Consumption Patterns of a Generalist Omnivore: Eastern Box Turtle Diets in the Long Island Pine Barrens
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freeland, W.J.; Janzen, D.H. Strategies in herbivory by mammals: The role of plant secondary compounds. Am. Nat. 1974, 108, 269–289. [Google Scholar] [CrossRef]
- Pulliam, H.R. On the theory of optimal diets. Am. Nat. 1974, 108, 59–74. [Google Scholar] [CrossRef]
- Huey, R.B.; Hertz, P.E. Is a jack-of-all-temperatures a master of none? Evolution 1984, 382, 441–444. [Google Scholar]
- Belovsky, G.E. Optimal foraging and community structure: Implications for a guild of generalist grassland herbivores. Oecologia 1986, 70, 35–52. [Google Scholar] [CrossRef]
- Abrams, P.A. Adaptive responses of generalist herbivores to competition: Convergence or divergence. Evol. Ecol. 1990, 4, 103–114. [Google Scholar] [CrossRef]
- Hailey, A.; Chidavaenzi, R.L.; Loveridge, J.P. Diet mixing in the omnivorous tortoise Kinixys spekii. Funct. Ecol. 1998, 1, 373–385. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Whalen, M.A.; Davenport, T.M.; Stone, J.P.; Duffy, J.E. Physiological effects of diet mixing on consumer fitness: A meta-analysis. Ecology 2013, 94, 565–572. [Google Scholar] [CrossRef]
- Groendahl, S.; Fink, P. The effect of diet mixing on a nonselective herbivore. PLoS ONE 2016, 11, e0158924. [Google Scholar] [CrossRef]
- Bjorndal, K.A. Diet mixing: Nonadditive interactions of diet items in an omnivorous freshwater turtle. Ecology 1991, 72, 1234–1241. [Google Scholar] [CrossRef]
- Saether, B.E. The impact of different growth pattern on the utilization of tree species by a generalist herbivore, the moose Alces alces: Implications of optimal foraging theory. In Behavioural Mechanisms of Food Selection; Hughes, R.N., Ed.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 323–341. [Google Scholar]
- Bowen, W.D.; Tully, D.; Boness, D.J.; Bulheier, B.M.; Marshall, G.J. Prey-dependent foraging tactics and prey profitability in a marine mammal. Mar. Ecol. Prog. Ser. 2002, 244, 235–245. [Google Scholar] [CrossRef]
- Vorel, A.; Válková, L.; Hamšíková, L.; Maloň, J.; Korbelová, J. Beaver foraging behaviour: Seasonal foraging specialization by a choosy generalist herbivore. Behav. Ecol. Sociobiol. 2015, 69, 1221–1235. [Google Scholar] [CrossRef]
- McKnight, S.K.; Hepp, G.R. Foraging-niche dynamics of gadwalls and American coots in winter. Auk 1998, 115, 670–683. [Google Scholar] [CrossRef]
- Clarke, J.A.; Johnson, R.E. Comparisons and contrasts between the foraging behaviors of two white-tailed ptarmigan (Lagopus leucurus) populations, Rocky Mountains, Colorado, and Sierra Nevada, California, USA. Arct. Antarct. Alp. Res. 2005, 37, 171–176. [Google Scholar] [CrossRef]
- Ríos, J.M.; Mangione, A.M.; Marone, L. Tolerance to dietary phenolics and diet breadth in three seed-eating birds: Implications for graminivory. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2012, 317, 425–433. [Google Scholar] [CrossRef]
- Dearing, M.D.; Schall, J.J. Testing models of optimal diet assembly by the generalist herbivorous lizard Cnemidophorus murinus. Ecology 1992, 73, 845–858. [Google Scholar] [CrossRef]
- van Marken Lichtenbelt, W.D. Optimal foraging of a herbivorous lizard, the green iguana in a seasonal environment. Oecologia 1993, 95, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Tilman, D. Predictions of species interactions from consumer-resource theory: Experimental tests with grasshoppers and plants. Oecologia 1993, 94, 516–527. [Google Scholar] [CrossRef]
- Pennings, S.C.; Nadeau, M.T.; Paul, V.J. Selectivity and growth of the generalist herbivore Dolabella auricularia feeding upon complementary resources. Ecology 1993, 74, 879–890. [Google Scholar] [CrossRef]
