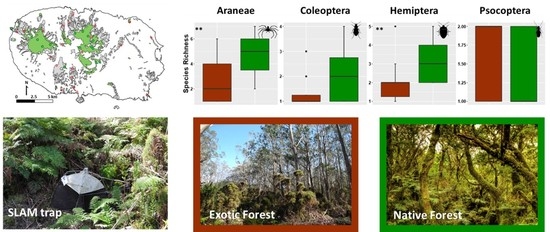
The Role of Small Lowland Patches of Exotic Forests as Refuges of Rare Endemic Azorean Arthropods
Abstract
1. Introduction
- (1)
- We predict that endemic and native non-endemic species should exhibit higher values of diversity and abundance in the native forests than in the exotic forests, while introduced species should display opposite patterns;
- (2)
- Given the differences in community dynamics of rare, common, or dominant species, we hypothesized that some dominant endemic and native non-endemic species with broad ecological needs will also find refuge in exotic forest sites, while some introduced species will be common in native forests if they are superior competitors in respect to endemic and native non-endemic species;
- (3)
- We predict that introduced species should show higher β-diversity between and within forests, while endemic and native non-endemic species should present high β-diversity between native and exotic forests and within exotic forest sites, but lower β-diversity values within native forest sites [20,34].
2. Materials and Methods
2.1. Study Area and Site Selection
2.2. Arthropod Sampling and Identification
2.3. Data Analysis
3. Results
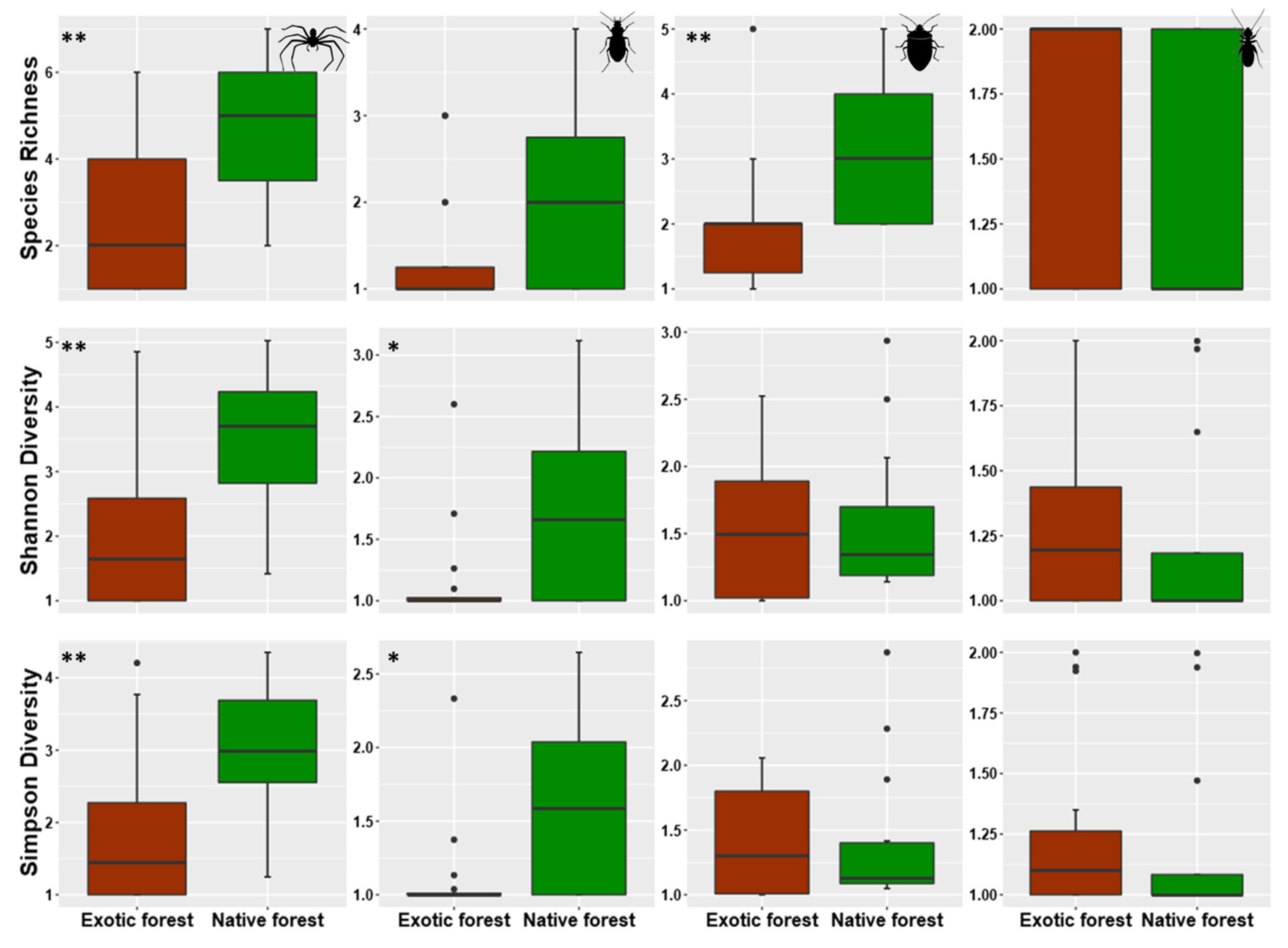
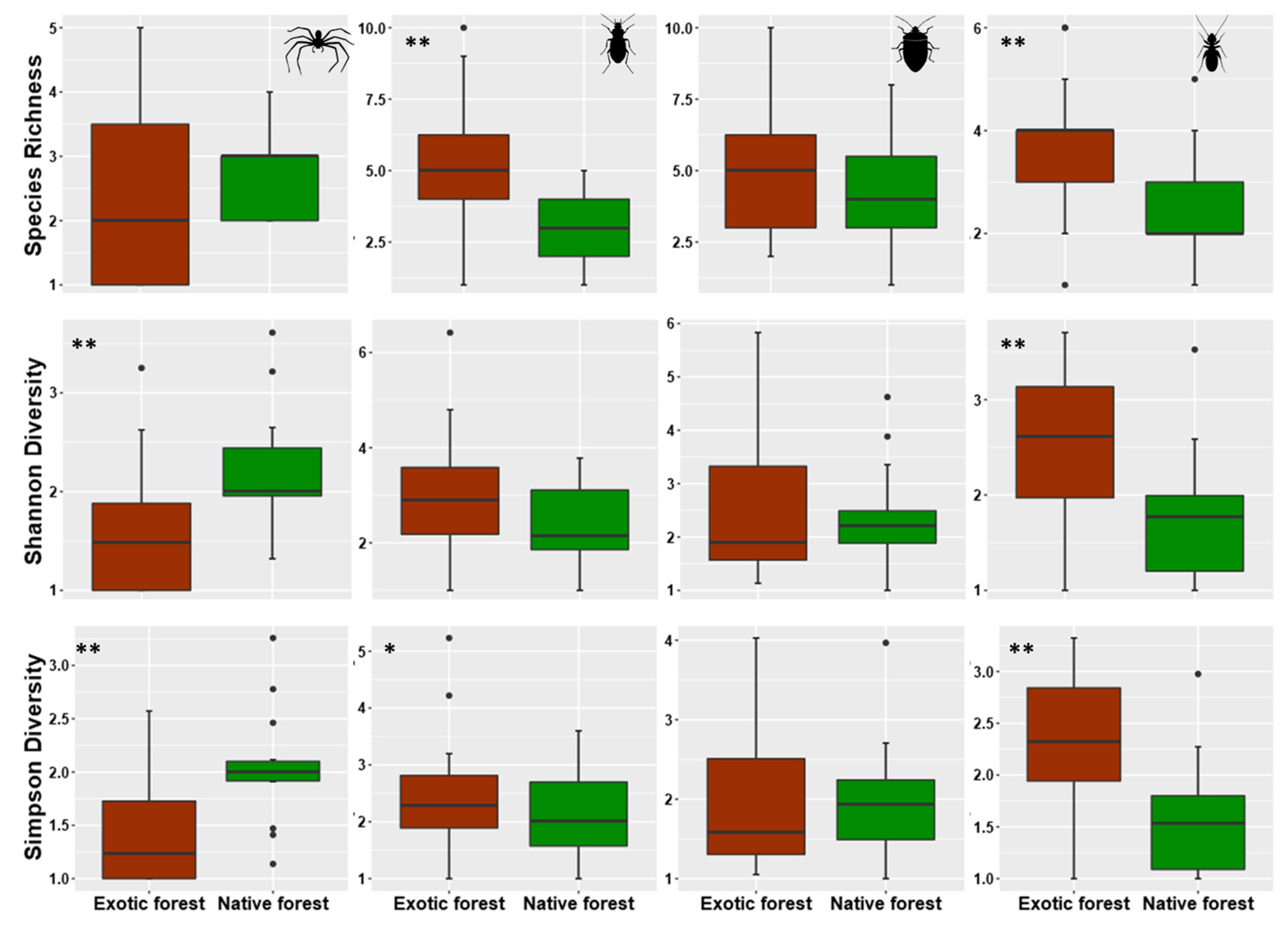
3.1. Overall Patterns of Abundances and Species Richness of Arthropods Communities in Native and Exotic Forests
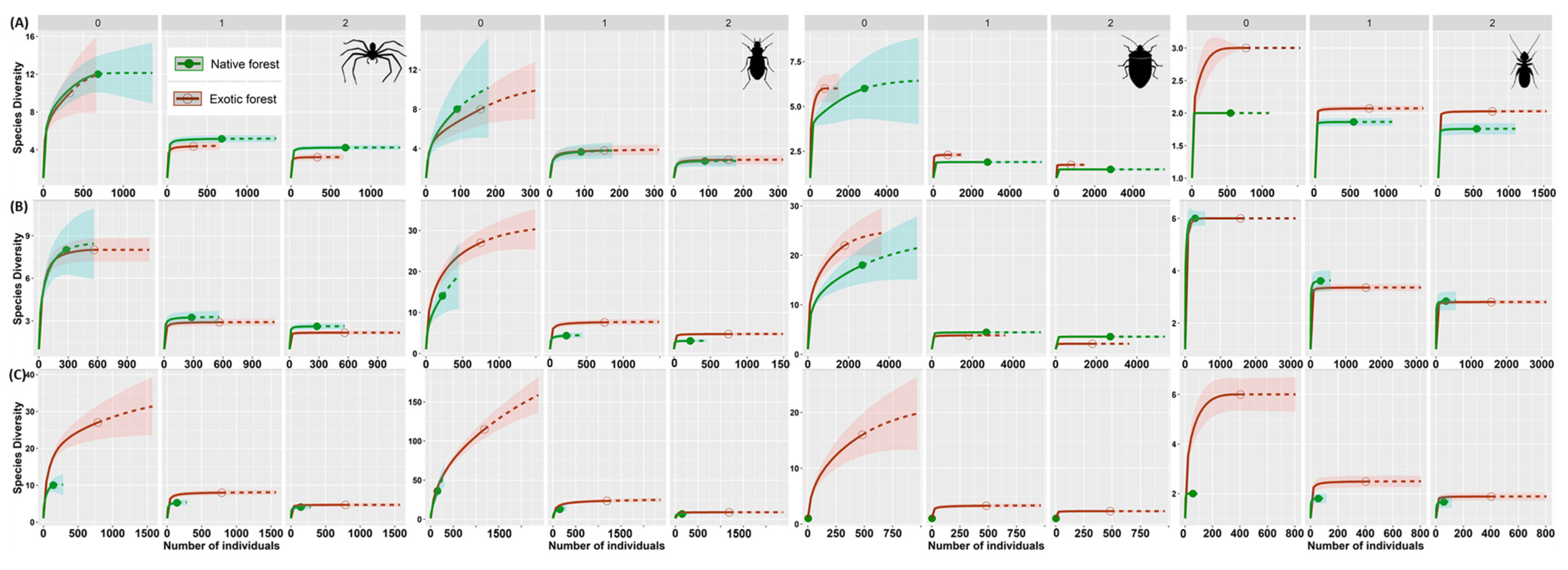
3.2. Assessing Local Diversity: α-Diversity in Native and Exotic Forests
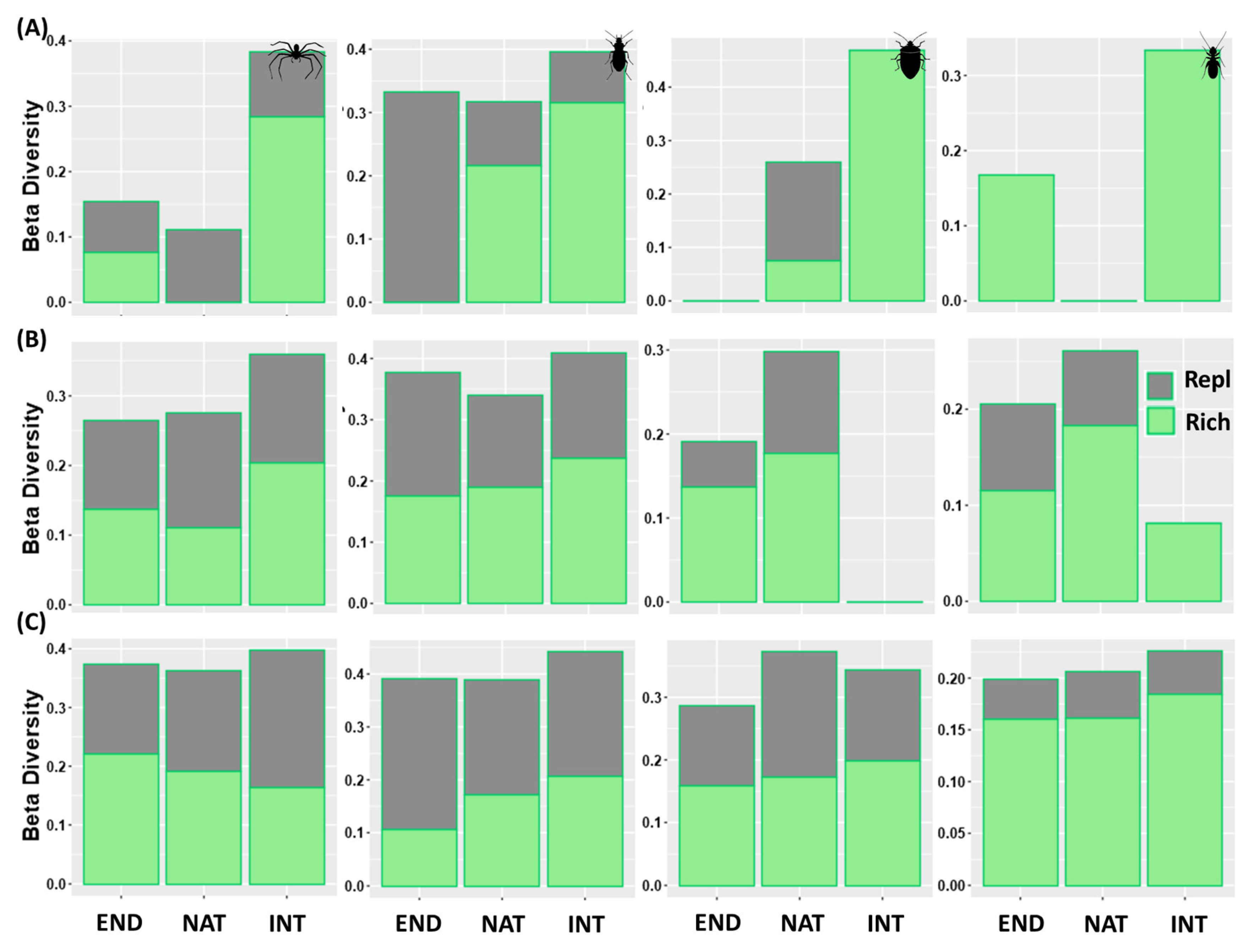
3.3. Detecting Diversity Change between Native and Exotic Forests and within Sites of Each Type of Forest: β-Diversity and Its Components
3.3.1. Change in Diversity between Native and Exotic Forest
3.3.2. Change in Diversity between Sites (Inside) of Each Forest Type
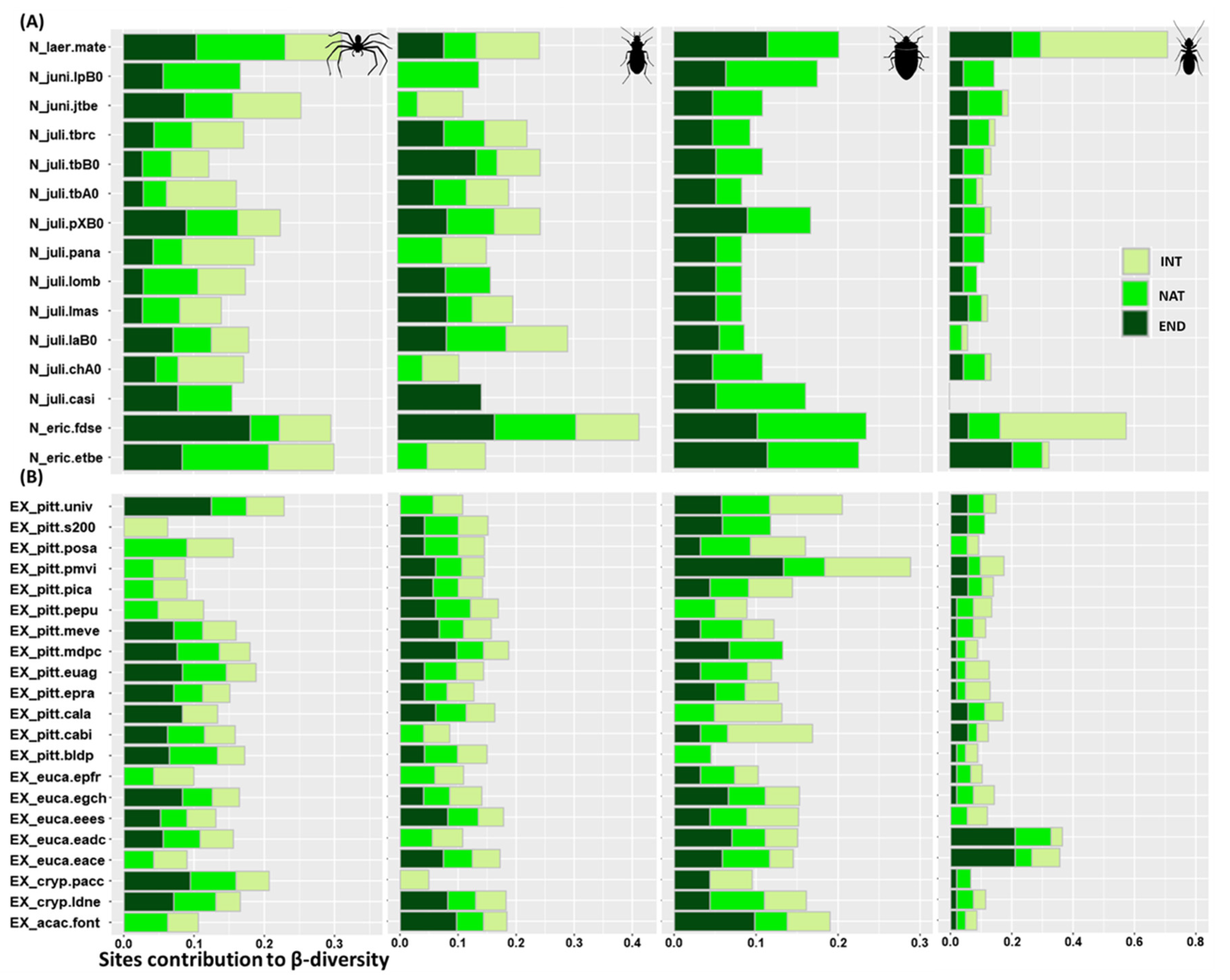
3.4. Sites Contribution to β-Diversity
4. Discussion
4.1. Abundance and Species Richness General Patterns within and between Native and Exotic Forests
4.2. Alpha Diversity Patterns within Rare, Common, and Dominant Species
