Population Genetic Structure and Phylogeography of Co-Distributed Pachymeniopsis Species (Rhodophyta) along the Coast of Korea and Japan
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coyer, J.A.; Hoarau, G.; Stam, W.T.; Olsen, J.L. Geographically specific heteroplasmy of mitochondrial DNA in the seaweed, Fucus serratus (Heterokontophyta: Phaeophyceae). Mol. Ecol. 2003, 13, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-M.; Li, J.-J.; Sun, Z.-M.; Oak, J.-H.; Zhang, J.; Fresia, P.; Grant, S.; Duan, D.-L. Phylogeographic structure and deep lineage diversification of the red alga Chondrus ocellatus Holmes in the Northwest Pacific. Mol. Ecol. 2015, 24, 5020–5033. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, J.; Hu, Z.-M.; Jueterbock, A.; Yotsukura, N.; Krupnova, T.N.; Nagasato, C.; Duan, D. Phylogeographic diversification and postglacial range dynamics shed light on the conservation of the kelp Saccharina japonica. Evol. Appl. 2018, 12, 791–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provan, J.; Bennett, K.D. Phylogeographic insights into cryptic glacial refugia. Trends Ecol. Evol. 2008, 23, 564–571. [Google Scholar] [CrossRef]
- Hewitt, G.M. Post-glacial re-colonization of European biota. Biol. J. Linn. Soc. 1999, 68, 87–112. [Google Scholar] [CrossRef]
- Hewitt, G. The genetic legacy of the Quaternary ice ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef]
- Provan, J.; Maggs, C.A. Unique genetic variation at a species’ rear edge is under threat from global climate change. Proc. R. Soc. B 2012, 279, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Neiva, J.; Assis, J.; Coelho, N.C.; Fernandes, F.; Pearson, G.A.; Serrão, E.A. Genes left behind: Climate change threatens cryptic genetic diversity in the canopy-forming seaweed Bifurcaria bifurcata. PLoS ONE 2015, 10, e0131530. [Google Scholar] [CrossRef]
- Assis, J.; Araújo, M.B.; Serrão, E.A. Projected climate changes threaten ancient refugia of kelp forests in the North Atlantic. Glob. Chang. Biol. 2018, 24, e55–e66. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.; Laepple, T.; Bartsch, I.; Wiencke, C. Impacts of oceanic waring on the distribution of seaweeds in polar and cold-temperate waters. Bot. Mar. 2009, 52, 617–638. [Google Scholar] [CrossRef]
- Jueterbock, A.; Tyberghein, L.; Verbruggen, H.; Coyer, J.A.; Olsen, J.L.; Hoarau, G. Climate change impact on seaweed meadow distribution in the North Atlantic rocky intertidal. Ecol. Evol. 2013, 3, 1356–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neiva, J.; Assis, J.; Fernandes, F.; Pearson, G.A.; Serrão, E.A. Species distribution models and mitochondrial DNA phylogeography suggest an extensive biogeographical shift in the high-intertidal seaweed Pelvetia canaliculata. J. Biogeogr. 2014, 41, 1137–1148. [Google Scholar] [CrossRef]
- Smale, D.A.; Wernberg, T. Extreme climatic event drives range contraction of a habitat-forming species. Proc. R. Soc. B 2013, 280, 20122829. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Bowes, G.; Ross, C.; Zhang, X.-H. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob. Chang. Biol. 2013, 19, 103–132. [Google Scholar] [CrossRef]
- Kerswell, A.P. Global biodiversity patterns of benthic marine algae. Ecology 2006, 87, 2479–2488. [Google Scholar] [CrossRef]
- Ni, G.; Li, Q.; Kong, L.; Yu, H. Comparative phylogeography in marginal seas of the northwestern Pacific. Mol. Ecol. 2014, 23, 534–548. [Google Scholar] [CrossRef]
- Hu, Z.-M.; Li, J.-J.; Sun, Z.-M.; Gao, X.; Yao, J.-T.; Choi, H.-G.; Endo, H.; Duan, D.-L. Hidden diversity and phylogeographic history provide conservation insights for the edible seaweed Sargassum fusiforme in the Northwest Pacific. Evol. Appl. 2017, 10, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Barkely, R.A. The Kuroshio Current. Science 1970, 6, 54–60. [Google Scholar]
