The Effect of Cutting and Waterlogging on Plant-Related CO2 and N2O Fluxes Associated with the Invasive N-Fixing Species Gunnera tinctoria
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Determination of Greenhouse Gas Fluxes
2.3. CO2 and N2O Flux Calculations
2.4. Environmental Variables
2.5. Plant Parameters
2.6. Statistical Analysis
3. Results
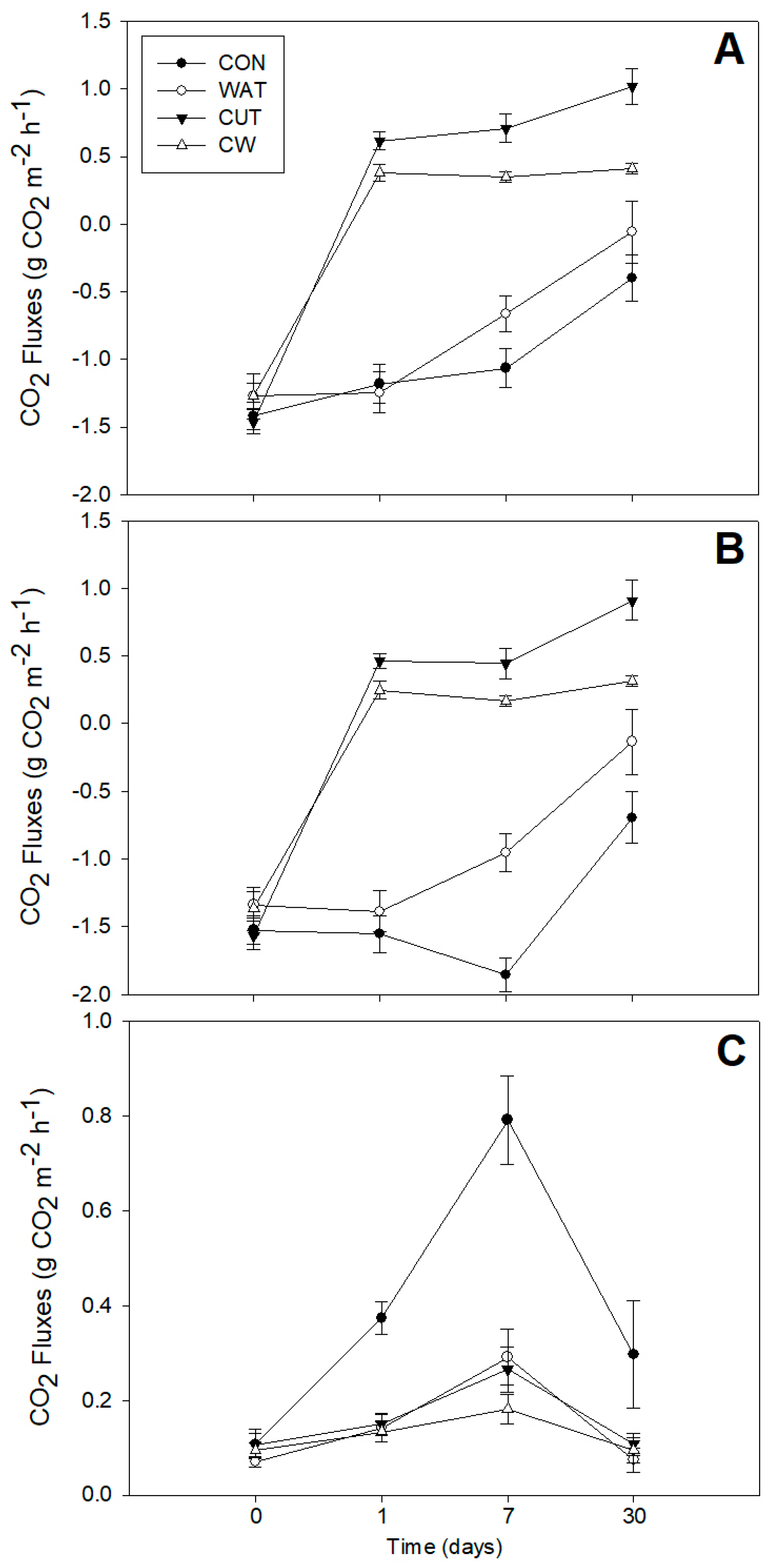
3.1. CO2 Fluxes
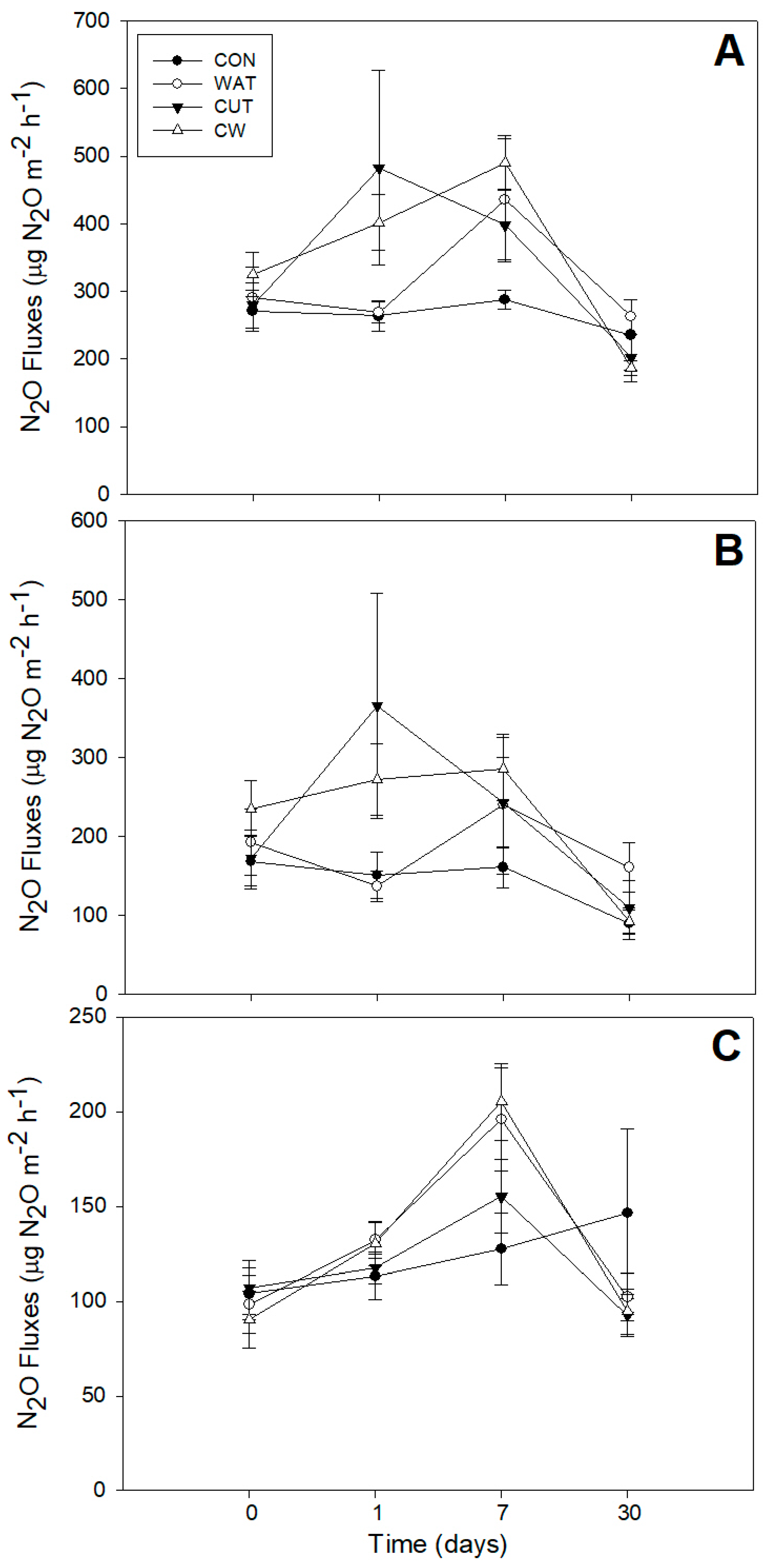
3.2. N2O Fluxes
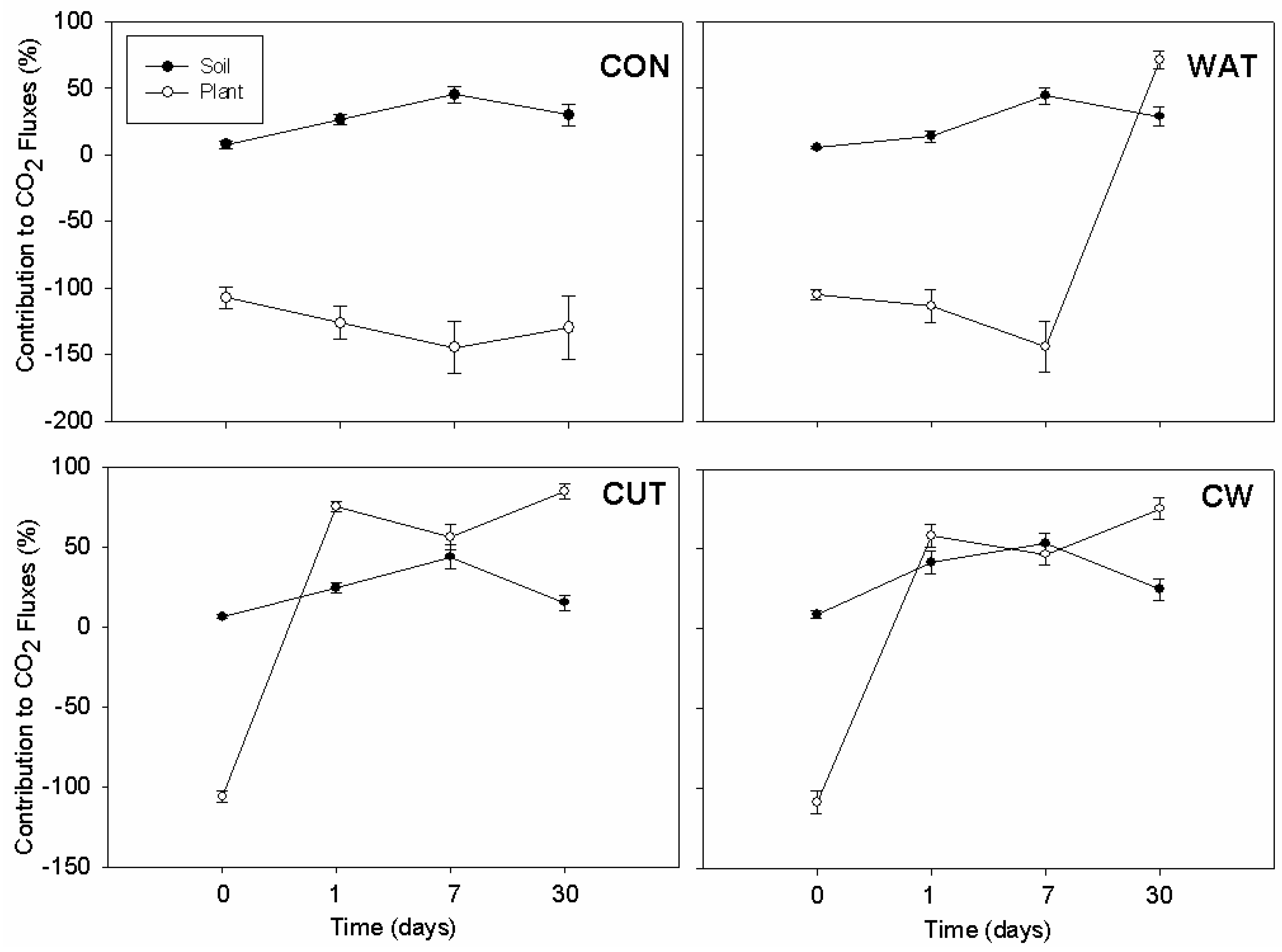
3.3. Plant Data and Correlations with CO2 and N2O Fluxes
4. Discussion
4.1. CO2 Fluxes
4.2. N2O Fluxes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woodward, F.I.; Quaife, T.; Lomas, M.R. Changing climate and the Irish landscape. In Biology and Environment: Proceedings of the Royal Irish Academy; Royal Irish Academy: Dublin, Ireland, 2010; Volume 110B, pp. 1–16. [Google Scholar]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 2003, 6, 503–523. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Ecosystem consequences of biological invasions. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef]
- Aerts, R.; Ewald, M.; Nicolas, M.; Piat, J.; Skowronek, S.; Lenoir, J.; Hattab, T.; Garzón-López, C.X.; Feilhauer, H.; Schmidtlein, S.; et al. Invasion by the alien tree Prunus serotina alters ecosystem functions in a temperate deciduous forest. Front. Plant Sci. 2017, 8, 179. [Google Scholar] [CrossRef]
- Tamura, M.; Tharayil, N. Plant litter chemistry and microbial priming regulate the accrual, composition and stability of soil carbon in invaded ecosystems. New Phytol. 2014, 203, 110–124. [Google Scholar] [CrossRef]
- Qiu, J. A global synthesis of the effects of biological invasions on greenhouse gas emissions. Glob. Ecol. Biogeogr. 2015, 24, 1351–1362. [Google Scholar] [CrossRef]