4.3. Change in Diversity between Native and Exotic Forests
4.4. Relative Importance of Sites in Supporting the Different Biogeographic Groups
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardoso, P.; Barton, P.S.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; Gaigher, R.; Habel, J.C.; Hallmann, C.A.; et al. Scientists’ warning to humanity on insect extinctions. Biol. Conserv. 2020, 242, 108426. [Google Scholar] [CrossRef]
- Harvey, J.A.; Heinen, R.; Armbrecht, I.; Basset, Y.; Baxter-Gilbert, J.H.; Bezemer, T.M.; Böhm, M.; Bommarco, R.; Borges, P.A.V.; Cardoso, P.; et al. International scientists formulate a roadmap for insect conservation and recovery. Nat. Ecol. Evol. 2020, 4, 174–176. [Google Scholar] [CrossRef]
- Samways, M.J.; Barton, P.S.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; Gaigher, R.; Habel, J.C.; Hallmann, C.A.; et al. Solutions for humanity on how to conserve insects. Biol. Conserv. 2020, 242, 108427. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Habel, J.C.; Trusch, R.; Schmitt, T.; Ochse, M.; Ulrich, W. Long-term large-scale decline in relative abundances of butterfly and burnet moth species across south-western Germany. Sci. Rep. 2019, 9, 14921. [Google Scholar] [CrossRef] [PubMed]
- Homburg, K.; Drees, C.; Boutaud, E.; Nolte, D.; Schuett, W.; Zumstein, P.; von Ruschkowski, E.; Assmann, T. Where have all the beetles gone? Long-term study reveals carabid species decline in a nature reserve in Northern Germany. Insect Conserv. Divers. 2019, 12, 268–277. [Google Scholar] [CrossRef]
- Seibold, S.; Gossner, M.M.; Simons, N.K.; Blüthgen, N.; Müller, J.; Ambarlı, D.; Ammer, C.; Bauhus, J.; Fischer, M.; Habel, J.C.; et al. Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 2019, 574, 671–674. [Google Scholar] [CrossRef]
- Terzopoulou, S.; Rigal, F.; Whittaker, R.J.; Borges, P.A.V.; Triantis, K.A. Drivers of extinction: The case of Azorean beetles. Biol. Lett. 2015, 11, 20150273. [Google Scholar] [CrossRef]
