Systematics of the Arboreal Neotropical ‘thorellii’ Clade of Centruroides Bark Scorpions (Buthidae) and the Efficacy of Mini-Barcodes for Museum Specimens
Abstract
:1. Introduction
2. Materials and Methods
- Taxon Sampling
- Material Examined
- DNA Sequencing
- Morphological Character Matrix
- Phylogenetic Analysis
| Outgroup |
|---|
| Heteroctenus junceus |
| 2101131122 1120211321 0100011011 1212101013 0001112120 2000121000 0111020011 01120021?? 0101021011 1011001001 1111211110 11 |
| Centruroides arctimanus |
| 1010010011 0120221221 0000010?1? 111111210? 00111???20 2121000010 0110102?10 0111000??? ???1100000 1210000100 0110011110 00 |
| Centruroides bani |
| 0110021222 1120011121 0001010001 1011112000 0110011222 2112110000 0110101010 01110031?? ???1101001 1[01]10000 0011001111 11201 |
| Centruroides exilicauda |
| 0100030202 1120200010 0000010111 0000200000 0010113110 2110220011 0000201010 11110130?? 0111021111 1011101010 1101211110 11 |
| Centruroides gracilis |
| 1000011112 1120201321 0100111111 1212202000 0110003120 2120220011 0110201020 1102100100 0111001111 101?001011 1110011012 11 |
| Centruroides hentzi |
| 2010030122 0120200020 0000014110 1010102000 0101101112 2111010010 0111202020 00110000?? ???1101011 1210000000 1101211000 01 |
| Centruroides infamatus |
| 1000031212 1120200011 0000010111 1010102000 0010111111 2110121010 0110100010 01010020?? ???1001111 1210101011 1101011?12 11 |
| Centruroides ochraceus |
| 0000031202 1100211111 01000101?1 0000102000 00102???22 2110221011 0110101?21 01010100?? ???1101111 1010011011 1101211110 11 |
| Centruroides thorellii |
| 0000021101 1120200000 0100010110 0101102000 0010112301 2100120011 0110201020 01011001?? ???1100011 1301100010 0110000002 01 |
| Centruroides tuxtla |
| 0100131222 1120100021 0000010111 1011102005 0110202322 2111120011 0101102010 1112010??? ???1000011 1210100110 0010000012 01 |
| Ingroup |
| Centruroides berstoni |
| 1110010111 1000000010 00010101?0 1010012115 0110002111 2112220011 0?00202?10 01110100?? ???1100000 0110000000 01110101?0 01 |
| Centruroides catemacoensis |
| 1110010222 1021200010 0001010110 1010010115 0010202111 2110020010 0110202120 00100100?? ???1100000 0110000000 0111210000 01 |
| Centruroides chanae |
| 1110010111 1021200000 0001314110 1111010005 00112???12 2102220011 0?10202120 0111010??? ???1110000 0010000000 0111211010 01 |
| Centruroides cuauhmapan |
| 1110010110 1120200010 0000314110 1010012010 0111202121 2103120011 0?10202010 01110100?? ???1100000 0010000000 0111010000 01 |
| Centruroides hamadryas |
| 1110010111 1000000010 00010101?0 1010012115 0110002111 2112220011 0?00202?10 01110100?? ???1100000 0110000000 01110101?0 01 |
| Centruroides hoffmanni |
| 0100010111 1121000010 0000010110 1111012005 1110112110 2103220011 0?00202020 01110100?? ???1100000 0210000010 0110211010 01 |
| Centruroides rileyi |
| 1110010110 1120200010 0000314110 1010012010 0111202121 2103120011 0?10202010 01110100?? ???1100000 0010000000 0111010000 01 |
| Centruroides schmidti |
| 1110010111 0120200010 0101010110 1010012010 0100012122 2102220011 0000202120 11110101?? 0111100000 0010000000 0101211010 00 |
| Centruroides yucatanensis |
| 1110010111 1120000010 0001014110 1010012105 0010012021 2103020011 0?10201120 0111010??? ???1100000 0010000000 0110010112 01 |
- Species Delimitation
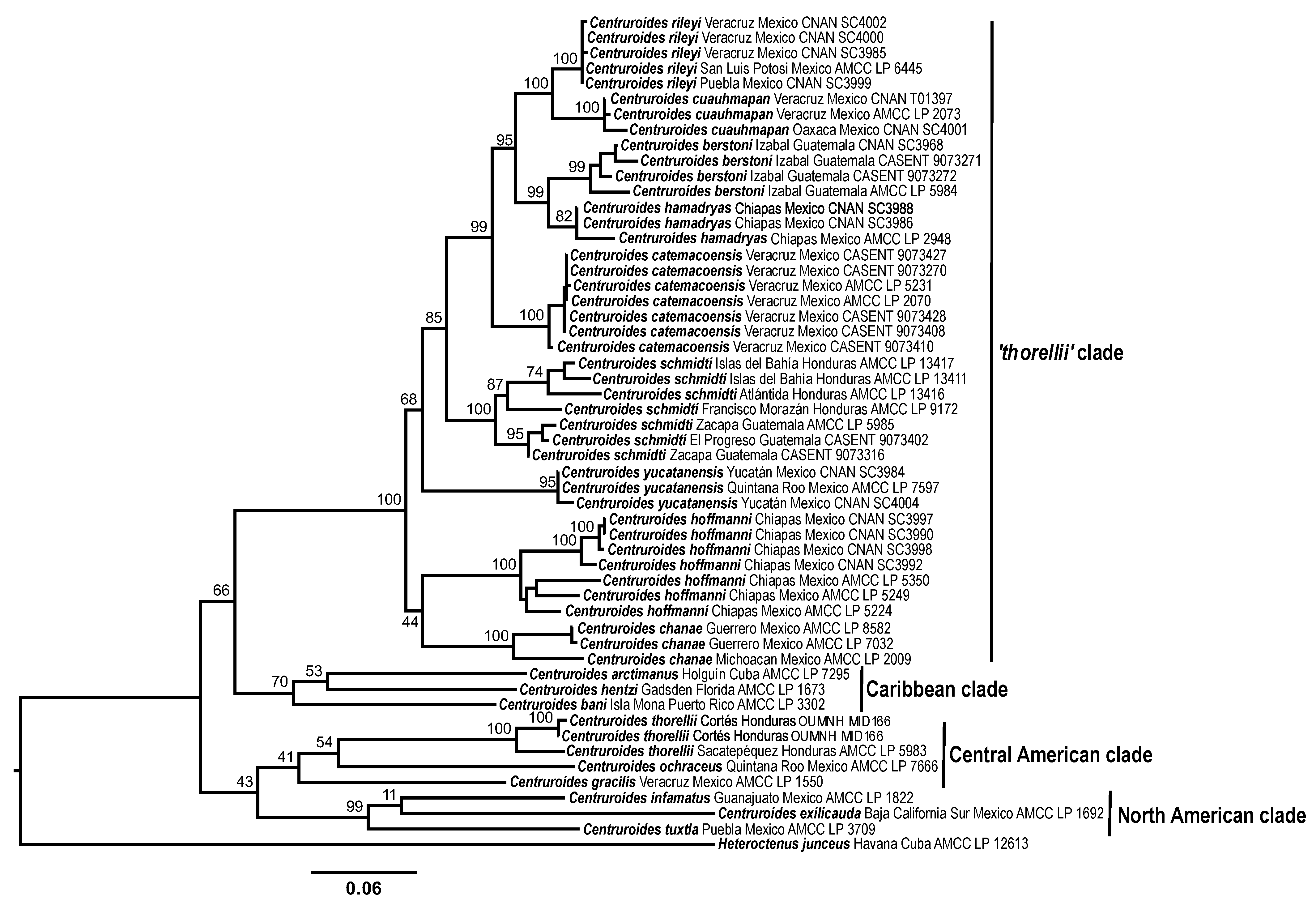