- Pennings, S.C.; Carefoot, T.H.; Siska, E.L.; Chase, M.E.; Page, T.A. Feeding preferences of a generalist salt-marsh crab: Relative importance of multiple plant traits. Ecology 1998, 79, 1968–1979. [Google Scholar] [CrossRef]
- Meek, R. Nutritional selection in Hermann’s tortoise, Testudo hermanni, in Montenegro and Croatia. Testudo 2010, 7, 88–95. [Google Scholar]
- Surface, H.A. First Report on the Economic Features of Turtles of Pennsylvania; Harrisburg Publishing Co.: Harrisburg, Pennsylvania, USA, 1908. [Google Scholar]
- Barbour, R.W. The reptiles of big black mountain, Harlan County, Kentucky. Copeia 1950, 1950, 100–107. [Google Scholar] [CrossRef]
- Bush, F.M. Foods of some Kentucky herptiles. Herpetologica 1959, 15, 73–77. [Google Scholar]
- Klimstra, W.D.; Newsome, F. Some observations on the food coactions of the common box turtle, Terrapene c. carolina. Ecology 1960, 41, 639–647. [Google Scholar] [CrossRef]
- Strang, C.A. Spatial and temporal activity patterns in two terrestrial turtles. J. Herpetol. 1983, 17, 43–47. [Google Scholar] [CrossRef]
- Stuart, M.D.; Miller, G.C. The eastern box turtle, Terrapene c. carolina (Testudines, Emydidae), in North Carolina. Brimleyana 1987, 13, 123–131. [Google Scholar]
- Dodd, K.C., Jr. North American Box Turtles: A Natural History; University of Oklahoma Press: Norman, OK, USA, 2002; Volume 6. [Google Scholar]
- Ernst, C.H.; Lovich, J.E. Turtles of the United States and Canada; The John Hopkins University Press: Baltimore, MD, USA, 2009. [Google Scholar]
- Anton, T.G. Predation on the house sparrow, Passer domesticus, by the Gulf Coast box turtle, Terrapene carolina major, under seminatural conditions. Bull. Chic. Herpetol. Soc. 1990, 25, 143–144. [Google Scholar]
- Beyer, N.W.; Connor, E.E.; Gerould, S. Estimates of soil ingestion by wildlife. J. Wildl. Manag. 1994, 58, 375–382. [Google Scholar] [CrossRef]
- Platt, S.G.; Hall, C.; Liu, H.; Borg, C.K. Wet-season food habits and intersexual dietary overlap of Florida box turtles (Terrapene carolina bauri) on National Key Deer Wildlife Refuge, Florida. Southeast. Nat. 2009, 8, 335–346. [Google Scholar] [CrossRef]
- Allard, H.A. The natural history of the box turtle. Sci. Mon. 1935, 41, 325–338. [Google Scholar]
- DeVault, T.L.; Brisbin, I.L., Jr.; Rhodes, O.E., Jr. Factors influencing the acquisition of rodent carrion by vertebrate scavengers and decomposers. Can. J. Zool. 2004, 82, 502–509. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen–Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Edinger, G.J.; Evans, D.J.; Gebauer, S.; Howard, T.G.; Hunt, D.M.; Olivero, A.M. (Eds.) Ecological communities of New York state. In A Revised and Expanded Edition of Carol Reschke’s Ecological Communities of New York State, 2nd ed.; (Draft for Review); New York Natural Heritage Program, New York State Department of Environmental Conservation: Albany, NY, USA, 2002. [Google Scholar]
- Jordan, M.J.; Patterson III, W.A.; Windisch, A.G. Conceptual ecological models for the Long Island pitch pine barrens: Implications for managing rare plant communities. For. Ecol. Manag. 2003, 185, 151–168. [Google Scholar] [CrossRef]
- Martin, A.C.; Barkley, W.D. Seed Identification Manual; The Blackburn Press: Caldwell, NJ, USA, 2000. [Google Scholar]
- Cappers, R.T.J.; Bekker, R.M. A Manual for the Identification of Plant Seeds and Fruits; Barkhuis and University of Groningen: Groningen, The Netherlands, 2014. [Google Scholar]
- Evans, A.V. Beetles of Eastern North America; Princeton University Press: Princeton, NJ, USA, 2014. [Google Scholar]
- United States Department of Agriculture (USDA). The Plants Database, National Plant Data Team, USA. 2018. Available online: http://plants.usda.gov (accessed on 30 April 2018).