- Triantis, K.A.; Borges, P.A.V.; Ladle, R.J.; Hortal, J.; Cardoso, P.; Gaspar, C.; Dinis, F.; Mendonça, E.; Silveira, L.M.A.; Gabriel, R.; et al. Extinction debt on oceanic islands. Ecography 2010, 33, 285–294. [Google Scholar] [CrossRef]
- Copsey, J.A.; Black, S.A.; Groombridge, J.J.; Jones, C.G. (Eds.) Species Conservation: Lessons from Islands; Ecology, Biodiversity and Conservation; Cambridge University Press: Cambridge, UK, 2018; ISBN 978-0-521-89939-0. [Google Scholar]
- Jupiter, S.; Mangubhai, S.; Kingsford, R.T. Conservation of Biodiversity in the Pacific Islands of Oceania: Challenges and Opportunities. Pac. Conserv. Biol. 2014, 20, 206–220. [Google Scholar] [CrossRef]
- Pétillon, J.; Privet, K.; Roderick, G.K.; Price, D. Non-native spiders change assemblages of Hawaiian forest fragment kipuka over space and time. NeoBiota 2020, 55, 1–9. [Google Scholar] [CrossRef]
- Raven, P.H.; Wagner, D.L. Agricultural intensification and climate change are rapidly decreasing insect biodiversity. Proc. Natl. Acad. Sci. USA 2021, 118, e2002548117. [Google Scholar] [CrossRef] [PubMed]
- Causton, C.E.; Peck, S.B.; Sinclair, B.J.; Roque-Albelo, L.; Hodgson, C.J.; Landry, B. Alien Insects: Threats and Implications for Conservation of Galápagos Islands. Ann. Entomol. Soc. Am. 2006, 99, 121–143. [Google Scholar] [CrossRef]
- Cronk, Q.C.B. Islands: Stability, diversity, conservation. Biodivers. Conserv. 1997, 6, 477–493. [Google Scholar] [CrossRef]
- Sax, D.F.; Gaines, S.D.; Brown, J.H. Species Invasions Exceed Extinctions on Islands Worldwide: A Comparative Study of Plants and Birds. Am. Nat. 2002, 160, 766–783. [Google Scholar] [CrossRef]
- Silva, L.; Smith, C.W. A Quantitative Approach to the Study of Non-indigenous Plants: An Example from the Azores Archipelago. Biodivers. Conserv. 2006, 15, 1661–1679. [Google Scholar] [CrossRef]
- Borges, P.A.V.; Gabriel, R.; Arroz, A.M.; Costa, A.; Cunha, R.T.; Silva, L.; Mendonça, E.; Martins, A.M.F.; Reis, F.; Cardoso, P. The Azorean Biodiversity Portal: An internet database for regional biodiversity outreach. Null 2010, 8, 423–434. [Google Scholar] [CrossRef]
