Abstract
Mediterranean islands host unique ecosystems that are particularly vulnerable to invasive species. However, knowledge regarding the precise impact of invasive species on local biodiversity remains limited for many of these systems. Here we report on the negative impacts of invasive wild boars (Sus scrofa) on native snakes on islands in the Mediterranean basin. Capture-mark-recapture was initiated in 2012 on two snake species (Montpellier snake, Malpolon monspessulanus and Ladder snake, Zamenis scalaris) across two islands of Port-Cros National Park. Several wild boars, an invasive species, reached the islands in 2007. They remained confined to small areas of the islands for several years. In Port-Cros, the numbers of wild boars suddenly increased in 2015, and rapidly colonized the whole island damaging vast land surfaces. In Porquerolles, wild boars did not proliferate. This offered an opportunity to examine the impact of wild boar outbreak with a Before-After Control-Impact design (BACI). Snake counts and mark-recapture modeling showed that demographic traits were stable before 2016 for both snake species on both islands. As well as abundance, recruitment, and population growth rate of Montpellier snakes significantly declined where wild boars proliferated but remained constant on the island where they did not. Wild boars probably impacted snake numbers through habitat destruction and direct killing. The rapid decline of snakes (apex predators) and intensive uprooting that strongly damage ground dwelling species (plants, animals) suggest that wild boars represent a serious threat to island biodiversity. As elsewhere around the world, these invasive ungulates proliferate in the Mediterranean basin, they are proficient swimmers and exhibit a remarkably high invasive potential. We recommend vigilance and fast eradication to prevent population outburst; even a few a localized non-proliferating individuals contain the latent potential for devastating outbreaks.
1. Introduction
Island ecosystems are relatively isolated from other landmasses, they shelter unique biodiversity [1]. They also offer refuges for species that are threatened on the mainland [2]. However, the small surface of many islands limits the possibility for threatened organisms to escape threats, and increases contact between native and invasive species, exacerbating their vulnerability to global changes [3]. Worldwide, islands have been directly and indirectly devastated by human activity, such as urbanization, intensive agriculture, pollution, or climate change [1,3]. Protecting islands is crucial to limit the decline of world biodiversity, but also to conserve their extraordinary dynamic ecosystems and their adaptive potential [1].
Introduced predators are major drivers of extinction on islands. Indeed, insular species thrive under relaxed predatory constraints compared to mainland ecosystems, individual tend to be less alert and defenseless against invasive species [4]. Alien herbivores can have devastating effects on the vegetation cover on islands, with cascading negative impact on pelagic birds for example [5]. Invasive species are responsible for the loss of greater numbers of species on islands compared to continental areas [1,6]. Thus, to guide conservation action, it is important to survey alien species on islands and to monitor their possible impacts on native species.
In Europe, wild boar (Sus scrofa) is a native species not officially classified as “Invasive Alien Species” [7]. This classification can be misleading, however. Wild boars are considered among the worst invasive species, across their broad distribution range [8,9,10,11]. Wild boars are robust and highly fertile ungulates that can colonize various habitats and exploit a wide range of resources, rapidly proliferating, especially when large predators are lacking [12] (including human hunters). Colonization of new areas might be natural (dispersal), but anthropogenic disturbances such as climate warming that limits winter mortality, predator eradication (e.g., wolves), and intensive agriculture promote spreading of wild boars [13]. This ungulate opportunistically feeds on a wide range of plants and animals (invertebrates, amphibians, reptiles, and mammals). Sounders (groups of wild boars) deeply dig out the soil and destroy large ground surfaces [14]. During outbreaks, they can entail strong demographic decline in the native biodiversity, causing multiple local population extinctions, notably in snakes [15].
Snakes are important predators that occupy high trophic levels and thus play important roles in the functioning of ecosystems [16]. More generally, it has been suggested that reptiles represent the most effective taxon when using animal counts as a surrogate of local diversity [17]. Snakes depend on the very microhabitats destroyed by wild boars, and they are regularly consumed by them [18]. In continental areas, large and dense populations of wild boars have a devastating effect on local populations of snakes [15,19]. Thus, snake population size changes might be used to monitor the impact of invasive wild boars on island ecosystems. The relationship between invasive species on island snakes, although likely recurrent, is insufficiently quantified [20,21].
Islands represent relatively small and simplified ecosystems where populations are relatively closed (limited migration across populations) [1]; therefore, they provide appropriate sites to precisely monitor the effects of invasive species, providing that native populations have been surveyed in the long term. One of the best-documented examples is found in Australia where populations of different animal species (mostly reptiles) have been monitored over decades before and after cane toad invasions, offering suitable data to examine large scale spatial and temporal effects [22]. However, the arrival of invasive species is not often predictable, and it is important to disentangle the effects of invasive species from other threats (e.g., climatic changes, pollution, diseases) or from natural variations. Comparative data among sites subjected to different degree of exposure to invasive species are thus needed to allow for temporal and spatial comparisons (i.e., without/with the invasive species). In such case, Before-After Control-Impact design (BACI) analyses are well suited to monitor possible negative impact on native species [23,24]. To the best of our knowledge, the possible impact of wild boars has never been assessed using such an approach.
Due to their isolation and to the implementation of strict protection measures, small islands are some of the last pristine landscapes in Europe. They host exceptional ecosystems characterized by high animal and plant diversity and important level of endemism [25,26]. Nonetheless, many invasive species such as the black (Rattus rattus) pose serious conservation issues. Small Mediterranean islands are relatively spared from wild boars; however. This was notably the case for two small islands that are part of Port-Cros National Park (PNPC; Southeast France). Limited human disturbance (especially on Port-Cros) and very low road traffic volumes offer favorable conditions to snakes, promoting adult survival and prolonged growth (unpublished data) that may explain exceptionally large sizes in both species and in both islands [27]. Indeed, road mortality mostly impacts large and presumably old snake individuals [28]. First signs of wild boar’s presence on Porquerolles and Port-Cros islands were recorded in 2007 [29]. From 2008 to 2015, in both islands, very few individuals were observed and remained confined to small areas (likely, because only one sex was present). However, an outbreak of invasive wild boars occurred in one island (Port-Cros) in 2016 possibly triggered by the arrival of novel individuals associated with the lack of predation pressure (no natural predator and no hunting). No wild boar outbreak occurred in Porquerolles [29]. Importantly, snake populations were monitored prior to wild boar outbreak. Therefore, the contrast between the two islands provided an opportunity to use a BACI approach: one strongly affected island, one largely spared island (control site), both monitored before and after wild boar outbreak.