3. Results
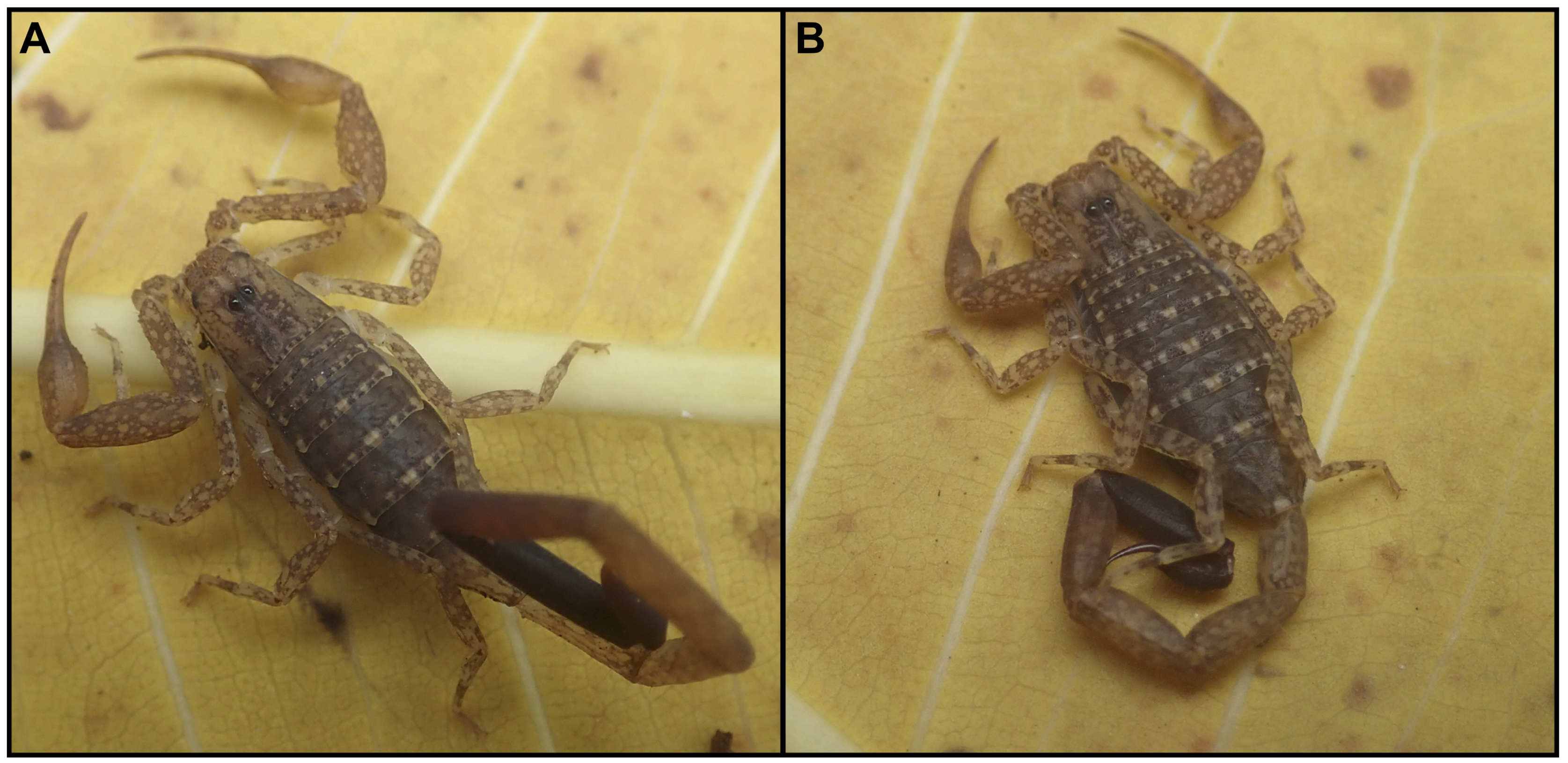
4. Discussion
- Systematics

| Putative Species | Specimens | Closest Species | Intra Dist | Inter Dist | Intra/Inter | P ID (Strict) | P ID (Liberal) | Av (MRCA-Tips) | P (RD) | P (AB) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1:C. rileyi | 5 | 2:C. cuauhmapan | 0.007 | 0.055 | 0.12 | 0.85 (0.73, 0.98) | 0.97 (0.87, 1.0) | 0.003 | 0.05 | 0.01 |
| 2:C. cuauhmapan | 3 | 1:C. rileyi | 0.002 | 0.055 | 0.36 | 0.55 (0.37, 0.73) | 0.80 (0.66, 0.95) | 0.010 | 0.19 | 0.01 |
| 3:C. berstoni | 4 | 4:C. hamadryas | 0.035 | 0.075 | 0.47 | 0.55 (0.41, 0.70) | 0.83 (0.72, 0.94) | 0.022 | 0.93 | 0.01 |
| 4:C. hamadryas | 3 | 3:C. berstoni | 0.016 | 0.075 | 0.22 | 0.65 (0.47, 0.82) | 0.88 (0.74, 1.0) | 0.081 | 0.13 | 0.01 |
| 5:C. catemacoensis | 7 | 1:C. rileyi | 0.007 | 0.110 | 0.06 | 0.92 (0.81, 1.0) | 0.98 (0.92, 1.0) | 0.010 | 0.05 | 5.50 × 10−7 |
| 6:C. schmidti | 7 | 5:C. catemacoensis | 0.073 | 0.161 | 0.46 | 0.71 (0.61, 0.82) | 0.90 (0.84, 0.96) | 0.045 | 0.59 | 4.50 × 10−8 |
| 7:C. hoffmanni | 7 | 5:C. chanae | 0.069 | 0.251 | 0.28 | 0.81 (0.71, 0.92) | 0.93 (0.87, 0.99) | 0.05 | 1.0 | 1.85 × 10−3 |
| 8:C. chanae | 3 | 9:C. schmidti | 0.058 | 0.251 | 0.23 | 0.64 (0.46, 0.81) | 0.88 (0.73, 1.0) | 0.042 | 1.0 | 1.85 × 10−3 |
| 9:C. yucatanensis | 3 | 8:C. schmidti | 0.014 | 0.197 | 0.07 | 0.74 (0.57, 0.92) | 0.97 (0.82, 1.0) | 0.008 | 0.05 | 4.20 × 10−6 |
- Diversification
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Outgroup
- Centruroides arctimanus Armas, 1976: CUBA:Holguín: Município Holguín: 20°53′32.8″ N 76°17′04.7″ W, 3.x.2007, A. Tietz, 2 juv. (AMCC [LP 7295]).
- Centruroides bani Armas and Marcano Fondeur, 1987: U.S.A.: Puerto Rico: Isla Mona, road between Sardiniera and airport: 18°03′48.4″ N 67°53′14.3″ W, 18.x.2009, L.A. Esposito and H. Yamaguti, 1 ♂ (AMCC [LP 3302]).
- Centruroides exilicauda (Wood, 1863): MEXICO:Baja California Sur: Município Los Cabos: Cabo San Lucas, 15 mi. E, 12°53′23″ N 109°54′56″ W, 1.vi.1999, M.E. Soleglad, 1 ♂ (AMCC [LP 1692]).
- Centruroides gracilis (Latreille, 1804): MEXICO: Veracruz: Município Actopan: Puente Nacional, Los Idolos, 19°25′44.9″ N 96°32′12.4″ W, 5.v.2006, O.F. Francke, P. Berea, and A. Ballesteros, 1 ex. (AMCC [LP 1550]).
- Centruroides hentzi (Banks, 1900): U.S.A.: Florida: Gadsden County, 16.v.2000, T. Gearheart, 1 ♂ (AMCC [LP 1673]).
- Centruroides infamatus (C.L. Koch, 1844): MEXICO: Guanajuato: Município Acámbaro: 2 km N Pueblo San Luis de los Agustinos, 20°12′27.9″ N 100°41′22.2″ W, 25.III.2006, O.F. Francke, A. Valdez, G. Villegas, H. Montaño, and C. Santibañez, 2 ♂ (AMCC [LP 1822]).
- Centruroides ochraceus (Pocock, 1898): MEXICO: Quintana Roo: Município Benito Juárez: Puerto Morelos, Botanical Gardens, 20°50′42.1″ N 86°54′12.9″ W, 4.VIII.2007, G. Montiel and R. Paredes, 1 subad. ♂ (AMCC [LP 7666]).
- Centruroides thorellii (Kraepelin, 1891): GUATEMALA: Sacatepéquez: Município Antigua: Parque Senderos de Alux, 15–20 km W of Guatemala City, 14°36′38.9″ N 90°38′20.8″ W, 30.VII.2008, J. Huff, C. Víquez, E. Agreda, and D. Ortíz, 1 ♀, 4 ex. (AMCC [LP 5983]). HONDURAS: Cortés: Município San Pedro Sula: Parque Nacional Cusuco, Cantilles Site, CA2 (Transect 2, subsite 3), 15°30′48″ N 88°14′23″ W, M. D’Sousa, K. Sagastume, and S. Longhorn, 2 ♀ (OUMNH [MID166]).