- Borges, P.A.V.; Rigal, F.; Ros-Prieto, A.; Cardoso, P. Increase of insular exotic arthropod diversity is a fundamental dimension of the current biodiversity crisis. Insect Conserv. Divers. 2020, 13, 508–518. [Google Scholar] [CrossRef]
- Borges, P.A.V.; Aguiar, C.; Amaral, J.; Amorim, I.R.; André, G.; Arraiol, A.; Baz, A.; Dinis, F.; Enghoff, H.; Gaspar, C.; et al. Ranking protected areas in the Azores using standardised sampling of soil epigean arthropods. Biodivers. Conserv. 2005, 14, 2029–2060. [Google Scholar] [CrossRef]
- Gaspar, C.; Gaston, K.J.; Borges, P.A.V.; Cardoso, P. Selection of priority areas for arthropod conservation in the Azores archipelago. J. Insect Conserv. 2011, 15, 671–684. [Google Scholar] [CrossRef]
- Fattorini, S. Endemism in historical biogeography and conservation biology: Concepts and implications. Biogeogr. J. Integr. Biogeogr. 2017, 32, 47–75. [Google Scholar] [CrossRef]
- Ferreira, M.T.; Cardoso, P.; Borges, P.A.V.; Gabriel, R.; de Azevedo, E.B.; Reis, F.; Araújo, M.B.; Elias, R.B. Effects of climate change on the distribution of indigenous species in oceanic islands (Azores). Clim. Chang. 2016, 138, 603–615. [Google Scholar] [CrossRef]
- Elias, R.B.; Gil, A.; Silva, L.; Fernández-Palacios, J.M.; Azevedo, E.B.; Reis, F. Natural zonal vegetation of the Azores Islands: Characterization and potential distribution. Phytocoenologia 2016, 46, 107–123. [Google Scholar] [CrossRef]
- Cardoso, P.; Aranda, S.C.; Lobo, J.M.; Dinis, F.; Gaspar, C.; Borges, P.A.V. A spatial scale assessment of habitat effects on arthropod communities of an oceanic island. Acta Oecologica 2009, 35, 590–597. [Google Scholar] [CrossRef]
- Borges, P.A.V.; Ugland, K.I.; Dinis, F.O.; Gaspar, C. Insect and spider rarity in an oceanic island (Terceira, Azores): True rare and pseudo-rare species. In Insect Ecology and Conservation; Fattorini, S., Ed.; Research Signpost: Kerala, India, 2008; pp. 47–70. ISBN 978-81-308-0297-8. [Google Scholar]
- Florencio, M.; Rigal, F.; Borges, P.A.V.; Cardoso, P.; Santos, A.M.C.; Lobo, J.M. The role of plant fidelity and land-use changes on island exotic and indigenous canopy spiders at local and regional scales. Biol. Invasions 2016, 18, 2309–2324. [Google Scholar] [CrossRef]