In this study, we evaluated the impact of wild boar outbreak on demographic traits of two native snake species. Since 2012, using visual counting and CMR, we monitored native populations of the Montpellier snake (Malpolon monspessulanus) and of the Ladder snake (Zamenis scalaris) [27]. These large Mediterranean snakes are terrestrial and abundant in PNPC islands [27]. They are oviparous and exhibit an eclectic diet that includes ectothermic and endothermic prey [30,31]. Both species are active foragers; M. monspessulanus is mostly diurnal and conspicuous while Z. scalaris is more secretive and tends to be nocturnal [30,32,33,34]. Monitoring simultaneously two species of native snake species using capture-mark-recapture protocol, in two protected islands before and after the proliferation of an invasive species in one site only, offered a unique opportunity to perform a robust analyse. Overall, this study provided a unique occasion to observe in real time the consequences of wild boar invasion in fragile island ecosystems.
2. Materials and Methods
2.1. Study Areas
Port-Cros (PC) and Porquerolles (PQL) are situated off the southeast coast of France (Figure 1). Port-Cros (700 ha, maximal altitude 199 m) is located 8.3 km from the mainland, while Porquerolles (1.254 ha, maximal altitude 142 m) is located 2.6 km from the mainland. In both islands, the landscape is hilly and mostly composed of dense forests (Aleppo pines and holly oaks). In Port-Cros, open areas include roads, several hiking trails, old fortresses, three small meadows, and scattered small rocky patches. In Porquerolles, the landscape is more open, composed of a mosaic of cultivated plains (25%), including vineyards and orchards. Both islands experience intensive summer tourism (peak ~90.000 visitors per month in summer). Continuous management efforts are required to preserve local biodiversity, notably in Port-Cros to combat highly invasive plants and animals such as the Hottentot fig (Carpobrotus edulis) and the black rat (Rattus rattus) [35,36,37].
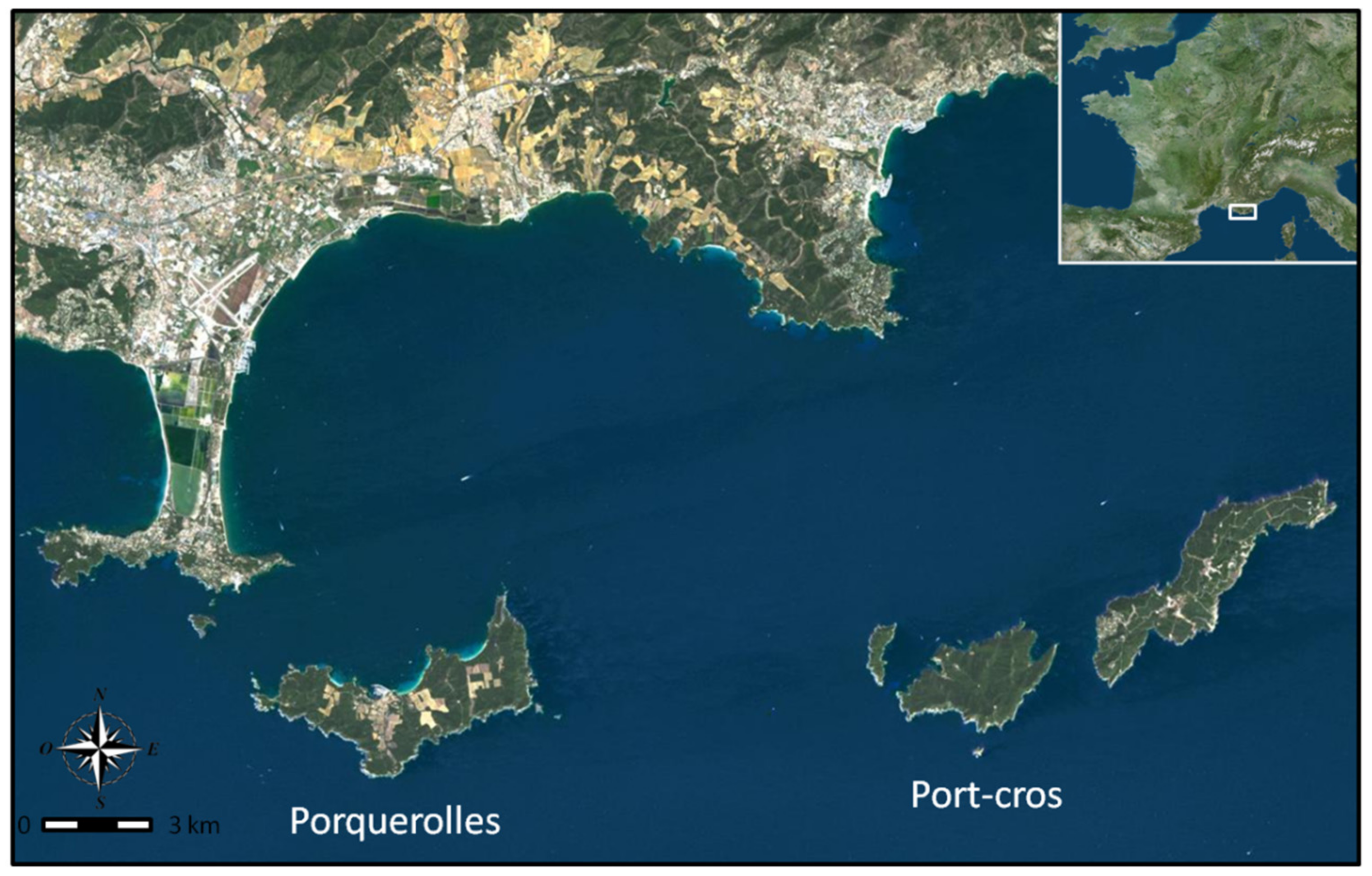
Figure 1.
Study location Port-Cros and Porquerolles are situated off the south-east coast of France’ located respectively 8.3 km and 2.6 km from the coast which are 9 km apart (google-earth© image).
2.2. Wild Boar Arrival and Proliferation
Wild boars were first recorded in 2007 on Port-Cros and in 2011 on Porquerolles [29]. They have been observed in the sea, halfway between mainland and PNPC islands, suggesting they are able to colonize naturally islands that are close to the shore. On Port-Cros, before 2015, only one or two wild boars were localized to the south-east of the island. Reproduction was detected and evidence of rooting was found across the whole island, both elements suggesting rapid population growth. In 2016 and following years, we estimated that 60% to 80% of the monitored area was uprooted, reflecting a demographic explosion [29]. Park authorities set up wild boar regulations (boar beating, cage trap) in late 2016. Many individuals were removed, demonstrating that wild boars were indeed proliferating (as indicated by high number of boars killed between 2017 and 2019, n = 200; [29]). Unfortunately, uprooting did not decrease, and wild boar impact remained strong on the whole landscape. Currently, uprooting occurs across ~60% of the areas where snakes are monitored (Figure 2). Most spots where snakes are regularly observed have been devastated (e.g., scrubs destroyed and stones returned). In Porquerolles, the number of wild boars remained low and rooting concerned less than 5% of the surface monitored (rooting slightly increased in 2019, reaching 10%) [29]. Throughout the study, no other alterations were noticed in the environment and tourist traffic did not change [38].