- Winemiller, K.O.; Pianka, E.R. Organization in natural assemblages of desert lizards and tropical fishes. Ecol. Monogr. 1990, 60, 27–55. [Google Scholar] [CrossRef]
- de León, L.F.; Podos, J.; Gardezi, T.; Herrel, A.; Hendry, A.P. Darwin’s finches and their diet niches: The sympatric coexistence of imperfect generalists. J. Evol. Biol. 2014, 27, 1093–1104. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Silva, A.D.O.; da Conceicao, B.M.; Faria, R.G. The diet of six species of lizards in an area of Caatinga, Brazil. Herpetol. J. 2017, 27, 151–160. [Google Scholar]
- Erazmus, K.R.; Figueras, M.; Luiselli, L.; Burke, R.L. Do diets vary over large spatial or temporal ranges? A test using inter-annual and inter-population data on Diamondback Terrapins (Malaclemys terrapin) diets. Can. J. Zool. 2019, 97, 251–257. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Hart, E.M.; Ellison, A.M. EcoSimR: Null Model Analysis for Ecological Data, R Package Version 0.1.0. 2015. Available online: http://github.com/gotellilab/EcoSimR (accessed on 4 January 2018). [CrossRef]
- R Foundation for Statistical Computing. A Language and Environment for Statistical Computing. Vienna, Austria. 2016. Available online: http://www.R-project.org (accessed on 11 October 2018).
- RStudio Team. RStudio Team (2020). RStudio: Integrated Development for R. RStudio, PBC, Boston, MA. 2016. Available online: http://www.rstudio.com/ (accessed on 11 October 2018).
- Huey, R.B.; Pianka, E.R. Temporal separation of activity and interspecific dietary overlap. In Lizard Ecology Studies of a Model Organism; Huey, R.B., Pianka, E.R., Schoener, T.W., Eds.; Harvard University Press: Cambridge, MA, USA, 1983; pp. 281–296. [Google Scholar]
- Barwell, L.J.; Isaac, N.J.B.; Kunin, W.E. Measuring β-diversity with species abundance data. J. Anim. Ecol. 2015, 84, 1112–1122. [Google Scholar] [CrossRef]
- Bergman, C.M.; Krebs, C.J. Diet overlap of collared lemmings and tundra voles at Pearce Point, Northwest Territories. Can. J. Zool. 1993, 71, 1703–1709. [Google Scholar] [CrossRef]
- Potier, M.; Menard, F.; Cherel, Y.; Lorrain, A.; Sabatié, R.; Marsac, F. Role of pelagic crustaceans in the diet of the longnose lancetfish Alepisaurus ferox in the Seychelles waters. Afr. J. Mar. Sci. 2007, 29, 113–122. [Google Scholar] [CrossRef]
