–681. [Google Scholar] [CrossRef]
- Naveh, D.; Bird-David, N. How Persons Become Things: Economic and Epistemological Changes among N Ayaka Hunter-Gatherers. J. R. Anthropol. Inst. 2014, 20, 74–92. [Google Scholar] [CrossRef]
- Mumby, H.S.; Plotnik, J.M. Taking the Elephants’ Perspective: Remembering Elephant Behavior, Cognition and Ecology in Human-Elephant Conflict Mitigation. Front. Ecol. Evol. 2018, 6, 122. [Google Scholar] [CrossRef] [Green Version]
- Torre, J.A.d.l.; Wong, E.P.; Lechner, A.M.; Zulaikha, N.; Zawawi, A.; Abdul-Patah, P.; Saaban, S.; Goossens, B.; Campos-Arceiz, A. There Will Be Conflict—Agricultural Landscapes Are Prime, Rather than Marginal, Habitats for Asian Elephants. Anim. Conserv. 2020. [Google Scholar] [CrossRef]


| Activity Pattern (Section 4.4) | ||
|---|---|---|
| Habitat (Section 4.1) |  Day Day |  Night Night |
 Arboreal (Section 4.3) Arboreal (Section 4.3) |  | 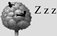 |
 Terrestrial (Section 4.2) Terrestrial (Section 4.2) | 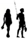 |  |
| Food Source (Section 5 of This Paper) |  Elephants |  Humans |  Tigers |
|---|---|---|---|
| Plants | |||
 Fruit (e.g., durian: Section 5.5) Fruit (e.g., durian: Section 5.5) | ⚫ | ⚫ | ● |
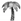 Leaves, stems (palms: Section 5.2, grass: Section 5.6) Leaves, stems (palms: Section 5.2, grass: Section 5.6) | ⚫ | ● | ○ |
 Roots (tubers: Section 5.3) Roots (tubers: Section 5.3) | ● | ⚫ | ○ |
| Animals (Section 5.7) | |||
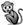 In trees (birds, squirrels, monkeys, honey (Section 5.4)) In trees (birds, squirrels, monkeys, honey (Section 5.4)) | ○ | ⚫ | ● |
 On the ground (pigs, deer, bovids) On the ground (pigs, deer, bovids) | ○ | ● | ⚫ |
 In holes*; ‘lots of little things’ (grubs, snails, fish) In holes*; ‘lots of little things’ (grubs, snails, fish) | ○ | ⚫ | ● |
| Group | Height Above Ground | Rationale Given | Period & Ref. |
|---|---|---|---|
| Korowai, New Guinea | 35 m | slavers, “cannibals” | 20th century [74] |
| Illongot, Luzon | 18 m | head-hunters | 20th century [75] |
| Temuan, Selangor | 9–12 m | elephants | ca. 1900 [76] |
| Aboriginal Malays | 9 m | not given | 19th century [77] |
| Semai, Pahang | 4.6–6 m | not given | 19th century [78] |
| Semelai, Pahang | 4 m | tigers, elephants | 1980 (R. Gianno pers. comm., 24 July 2021) |
| Temuan), Selangor | 3.7 m | elephants | 19th century [79,80] |
| Temiar, Perak/Kelantan | 3 m | tigers, slavers, elephants | 20th century [64,81] |
| Jakun, Johor | 1.5–2.7 m* | elephants, tigers | ca. 1900 [76,82] |
| Menraq (in cliffs and “large” trees) | elephants, tigers | ca. 1900 [51,76,83] | |
| Semai (in the “sturdiest longhouse”) | elephants, slavers | ca. 1960 [84] | |
| Energy | Protein | |
|---|---|---|
| Elephant (1) | 290.3 MJ (2) | 2400 g (6.7 g per kg BW) |
| Human (3) | 10.9 MJ | 46 g (0.7 g per kg BW) |
| Food source | Gross MJ (1) | Nett MJ (2) | Protein | Ref. | Section |
|---|---|---|---|---|---|
 Elephant (3605 kg whole animal) (3) Elephant (3605 kg whole animal) (3) | 9361 | 8392 | 283,738 g | [4] | Section 5.7 |
 Sago (90 kg starch from 1 tree) Sago (90 kg starch from 1 tree) | 1314 | 1149 | 180 g | [96,97] | Section 5.2 |
 Pig (50 kg meat from 1 animal) (4) Pig (50 kg meat from 1 animal) (4) | 310 | 246 | 8195 g | [97] | Section 5.7 |
 Yam (14.4 kg @ 0.8 kg per hr) Yam (14.4 kg @ 0.8 kg per hr) | 71 | 31 | 220 g | [53] | Section 5.3 |
 Honey (4.2 kg) Honey (4.2 kg) | 53 | 15 | 13 g | [98,99] | Section 5.4 |
 Arboreal game (7 kg) Arboreal game (7 kg) | 51 | 13 | 2170 g | [53] | Section 5.7 |
 Durian (27 kg fruit; 5.4 kg pulp) (5) Durian (27 kg fruit; 5.4 kg pulp) (5) | 33 | -3 | 79 g | – | Section 5.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, T.; Campos-Arceiz, A. A Review of Human-Elephant Ecological Relations in the Malay Peninsula: Adaptations for Coexistence. Diversity 2022, 14, 36. https://doi.org/10.3390/d14010036
Lim T, Campos-Arceiz A. A Review of Human-Elephant Ecological Relations in the Malay Peninsula: Adaptations for Coexistence. Diversity. 2022; 14(1):36. https://doi.org/10.3390/d14010036
Chicago/Turabian StyleLim, Teckwyn, and Ahimsa Campos-Arceiz. 2022. "A Review of Human-Elephant Ecological Relations in the Malay Peninsula: Adaptations for Coexistence" Diversity 14, no. 1: 36. https://doi.org/10.3390/d14010036
APA StyleLim, T., & Campos-Arceiz, A. (2022). A Review of Human-Elephant Ecological Relations in the Malay Peninsula: Adaptations for Coexistence. Diversity, 14(1), 36. https://doi.org/10.3390/d14010036