- Florencio, M.; Lobo, J.M.; Cardoso, P.; Almeida-Neto, M.; Borges, P.A.V. The Colonisation of Exotic Species Does Not Have to Trigger Faunal Homogenisation: Lessons from the Assembly Patterns of Arthropods on Oceanic Islands. PLoS ONE 2015, 10, e0128276. [Google Scholar] [CrossRef] [PubMed]
- Florencio, M.; Cardoso, P.; Lobo, J.M.; de Azevedo, E.B.; Borges, P.A.V. Arthropod assemblage homogenization in oceanic islands: The role of indigenous and exotic species under landscape disturbance. Divers. Distrib. 2013, 19, 1450–1460. [Google Scholar] [CrossRef]
- Meijer, S.S.; Whittaker, R.J.; Borges, P.A.V. The effects of land-use change on arthropod richness and abundance on Santa Maria Island (Azores): Unmanaged plantations favour endemic beetles. J. Insect Conserv. 2011, 15, 505–522. [Google Scholar] [CrossRef]
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C.-H.; Jost, L. Unifying Species Diversity, Phylogenetic Diversity, Functional Diversity, and Related Similarity and Differentiation Measures Through Hill Numbers. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 297–324. [Google Scholar] [CrossRef]
- Matthews, T.J.; Sadler, J.; Carvalho, R.; Nunes, R.; Borges, P.A.V. Differential temporal beta-diversity patterns of native and non-native arthropod species in a fragmented native forest landscape. Ecography 2019, 42, 45–54. [Google Scholar] [CrossRef]
- Artega, A.; Malumbres-Olarte, J.; Gabriel, R.; Ros-Prieto, A.; Casimiro, P.; Sanchez, A.; Albergaria, I.; Borges, P.A.V. Arthropod diversity in two Historic Gardens in the Azores, Portugal. Biodivers. Data J. 2020, 8, e54749. [Google Scholar] [CrossRef] [PubMed]
- Borges, P.; Gabriel, R.; Pimentel, C.; Brito, M.; Serrano, A.; Crespo, L.; Assing, V.; Stüben, P.; Fattorini, S.; Soares, A.; et al. Biota from the coastal wetlands of Praia da Vitória (Terceira, Azores, Portugal): Part 1—Arthropods. Biodivers. Data J. 2018, 6, e27194. [Google Scholar] [CrossRef] [PubMed]