- Estupiñán-Montaño, C.; Pacheco-Triviño, F.; Cedeño-Figueroa, L.G.; Galván-Magaña, F.; Estupiñán-Ortiz, J.F. Diet of three shark species in the Ecuadorian Pacific, Carcharhinus falciformis, Carcharhinus limbatus and Nasolamia velox. J. Mar. Biol. Assoc. U. K. 2018, 98, 927–935. [Google Scholar] [CrossRef]
- Smith, E.P.; Zaret, T.M. Bias in estimating niche overlap. Ecology 1982, 63, 1248–1253. [Google Scholar] [CrossRef]
- Braun, J.; Brooks, G.R., Jr. Box turtles (Terrapene carolina) as potential agents for seed dispersal. Am. Midl. Nat. 1987, 117, 312–318. [Google Scholar] [CrossRef]
- Budischak, S.A.; Hester, J.M.; Price, S.J.; Dorcas, M.E. Natural history of Terrapene carolina (box turtles) in an urbanized landscape. Southeast. Nat. 2006, 5, 191–204. [Google Scholar] [CrossRef]
- Fields, J.R.; Simpson, T.R.; Manning, R.W.; Rose, F.L. Modifications to the stomach flushing technique for turtles. Herpetol. Rev. 2000, 31, 32. [Google Scholar]
- Bell, R.H.V. A grazing ecosystem in the Serengeti. Sci. Am. 1971, 225, 86–93. [Google Scholar] [CrossRef]
- Geist, V. On the relationship of social evolution and ecology in ungulates. Am. Zool. 1974, 14, 205–220. [Google Scholar] [CrossRef]
- Jarman, P.J. The social organization of antelope in relation to their ecology. Behaviour 1974, 48, 215–266. [Google Scholar] [CrossRef]
- Hailey, A. Digestive efficiency and gut morphology of omnivorous and herbivorous African tortoises. Can. J. Zool. 1997, 75, 787–794. [Google Scholar] [CrossRef]
- Barboza, P.S. Digesta passage and functional anatomy of the digestive tract in the desert tortoise (Xerobates agassizii). J. Comp. Physiol. B. 1995, 165, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.E.; Hume, I.D. Comparative Physiology of the Vertebrate Digestive System; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Stone, M.D.; Moll, D. Diet-dependent differences in digestive efficiency in two sympatric species of box turtles, Terrapene carolina and Terrapene ornata. J. Herpetol. 2006, 40, 364–371. [Google Scholar] [CrossRef]
- Yuan, M.L.; Dean, S.H.; Longo, A.V.; Rothermel, B.B.; Tuberville, T.D.; Zamudio, K.R. Kinship, inbreeding and fine-scale spatial structure influence gut microbiota in a hindgut-fermenting tortoise. Mol. Ecol. 2015, 24, 2521–2536. [Google Scholar] [CrossRef] [PubMed]