- Centruroides tuxtla Armas, 1999: MEXICO: Chiapas: Município Suchiapa: La Vuelta del Alacran, 4.8 km from km 27 on road from Ocozocuautla to Villa Flores, 16°32′35.2″ N 93°12′09.8″ W, x.2004, R. Paredes, O.F. Francke, and G. Villegas, 1 ♂, 1 ♀ (AMCC [LP 3709]).
- Heteroctenus junceus (Herbst, 1800): CUBA: Guantánamo: Município Baracoa: Alejandro Humboldt National Park, near El Yunque de Baracoa, 20°20′42.1″ N 74°33′59.1″ W, 370 m, 5.iv.2012, CarBio team, 1 ♂ (AMCC [LP 12613]).
- Ingroup
- Centruroides berstoni Goodman et al., 2021: GUATEMALA: Departamento Izabal: Município Livingston: Biotopo Chocón Machacas, 15°44′05.3″ N 88°54′57.2″ W, 15 m, 25.ix.2019, A.M. Goodman, 8 ♂, 1 ♀, 1 juv. ♂ (CASENT 9073271); Río Dulce, Hotel Tijax, 15°40′12.2″ N 89°00′27″ W, 49 m, 8.vii.2006, J. Huff, C. Víquez, and D. Ortíz, collected along trails through old secondary growth tropical forest using UV at night, 1 ♂ (AMCC [LP 5984]); 15°39′51.2″ N 89°00′14.6″ W, 17 m, 24.ix.2019, A.M. Goodman, collected along gravel road of Hacienda Tijax Parking Lot, flanked by bamboo groves and live fencing, 1 ♂ (CASENT 9073272); 15°44′05.3″ N 88°54′57.2″ W, 15 m, 25.ix.2019, A.M. Goodman, 8 ♂, 1 ♀, 1 juv. ♂ (CASENT 9073271). Município Morales: Morales, Finca Fiymeza, Sendero Anfibio, 15°24′24.1″ N 88°41′46.8″ W, 595 m, 17.viii.2017, D. Barrales and R. Monjaraz, 1 juv. ♀ (CNAN SC3968).
- Centruroides catemacoensis Goodman et al., 2021: MEXICO: Veracruz: Município Catemaco: Estacion Biología Los Tuxtlas, UNAM, 18°35′05.6″ N 95°04′29.9″ W, 134 m, 26.viii.2005, O.F. Francke, M. Córdova, A. Jaimes, A. Valdez, and H. Montaño, 1 ♂ (AMCC [LP 5231]); 18°34′54″ N 95°04′54.6″ W, 134 m, 19.vii.2002, J. Ponce and O.F. Francke, 1 ♀ (AMCC [LP 2070]), 74–416 m, 17.vii–25.vii.2018, A.M. Goodman, J. Gorneau, and M.K. Lippey, 2 ♂ (CASENT 9073270, 9073427), 4 juv. ♀ (CASENT 9073408, 9073410, 9073428).
- Centruroides chanae Goodman et al., 2021: MEXICO: Guerrero: Município Copala: Microondas Fogos, 16°33′59.5″ N 98°53′18.1″ W, 103 m, 22.vi.2007, O.F. Francke, M. Escalante, H. Montaño, and A. Ballesteros, 1 ♂ (AMCC [LP 7032]), 1 ♀ (AMCC [LP 8582]). Michoacán: Município Aquila: Faro de Bucerias, 18°21′08.3″ N 103°30′20.9″ W, 13 m, 10.iii.2002, J. Ponce, low deciduous forest, 3 ♂ (CNAN SC4005); 18°35′50.5″ N 103°30′04.3″ W, 221 m, 14.iv.2002, J. Ponce and E. González, low deciduous forest, 1 ♂ (AMCC [LP 2009]).
- Centruroides cuauhmapan Goodman et al., 2021: MEXICO: Oaxaca: Município San Juan Bautista Tuxtepec: 17 km from San Juan Bautista Tuxtepec, Cerro del Oro Dam, 17°59′55″ N 96°15′47.2″ W, 74 m, 23.v.1990, E. Barrera and A. Cadena, 1 ♂ (CNAN SC4001). Veracruz: Município Amatlán de los Reyes: Cañada Blanca, 18°55′43.5″ N 96°51′26″ W, 555 m, 18.vii.2002, E. González, found in coffee plantation in lowland rainforest, collected at night with UV light, 1 ♂ (AMCC [LP 2073]). Município Actopan: Los Idolos, 19°25′44.9″ N 96°32′12.4″ W, 112 m, 5.v.2006, O.F. Francke, P. Berea, and A.J. Ballesteros, collected with UV detection, 2 ♂ paratypes (CNAN T01397).
- Centruroides hamadryas Goodman et al., 2021: MEXICO: Chiapas: Municipío Ocosingo: La Galleta, 2 km SE of Frontera Corozal, 16°48′12.7″ N 90°52′11.1″ W, 132–150 m, 28.iv.2004, R. Paredes and J.L. Castelo, collected with UV light detection, 2 ♂ (AMCC [LP 2948]); 16°49′55″ N 90°56′08″ W, 146 m, 7.iv.2005, A. Valdez, O.F. Francke, and A. Ballesteros, collected at night with UV lamp, 1 juv. ♂ (CNAN SC3986); 16°48′18.5″ N 90°54′25″ W, 114 m, 28.iv.2005, A. Valdez, O.F. Francke, and A. Ballesteros, urban area towards blue water bridge, collected with UV light detection, 2 ♂, 1 ♀, 1 juv. ♂, 1 juv. ♀ (CNAN SC3988).
- Centruroides hoffmanni Armas, 1996: MEXICO: Chiapas: Município Ángel Albino Corzo: 8 km from Siltepec, 18°48′33″ N 92°40′30.6″ W, 663 m, 17.viii.2007, C. Mayorga, G. Ortega, and L. Cervantes, 1 juv. ♀ (CNAN SC3990). Município Comitan: Parque Nacional Lagunas de Montebelo, 16°17′17″ N 91°56′16″ W, 1473 m, 3.ix.2005, O.F. Francke, M. Córdova, A. Jaimes, A. Valdez, and H. Montaño, 1 juv. ♂ (CNAN SC3992). Município La Concordia: Villa Corzo La Tigrilla, San Julián, Revolución Mexicana, 16°00′00″ N 92°50′47″ W, 544 m, 17.iii.2007, C. Mayorga, G. Ortega, and L. Cervantes; 1 ♂, 1 ♀ (CNAN SC3998). Município Tuxtla Gutiérrez: Las Delicias, 16°45′31.1″ N 93°06′26.4″ W, 529 m, 2.iii.2005, O.F. Francke, M. Córdova, A. Jaimes, A. Valdez, and H. Montaño, 1 ♀ (AMCC [LP 5224]); Gutiérrez, San Julián, Revolución Mexicana, 16°11′41″ N 93°01′16″ W, 544 m, 16.iii.2007, G. Ortega and A. Cervantes, 2 ♂, 3 ♀ (CNAN SC3997). Município Tzimol: Carretera [Hwy] Comitán–Tzimol Santa Rosa, 16°11′03.4″ N 92°16′59.3″ W, 632–730 m, 2.ix.2005, O.F. Francke, M. Córdova, A. Jaimes, A. Valdez, and H. Montaño, 1 ♂, 1 ♀ (AMCC [LP 5249]). Município Villaflores: Reserva de La Biosfera La Sepultura, 1 km SE of Ejido California, 16°15′14.2″ N 93°35′46.4″ W, 1009–1132 m, 30.viii.2005, O.F. Francke, M. Córdova, A. Jaimes, A. Valdez, and H. Montaño, 1 ♂ (AMCC [LP 5350]).
- Centruroides rileyi Sissom, 1995: MEXICO: Puebla: Município Cuetzalan el Progreso: Cuetzalan, Santiago Yancuitlalpan, 18°54′42.2″ N 98°35′15.3″ W, 2554 m, 19.v.1995, G. Oclogaig Barrzia, juvs (CNAN SC3999). San Luis Potosí: Município Axtlan de Terrazas: Axtlan de Terrazas, 21°25′34.9″ N 98°52′42″ W, 100 m, 28.iv.2006, O.F. Francke, A. Valdez, G. Villegas, and R. Paredes, 1 ♂ (AMCC [LP 6445]). Veracruz: Município Paplanta: Papantla 20°27′24.1″ N 97°18′56.1″ W, 2197 m, iii.2000, J.L. Castelo, 1 ♂ (CNAN SC4000). Município Tamiahua: Moralillo, Cerro Azul, 21°11′03.8″ N 97°44′49.6″ W, 153 m, 27.ii.2007, E. Barrera and L. Cervantes, 2 ♀ (CNAN SC3985), 1 juv. ♀ (CNAN SC4002).