- Mantoani, M.C.; Osborne, B.A. Alien plant introductions and greenhouse gas emissions: Insights from Gunnera tinctoria invasions. Sci. Total Environ. 2021, 775, 145861. [Google Scholar] [CrossRef] [PubMed]
- Kao-Kniffin, J.; Zhu, B. A Microbial Link between Elevated CO2 and Methane Emissions that is Plant Species-Specific. Microb. Ecol. 2013, 66, 621–629. [Google Scholar] [CrossRef]
- Penton, C.R.; Deenik, J.L.; Popp, B.N.; Bruland, G.L.; Engstrom, P.; Louis, D.S.; Tiedje, J. Importance of sub-surface rhizosphere-mediated coupled nitrification-denitrification in a flooded agroecosystem in Hawaii. Soil Biol. Biochem. 2013, 57, 362–373. [Google Scholar] [CrossRef]
- Brix, H.; Sorrell, B.K.; Schierup, H. Gas fluxes achieved by in situ convective flow in Phragmites australis. Aquat. Bot. 1996, 54, 151–163. [Google Scholar] [CrossRef]
- Joabsson, A.; Christensen, T.R.; Wallén, B. Vascular plant controls on methane emissions from northern peat forming wetlands. Trends Ecol. Evol. 1999, 14, 385–388. [Google Scholar] [CrossRef]
- Baruah, K.K.; Gogoi, B.; Gogoi, P. Plant physiological and soil characteristics associated with methane and nitrous oxide emission from rice paddy. Physiol. Mol. Biol. Plants 2010, 16, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Dingemans, B.J.J.; Bakker, E.S.; Bodelier, P.L.E. Aquatic herbivores facilitate the emission of methane from wetlands. Ecology 2011, 92, 1166–1173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhullar, G.S.; Iravani, M.; Edwards, P.J.; Venterink, H.O. Methane transport and emissions from soil as affected by water table and vascular plants. BMC Ecol. 2013, 13, 32. [Google Scholar] [CrossRef]
- Jørgensen, C.J.; Struwe, S.; Elberling, B. Temporal trends in N2O flux dynamics in a Danish wetland—Effects of plant-mediated gas transport of N2O and O2 following changes in water level and soil mineral N availability. Glob. Chang. Biol. 2012, 18, 210–222. [Google Scholar] [CrossRef]