- Modica, B.P. Physically Effective Fiber Threshold, Apparent Digestibility, and Novel Fecal Microbiome Identification of the Leopard Tortoise (Stigmochelys pardalis). Master’s Thesis, California Polytechnic State University, San Luis Obispo, CA, USA, 2016. [Google Scholar]
- Abdelrhman, K.F.; Bacci, G.; Mancusi, C.; Mengoni, A.; Serena, F.; Ugolini, A. A first insight into the gut microbiota of the sea turtle Caretta caretta. Front. Microbiol. 2016, 7, 1060. [Google Scholar] [CrossRef] [PubMed]
- Spinks, P.Q.; Thomson, R.C.; McCartney-Melstad, E.; Shaffer, H.B. Phylogeny and temporal diversification of the New World pond turtles (Emydidae). Mol. Phylogenetics Evol. 2016, 103, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Habeck, C.W.; Figueras, M.P.; Deo, J.E.; Burke, R.L. A surfeit of studies: What have we learned from all the box turtle (Terrapene carolina and T. ornata) home range studies? Diversity 2019, 11, 68. [Google Scholar] [CrossRef]
- Stickel, L.F. Home range behavior among box turtles (Terrapene c. carolina) of a bottomland forest in Maryland. J. Herpetol. 1989, 23, 40–44. [Google Scholar] [CrossRef]
- Gould, E. Orientation in box turtles, Terrapene c. carolina (Linnaeus). Biol. Bull. 1957, 112, 336–348. [Google Scholar] [CrossRef]
- DeRosa, C.T.; Taylor, D.H. Homeward orientation mechanisms in three species of turtles (Trionyx spinifer, Chrysemys picta, and Terrapene carolina). Behav. Ecol. Sociobiol. 1980, 7, 15–23. [Google Scholar] [CrossRef]
- Valentini, A.; Miquel, C.; Nawaz, M.A.; Bellemain, E.; Coissac, E.; Pompanon, F.; Gielly, L.; Cruaud, C.; Nascetti, G.; Wincker, P.; et al. New perspectives in diet analysis based on DNA barcoding and parallel pyrosequencing: The trnL approach. Mol. Ecol. Resour. 2009, 9, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Valentini, A.; Pompanon, F.; Taberlet, P. DNA barcoding for ecologists. Trends Ecol. Evol. 2009, 24, 110–117. [Google Scholar] [CrossRef]
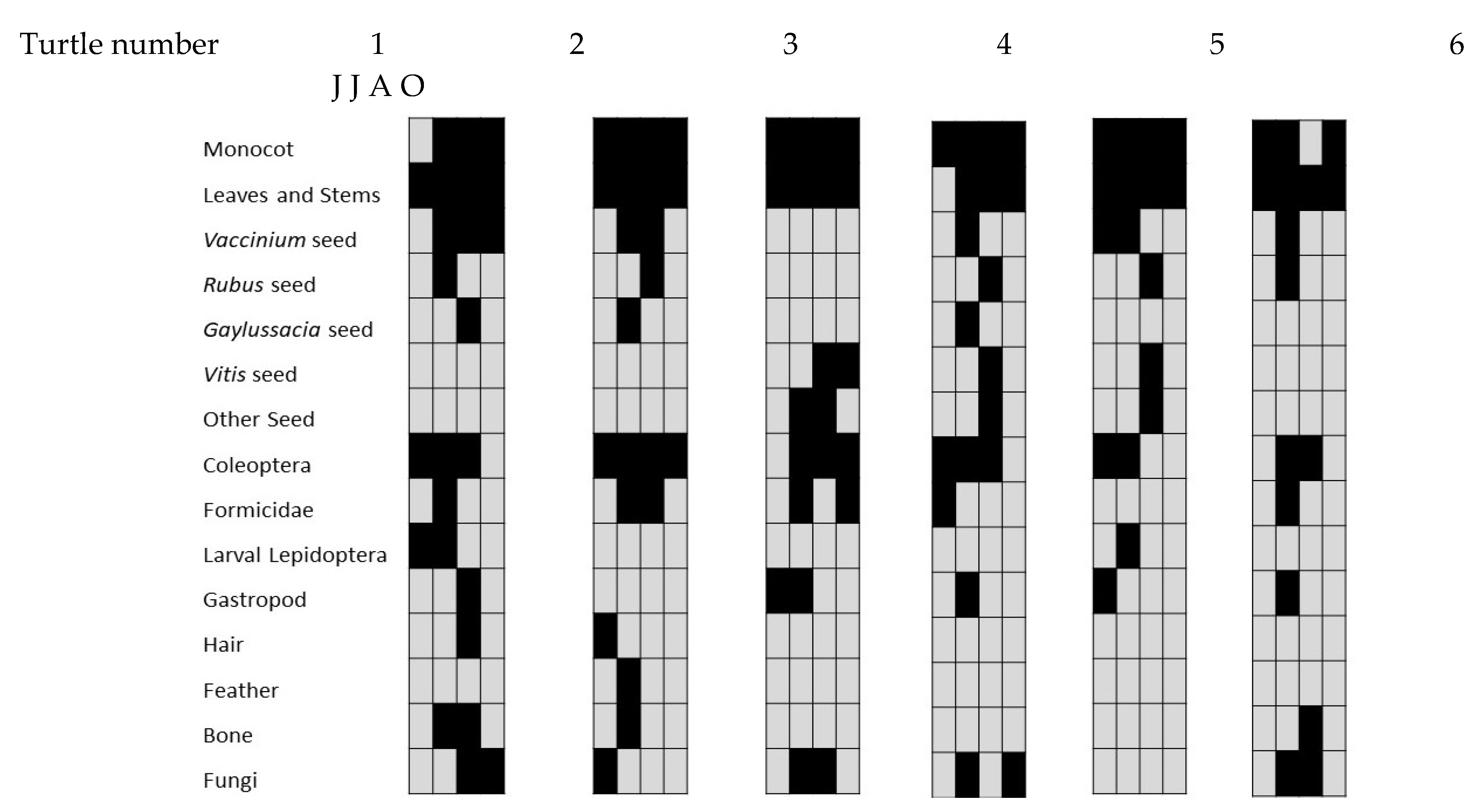
| 15 June | 15 July | 16 August | 16 October | Total (62) | ||
|---|---|---|---|---|---|---|
| Monocots | 80.00 | 80.00 | 87.50 | 68.75 | 79.03 | |
| Dicots | 86.67 | 100.00 | 100.00 | 100.00 | 96.77 | |
| Leaves and stems | 86.67 | 100.00 | 100.00 | 100.00 | 96.77 | |
| Vaccinium seeds | 26.67 | 80.00 | 43.75 | 6.25 | 38.71 | |
| Rubus seeds | 0.00 | 40.00 | 37.50 | 0.00 | 19.35 | |
| Gaylussacia seeds | 0.00 | 33.33 | 37.50 | 0.00 | 17.74 | |
| Vitis seeds | 0.00 | 0.00 | 37.50 | 12.50 | 12.90 | |