- Centruroides schmidti Sissom, 1995: GUATEMALA: Departamento El Progreso: Município Rio Hondo: San Francisco Zapotitlan: Finca El Olvido, Las Minas, 15°02′04.8″ N 89°52′26.7″ W, 1214 m, 18.ix.2019, A.M. Goodman, L.A. Esposito, and L. Allen, 5 ♀, 1 juv. ♂, 1 juv. ♀ (CASENT 9073402). Departamento Zacapa: Município Rio Hondo: Bosque Pino, Guadalupe, Manta de Golpeo, 14°58′04.7″ N 89°24′47″ W, 751 m, 20.ix.2019, A.M. Goodman, M. Barrios, and M. van Dam, UV hand collection, found on oak and pine trees, 2–3 m high, 7 ♂, 1 ♀, 1 juv. ♂, 1 juv. ♀ (CASENT 9073278); Aldea Casas de Pinto, near turnoff for Zacapa at Rio Hondo, 15°01′38.2″ N 89°36′57.2″ W, 77 m, 13.vii.2006, J.H. Huff, semi-arid region with scrub forest and cacti, collected under rocks in shaded areas and at night using UV, 1 ad. (AMCC [LP 5985]). HONDURAS: Departamento Atlántida: Município La Ceiba: Parque Nacional Pico Bonito, Pico Bonito, trails from Visitor Centre and park entrance, 14°43′30.6″ N 86°44′11.5″ W, 184 m, 30.viii.2013, S. Longhorn, dense wet lowland tropical forest near large river, sweeping and beating, may have been on or under wood, day search, 1 juv. ♂ (AMCC [LP 13416]). Departmento Francisco Morazán: Município San Antonio de Oriente: E.A.P. Zamorano, Monte Redondo, Acuacultura, 13°39′59.6″ N 86°59′21″ W, 773 m, 23.ix.2008, C. Víquez, UV at night, 1 ♀ (AMCC [LP 9172]). Departamento Islas de la Bahía: Município Roatán: Cayos Cochinos, Cayos Menor, forest trails, 15°57′26.9″ N 86°30′03.3″ W, 101 m, 2.viii.2012, S. Longhorn, scrub oak forest, 1 ♀ (AMCC [LP 13411]); Isla Utila, 16°06′22.1″ N 86°54′08.1″ W, 12 m, 21.vii.2012, S. Longhorn, scrub forest/wet savannah, 1 ♀ (AMCC LP [13417]).
- Centruroides yucatanensis Goodman et al., 2021: MEXICO: Quintana Roo: Município Benito Juarez: Puerto Morelos, Jardin Botanico Alfredo Barrera, 20°50′42.1″ N 86°54′12.9″ W, 38 m, 4.vii.2007, G. Montiel, R. Paredes, M. Ramírez, D. Chibras, and G. Bonilla, 1 ♂ (AMCC [LP 7597]), 2 juv. ♂ (CNAN SC3984). Yucatán: Município Felipe Carrillo Puerto: Cenote Chac-ha, 3.5 km N and 3 km E of Kalacmul, 20°04′40.3″ N 88°08′27.9″ W, 23 m, 9.vii.2007, R. Paredes, D. Chibras, and G. Montiel, 1 ♀ (CNAN SC4004).
Appendix B
| Species | Coll. Number | Locality | Length | GenBank Code |
| Outgroup | ||||
| H. junceus | AMCC [LP 12613] | Cuba: Guantánamo: Humboldt N. P. | 1078 | KY982192.1 |
| C. bani | AMCC [LP 3302] | Puerto Rico: Isla Mona | 1078 | MK479164.1 |
| C. exilicauda | AMCC [LP 1692] | Mexico: Baja California Sur: Cabo San Lucas | 1078 | KY982179.1 |
| C. gracilis | AMCC [LP 1550] | Mexico: Veracruz: Los Idolos | 1078 | MK479175.1 |
| C. hentzi | AMCC [LP 1673] | U.S.: Florida: Gadsden Co. | 1078 | MK479177.1 |
| C. infamatus | AMCC [LP 1822] | Mexico: Guanajuato: Acámbaro | 1078 | KY982181.1 |
| C. ochraceus | AMCC [LP 7666] | Mexico: Morelos: Puerto Morelos Bot. Gard. | 1078 | MK479194.1 |
| C. thorellii | AMCC [LP 5983] | Guatemala: Sacatepéquez: Parque Alux | 1078 | MK479208.1 |
| OUMNH [MID166] | Honduras: Cortés: San Pedro Sula | 642 | MZ366335 | |
| 658 | MZ366336 | |||
| C. tuxtla | AMCC [LP 3709] | Mexico: Chiapas: La Vuelta de Alacran | 1078 | MK479209.1 |
| Ingroup | ||||
| C. berstoni | CASENT 9073271 | Guatemala: Izabal: Biotopo Chocón Machacas | 430 | MZ366346 |
| CASENT 9073272 | Guatemala: Izabal: Hotel Tijax | 621 | MZ366345 | |
| CNAN SC3968 | Guatemala: Izabal: Morelos | 658 | MZ366344 | |
| C. catemacoensis | AMCC [LP 2070] | Mexico: Veracruz: Los Tuxtlas | 1078 | MZ429054 |
| AMCC [LP 5231] | 1078 | MZ429055 | ||
| CASENT 9073270 | 659 | MZ366343 | ||
| CASENT 9073408 | 648 | MZ366342 | ||
| CASENT 9073410 | 658 | MZ366341 | ||
| CASENT 9073427 | 659 | MZ366340 | ||
| CASENT 9073428 | 648 | MZ366339 | ||
| C. chanae | AMCC [LP 2009] | Mexico: Michoacán: Faro de Bucerias | 1078 | MZ429056 |
| AMCC [LP 7032] | 1078 | MZ429057 | ||
| AMCC [LP 8582] | Mexico: Guerrero: Microondas Fogos | 1078 | MZ429058 | |
| C. cuauhmapan | AMCC [LP 2073] | Mexico: Veracruz: Cañada Blanca | 1078 | MZ429059 |
| CNAN SC4001 | Mexico: Oaxaca: Cerro del Oro | 40 | - | |
| CNAN T01397 | Mexico: Veracruz: Los Idolos | 127 | - | |
| C. hamadryas | AMCC [LP 2948] | Mexico: Chiapas: La Galleta | 1078 | MZ429060 |
| CNAN SC3986 | 127 | - | ||
| CNAN SC3988 | 127 | - | ||
| C. hoffmanni | AMCC [LP 5224] | Mexico: Chiapas: Las Delicias | 1078 | MZ429061 |
| AMCC [LP 5249] | Mexico: Chiapas: Santa Rosa | 1078 | MZ429062 | |
| AMCC [LP 5350] | Mexico: Chiapas: Res. Biosfera Sepultura | 1078 | MK479178.1 | |
| CNAN SC3990 | Mexico: Chiapas: Siltepec | 134 | - | |
| CNAN SC3992 | Mexico: Chiapas: P. N. Lagunas de Montebelo | 137 | - | |
| CNAN SC3997 | Mexico: Chiapas: Gutierrez | 134 | - | |
| CNAN SC3998 | Mexico: Chiapas: Villa Corzo | 138 | - | |
| C. rileyi | AMCC [LP 6445] | Mexico: San Luis Potosí: Axtlan de Terrazas | 1078 | KY982183.1 |
| CNAN SC3985 | Mexico: Veracruz: Cerro Azul | 127 | - | |
| CNAN SC3999 | Mexico: Puebla: Cuetzalan | 127 | - | |
| CNAN SC4000 | Mexico: Veracruz: Papantla | 127 | - | |
| CNAN SC4002 | Mexico: Veracruz: Cerro Azul | 127 | - | |
| C. schmidti | AMCC [LP 13416] | Honduras: Atlántida: Pico Bonito | 127 | - |
| AMCC [LP 13411] | Honduras: Isla del Bahía: Cayos Menor | 1078 | MZ429064 | |
| AMCC [LP 13417] | 1078 | MZ429065 | ||
| AMCC [LP 5985] | Guatemala: Zacapa: Aldea casas de Pinto | 1078 | MZ429063 | |
| AMCC [LP 9172] | Honduras: Francisco Morazán: E.A.P. Zamorano | 1078 | KY982184.1 | |
| CASENT 9073316 | Guatemala: Zacapa: Guadalupe | 606 | MZ366338 | |
| CASENT 9073402 | Guatemala: Zacapa: Las Minas | 620 | MZ366337 | |
| C. yucatanensis | AMCC [LP 7597] | Mexico: Quintana Roo: Puerto Morelos | 1078 | MK479201.1 |
| CNAN SC3984 | 127 | - | ||
| CNAN SC4004 | Mexico: Yucatán: Cenote Chac-ha | 127 | - |
Appendix C
- Carapace
- 1.