- Borges, P.; Amorim, I.; Terzopoulou, S.; Rigal, F.; Emerson, B.C.; Serrano, A. Cryptic diversity in the Azorean beetle genus Tarphius Erichson, 1845 (Coleoptera: Zopheridae): An integrative taxonomic approach with description of four new species. Zootaxa 2017, 4236, 401–449. [Google Scholar] [CrossRef] [PubMed]
- Borges, P.; Lamelas-López, L.; Amorim, I.; Nunes, R.; Serrano, A.; Boieiro, M.; Hochkirch, A.; Vieira, V. Conservation status of the forest beetles (Insecta, Coleoptera) from Azores, Portugal. Biodivers. Data J. 2017, 5, e14557. [Google Scholar] [CrossRef]
- Marcelino, J.; Borges, P.A.V.; Borges, I.; Pereira, E.; Santos, V.; Soares, A.O. Standardised arthropod (Arthropoda) inventory across natural and anthropogenic impacted habitats in the Azores archipelago. Biodivers. Data J. 2021, 9, e62157. [Google Scholar] [CrossRef]
- Rego, C.; Boieiro, M.; Rigal, F.; Ribeiro, S.P.; Cardoso, P.; Borges, P.A.V. Taxonomic and functional diversity of insect herbivore assemblages associated with the canopy-dominant trees of the Azorean native forest. PLoS ONE 2019, 14, e0219493. [Google Scholar] [CrossRef]
- Fernández-Palacios, J.M.; de Nascimento, L.; Otto, R.; Delgado, J.D.; García-del-Rey, E.; Arévalo, J.R.; Whittaker, R.J. A reconstruction of Palaeo-Macaronesia, with particular reference to the long-term biogeography of the Atlantic island laurel forests. J. Biogeogr. 2011, 38, 226–246. [Google Scholar] [CrossRef]
- Gaspar, C.; Borges, P.; Gaston, K. Diversity and distribution of arthropods in native forests of the Azores archipelago. Arquipel. Life Mar. Sci. 2008, 25, 1–30. [Google Scholar]
- Fattorini, S.; Cardoso, P.; Rigal, F.; Borges, P.A.V. Use of Arthropod Rarity for Area Prioritisation: Insights from the Azorean Islands. PLoS ONE 2012, 7, e33995. [Google Scholar] [CrossRef]
- Silva, L.; Ojeda Land, E.; Rodriguez Luengo, J.L.; Borges, P.A.V.; Oliveira, P.; Jardim, R. Invasive terrestrial Flora & Fauna of Macaronesia. In TOP 100 in Azores, Madeira and Canaries; ARENA: Ponta Delgada, Portugal, 2008; p. 546. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 5 July 2021).