Figure 2.
Sampling locations on Port-Cros before (A,B) and after wild boar outbreak in 2016 (C,D). Image (A) shows an intact dry stone wall. Image (B) shows corrugated fibrocement coverboard positioned along a hedge in a meadow. Image (C) shows rooting of the ground, leading to the disappearance of the herbaceous layer and of many shrubs. Image (D) shows the partial destruction of a stone wall. Overall a variety of habitat types was affected, we estimated that 60% to 80% of the whole area was plowed.
2.3. Snake Population Monitoring
Between 2012 and 2019 (8 years), we monitored snake populations using the capture-mark-recapture methodology. Surveys were conducted during the day from mid-April to mid-June by two experienced observers. We used two techniques: networks of corrugated fibrocement coverboards (80 × 120 cm and 80 × 90 cm, Figure 2) to detect and capture sheltered snakes, and Visual Encounter Surveys (VES) along transects to find not-sheltered snakes basking in screes or sun patches [27]. Coverboards were placed in different appropriates sites (e.g., open areas) varying in size, from one hectare to three hectares (20–30 cover boards per hectares) [27]. Distances between coverboards were approximately 10 to 20 m. Coverboards were not displaced over time, and similar transects were searched. In Port-Cros 25 boards were positioned along the hedges of a meadow and of an old orchard. VES were conducted along a 10 km trail that winds through forest and bush patches and that encloses 170 ha (25% of the island) [27]. The trail and the immediate area surrounding the trail were searched. Basking snakes can be spotted from a distance of ~10 m, thus the total surveyed area covered ~20 ha (3% of the island’s surface). In this island, most snakes were not found under shelters (95%). On Porquerolles, a total of 232 coverboards were positioned mostly along hedges while VES were conducted along tracks between places where coverboards were placed. On this island, most snakes were found sheltered (95%). Total surveyed area covers ~80 ha, representing 6% of the island’s surface [27]. Each year, on each island, we performed 6 to 8 survey sessions of two or three days each (except in 2012 with only two surveys). Two weeks separated each session. During each survey session, we attempted to capture each snake. Individuals spotted but not caught were described as precisely as possible (location, crude body size, color pattern that is sexually dimorphic in Malpolon monspessulanus). Captured individuals were measured for body size (snout-vent length, SVL, ±0.5 cm) by stretching the snake along a tape measure, weighed (±1 g) using an electronic scale (Ohaus, CL2000), sexed (using color, tail inspection, hemipenes eversion when necessary), and were permanently marked using superficial scale branding. Identity numbers were coded using unique combination of ventral scales plus adjacent lateral scale line [39]. Juveniles and adults were distinguished according to their size (snout to vent length, SVL) and/or using information on their reproductive status when available (i.e., presence of eggs) [32]. The size at which individuals attain maturity varies according to species and sex, we used the following criteria: juvenile (SVL < 55 cm) in male of M. monspessulanus, and females SVL < 65 cm [40]. All individuals were rapidly released at the exact place of capture.
2.4. Analyses
Counts of snakes detected along transects and under coverboards were pooled and used as a measure of yearly abundance. We modeled variations in snake abundances using the number of snake observations gathered at each field survey session as an index of snake abundance calculated per year and per site. This number includes captured and uncaptured individuals (i.e., snakes observed but escaped capture). Repeated observations of the same individual within the same session were counted only once; this happened rarely (individuals were observed less than once per year one average, and rarely more than twice, see results). For consistency among years, and to limit the effects of survival and recruitment on estimates (negligible in each short-term session), we used spring sessions only in this analysis. We also excluded data collected in 2012, given that field procedures were not yet standardized at that time. The effect of boar presence on snake abundance was analyzed using generalized linear modeling (GLMs; MASS package). In this model, the dependent variable was the total snake count per session. The independent variables were the islands and the timing of the invasion event (i.e., before or after wild boar outbreak, named time for conciseness).
We used a negative binomial distribution instead of a Poisson distribution to account for large over-dispersion in the counts (c-hat ≈ 1.5−2.0 for all datasets). As monitoring effort (number of searching days) varied per session, we used the number of searching days (log-transformed) as an offset in the model. To fulfill BACI analysis requirements [41], we fitted the model in which the rough number of snakes observed depends on the interaction between time (before 2016 versus after 2016; BA) and islands (CI). BACI analysis suggests an impact has occurred when the interactions between these factors is significant. We ran three models, first considering all species then a second, and a third for Z. scalaris and M. monspessulanus species respectively. All GLM analyses were performed under R statistical software, v3.6.1 [42].
To analyze capture-recapture data we used a Robust Design Pradel model, which is based on two nested temporal scales of sampling: the year and the repeated session intra-years [43]. Robust design schemes allow the population to be open between the primary sessions (birth, death, and dispersal are possible) but assume closure for the secondary sessions (no dispersal, birth or death between secondary sessions of the same primary session) [44]. Seven primary sessions were considered, corresponding to the years 2012 to 2019. Each secondary session corresponded to field survey sessions that occurred within each year (n = 6 to 8 depending of the year). This modeling framework allows estimating the survival probability (phi = ϕ), between primary sessions, the probability that an individual captured during a session was present in the population during the previous primary session (Gamma γ = seniority), the capture probability for each secondary sessions (p), the recapture probability (c) and the number of present but missed individuals (F (0)) at each primary session from which yearly population abundances can be derived. The simultaneous estimation of survival and seniority probabilities also allows deriving inter-annual population growth rate (GR). We only modeled captures of adults because immature snakes display low recapture probabilities so that their inclusion can lead to strong capture probability heterogeneity [45]. We simultaneously analyzed both species to increase statistical power, to compare them and both islands (we had no a priori regarding possible differential impact of wild boars on each of the two snake species). Capture-recapture analyses were performed using MARK software (8.0) [46]. All estimates are provided with their 95% confident interval.
We used a backward stepwise modeling approach to select the most parsimonious model. We first fitted a general model in which survival and seniority probabilities depend on the interaction between islands, time (before 2016 versus after 2016), and species. In this model capture-recapture probabilities were allowed to depend on the island, the species, and the year. From this general model, we first removed covariates fitted on capture-recapture parameters (e.g., removing year effect on ϕ), one by one (e.g., we tested three models with two covariates in each of them instead of three). Models were compared using AIC criteria. We considered that a model was significantly better when its AIC was two points lower compared to another, enabling a ranking of all the models. Then, from the best model, we simplified the survival probabilities by first removing the interaction between islands and time, then the covariates one by one. We performed the same procedure to model the seniority probabilities from the best model of the second step. Resulting model comparisons are summarized in Table 1.

Table 1.