| Other seeds | 0.00 | 6.67 | 31.25 | 18.75 | 14.52 | |
| Invertebrates | 93.33 | 93.33 | 93.75 | 81.25 | 90.32 | |
| Coleoptera | 73.33 | 73.33 | 81.25 | 25.00 | 62.90 | |
| Formicidae | 20.00 | 33.33 | 25.00 | 18.75 | 24.19 | |
| Larval Lepidoptera | 33.33 | 40.00 | 6.25 | 6.25 | 20.97 | |
| Gastropods | 20.00 | 20.00 | 12.50 | 6.25 | 14.52 | |
| Vertebrates | 40.00 | 26.67 | 31.25 | 6.25 | 25.81 | |
| Hair | 26.67 | 13.33 | 18.75 | 6.25 | 16.13 | |
| Feathers | 6.67 | 6.67 | 0.00 | 0.00 | 3.23 | |
| Bones | 6.67 | 20.00 | 18.75 | 0.00 | 11.29 | |
| Fungi | 13.33 | 33.33 | 75.00 | 31.25 | 38.71 | |
| Morisita-Horn Index | Pianka | |||||
|---|---|---|---|---|---|---|
| Index | Lower Quantile | Upper Quantile | O Value | P(OBS < NULL) | P(OBS > NULL) | |
| June vs. July | 0.89342 | 0.87382 | 0.91104 | 0.86836 * | 0.99 | <0.01 |
| June vs. August | 0.83779 | 0.80373 | 0.83905 | 0.81459 * | 0.98 | 0.02 |
| June vs. October | 0.86552 | 0.81272 | 0.89535 | 0.82907 * | 0.99 | 0.01 |
| July vs. August | 0.90982 * | 0.89894 | 0.90448 | 0.89258 * | 0.99 | <0.01 |
| July vs. October | 0.7673 | 0.67849 | 0.79705 | 0.75747 * | 0.98 | 0.02 |
| August vs. October | 0.82186 | 0.75479 | 0.8625 | 0.83145 * | 0.99 | <0.01 |
| Sample Size | Mean ± SD | Range | |
|---|---|---|---|
| June | 15 | 4.80 ± 1.47 | 2–6 |
| July | 15 | 6.13 ± 2.07 | 2–8 |
| August | 16 | 6.56 ± 1.50 | 4–10 |
| October | 16 | 3.75 ± 1.39 | 1–6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueras, M.P.; Green, T.M.; Burke, R.L. Consumption Patterns of a Generalist Omnivore: Eastern Box Turtle Diets in the Long Island Pine Barrens. Diversity 2021, 13, 345. https://doi.org/10.3390/d13080345
Figueras MP, Green TM, Burke RL. Consumption Patterns of a Generalist Omnivore: Eastern Box Turtle Diets in the Long Island Pine Barrens. Diversity. 2021; 13(8):345. https://doi.org/10.3390/d13080345
Chicago/Turabian StyleFigueras, Miranda P., Timothy M. Green, and Russell L. Burke. 2021. "Consumption Patterns of a Generalist Omnivore: Eastern Box Turtle Diets in the Long Island Pine Barrens" Diversity 13, no. 8: 345. https://doi.org/10.3390/d13080345
APA StyleFigueras, M. P., Green, T. M., & Burke, R. L. (2021). Consumption Patterns of a Generalist Omnivore: Eastern Box Turtle Diets in the Long Island Pine Barrens. Diversity, 13(8), 345. https://doi.org/10.3390/d13080345