- Pangala, S.R.; Gowing, D.J.; Hornibrook, E.R.C.; Gauci, V. Controls on methane emissions from Alnus glutinosa saplings. New Phytol. 2014, 201, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Machacova, K.; Bäck, J.; Vanhatalo, A.; Halmeenmäki, E.; Kolari, P.; Mammarella, I.; Pumpanen, J.; Acosta, M.; Urban, O.; Pihlatie, M. Pinus sylvestris as a missing source of nitrous oxide and methane in boreal forest. Sci. Rep. 2016, 6, 23410. [Google Scholar] [CrossRef] [PubMed]
- Schindler, T.; Mander, Ü.; Machacova, K.; Espenberg, M.; Krasnov, D.; Escuer-Gatius, J.; Veber, G.; Pärn, J.; Soosaar, K. Short-term flooding increases CH4 and N2O emissions from trees in a riparian forest soil-stem continuum. Sci. Rep. 2020, 10, 3204. [Google Scholar] [CrossRef]
- Carmichael, M.J.; Bernhardt, E.S.; Bräuer, S.L.; Smith, W.K. The role of vegetation in methane flux to the atmosphere: Should vegetation be included as a distinct category in the global methane budget? Biogeochemistry 2014, 119, 1–24. [Google Scholar] [CrossRef]
- Gebremichael, A.W.; Osborne, B.; Orr, P. Flooding-related increases in CO2 and N2O emissions from a temperate coastal grassland ecosystem. Biogeosciences 2017, 14, 2611–2626. [Google Scholar] [CrossRef]
- Jørgensen, C.J.; Elberling, B. Effects of flooding-induced N2O production, consumption and emission dynamics on the annual N2O emission budget in wetland soil. Soil Biol. Biochem. 2012, 53, 9–17. [Google Scholar] [CrossRef]
- Girkin, N.T.; Turner, B.L.; Ostle, N.; Craigona, J.; Sjögersten, S. Root exudate analogues accelerate CO2 and CH4 production in tropical peat. Soil Biol. Biochem. 2018, 117, 48–55. [Google Scholar] [CrossRef]