Model selection statistics for the capture-recapture models used to estimate the demographic parameters of snakes of Porquerolles and Port-Cros islands from 2012 to 2019. ϕ is the survival probability, γ the seniority probability, p is the capture and recapture probability. Year means that a yearly variation was fitted on the parameter, sp an effect of species, island and effect of island, BACI means an interaction between island and time (before 2016 vs. after 2016), BA means “before 2016 versus after 2016” and “cst PQL” means that only the estimates of Port-Cros after 2016 differ from all others transitions.
2.5. Ethics Statement
All data used in this analysis were collected using protocols approved by the Direction Départementale des Territoires et de la Mer (DDTM 83 2015-01). Snakes were gently handled and kept in a calico bag (one snake per bag) to minimize stress between capture and release. No individual was harmed or injured, we never observed any sign of disorder or distress.
3. Results
A total of 892 snakes were observed between 2012 and 2019 (PC, n = 539; PQL, n = 338). On both islands, Malpolon monspessulanus was more frequently observed than Zamenis scalaris (n = 631 observations vs. n = 261). The total number of individuals marked was 384 (PC, n = 231; PQL, n = 153). We obtained 246 recapture events (Supplementary Material Table S1). A total of 122 individuals were recaptured at least once (24 individuals recaptured within a given year; 64 recaptured one year later; 20 two years later; 9 three years later; 3 four years later; 1 four or six years later). A greater number of M. monspessulanus (n = 256) were marked than Z. scalaris (n = 128). Most snakes were adult (92% of marked individuals); we found 33 juveniles (8%; M. monspessulanus, n = 26; Z. scalaris, n = 7).
3.1. Index of Snake Abundance
After discounting 2012 and autumnal sessions, we analyzed 822 observations (258 Z. scalaris (PC, n = 141; PQL, n = 117) and 556 M. monspessulanus (PC, n = 351; PQL, n = 205)).
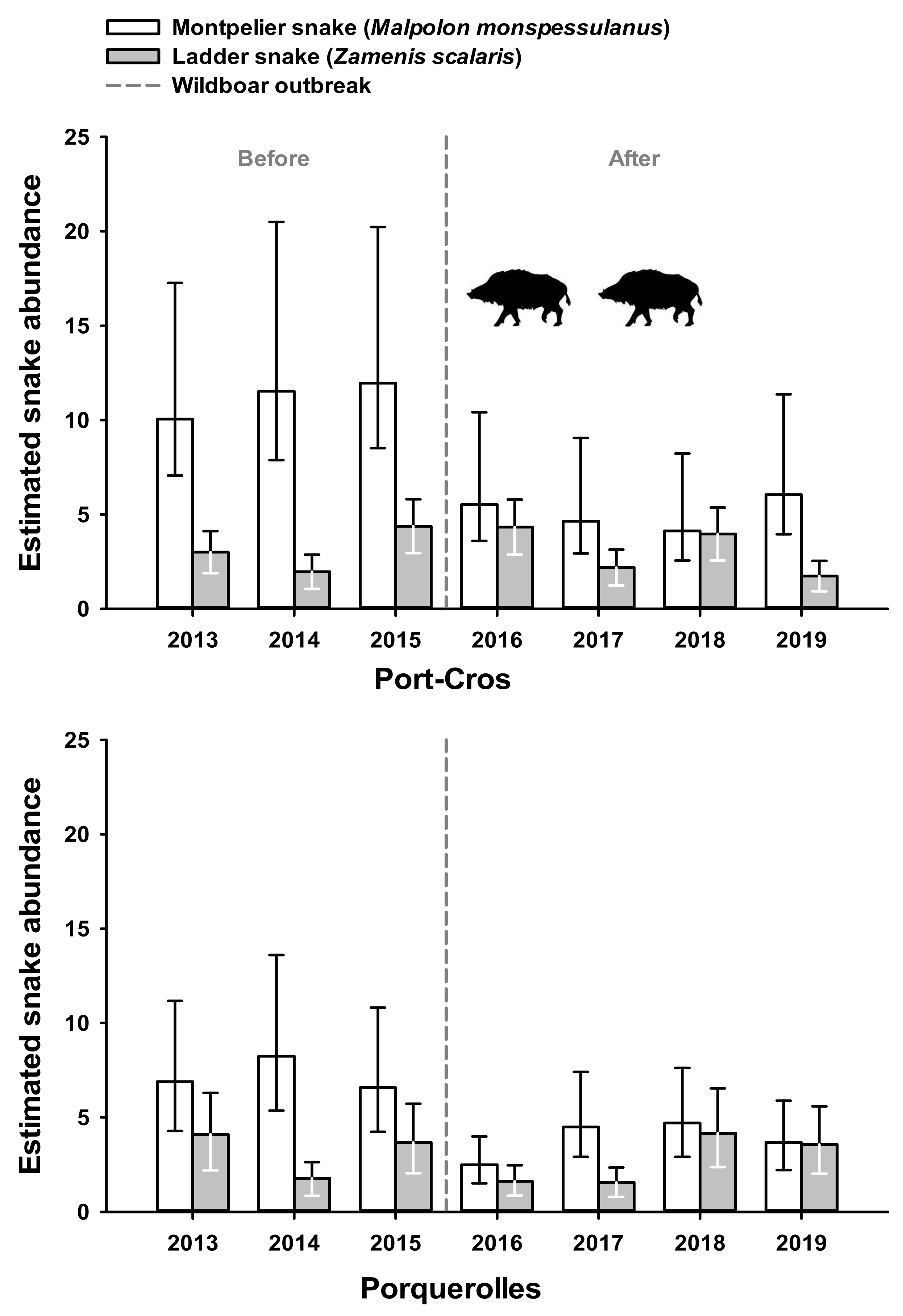
Pooling both species, estimated abundance of snakes was significantly affected by the interaction between time (before 2016 vs. after 2016) and site (Porquerolles vs. Port-Cros) (p < 0.05, Table 2). In Port-Cros, estimated abundance declined from 13.45 snakes per session before 2016 (95% CI: 11.17–16.19) to 7.82 snakes per session after 2016 (95% CI: 6.49–9.42). In Porquerolles, estimated abundance was stable over time (before 2016 = 8.32 (95% CI: 6.67–10.39); after = 7.88 (95% CI: 6.49–9.57)).

Table 2.
Results of the generalized linear models’ analysis testing the influence of time (before 2016 versus after 2016 = BA), site (islands, CI), and their interaction (*, BACI) on snake abundance.