- Lateral ocular carinae: 0, present, distinct; 1, reduced to several granules; 2, absent (E.S. Volschenk and L. Prendini, unpublished data; Esposito et al. 2017, 2018).
- 2.
- Centrolateral carinae: 0, present; 1, absent (E.S. Volschenk and L. Prendini, unpublished data; Esposito et al., 2017, 2018).
- 3.
- Anterior centrosubmedian carinae: 0, present; 1, absent (E.S. Volschenk and L. Prendini, unpublished data; Esposito et al., 2017, 2018).
- 4.
- Posterior centrosubmedian carinae: 0, present; 1, absent (E.S. Volschenk and L. Prendini, unpublished data; Esposito et al., 2017, 2018).
- 5.
- Anteromedian notch: 0, present; 1, absent (Esposito, 2011).
- 6.
- Surface granulation density: 0, smooth; 1, sparsely granular; 2, densely granular medially; 3, densely granular throughout (Esposito, 2011).
- 7.
- Surface granulation texture: 0, weakly granular, shagreened; 1, large, rounded granules; 2, large, conical granules (Esposito, 2011).
- 8.
- Anterior median ocular sulcus: 0, absent; 1, wide; 2, narrow, deep (Esposito, 2011).
- 9.
- Median ocular sulcus: 0, absent; 1, wide; 2, narrow, deep (Esposito, 2011).
- 10.
- Posteromedian sulcus: 0, absent; 1, wide; 2, narrow, deep (Esposito, 2011).
- 11.
- Posteromarginal sulci: 0, absent; 1, present (Esposito, 2011).
- 12.
- Posterolateral sulci: 0, absent; 1, present (Esposito, 2011).
- 13.
- Anterior margin, carina: 0, absent; 1, smooth; 2, granular (Esposito, 2011).
- 14.
- Lateral margins, carina: 0, absent; 1, smooth; 2, granular (Esposito, 2011).
- 15.
- Posterior margin, carina between posterior centrosubmedian carinae: 0, absent; 1, smooth; 2, granular (Esposito, 2011).
- Pedipalps
- 16.
- Chela prodorsal carina: 0, granular; 1, smooth; 2, absent (E.S. Volschenk and L. Prendini, unpublished data; Esposito, 2011; Esposito et al., 2017, 2018).
- 17.
- Chela retrodorsal carina: 0, granular; 1, smooth; 2, absent (E.S. Volschenk and L. Prendini, unpublished data; Esposito, 2011).
- 18.
- Chela retromedian carinae: 0, granular; 1, smooth; 2, absent; 3, vestigial, reduced to several sparse granules; ?, unknown (E.S. Volschenk and L. Prendini, unpublished data; Esposito, 2011; Esposito et al., 2017, 2018).
- 19.
- Chela retroventral accessory carina: 0, complete; 1, reduced; 2, absent (E.S. Volschenk and L. Prendini, unpublished data; Esposito, 2011).
- 20.
- Chela retroventral carinae: 0, granular; 1, smooth; ?, unknown (Esposito, 2011; Esposito et al., 2017, 2018).
- 21.
- Chela retrodorsal accessory carinae: 0, present; 1, absent (Esposito, 2011; Esposito et al., 2017, 2018).
- 22.
- Chela prodorsal accessory carinae: 0, present; 1, absent (Esposito, 2011; Esposito et al., 2017, 2018).
- 23.
- Patella dorsomedian carina: 0, present; 1, absent (Esposito, 2011).
- 24.
- Femur retromedian carinae: 0, small granules; 1, large, conical granules (Esposito, 2011).
- 25.
- Fixed finger, number of median denticle subrows: 0, eight; 1, nine; 2, ten or more; 3, seven plus fused proximal subrow; 4, six plus fused proximal subrow (Esposito, 2011; Esposito et al., 2017, 2018).
- 26.
- Fixed and movable fingers, supernumary granules; 0, absent; 1, present (Soleglad and Fet, 2003; Esposito, 2011; Esposito et al., 2017, 2018).
- 27.
- Movable finger, number of median denticle subrows: 0, eight; 1, nine; 2, eleven; 3, thirteen or more; 4, seven plus fused proximal subrow (Soleglad and Fet, 2003; Prendini, 2004; Esposito, 2011; Esposito et al., 2017, 2018).
- 28.
- Chela shape (♂): 0, incrassate (bulbous or swollen); 1, slender (Prendini, 2001, 2004; Esposito, 2011; Esposito et al., 2017, 2018).
- 29.
- Chela shape (♀): 0, incrassate (bulbous or swollen); 1, slender (Prendini, 2001; Esposito, 2011; Esposito et al., 2017, 2018).
- 30.
- Chela movable finger, proximal lobe (♂): 0, present; 1, absent (Prendini, 2001; Esposito, 2011; Esposito et al., 2017, 2018).
- 31.
- Patella prolateral surface, setation: 0, long, dense setae; 1, short, sparse setae (E.S. Volschenk and L. Prendini, unpublished data; Esposito, 2011).
- Legs
- 32.
- Leg I, tarsal setation: 0, short, dense setae; 1, long, dense setae; 2, sparse setae (E.S. Volschenk and L. Prendini, unpublished data; Esposito, 2011; Esposito et al., 2017, 2018).
- 33.
- Leg IV, tarsal setation: 0, short, dense setae; 1, long, dense setae; 2, sparse setae (Esposito, 2011; Esposito et al., 2017, 2018).
- 34.
- Legs I–IV, trochanter lateral margin carina: 0, absent; 1, smooth; 2, granular (Esposito, 2011).
- 35.
- Telotarsal ungues: 0, long, slightly curved; 1, hooked (Esposito, 2011).
- Pectines
- 36.
- Pectinal tooth shape: 0, elongate; 1, rounded, spade-like (Esposito, 2011; Esposito et al., 2017, 2018).
- 37.
- Proximal teeth, nodules on dorsal surface: 0, one; 1, multiple; 2, absent (Esposito, 2011; Esposito et al., 2017, 2018).
- 38.
- Dorsal fulcra: 0, present; 1, reduced (Esposito, 2011).
- 39.
- Tympanum-like expansion between lamellae and teeth: 0, absent; 1, present (Esposito, 2011).
- 40.
- Proximal fulcra, setal count: 0, one; 1, two; 2, three; 3, four; 4, six or more; 5, none; ?, unknown. (Esposito, 2011; Esposito et al., 2017, 2018).
- 41.
- Pectinal plate anterior margin, sulcus (♂): 0, present; 1, absent (Esposito, 2011; Esposito et al., 2017, 2018).
- 42.
- Pectinal plate, posterior margin (♂): 0, straight; 1, convex; 2, concave (Esposito, 2011; Esposito et al., 2017, 2018).
- 43.
- Median pectinal plate depression (♂): 0, present; 1, absent (Esposito, 2011; Esposito et al., 2017, 2018).
- 44.
- Lateral pectinal plate depression (♂): 0, present; 1, absent (Esposito, 2011).
- 45.
- Pectinal plate shape (♂): 0, square; 1, rectangular; 2, trapezoidal (Esposito, 2011).
- 46.
- Pectinal plate anterior margin, sulcus (♀): 0, present; 1, absent; ?, unknown (Esposito, 2011).
- 47.
- Pectinal plate posterior margin (♀): 0, straight; 1, slightly convex; 2, prominently rounded; 3, concave; ?, unknown (Esposito, 2011).
- 48.
- Pectinal plate depressions (♀): 0, absent; 1, single wide median depression; 2, paired lateral depressions; 3, single small, deep median depression (pinhole); ?, unknown (Esposito, 2011).