- Koop-Jakobsen, K.; Meier, R.J.; Mueller, P. Plant-mediated rhizosphere oxygenation in the native invasive salt marsh grass Elymus athericus. Front. Plant Sci. 2021, 12, 669751. [Google Scholar] [CrossRef]
- Kelker, D.; Chanton, J. The effect of clipping on methane emissions from Carex. Biogeochemistry 1997, 39, 37–44. [Google Scholar] [CrossRef]
- Mueller, P.; Granse, D.; Nolte, S.; Do, H.T.; Weingartner, M.; Hoth, S.; Jensen, K. Top-down control of carbon sequestration: Grazing affects microbial structure and function in salt marsh soils. Ecol. Appl. 2017, 27, 1435–1450. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Hou, L.; Yang, L.; Wu, D.; Zhang, L.; Li, L. Effects of grazing on CO2, CH4, and N2O fluxes in three temperate steppe ecosystems. Ecosphere 2017, 8, e01760. [Google Scholar] [CrossRef]
- Tong, C.; Wang, W.; Huang, J.; Gauci, V.; Zhang, L.; Zeng, C. Invasive alien plants increase CH4 emissions from a subtropical tidal estuarine wetland. Biogeochemistry 2012, 111, 677–693. [Google Scholar] [CrossRef]
- Wilgen, B.W.; van Khan, A.; Marais, C. Changing perspectives on managing biological invasions: Insights from South Africa and the working for Water Programme. In Fifty Years of Invasion Ecology: The Legacy of Charles Elton; Richardson, D.M., Ed.; Wiley-Blackwell Publishing: Hoboken, NJ, USA, 2011; pp. 377–394. [Google Scholar]
- Zavaleta, E.S.; Hobbs, R.J.; Mooney, H.A. Viewing invasive species removal in a whole-ecosystem context. Trends Ecol. Evol. 2001, 16, 454–459. [Google Scholar] [CrossRef]
- Gioria, M.; Osborne, B. Biological flora of the British Isles: Gunnera tinctoria. J. Ecol. 2013, 101, 243–264. [Google Scholar] [CrossRef]
- Timilsina, A.; Zhang, C.; Pandey, B.; Bizimana, F.; Dong, W.; Hu, C. Potential pathway of nitrous oxide formation in plants. Front. Plant Sci. 2020, 11, 1177. [Google Scholar] [CrossRef] [PubMed]