Temporal changes of estimated abundance differed between snake species and between sites (Figure 3). In M. monspessulanus, the interaction between time and site on estimated abundance was significant (p < 0.05, Table 2). In Port-Cros, estimated abundance declined from 10.45 before 2016 (95% CI: 8.66–12.063) to 4.76 after 2016 (95% CI: 3.86–5.88). In Porquerolles, estimated abundance remained stable (before = 5.68 (95% CI 4.47–7.22); after = 4.55 (95% CI 3.63–5.71)). In Z. scalaris, the interaction between time and site was not significant (p = 0.50; Table 2). Estimated abundance of Z. scalaris remained stable over time in Port-Cros (before = 3.01 (95% CI: 2.14–4.23); after = 2.87 (95% CI 2.12–3.89)) as well as in Porquerolles (before = 2.63 (95% CI: 1.80–3.84); after = 3.16 (95% CI: 2.32–4.31)).
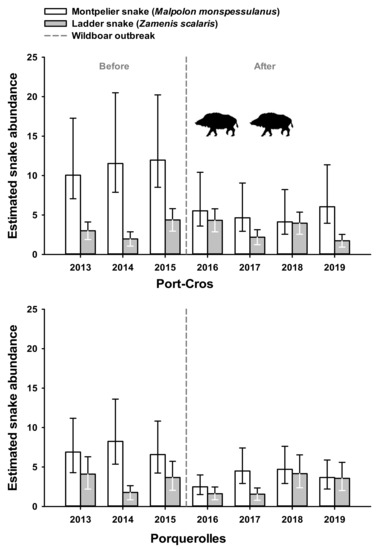
Figure 3.
Annual abundance of the species of snake (M. Monspessulanus or Z. scalaris) was assessed using a generalized linear model to estimate the number of snakes encountered during a daily transect (Y axis, see text for details). Error bars represent lower and upper 95% confident interval respectively. Vertical dashed lines indicate the time limit before and after wild boar outbreak that occurred in Port-Cros (upper panel), but not in Porquerolles (lower panel).
3.2. Demographic Parameters
The best Robust Design model included an effect of species on survival probability, an interaction between island and time with a significant difference after 2016 only in PC, a species effect in addition to seniority probability with the effects of islands, species, and year in addition to capture-recapture probabilities (Table 1).
Capture-recapture probabilities were low, ranging from 0.07 to 0.14. They were lower for Z. scalaris than for M. monspessulanus. They were lower in PQL than in PC. They differed among years, for instance in PQL for M. monspessulanus, varying between 0.01 and 0.07. Survival probabilities were higher in Z. scalaris (ϕ = 0.75 (0.66–0.82)) than in M. monspessulanus (ϕ = 0.62 (0.53–0.39)), were constant through time and equal between islands. Before 2016, “seniority” (i.e., probability that a captured individual was present during the previous session) were higher in Z. scalaris than in M. monspessulanus in PQL as well as in PC (Z. scalaris, γ = 0.72 (0.62–0.80]) vs. M. monspessulanus, γ = 0.63 (0.54–0.71)). Seniority probability was constant across years in PQL. Estimated gammas were not significantly different between PQL and PC before 2016; γ = 0.63 (0.54–0.71) for M. monspessulanus and γ = 0.72 (0.62–0.80 for Z. scalaris). After 2016, in PC, estimated values increased for both snake species (γ = 0.81 (0.64–0.91) for M. monspessulanus; γ = 0.87 (0.75–0.93) for Z. scalaris) while they remained constant on PQL.
These estimates translate into annual population growth rates close to one at PQL (all years) and PC before 2016 for both species (M. monspessulanus, GR = 0.98 (0.90–1.08); Z. scalaris, GR = 1.04 (0.94–1.14)). After 2016, the population growth rates were however below one on PC (M. monspessulanus, GR = 0.76 (0.66–0.88); Z. scalaris, GR = 0.86 (0.77–0.97)).
4. Discussion
This study has shown that wild boar (Sus scrofa) outbreaks can clearly have devastating impacts on mainland snakes [15] with snake counts and mark-recapture demographic analyses both revealing a strong negative effect of wild boars on snakes on one island, especially on Montpellier snakes (Malpolon monspessulanus), and to lesser extent on ladder snakes (Zamenis scalaris). The combination of snake abundance estimates and demographic trends performed in two islands, before and after the proliferation of wild boars that occurred in one site but not in the other, offered comparative information. After 2016, and only on the island affected by wild boars, did we find a significant decline in snake counts, recruitment, and annual population growth rates in both species. These results provide further evidence that wild boars can be destructive when they proliferate, notably in the absence of predatory pressures (wolf or hunters) [14,47,48,49].
Snakes are experiencing a worrying rate of global decline worldwide, and hence the maintenance of sanctuaries such as the Port-Cros and Porquerolles islands is important; in this context, the population decrease we observed is particularly damageable [50]. Small islands offer exceptional conditions for many snake species; endemism and extremely high densities are commonly documented [21,51]. The recent invasion of wild boars may compromise their viability through different mechanisms [15]. Guided by a fine sense of smell, wild boars are able to pursue snakes in the open and in thick vegetation. We observed intensive rooting in the places frequently used by M. monspessulanus snakes (e.g., screes in open areas), which suggests that snakes were also found in their refuge [18]. Large shelter rocks were frequently upturned (elsewhere this problem also concerns coverboards; unpublished XB data). Small snakes and eggs are easy prey for wild boars; this may explain the decrease of recruitment observed in the population of M. monspessulanus in Port-Cros. There was no decline in survival between years and high recapture rate (>50%, unpublished data) of the largest individuals (M. Monspessulanus: SVL > 1.50 m; Z. scalaris SVL > 1.30 m) suggesting that population decrease was principally caused by predation targeting small individuals (including juveniles) rather than the result of emigration of large snakes outside the prospected areas which would have induced a drop of apparent survival. In other words, this suggests that snake above a certain size (e.g., SVL > 1.20 m) might be less impacted. Field management may focus on the protection of youngest age classes (e.g., fencing nesting area or creation of heavy shelters) so that they can reach a size where the boars will not affect them.
The abundance of M. monspessulanus and Z. scalaris on Port-Cros and Porquerolles is associated with the large body size (SVL) attained by many individuals in both sites [27]. Large and presumably old individuals are essential for population viability in long-lived reptiles with indeterminate growth, notably because reproductive output and survival probability increase with body size [52]. Both snake species (M. monspessulanus and Z. scalaris) are long-lived animals (>14 years) [40] with delayed maturity and relatively low fecundity [31]. The sharp decline in M. monspessulanus snake numbers in Port-Cros is worrying, adult numbers will decrease if wild boar pressure is not relaxed, therefore entailing population collapse. Moreover, snakes are highly susceptible to the destruction of their habitat for which they express fine scale fidelity [53]. In Port-Cros, wild boars shift between forest refuges, open areas, and low maquis where they destroy microhabitats, and shelters [19]. All areas frequently used by snakes are plowed. Snakes might also be impacted by reduced abundance of their main prey, micromammals notably, that are destroyed during rooting [54].