- Sternites
- 49.
- Sternite VI, ventromedian carina: 0, absent; 1, granular; 2, smooth (E.S. Volschenk and L. Prendini, unpublished data; Esposito, 2011; Esposito et al., 2017, 2018).
- 50.
- Sternite V, setation (♂): 0, absent; 1, present, setal base not situated in pits (surface smooth); 2, present, setal base situated in pits (surface punctate) (Esposito, 2011).
- 51.
- Sternite VI, ventrolateral carinae: 0, absent; 1, reduced to single granule; 2, present, more than one granule (E.S. Volschenk and L. Prendini, unpublished data; Esposito, 2011; Esposito et al., 2017, 2018).
- Tergites
- 52.
- Tergites III–VI, dorsolateral carinae: 0, present; 1, absent (E.S. Volschenk and L. Prendini, unpublished data; Esposito, 2011; Esposito et al., 2017, 2018).
- 53.
- Tergites III–VI, dorsosubmedian carinae: 0, absent; 1, vestigial; 2, distinct (Prendini, 2004; Esposito, 2011; Esposito et al., 2017, 2018).
- 54.
- Tergite VII, median carina: 0, narrow, granular carina; 1, broad, granular carina; 2, broad, smooth carina; 3, vestigial (E.S. Volschenk and L. Prendini, unpublished data; Esposito, 2011; Esposito et al., 2017, 2018).
- Metasoma
- 55.
- Segment II, median lateral carinae: 0, complete; 1, posteriorly restricted; 2, absent (Esposito, 2011; Esposito et al., 2017, 2018).
- 56.
- Segment III, median lateral carinae: 0, complete; 1, posteriorly restricted; 2, absent (Esposito, 2011; Esposito et al., 2017, 2018).
- 57.
- Segment III, dorsolateral carinae, posterior granules: 0, similar to preceding granules; 1, larger than preceding granules, acuminate (E.S. Volschenk and L. Prendini, unpublished data; Esposito, 2011; Esposito et al., 2017, 2018).
- 58.
- Segment IV, median lateral carinae: 0, absent or obsolete; 1, present (Esposito, 2011; Esposito et al., 2017, 2018).
- 59.
- Segment V, posterior margin (anal rim) granulation: 0, present; 1, absent (Esposito, 2011; Esposito et al., 2017, 2018).
- 60.
- Segment V, dorsolateral carinae: 0, present; 1, absent (Esposito, 2011; Esposito et al., 2017, 2018).
- 61.
- Segment V, median lateral carinae: 0, present; 1, absent (Esposito 2011; Esposito et al., 2017, 2018).
- 62.
- Segment V, ventrolateral carinae: 0, present; 1, absent; ?, unknown (Esposito, 2011).
- 63.
- Segment V, ventromedian carinae: 0, absent or obsolete; 1, present (Esposito, 2011; Esposito et al., 2017, 2018)
- 64.
- Segment V, ventrosubmedian carinae: 0, absent or obsolete; 1, present (Esposito, 2011; Esposito et al., 2017, 2018).
- 65.
- Segment V, ratio of segment length to width (♂): 0, slightly elongated, length less than 2x width; 1, moderately elongated, length 2.5–3x width; 2, markedly elongated, length more than 3x width (Esposito, 2011; Esposito et al., 2017, 2018).
- 66.
- Segments I–IV, width: 0, narrowing posteriorly, segment I slightly wider than IV; 1, slightly widening posteriorly, segment I slightly narrower than IV; 2, markedly widening posteriorly, segment I much narrower than IV (Esposito, 2011; Esposito et al., 2017, 2018).
- 67.
- Metasoma length relative to length of prosoma and mesosoma (♂): 0, similar or slightly greater; 1, 1.5–2x; 2, more than 2x (Esposito, 2011; Esposito et al., 2017, 2018).
- 68.
- Metasoma length relative to length of prosoma and mesosoma (♀): 0, similar or slightly greater; 1, 1.5–2x; 2, more than 2x; ?, unknown (Lamoral, 1978; Prendini 2001, 2003; Esposito, 2011; Esposito et al., 2017, 2018).
- Telson
- 69.
- Telson shape and length (♂): 0, spherical, length similar to width; 1, slightly ovate, length approximately 1.5x width; 2, ovate, length more than 2x width (Esposito, 2011; Esposito et al., 2017, 2018).
- 70.
- Telson vesicle, width in relation to width of metasomal segment V (♂): 0, similar; 1, narrower; 2, much narrower, less than half (Lamoral, 1978; Prendini 2001, 2003; Esposito, 2011; Esposito et al., 2017, 2018).
- 71.
- Telson vesicle, ventromedian carinae: 0, present; 1, absent (E.S. Volschenk and L. Prendini, unpublished data; Esposito, 2011; Esposito et al., 2017, 2018).
- 72.
- Telson vesicle, ventrolateral carinae: 0, present; 1, absent (E.S. Volschenk and L. Prendini, unpublished data; Esposito, 2011; Esposito et al., 2017, 2018).
- 73.
- Telson aculeus angle: 0, angled approximately 90° to vesicle; 1, angled less than 90° to vesicle (Esposito, 2011).
- 74.
- Telson vesicle, proximal margin, notch: 0, absent; 1, present, unmodified; 2, present, projecting vertically (Esposito, 2011).
- 75.
- Telson vesicle, lateral lobes: 0, absent; 1, present (Esposito, 2011).
- 76.
- Telson ventral surface: 0, granular; 1, smooth (Esposito, 2011).
- 77.
- Telson subaculear tubercle: 0, distinct, singular; 1, distinct, bifurcate; 2, obsolete, slight protuberance; 3, absent (Lamoral, 1980; Stockwell, 1989; Prendini, 2001, 2003; Esposito, 2011; Esposito et al., 2017, 2018).
- Total length
- 78.
- Total length, sexual dimorphism: 0, male shorter than or similar to female; 1, male much longer than female; ?, unknown (Esposito, 2011; Esposito et al., 2017, 2018).
- Ovariuterus
- 79.
- Number of loops (‘cells’): 0, eight; 1, nine; ?, unknown (Volschenk et al., 2008; Esposito, 2011; Esposito et al., 2017, 2018).
- 80.
- Loop shape: 0, simple; 1, complex bridged; ?, unknown (Volschenk et al., 2008; Esposito, 2011; Esposito et al., 2017, 2018).
- Book lungs
- 81.
- Lamellar surface: 0, slender venation; 1, ribbed venation; ?, unknown (Kamenz and Prendini, 2008; Esposito, 2011; Esposito et al., 2017, 2018).
- 82.
- Lamellar edge: 0, thorns; 1, smooth or wrinkled; ?, unknown (Kamenz and Prendini, 2008; Esposito, 2011; Esposito et al., 2017, 2018).
- 83.
- Spiracle, posterior margin: 0, hilocks; 1, subconical; ?, unknown (Kamenz and Prendini, 2008; Esposito, 2011; Esposito et al., 2017, 2018).
- Color and infuscation
- 84.
- Cheliceral manus, reticulate infuscation: 0, present; 1, laterally restricted; 2, absent (Esposito, 2011).
- 85.
- Pedipalp segments, color pattern: 0, chela manus and patella similar to femur; 1, chela manus darker than femur; 2, chela manus and patella darker than femur; 3, chela manus paler than femur (Esposito, 2011).
- 86.
- Pedipalp femur, mottled infuscation: 0, present; 1, absent (Esposito, 2011).
- 87.
- Pedipalp patella, mottled infuscation: 0, present; 1, absent (Esposito, 2011).
- 88.
- Pedipalp chela manus, mottled infuscation: 0, present; 1, absent (Esposito, 2011).
- 89.
- Pedipalp chela fingers, mottled infuscation: 0, present; 1, absent (Esposito, 2011).
- 90.
- Tergites I–VI, mottled infuscation: 0, present; 1, absent (Esposito, 2011).
- 91.
- Tergites I–VI, lateral band of infuscation, shape: 0, absent; 1, narrow; 2, distinct, rectangular; 3, wide, almost touching lateral margins (Esposito, 2011).
- 92.
- Tergites I–VI, lateral band of infuscation, intensity: 0, absent; 1, faint; 2, mottled; 3, distinct (Esposito, 2011).
- 93.
- Tergites I–VI, infuscation: 0, eye-shaped pattern; 1, absent (Esposito, 2011).
- 94.