- Osborne, B.A.; Doris, F.; Cullen, A.; McDonald, R.; Campbell, G.; Steer, M. Gunnera tinctoria: An unusual nitrogen-fixing invader. BioScience 1991, 41, 224–234. [Google Scholar] [CrossRef]
- Osborne, B.A.; Sprent, J.I. Ecology of the Nostoc-Gunnera symbiosis. In Cyanobacteria in Symbiosis; Rai, A.N., Bergman, B., Rasmussen, U., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 233–251. [Google Scholar]
- Mantoani, M.C.; González, A.B.; Sancho, L.G.; Osborne, B.A. Growth, phenology and N-utilization by invasive populations of Gunnera tinctoria. J. Plant Ecol. 2020, 13, 589–600. [Google Scholar] [CrossRef]
- Carter, M.S.; Ambus, P. Biologically fixed N2 as a source for N2O productionin a grass-clover mixture, measured by 15N2. Nutr. Cycl. Agroecosystems 2006, 74, 13–26. [Google Scholar] [CrossRef]
- Easlon, H.M.; Bloom, A.J. Easy Leaf Area: Automated digital image analysis for rapid and accurate measurement of leaf area. Appl. Plant Sci. 2014, 2, 1400033. [Google Scholar] [CrossRef]
- IBM® SPSS® Statistics for Windows; v. 24; IBM Corp.: Armonk, NY, USA, 2016.
- Tang, J.; Baldocchi, D.D.; Xu, L. Tree photosynthesis modulates soil respiration on a diurnal time scale. Glob. Chang. Biol. 2005, 11, 1298–1304. [Google Scholar] [CrossRef]
- Xuma, T.; Naidoo, G. Responses of an ethnobotanically important wetland species, Gunnera perpensa L. to soil waterlogging. Wetlands 2007, 27, 928–935. [Google Scholar] [CrossRef]
- Weathers, P.J.; Niedzielski, J.J. Nitrous oxide production by cyanobacteria. Arch. Microbiol. 1986, 146, 204–206. [Google Scholar] [CrossRef]
- Henry, S.; Texier, S.; Hallet, S.; Bru, D.; Dambreville, C.; Chèneby, D.; Bizouard, F.; Germon, J.C.; Philippot, L. Disentangling the rhizosphere effect on nitrate reducers and denitrifiers: Insight into the role of root exudates. Environ. Microbiol. 2008, 10, 3082–3092. [Google Scholar] [CrossRef]