Compared to M. monspessulanus, the ladder snake (Z. scalaris) appears to be less impacted. The ladder snake is a particularly cryptic species with marked fossorial and nocturnal habits [33,34]. This explains the difficulties in finding this snake using visual surveys (e.g., VES). In contrast M. monspessulanus is a conspicuous species often observed basking in the open or during displacements. Snakes are more exposed to predation during displacements in the open and mortality is high in large racers such as whip snakes (Hierophis viridiflavus) or M. monspessulanus [28]. We assume that he highly secretive and fossorial lifestyle of Z. scalaris may have limited the impact of wild boars.
The low detectability of Z. scalaris and immature snakes also limited the statistical power of several analyses. More generally, snakes are secretive organisms notoriously difficult to study in the field. Demographic changes are not easily accessible to evaluation [55,56]. Strong decreases may escape observation and their possible causes might remain unnoticed. Mark-recapture studies (CMR) enable circumvention of most of these difficulties. In the current study, important field effort (>15 days per year per site), and the combination of visual searching with the systematic inspection of two networks of artificial refuges (coverboards) for 8 years allowed us to gather abundant count and CMR data. CMR data allowed taking into account spatial and temporal heterogeneity of detectability. They indicate that the decline in snake abundance cannot be attributed to increasing snake’s vigilance or discretion as a possible response of wild boar impact (i.e., detectability is factored out). In addition, M. monspessulanus is a typical thermophilic racer species that frequently basks in the sun to meet its physiological demands, and thus cannot easily adopt fossorial/diurnal habits and escape boars. Overall, the strong consistency between visual counts and CMR demographic parameters suggests that the negative impact of wild boars in M. monspessulanus was not a methodological artifact.
Wild boars were detected almost 10 years before the outbreak in Port-Cros. However, control operations were initiated only in 2016; thus, too late to prevent population outbreak. This decision was based on the erroneous notion that small numbers of wild boars (possibly one sex only) did not necessarily represent a threat. This was true for a while but for unknown reasons (e.g., arrival of new individual(s) of the other sex, behavioral change, novel capacity to exploit local resources) these fertile ungulates suddenly expressed their capacity to proliferate. Wild boars should be removed from newly colonized areas, especially in small and fragile ecosystems characterized by many Mediterranean islands where the lack of predators demographic explosions of invasive species [10,57]. Successful eradication operations of wild boars have been achieved on different islands [47,58] but to be effective, adult females and piglets must be targeted and early controls are essential [59,60,61]. Unfortunately, wild boars are not easy to capture in rocky and very bushy landscapes while hunters are reluctant to target reproductive females. In Port-Cros, during eradication operations, tens of wild boars are killed each year but a large population persists [29]. Control programs therefore should be intensified, hunting could be supported by methods such as poisoning that are efficient but that must be performed very cautiously [47,62].
5. Conclusions
Wild boar (Sus scrofa) poses serious challenges on the two islands surveyed. Intensification of control operations would require important logistical and financial investments, typically in the range of those undertaken to eradicate other less popular invasive species (e.g., black rats). Unfortunately, due to strong opposition from the public, setting up total eradication programs is controversial. The common misconception of what is an invasive species puts threatened species and fragile ecosystems at risk of extinction. Wild boar numbers are increasing all over the world; this increases invasion risks in yet untouched small islands. Prevention is better than cure, vigilance is thus needed to control wild boars as soon as they are detected.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/d13100498/s1, Table S1: Number of observation (Obs), individual captured (C) and recaptured (R) (adults and immatures) each year in Port-Cros and Porquerolles islands according to snake species.
Author Contributions
Conceptualization of the study J.-M.B. and X.B.; Methodology J.-M.B. and X.B.; Formal analyses J.-M.B., C.K. and A.B.; Final statistical analyses A.B. and J.-M.B.; Field work J.-M.B., C.K., M.A. (Manon Amiguet), M.A. (Mathieu Ausanneau), S.C., G.B., T.F., G.F., G.G., L.M., F.D., V.M. and D.G.; Data curation J.-M.B.; Writing J.-M.B., X.B., C.K. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All data used in this analysis was collected using protocols approved by the Direction Départementale des Territoires et de la Mer (DDTM 83 2015-01).
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://silene.eu/.
Acknowledgments
We thank the Parc National de Port-Cros for the partnership and providing accessibility to the study sites. We thank all students not involved as co-authors for helping us to collect data. We thank Roger Meek for helping us to improve the English.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Losos, J.B.; Ricklefs, R.E. Adaptation and diversification on islands. Nature 2009, 457, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.C.; Kueffer, C. Island biodiversity in the Anthropocene. Annu. Rev. Environ. Resour. 2019, 44, 31–60. [Google Scholar] [CrossRef]
- Courchamp, F.; Hoffmann, B.D.; Russell, J.C.; Leclerc, C.; Bellard, C. Climate change, sea-level rise, and conservation: Keeping island biodiversity afloat. Trends Ecol. Evol. 2014, 29, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Courchamp, F.; Chapuis, J.L.; Pascal, M. Mammal invaders on islands: Impact, control and control impact. Biol. Rev. 2003, 78, 347–383. [Google Scholar] [CrossRef]
- Chapuis, J.L.; Boussès, P.; Barnaud, G. Alien mammals, impact and management in the French subantarctic islands. Biol. Conserv. 1994, 67, 97–104. [Google Scholar] [CrossRef]
- Spatz, D.R.; Zilliacus, K.M.; Holmes, N.D.; Butchart, S.H.; Genovesi, P.; Ceballos, G.; Tershy, B.R.; Croll, D.A. Globally threatened vertebrates on islands with invasive species. Sci. Adv. 2017, 3, e1603080. [Google Scholar] [CrossRef]
- Colautti, R.I.; MacIsaac, H.J. A neutral terminology to define ‘invasive’ species. Divers. Distrib. 2004, 10, 135–141. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; ISSG: Auckland, New Zealand, 2000; Available online: www.issg.org/booklet.pdf (accessed on 21 June 2021).