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Carvalho, J.C.; Cardoso, P.; Borges, P.A.V.; Schmera, D.; Podani, J. Measuring fractions of beta diversity and their relationships to nestedness: A theoretical and empirical comparison of novel approaches. Oikos 2013, 122, 825–834. [Google Scholar] [CrossRef]
- Dray, S.; Bauman, G.; Bocard, D.; Clappe, S.; Guenard, G. Adespatial: Multivariate Multiscale Spatial Analysis. 2019. Available online: https://cran.r-project.org/web/packages/adespatial (accessed on 5 July 2021).
- Legendre, P.; De Cáceres, M. Beta diversity as the variance of community data: Dissimilarity coefficients and partitioning. Ecol. Lett. 2013, 16, 951–963. [Google Scholar] [CrossRef]
- Legendre, P. Interpreting the replacement and richness difference components of beta diversity. Glob. Ecol. Biogeogr. 2014, 23, 1324–1334. [Google Scholar] [CrossRef]
- Searcy, K.B.; Pucko, C.; McClelland, D. The distribution and habitat preferences of introduced species in the mount Holyoke range, Hampshire co., Massachusetts. Rhodora 2006, 108, 43–61. [Google Scholar] [CrossRef]
- Gaston, K.J.; Borges, P.A.V.; He, F.; Gaspar, C. Abundance, spatial variance and occupancy: Arthropod species distribution in the Azores. J. Anim. Ecol. 2006, 75, 646–656. [Google Scholar] [CrossRef]
- Rigal, F.; Whittaker, R.J.; Triantis, K.A.; Borges, P.A.V. Integration of non-indigenous species within the interspecific abundance–occupancy relationship. Acta Oecologica 2013, 48, 69–75. [Google Scholar] [CrossRef]
- Ribeiro, S.P.; Borges, P.A.V.; Gaspar, C.; Melo, C.; Serrano, A.R.M.; Amaral, J.; Aguiar, C.; André, G.; Quartau, J.A. Canopy insect herbivores in the Azorean Laurisilva forests: Key host plant species in a highly generalist insect community. Ecography 2005, 28, 315–330. [Google Scholar] [CrossRef]
- Quartau, J.A.; Borges, P.A.V. A new species of the genus Aphrodes Curtis from the Azores (Hemiptera, Cicadellidae). Bocagiana 2003, 213, 1–11. [Google Scholar]
- Borges, P.A.V.; Wunderlich, J. Spider biodiversity patterns and their conservation in the Azorean archipelago, with descriptions of new species. Null 2008, 6, 249–282. [Google Scholar] [CrossRef][Green Version]
- Broadhead, E.; Wolda, H. The Diversity of Psocoptera in Two Tropical Forests in Panama. J. Anim. Ecol. 1985, 54, 739–754. [Google Scholar] [CrossRef]
- Jaenike, J. Host Specialization in Phytophagous Insects. Annu. Rev. Ecol. Syst. 1990, 21, 243–273. [Google Scholar] [CrossRef]
- Farnon Ellwood, M.D.; Manica, A.; Foster, W.A. Stochastic and deterministic processes jointly structure tropical arthropod communities. Ecol. Lett. 2009, 12, 277–284. [Google Scholar] [CrossRef]
- Lessard, J.-P.; Borregaard, M.K.; Fordyce, J.A.; Rahbek, C.; Weiser, M.D.; Dunn, R.R.; Sanders, N.J. Strong influence of regional species pools on continent-wide structuring of local communities. Proc. R. Soc. B Biol. Sci. 2012, 279, 266–274. [Google Scholar] [CrossRef]
- Segre, H.; Ron, R.; De Malach, N.; Henkin, Z.; Mandel, M.; Kadmon, R. Competitive exclusion, beta diversity, and deterministic vs. stochastic drivers of community assembly. Ecol. Lett. 2014, 17, 1400–1408. [Google Scholar] [CrossRef]
- Gillespie, R.G.; Roderick, G.K. Arthropods on Islands: Colonization, Speciation, and Conservation. Annu. Rev. Entomol. 2002, 47, 595–632. [Google Scholar] [CrossRef]
- Kremen, C.; Colwell, R.K.; Erwin, T.L.; Murphy, D.D.; Noss, R.F.; Sanjayan, M.A. Terrestrial Arthropod Assemblages: Their Use in Conservation Planning. Conserv. Biol. 1993, 7, 796–808. [Google Scholar] [CrossRef]
- McNeill, S.E.; Fairweather, P.G. Single Large or Several Small Marine Reserves? An Experimental Approach with Seagrass Fauna. J. Biogeogr. 1993, 20, 429–440. [Google Scholar] [CrossRef]
- Tjørve, E. How to resolve the SLOSS debate: Lessons from species-diversity models. J. Theor. Biol. 2010, 264, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Capano, G.C.; Toivonen, T.; Soutullo, A.; Di Minin, E. The emergence of private land conservation in scientific literature: A review. Biol. Conserv. 2019, 237, 191–199. [Google Scholar] [CrossRef]
- Kamal, S.; Grodzinska-Jurczak, M.; Kaszynska, A.P. Challenges and opportunities in biodiversity conservation on private land: An institutional perspective from Central Europe and North America. Biodivers. Conserv. 2015, 24, 1271–1292. [Google Scholar] [CrossRef]