- Le Roux, X.; Bardy, M.; Loiseau, P.; Louault, F. Stimulation of soil nitrification and denitrification by grazing in grasslands: Do changes in plant species composition matter? Oecologia 2003, 137, 417–425. [Google Scholar] [CrossRef]
- Norton, U.R.S.Z.U.L.A.; Mosier, A.R.; Morgan, J.A.; Derner, J.D.; Ingram, L.J.; Stahl, P.D. Moisture pulses, trace gas emissions and soil C and N in cheatgrass and native grass-dominated sagebrush-steppe in Wyoming, USA. Soil Biol. Biochem. 2008, 40, 1421–1431. [Google Scholar] [CrossRef]
- Drew, M.C. Oxygen deficiency and root metabolism: Injury and acclimation under hypoxia and anoxia. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Visser, E.J.W.; Voesenek, L.A.C.J.; Vartapetian, B.B.; Jackson, M.B. Flooding and plant growth. Ann. Bot. 2003, 91, 107–109. [Google Scholar] [CrossRef]
- Osborne, B.A.; Cullen, A.; Jones, P.W.; Campbell, G.J. Use of nitrogen by the Nostoc—Gunnera tinctoria (Molina) Mirbel symbiosis. New Phytol. 1992, 120, 481–487. [Google Scholar] [CrossRef]
- Mantoani, M.C. Gunnera tinctoria Invasions: Phenology, Ecosystem Impacts and Environmental Interactions. Ph.D. Thesis, University College Dublin, Dublin, Ireland, 2019. [Google Scholar]

| Time (Days) | CO2 Fluxes | N2O Fluxes | r2 | p |
|---|---|---|---|---|
| 0 | y = 1.001x − 0.094 | 0.965 | *** | |
| y = 0.96x − 88.27 | 0.850 | *** | ||
| 1 | y = 1.047x − 0.184 | 0.983 | *** | |
| y = 1.04x − 124.43 | 0.983 | *** | ||
| 7 | y = 1.17x − 0.354 | 0.917 | *** | |
| y = 0.904x − 132.02 | 0.851 | *** | ||
| 30 | y = 1.052x − 0.156 | 0.929 | *** | |
| y = 0.955x − 88.20 # | 0.837 | *** |
| Time (Days) | Treatment | Variable | Total CO2 | Plant-Mediated CO2 | Total N2O | Plant-Mediated N2O | r2 | p |
|---|---|---|---|---|---|---|---|---|
| 1 | WAT | Petiole Area | y = 1090x + 187 | 0.35 | * | |||