- Ickes, K.; Paciorek, C.J.; Thomas, S.C. Impacts of nest construction by native pigs (Sus scrofa) on lowland Malaysian rain forest saplings. Ecology 2005, 86, 1540–1544. [Google Scholar] [CrossRef]
- Massei, G.; Kindberg, J.; Licoppe, A.; Gačić, D.; Šprem, N.; Kamler, J.; Baubet, E.; Hohmann, U.; Monaco, A.; Ozoliņš, J.; et al. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Manag. Sci. 2014, 71, 492–500. [Google Scholar] [CrossRef]
- Tack, J. Wild boar (Sus scrofa) populations in Europe. In A Scientific Review of Population Trends and Implications for Management; European Landowners’ Organization: Brussels, Belgium, 2018; p. 56. [Google Scholar]
- Sáaez--Royuela, C.; Telleriia, J.L. The increased population of the wild boar (Sus scrofa L.) in Europe. Mammal. Rev. 1986, 16, 97–101. [Google Scholar] [CrossRef]
- Vetter, S.G.; Puskas, Z.; Bieber, C.; Ruf, T. How climate change and wildlife management affect population structure in wild boars. Sci. Rep. 2020, 10, 7298. [Google Scholar] [CrossRef]
- Barrios-Garcia, M.N.; Ballari, S.A. Impact of wild boar (Sus scrofa) in its introduced and native range: A review. Biol. Invasions 2012, 14, 2283–2300. [Google Scholar] [CrossRef]
- Graitson, E.; Barbraud, C.; Bonnet, X. Catastrophic impact of wild-boars: Insufficient hunting pressure pushes snakes to the brink. Anim. Conserv. 2018, 22, 165–176. [Google Scholar] [CrossRef]
- Kupfer, A.; Langel, R.; Scheu, S.; Himstedt, W.; Maraun, M. Trophic ecology of a tropical aquatic and terrestrial food web: Insights from stable isotopes (15N). J. Trop. Ecol. 2006, 22, 469–476. [Google Scholar] [CrossRef]
- Lewandowski, A.S.; Noss, R.F.; Parsons, D.R. The effectiveness of surrogate taxa for the representation of biodiversity. Conserv. Biol. 2010, 24, 1367–1377. [Google Scholar] [CrossRef]
- Jolley, D.B.; Ditchkoff, S.S.; Sparklin, B.D.; Hanson, L.B.; Mitchell, M.S.; Grand, J.B. Estimate of herpetofauna depredation by a population of wild pigs. J. Mammal. 2010, 91, 519–524. [Google Scholar] [CrossRef]
- Filippi, E.; Luiselli, L. Negative effect of the wild boar (Sus scrofa) on the populations of snakes at a protected mountainous forest in central Italy. Ecol. Mediterr. 2002, 28, 93–98. [Google Scholar] [CrossRef]
- Daltry, J.C.; Lindsay, K.; Lawrence, S.N.; Morton, M.N.; Otto, A.; Thibou, A. Successful reintroduction of the Critically Endangered Antiguan racer Alsophis antiguae to offshore islands in Antigua, West Indies. Int. Zoo Yearb. 2017, 51, 97–106. [Google Scholar] [CrossRef]
- Lillywhite, H.; Martins, M. (Eds.) Islands and Snakes: Isolation and Adaptive Evolution; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Shine, R. The ecological impact of invasive cane toads (Bufo marinus) in Australia. Q. Rev. Biol. 2010, 85, 253–291. [Google Scholar] [CrossRef]
- Smokorowski, K.E.; Randall, R.G. Cautions on using the Before-After-Control-Impact design in environmental effects monitoring programs. Facets 2017, 2, 212–232. [Google Scholar] [CrossRef]
- Guareschi, S.; Laini, A.; England, J.; Johns, T.; Winter, M.; Wood, P.J. Invasive species influence macroinvertebrate biomonitoring tools and functional diversity in British rivers. J. Appl. Ecol. 2021, 58, 135–147. [Google Scholar] [CrossRef]
- Médail, F.; Quézel, P. Biodiversity hotspots in the Mediterranean Basin: Setting global conservation priorities. Conserv. Biol. 1999, 13, 1510–1513. [Google Scholar] [CrossRef]
- Migheli, Q.; Balmas, V.; Komoñ-Zelazowska, M.; Scherm, B.; Fiori, S.; Kopchinskiy, A.G.; Kubicek, C.P.; Druzhinina, I.S. Soils of a Mediterranean hot spot of biodiversity and endemism (Sardinia, Tyrrhenian Islands) are inhabited by pan—European, invasive species of Hypocrea/Trichoderma. Environ. Microbiol. 2009, 11, 35–46. [Google Scholar] [CrossRef]
- Ballouard, J.M.; Ferrari, T.; Bonnet, X.; Caron, S.; Maxime, L.; Garnier, G.; Gillet, P.; Ausanneau, M. Snakes of Port-Cros National Park islands: Capture-Mark-Recapture study of Malpolon monspessulanus and Rhinechis scalaris. Sci. Rep. Port-Cros Natl. Park 2016, 30, 23–44. [Google Scholar]
- Bonnet, X.; Naulleau, G.; Shine, R. The dangers of leaving home: Dispersal and mortality in snakes. Biol. Conserv. 1999, 89, 39–50. [Google Scholar] [CrossRef]
- Cheylan, G.; Geoffroy, D. Colonisation des îles d’Hyères (Var, sud de la France) par le sanglier Sus scrofa. Sci. Rep. Port-Cros Natl. Park 2020, 34, 45–56. [Google Scholar]
- Pleguezuelos, J.M.; Fernández-Cardenete, J.R.; Honrubia, S.; Feriche, M.; Villafranca, C. Correlates between morphology, diet and foraging mode in the Ladder Snake Rhinechis scalaris (Schinz, (Schinz, 1822). Contrib. Zool. 2007, 76, 179–186. [Google Scholar] [CrossRef]
- Feriche, M.; Pleguezuelos, J.M.; Santos, X. Reproductive ecology of the Montpellier snake, Malpolon monspessulanus (Colubridae), and comparison with other sympatric colubrids in the Iberian Peninsula. Copeia 2008, 2, 279–285. [Google Scholar] [CrossRef]
- Deso, G.; Bonnet, X.; De Haan, C.; Garnier, G.; Dubos, N.; Ballouard, J.M. Snake overboard! Observations of marine swimming in Malpolon monspessulanus. Herpet. Notes 2021, 14, 593–596. [Google Scholar]
- Cheylan, M. Mise en évidence d’une activité nocturne chez le serpent méditerranéen Elaphe scalaris (Ophidia, Colubridae). Amphib.-Reptil. 1986, 7, 181–186. [Google Scholar] [CrossRef]
- Martinez-Freiria, F.; Lorenzo, M.; Lizana, M. Zamenis scalaris prefers abandoned citrus orchards in Eastern Spain. Ecological insights from a radio-tracking survey. Amphib.-Reptil. 2019, 40, 113–119. [Google Scholar] [CrossRef]
- Suehs, C.M.; Affre, L.; Médail, F. Invasion dynamics of two alien Carpobrotus (Aizoaceae) taxa on a Mediterranean island: I. Genetic diversity and introgression. Heredity 2004, 92, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Hulme, P.E.; Brundu, G.; Camarda, I.; Dalias, P.; Lambdon, P.; Lloret, F.; Medail, F.; Moragues, E.; Suehs, C.; Traveset, A.; et al. Assessing the risks to Mediterranean islands ecosystems from alien plant introductions. In Plant Invasions: Human Perception, Ecological Impacts and Management; Backhuys Publishers: Leiden, The Netherlands, 2008; pp. 39–56. [Google Scholar]
- Capizzi, D.; Baccetti, N.; Sposimo, P. Fifteen years of rat eradication on Italian islands. In Problematic Wildlife; Angelici, F.M., Ed.; Springer: Cham, Germany, 2016; pp. 205–227. [Google Scholar]
- Brécard, D.; De Luigi, C. Fréquentation touristique de Port-Cros et Porquerolles: Les enseignements de la base de données Bount îles. Sci. Rep. Port-Cros Natl. Park 2016, 30, 65–94. [Google Scholar]
- Brown, W.S.; Parker, W.S. A ventral scale clipping system for permanently marking snakes (Reptilia, Serpentes). J. Herpetol. 1976, 10, 247–249. [Google Scholar] [CrossRef]
- López-Calderón, C.; Feriche, M.; Alaminos, E.; Pleguezuelos, J.M. Loss of largest and oldest individuals of the Montpellier snake correlates with recent warming in the southeastern Iberian Peninsula. Cur. Zool. 2017, 63, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.P.; Orvos, D.R.; Cairns, J., Jr. Impact assessment using the before-after-control-impact (BACI) model: Concerns and comments. Can. J. Fish. Aquat. Sci. 1993, 50, 627–637. [Google Scholar] [CrossRef]
- Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. Available online: https://www.R-project.org/ (accessed on 21 June 2021).