- Tergites I–VI, lateral margins infuscation: 0, distinct black line; 1, absent; ?, unknown (Esposito, 2011).
- 95.
- Tergites I–VI, median stripe of infuscation: 0, absent; 1, present (Esposito, 2011).
- 96.
- Tergite VII, color pattern: 0, similar to other tergites; 1, paler than other tergites (Esposito, 2011).
- 97.
- Carapace, mottled infuscation: 0, present; 1, absent (Esposito, 2011).
- 98.
- Carapace, bands of infuscation: 0, absent; 1, two broad bands; 2, four narrow lines (Esposito, 2011).
- 99.
- Carapace, interocular triangle infuscation: 0, present; 1, absent (Esposito, 2011).
- 100.
- Metasomal segments I–V, ventral surfaces, mottled infuscation: 0, present; 1, absent (Esposito, 2011).
- 101.
- Metasomal segments I–V, lateral surfaces, mottled infuscation: 0, present; 1, absent (Esposito, 2011).
- 102.
- Metasomal segments I–V, ventromedian stripe of infuscation: 0, present; 1, absent (Esposito, 2011).
- 103.
- Metasomal segment V, color pattern: 0, similar to preceding segments; 1, darker than preceding segments (Esposito, 2011; Esposito et al., 2017, 2018).
- 104.
- Telson, lateral bands of infuscation: 0, present; 1, absent (Esposito, 2011).
- 105.
- Telson, median stripe of infuscation: 0, complete; 1, posteriorly confined; 2, absent (Esposito, 2011).
- 106.
- Telson, mottled infuscation: 0, present; 1, absent (Esposito, 2011).
- 107.
- Sternites III–VI, mottled infuscation: 0, present; 1, absent (Esposito, 2011).
- 108.
- Sternite V, pale surface (♂): 0, present; 1, absent; ?, unknown (Esposito, 2011).
- 109.
- Sternite V, pale surface (♀): 0, present; 1, absent; ?, unknown (Esposito, 2011).
- 110.
- Sternite VII, color pattern: 0, similar to other sternites; 0, paler than preceding sternites; 2, darker than preceding sternites (Esposito, 2011).
- 111.
- Legs I–IV, dorsal surfaces, mottled infuscation: 0, present; 1, absent (Esposito, 2011).
- 112.
- Legs I–IV, ventral surfaces, mottled infuscation: 0, present; 1, absent (Esposito, 2011).
References
- Wandeler, P.; Hoeck, P.E.; Keller, L.F. Back to the future: Museum specimens in population genetics. Trends Ecol. Evol. 2007, 22, 634–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, J.; Hajibabaei, M.; Blackburn, D.C.; Hanken, J.; Cantin, E.; Posfai, J.; Evans, T.C. DNA damage in preserved specimens and tissue samples: A molecular assessment. Front. Zool. 2008, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Dean, M.D.; Ballard, J.W. Factors affecting mitochondrial DNA quality from museum preserved Drosophila simulans. Entomol. Exp. Appl. 2001, 98, 279–283. [Google Scholar] [CrossRef] [Green Version]
- Evans, T. DNA damage. NEB Expr. 2007, 2, 1–3. [Google Scholar]
- Cárdenas, P.; Moore, J.A. First records of Geodia demosponges from the New England seamounts, an opportunity to test the use of DNA mini-barcodes on museum specimens. Mar. Biodivers. 2019, 49, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Francis, C.M.; Borisenko, A.V.; Ivanova, N.V.; Eger, J.L.; Lim, B.K.; Guillén-Servent, A.; Kruskop, S.V.; Mackie, I.; Hebert, P.D. The role of DNA barcodes in understanding and conservation of mammal diversity in Southeast Asia. PLoS ONE 2010, 5, e12575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hebert, P.D.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. USA 2004, 101, 14812–14817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meusnier, I.; Singer, G.A.; Landry, J.F.; Hickey, D.A.; Hebert, P.D.; Hajibabaei, M. A universal DNA mini-barcode for biodiversity analysis. BMC Genom. 2008, 9, 214. [Google Scholar] [CrossRef] [Green Version]
- Alberdi, A.; Garin, I.; Aizpurua, O.; Aihartza, J. The foraging ecology of the mountain long-eared bat Plecotus macrobullaris revealed with DNA mini-barcodes. PLoS ONE 2012, 7, e35692. [Google Scholar] [CrossRef] [Green Version]
- Bagley, J.C.; De Aquino, P.D.; Breitman, M.F.; Langeani, F.; Colli, G.R. DNA barcode and minibarcode identification of freshwater fishes from Cerrado headwater streams in central Brazil. J. Fish Biol. 2019, 95, 1046–1060. [Google Scholar] [CrossRef]
- Dong, W.; Liu, H.; Xu, C.; Zuo, Y.; Chen, Z.; Zhou, S. A chloroplast genomic strategy for designing taxon specific DNA mini-barcodes: A case study on ginsengs. BMC Genet. 2014, 15, 138. [Google Scholar] [CrossRef] [Green Version]
- Dubey, B.; Meganathan, P.R.; Haque, I. DNA mini-barcoding: An approach for forensic identification of some endangered Indian snake species. Forensic Sci. Int. Genet. 2011, 5, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Ali, M.E.; Hossain, M.M.; Naquiah, N.; Zaidul, I.S. Universal mini COI barcode for the identification of fish species in processed products. Food Res. Int. 2018, 105, 19–28. [Google Scholar] [CrossRef]
- Wannell, G.J.; Griffiths, A.M.; Spinou, A.; Batista, R.; Mendonça, M.B.; Wosiacki, W.B.; Fraser, B.; Wintner, S.; Papadopoulos, A.I.; Krey, G.; et al. A new minibarcode assay to facilitate species identification from processed, degraded or historic ray (Batoidea) samples. Conserv. Genet. Resour. 2020, 18, 659–668. [Google Scholar] [CrossRef]
- Yang, F.; Ding, F.; Chen, H.; He, M.; Zhu, S.; Ma, X.; Jiang, L.; Li, H. DNA barcoding for the identification and authentication of animal species in traditional medicine. Evid.-Based Complement. Altern. Med. 2018, 2018, 5160254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Armas, L.F. Presencia de Centruroides schmidti Sissom en el sureste de Mexico y descripcion de dos especies nuevas (Scorpiones: Buthidae). Rev. Nicar. Entomol. 1996, 36, 21–33. [Google Scholar]
- Hoffmann, C.C. Monografias para la entomología médica de México. Monografia No. 2, Los escorpiones de México. Segunda parte: Buthidae. An. Inst. Biol. Univ. Nac. Autón. México 1932, 3, 243–361. [Google Scholar]
- Martín-Frías, E.; de Armas, L.F.; Paniagua-Solís, J.F. Redescription of the Mexican scorpion Centruroides hoffmanni Armas, 1996 (Scorpiones: Buthidae). Euscorpius 2005, 2005, 1–7. [Google Scholar] [CrossRef]
- Sissom, W.D. Redescription of the scorpion Centruroides thorelli Kraepelin (Buthidae) and description of two new species. J. Arachnol. 1995, 23, 91–99. [Google Scholar]
- Ponce-Saavedra, J.; Moreno-Barajas, R.J. El género Centruroides Marx 1890 (Scorpiones: Buthidae) en México. Biológicas 2005, 7, 42–51. [Google Scholar]
- Esposito, L.A.; Prendini, L. Island ancestors and New World biogeography: A case study from the scorpions (Buthidae: Centruroidinae). Sci. Rep. 2019, 9, 3500. [Google Scholar] [CrossRef] [Green Version]
- Esposito, L. Systematics and Biogeography of the New World Scorpion Genus Centruroides Marx, 1890 (Scorpiones: Buthidae). Ph.D. Dissertation, City University of New York, New York, NY, USA, 2011. [Google Scholar]
- Goodman, A.; Prendini, L.; Francke, O.F.; Esposito, L.A. Systematic revision of the arboreal Neotropical “thorellii” clade of Centruroides Marx, 1890, bark scorpions (Buthidae C.L. Koch, 1837) with descriptions of six new species. Bull. Am. Mus. Nat. Hist. 2021, 452, 1–92. [Google Scholar]