| y = 1357x + 34.88 | 0.38 | * | ||||||
| Rhizome + Petiole Area | y = 981x + 164 | 0.36 | * | |||||
| y = 1341x − 5.83 | 0.47 | * | ||||||
| CUT | Petiole Area | y = 6.08x + 0.148 | 0.58 | ** | ||||
| y = 5.23x + 0.064 | 0.57 | ** | ||||||
| y = 13,573x − 558 | 0.59 | ** | ||||||
| y = 13,376x − 660 | 0.57 | ** | ||||||
| Rhizome Area | y = 20.14x − 0.069 | 0.50 | ** | |||||
| Rhizome + Petiole Area | y = 5.53x + 0.003 | 0.66 | ** | |||||
| y = 4.52x − 0.035 | 0.59 | ** | ||||||
| y = 10,940x − 727 | 0.53 | ** | ||||||
| y = 10,665x − 814 | 0.51 | ** | ||||||
| 7 | WAT | Rhizome Area | y = 18,914x − 350 | 0.35 | * | |||
| CUT | Petiole Area | y = 7.44x + 0.133 | 0.41 | * | ||||
| y = 7.55x − 0.141 | 0.36 | * | ||||||
| CW | Rhizome + Petiole Area | y = 2.001x + 0.108 | 0.35 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantoani, M.C.; Osborne, B.A. The Effect of Cutting and Waterlogging on Plant-Related CO2 and N2O Fluxes Associated with the Invasive N-Fixing Species Gunnera tinctoria. Diversity 2021, 13, 427. https://doi.org/10.3390/d13090427
Mantoani MC, Osborne BA. The Effect of Cutting and Waterlogging on Plant-Related CO2 and N2O Fluxes Associated with the Invasive N-Fixing Species Gunnera tinctoria. Diversity. 2021; 13(9):427. https://doi.org/10.3390/d13090427
Chicago/Turabian StyleMantoani, Mauricio C., and Bruce A. Osborne. 2021. "The Effect of Cutting and Waterlogging on Plant-Related CO2 and N2O Fluxes Associated with the Invasive N-Fixing Species Gunnera tinctoria" Diversity 13, no. 9: 427. https://doi.org/10.3390/d13090427
APA StyleMantoani, M. C., & Osborne, B. A. (2021). The Effect of Cutting and Waterlogging on Plant-Related CO2 and N2O Fluxes Associated with the Invasive N-Fixing Species Gunnera tinctoria. Diversity, 13(9), 427. https://doi.org/10.3390/d13090427