- Pradel, R. Utilization of capture-mark-recapture for the study of recruitment and population growth rate. Biometrics 1996, 52, 703–709. [Google Scholar] [CrossRef]
- Kendall, W.L.; Nichols, J.D.; Hines, J.E. Estimating temporary emigration using capture recapture data with Pollock’s robust design. Ecology 1997, 78, 563–578. [Google Scholar]
- Arsovski, D.; Olivier, A.; Bonnet, X.; Drilholle, S.; Tomovic, L.; Béchet, A.; Golubovic, A.; Besnard, A. Covariates streamline age-specific early life survival estimates of two chelonian species. J. Zool. 2018, 306, 223–234. [Google Scholar] [CrossRef]
- White, G.C.; Burnham, K.P. Program MARK: Survival estimation from populations of marked animals. Bird Study 1999, 46, 120–138. [Google Scholar] [CrossRef]
- Cruz, F.; Donlan, C.J.; Campbell, K.; Carrion, V. Conservation action in the Galapagos: Feral pig (Sus scrofa) eradication from Santiago Island. Biol. Conserv. 2005, 121, 473–478. [Google Scholar] [CrossRef]
- Hegel, C.G.Z.; Santos, L.R.; Marinho, J.R.; Marini, M.Â. Is the wild pig the real “big bad wolf”? Negative effects of wild pig on Atlantic Forest mammals. Biol. Invasions 2019, 21, 3561–3574. [Google Scholar] [CrossRef]
- Mori, E.; Lazzeri, L.; Ferretti, F.; Gordigiani, L.; Rubolini, D. The wild boar Sus scrofa as a threat to ground--nesting bird species: An artificial nest experiment. J. Zool. 2021, 314, 311–320. [Google Scholar] [CrossRef]
- Reading, C.J.; Luiselli, L.M.; Akani, G.C.; Bonnet, X.; Amori, G.; Ballouard, J.M.; Philippi, E.; Naulleau, G.; Pearson, D.; Rugiero, L. Are snake populations in widespread decline? Biol. Lett. 2010, 6, 777–780. [Google Scholar] [CrossRef]
- Ajtić, R.; Tomović, L.; Sterijovski, B.; Crnobrnja-Isailović, J.; Djordjević, S.; Djurakić, M.; Bonnet, X. Unexpected life history traits in a very dense population of dice snakes. Zool. Anz. 2013, 252, 350–358. [Google Scholar] [CrossRef]
- Folt, B.; Goessling, J.M.; Tucker, A.; Guyer, C.; Hermann, S.; Shelton--Nix, E.; McGowan, C. Contrasting patterns of demography and population viability among gopher tortoise populations in Alabama. J. Wildl. Manag. 2021, 85, 617–630. [Google Scholar] [CrossRef]
- Brischoux, F.; Bonnet, X.; Pinaud, D. Fine scale site fidelity in sea kraits: Implications for conservation. Biodivers. Conserv. 2009, 18, 2473–2481. [Google Scholar] [CrossRef]
- Wilcox, J.T. Implications of predation by wild pigs on native vertebrates: A case study. Calif. Fish Game 2015, 101, 72–77. [Google Scholar]
- Shine, R.; Bonnet, X. Snakes: A new ‘model organism’in ecological research? Trends Ecol. Evol. 2000, 15, 221–222. [Google Scholar] [CrossRef]
- Ward, R.J.; Griffiths, R.A.; Wilkinson, J.W.; Cornish, N. Optimising monitoring efforts for secretive snakes: A comparison of occupancy and N-mixture models for assessment of population status. Sci. Rep. 2017, 7, 18074. [Google Scholar] [CrossRef]
- Kaminski, G.; Brandt, S.; Baubet, E.; Baudoin, C. Life-history patterns in female wild boars (Sus scrofa): Mother–daughter postweaning associations. Can. J. Zool. 2005, 83, 474–480. [Google Scholar] [CrossRef]
- Lombardo, C.A.; Faulkner, K.R. Eradication of feral pigs (Sus scrofa) from Santa Rosa Island, Channel Islands National Park, California. In Proceedings of the Fifth California Islands Symposium; Santa Barbara Museum of Natural History: Santa Barbara, CA, USA, 2000; pp. 300–306. [Google Scholar]
- Servanty, S.; Gaillard, J.M.; Ronchi, F.; Focardi, S.; Baubet, E.; Gimenez, O. Influence of harvesting pressure on demographic tactics: Implications for wildlife management. J. Appl. Ecol. 2011, 48, 835–843. [Google Scholar] [CrossRef]
- Keuling, O.; Baubet, E.; Duscher, A.; Ebert, C.; Fischer, C.; Monaco, A.; Podgórski, T.; Prevot, C.; Ronnenberg, K.; Sodeikat, G.; et al. Mortality rates of wild boar Sus scrofa L. in central Europe. Eur. J. Wildl. Res. 2013, 59, 805–814. [Google Scholar] [CrossRef]
- Vajas, P.; Calenge, C.; Richard, E.; Fattebert, J.; Rousset, C.; Saïd, S.; Baubet, E. Many, large and early: Hunting pressure on wild boar relates to simple metrics of hunting effort. Sci. Total Environ. 2020, 698, 134251. [Google Scholar] [CrossRef] [PubMed]
- Donlan, C.J.; Howald, G.R.; Tershy, B.R.; Croll, D.A. Evaluating alternative rodenticides for island conservation: Roof rat eradication from the San Jorge Islands, Mexico. Biol. Conserv. 2003, 114, 29–34. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).