- Shumba, T.; De Vos, A.; Biggs, R.; Esler, K.J.; Ament, J.M.; Clements, H.S. Effectiveness of private land conservation areas in maintaining natural land cover and biodiversity intactness. Glob. Ecol. Conserv. 2020, 22, e00935. [Google Scholar] [CrossRef]

| Number of Species | Abundance of Species | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| END | NAT | INT | χ2 | p | END | NAT | INT | χ2 | p | |
| Araneae | 22 | 16 | 37 | 9.36 | <0.01 | 1013 | 845 | 931 | 15.183 | <0.001 |
| Coleoptera | 16 | 41 | 151 | 148.8 | <0.001 | 247 | 971 | 1340 | 725.17 | <0.001 |
| Hemiptera | 12 | 40 | 17 | 19.391 | <0.001 | 3594 | 4498 | 487 | 3095.8 | <0.001 |
| Psocoptera | 5 | 12 | 9 | 2.8462 | 0.241 | 1318 | 1852 | 461 | 813.68 | <0.001 |
| Total | 55 | 109 | 214 | 103.76 | <0.001 | 6172 | 8166 | 3219 | 2117.1 | <0.001 |
| END | NAT | INT | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Native Forest | Exotic Forest | p | Native Forest | Exotic Forest | p | Native Forest | Exotic Forest | p | |
| Number of morpho(species) | |||||||||
| Araneae | 12 | 7 | 0.359 | 8 | 6 | 0.791 | 10 | 19 | 0.136 |
| Coleoptera | 8 | 6 | 0.791 | 14 | 19 | 0.487 | 36 | 82 | <0.001 |
| Hemiptera | 6 | 4 | 0.754 | 18 | 16 | 0.864 | 1 | 11 | 0.006 |
| Psocoptera | 2 | 2 | 1 | 6 | 4 | 0.754 | 2 | 5 | 0.453 |
| Total | 28 | 19 | 0.243 | 46 | 45 | 1 | 49 | 118 | <0.001 |
| Number of individuals | |||||||||
| Araneae | 683 | 236 | <0.001 | 281 | 403 | <0.001 | 143 | 563 | <0.001 |
| Coleoptera | 90 | 112 | 0.139 | 225 | 533 | <0.001 | 151 | 849 | <0.001 |
| Hemiptera | 2835 | 542 | <0.001 | 2686 | 1294 | <0.001 | 4 | 345 | <0.001 |
| Psocoptera | 548 | 550 | 0.976 | 285 | 1119 | <0.001 | 58 | 288 | <0.001 |
| Total | 4156 | 1440 | <0.001 | 3477 | 3349 | 0.124 | 356 | 2045 | <0.001 |
| END | NAT | INT | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Native Forest | Exotic Forest | Total | Native Forest | Exotic Forest | Total | Native Forest | Exotic Forest | |
| Araneae | 0.154 (0.077/0.077) | 0.265 (0.128/0.136) | 0.375 (0.154/0.221) | 0.111 (0.111/0.000) | 0.276 (0.166/0.111) | 0.364 (0.173/0.191) | 0.383 (0.100/0.283) | 0.360 (0.205/0.155) | 0.397 (0.235/0.163) |
| Coleoptera | 0.333 (0.333/0.000) | 0.377 (0.201/0.176) | 0.391 (0.285/0.106) | 0.317 (0.100/0.217) | 0.339 (0.149/0.190) | 0.388 (0.217/0.171) | 0.396 (0.08/0.316) | 0.409 (0.238/0.171) | 0.442 (0.236/0.206) |
| Hemiptera | 0.000 | 0.191 (0.053/0.137) | 0.287 (0.130/0.158) | 0.259 (0.125/0.074) | 0.298 (0.122/0.177) | 0.373 (0.201/0.172) | 0.469 (0.000/0.469) | 0.000 | 0.343 (0.144/0.199) |
| Psocoptera | 0.167 (0.000/0.167) | 0.205 (0.090/0.115) | 0.199 (0.039/0.160) | 0.000 | 0.261 (0.078/0.183) | 0.207 (0.046/0.161) | 0.333 (0.000/0.333) | 0.082 (0.082/0.000) | 0.226 (0.042/0.184) |
| Total | 0.191 (0.176/0.015) | 0.278 (0.198/0.079) | 0.373 (0.226/0.147) | 0.243 (0.125/0.118) | 0.316 (0.168/0.148/) | 0.358 (0.217/0.141) | 0.398 (0.073/0.325) | 0.386 (0.194/0.192) | 0.417 (0.230/0.187) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsafack, N.; Fattorini, S.; Boieiro, M.; Rigal, F.; Ros-Prieto, A.; Ferreira, M.T.; Borges, P.A.V. The Role of Small Lowland Patches of Exotic Forests as Refuges of Rare Endemic Azorean Arthropods. Diversity 2021, 13, 443. https://doi.org/10.3390/d13090443
Tsafack N, Fattorini S, Boieiro M, Rigal F, Ros-Prieto A, Ferreira MT, Borges PAV. The Role of Small Lowland Patches of Exotic Forests as Refuges of Rare Endemic Azorean Arthropods. Diversity. 2021; 13(9):443. https://doi.org/10.3390/d13090443
Chicago/Turabian StyleTsafack, Noelline, Simone Fattorini, Mário Boieiro, François Rigal, Alejandra Ros-Prieto, Maria Teresa Ferreira, and Paulo A. V. Borges. 2021. "The Role of Small Lowland Patches of Exotic Forests as Refuges of Rare Endemic Azorean Arthropods" Diversity 13, no. 9: 443. https://doi.org/10.3390/d13090443
APA StyleTsafack, N., Fattorini, S., Boieiro, M., Rigal, F., Ros-Prieto, A., Ferreira, M. T., & Borges, P. A. V. (2021). The Role of Small Lowland Patches of Exotic Forests as Refuges of Rare Endemic Azorean Arthropods. Diversity, 13(9), 443. https://doi.org/10.3390/d13090443