- Folmer, R.H.; Nilges, M.; Folkers, P.J.; Konings, R.N.; Hilbers, C.W. A model of the complex between single-stranded DNA and the single-stranded DNA binding protein encoded by gene V of filamentous bacteriophage M13. J. Mol. Biol. 1994, 240, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Arango, C.P.; Wheeler, W.C. Phylogeny of the sea spiders (Arthropoda, Pycnogonida) based on direct optimization of six loci and morphology. Cladistics 2007, 23, 255–293. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Thierer, T. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. J. Bioinform. 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Esposito, L.A.; Yamaguti, H.Y.; Pinto-da-Rocha, R.; Prendini, L. Plucking with the plectrum: Phylogeny of the New World buthid scorpion subfamily Centruroidinae Kraus, 1955 (Scorpiones: Buthidae) reveals evolution of three pecten-sternite stridulation organs. Arthropod Syst. Phylogeny 2018, 76, 87–122. [Google Scholar]
- Hjelle, J.T. Anatomy and morphology. In The Biology of Scorpions; Polis, G.A., Ed.; Stanford University Press: Stanford, CA, USA, 1990; pp. 9–63. [Google Scholar]
- Sissom, W.D. Systematics, biogeography and paleontology. In The Biology of Scorpions; Polis, G.A., Ed.; Stanford University Press: Stanford, CA, USA, 1990; pp. 64–160. [Google Scholar]
- Vachon, M. Études sur les Scorpions; Institut Pasteur d’Algérie: Algiers, Algeria, 1952; 482p. [Google Scholar]
- Vachon, M. Étude des caractères utilisés pour classer les familles et les genres de scorpions (Arachnides). 1. La trichobothriotaxie en arachnologie. Sigles trichobothriaux et types de trichobothriotaxie chez les scorpions. Bull. Mus. Hist. Nat. Paris 1974, 140, 857–958. [Google Scholar]
- Prendini, L. Phylogeny and classification of the superfamily Scorpionoidea Latreille 1802 (Chelicerata, Scorpiones): An exemplar approach. Cladistics 2000, 16, 1–78. [Google Scholar] [CrossRef]
- Kamenz, C.; Prendini, L. An atlas of book lung fine structure in the order Scorpiones (Arachnida). Bull. Am. Mus. Nat. Hist. 2008, 316, 1–359. [Google Scholar] [CrossRef]
- Volschenk, E.S.; Mattoni, C.I.; Prendini, L. Comparative anatomy of the mesosomal organs of scorpions (Chelicerata, Scorpiones), with implications for the phylogeny of the order. Zool. J. Linn. Soc. 2008, 154, 651–675. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Thompson, J.D. Clustal W and Clustal X version 2.0. J. Bioinform. 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids. Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maddison, W.P.; Maddison, D.D. Mesquite: A Modular System for Evolutionary Analysis. Version 3.4. Available online: https://www.mesquiteproject.org (accessed on 20 June 2020).
- Ronquist, F.; Huelsenbeck, J.P. MrBayes, 3, Bayesian phylogenetic inference under mixed models. J. Bioinform. 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, A. RAxML version, 8, A tool for phylogenetic analysis and post-analysis of large phylogenies. J. Bioinform. 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder, 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, P.O. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst. Biol. 2001, 50, 913–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfaro, M.E.; Zoller, S.; Lutzoni, F. Bayes or bootstrap? A simulation study comparing the performance of Bayesian Markov Chain Monte Carlo sampling and bootstrapping in assessing phylogenetic confidence. Mol. Biol. Evol. 2003, 20, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Masters, B.C.; Fan, V.; Ross, H.A. Species delimitation—A Geneious plugin for the exploration of species boundaries. Mol. Ecol. Resour. 2011, 11, 154–157. [Google Scholar] [CrossRef]
- Rosenberg, N.A. Statistical tests for taxonomic distinctiveness from observations of monophyly. J. Evol. 2007, 61, 317–323. [Google Scholar] [CrossRef]
- Moritz, C.; Cicero, C.; Godfray, C. DNA barcoding: Promise and pit-falls. PLoS Biol. 2004, 2, e354. [Google Scholar] [CrossRef] [Green Version]
- Yeo, D.; Srivathsan, A.; Meier, R. Longer is not always better: Optimizing barcode length for large-scale species discovery and identification. Syst Biol. 2020, 69, 999–1015. [Google Scholar] [CrossRef] [PubMed]
- Larena, I.; Salazar, O.; Gonzalez, V.; Julian, M.C.; Rubio, V. Design of a primer for ribosomal DNA internal transcribed spacer with enhanced specificity for ascomycetes. J. Biotechnol. 1999, 75, 187–194. [Google Scholar] [CrossRef]
- Colgan, D.J.; McLauchlan, A.; Wilson, G.D.F.; Livingston, S.P.; Edgecombe, G.D.; Macaranas, J.; Cassis, G.; Gray, M.R. Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution. Aust. J. Zool. 1998, 46, 419–437. [Google Scholar] [CrossRef]
- Johnson, N.K.; Cicero, C. New mitochondrial DNA data affirm the importance of Pleistocene speciation in North American birds. Evolution 2004, 58, 1122–1130. [Google Scholar] [CrossRef]
- Rodrigo, A.; Bertels, F.; Heled, J.; Noder, R.; Shearman, H.; Tsai, P. The perils of plenty: What are we going to do with all these genes? Philos. Trans. R. Soc. B 2008, 363, 3893–3902. [Google Scholar] [CrossRef] [Green Version]
- Goodman, A.; Esposito, L. Niche partitioning in congeneric scorpions. Invertebr. Biol. 2020, 139, e12280. [Google Scholar] [CrossRef]
- Johnson, J.D.; Mata-Silva, V.; García-Padilla, E.; Wilson, L.D. The herpetofauna of Chiapas, Mexico: Composition, physiographic distribution, and conservation status. Mesoam. Herpetol. 2015, 2, 272–329. [Google Scholar]
- Towler, W.I. Phylogenetic Structure of Two Central Mexican Centruroides Species Complexes. Master’s Thesis, Marshall University, Huntington, WV, USA, 2002. [Google Scholar]
- Ponce Saavedra, J.; Francke, O.F. Una nueva especie de alacrán del género Centruroides Marx 1890 (Scorpiones, Buthidae) de la depresión del Balsas, México. Acta Zool. Mex. 2004, 20, 221–232. [Google Scholar]
- Saavedra, J.P.; Sissom, W.D. A new species of the genus Vaejovis (Scorpiones, Vaejovidae) endemic to the Balsas basin of Michoacán, Mexico. J. Arachnol. 2004, 32, 539–544. [Google Scholar] [CrossRef]
- Mendoza, J.; Francke, O.F. Systematic revision of Brachypelma red-kneed tarantulas (Araneae: Theraphosidae), and the use of DNA barcodes to assist in the identification and conservation of CITES-listed species. Invert. Syst. 2017, 31, 157–179. [Google Scholar] [CrossRef]
- Meyer, S.E.; García-Moya, E.; Lagunes-Espinoza, L.D.C. Topographic and soil surface effects on gypsophile plant community patterns in central Mexico. J. Veg. Sci. 1992, 3, 429–438. [Google Scholar] [CrossRef]
- Ødegaard, F. How many species of arthropods? Erwin’s estimate revised. Biol. J. Linn. Soc. 2000, 71, 583–597. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goodman, A.M.; Prendini, L.; Esposito, L.A. Systematics of the Arboreal Neotropical ‘thorellii’ Clade of Centruroides Bark Scorpions (Buthidae) and the Efficacy of Mini-Barcodes for Museum Specimens. Diversity 2021, 13, 441. https://doi.org/10.3390/d13090441
Goodman AM, Prendini L, Esposito LA. Systematics of the Arboreal Neotropical ‘thorellii’ Clade of Centruroides Bark Scorpions (Buthidae) and the Efficacy of Mini-Barcodes for Museum Specimens. Diversity. 2021; 13(9):441. https://doi.org/10.3390/d13090441
Chicago/Turabian StyleGoodman, Aaron M., Lorenzo Prendini, and Lauren A. Esposito. 2021. "Systematics of the Arboreal Neotropical ‘thorellii’ Clade of Centruroides Bark Scorpions (Buthidae) and the Efficacy of Mini-Barcodes for Museum Specimens" Diversity 13, no. 9: 441. https://doi.org/10.3390/d13090441
APA StyleGoodman, A. M., Prendini, L., & Esposito, L. A. (2021). Systematics of the Arboreal Neotropical ‘thorellii’ Clade of Centruroides Bark Scorpions (Buthidae) and the Efficacy of Mini-Barcodes for Museum Specimens. Diversity, 13(9), 441. https://doi.org/10.3390/d13090441