- Avise, J.C. What is the field of biogeography, and where is it going? Taxon 2004, 53, 893–898. [Google Scholar] [CrossRef]
- Kim, K.M.; Hoarau, G.G.; Boo, S.M. Genetic structure and distribution of Gelidium elegans (Gelidiales, Rhodophyta) in Korea based on mitochondrial cox1 sequence data. Aquat. Bot. 2012, 98, 27–33. [Google Scholar] [CrossRef]
- Hanyuda, T.; Yamamura, K.; Boo, G.H.; Miller, K.A.; Vinogradova, K.L.; Kawai, H. Identification of true Gloiopeltis furcata (Gigartinales, Rhodophyta) and preliminary analysis of its biogeography. Phycol. Res. 2019, 68, 161–168. [Google Scholar] [CrossRef]
- Yang, M.Y.; Yang, E.C.; Kim, M.S. Genetic diversity hotspot of the amphi-Pacific macroalga Gloiopeltis furcata sensu lato (Gigartinales, Florideophyceae). J. Appl. Phycol. 2020, 32, 2515–2522. [Google Scholar] [CrossRef]
- Yang, M.Y.; Fujita, D.; Kim, M.S. Phylogeography of Gloiopeltis furcata sensu lato (Gigartinales, Rhodophyta) provides the evidence of glacial refugia in Korea and Japan. Algae 2021, 36, 13–24. [Google Scholar] [CrossRef]
- Zhong, K.-L.; Song, X.-H.; Choi, H.-G.; Satochi, S.; Weinberger, F.; Draisma, S.G.A.; Duan, D.-L.; Hu, Z.-M. MtDNA-based phylogeography of the red alga Agarophyton vermiculophyllum (Gigartinales, Rhodophyta) in the native northwest Pacific. Front. Mar. Sci. 2020, 7, 366. [Google Scholar] [CrossRef]
- Gargiulo, G.M.; Marobito, M.; Manghisi, A. A re-assessment of reproductive anatomy and postfertilization development in the systematics of Grateloupia (Halymeniales, Rhodophyta). Cryptogam Algol. 2013, 34, 3–35. [Google Scholar] [CrossRef]
- Okamura, K. Icons of Japanese Algae; Kazamashobo: Tokyo, Japan, 1934; Volume 7, No 5; pp. 39–48, pls 321–325. [Google Scholar]
- Kawaguchi, S. Taxonomic notes on the Halymeniaceae (Gigartinales, Rhodophyta) from Japan. III. Synonymization of Pachymeniopsis Yamada in Kawabata with Grateloupia C. Agarch. Phycol. Res. 1997, 45, 9–21. [Google Scholar] [CrossRef]
- Wang, H.W.; Kawaguchi, S.; Horiguchi, T.; Masuda, M. A morphological and molecular assessment of the genus Prionitis J. Agardh (Halymeniaceae, Rhodophyta). Phycol. Res. 2001, 49, 251–261. [Google Scholar] [CrossRef]
- Wilkes, R.J.; Morabito, M.; Gargiulo, G.M. Taxonomic considerations of a foliose Grateloupia species from the Strait of Messina. J. Appl. Phycol. 2006, 18, 663–669. [Google Scholar] [CrossRef]
- De Clerck, O.; Gavio, B.; Fredericq, S.; Bárbara, I.; Coppejans, E. Systematics of Grateloupia filicina (Halymeniaceae, Rhodophyta), based on rbcL sequence analyses and morphological evidence, including the reinstatement of G. minima and the description of G. capensis sp. nov. J. Phycol. 2005, 41, 391–410. [Google Scholar] [CrossRef]
- Calderon, M.S.; Boo, G.H.; Boo, S.M. Neorubra decipiens gen. & comb. nov. and Phyllymenia lancifolia comb. nov. (Halymeniales, Rhodophyta) from South America. Phycologia 2014, 53, 409–422. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: http://www.algaebase.org (accessed on 2 March 2021).
- Yoshida, T. Marine Algae of Japan; Uchida Rokakuho Publishing Co., Ltd.: Tokyo, Japan, 1998; pp. 1–1222. [Google Scholar]
- Lee, Y. Marine algae of Jeju; Academy Publication: Seoul, Korea, 2008; pp. 1–477. [Google Scholar]
- Kim, S.Y.; Manghisi, A.; Morabito, M.; Yang, E.C.; Yoon, H.S.; Miller, K.A.; Boo, S.M. Genetic diversity and haplotype distribution of Pachymeniopsis gargiuli sp. nov. and P. lanceolata (Halymeniales, Rhodophyta) in Korea, with notes on their non-native distributions. J. Phycol. 2014, 50, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.B.; Lee, I.K. A taxonomic study on the genus Pachymeniopsis (Halymeniaceae Rhodophyta) in Korea. Korean J. Phycol. 1993, 8, 55–65. [Google Scholar]
- Miller, K.A.; Hughey, J.R.; Gabrielson, P.W. First report of the Japanese species Grateloupia lanceolata (Halymeniaceae, Rhodophyta) from California, USA. Phycol. Res. 2009, 57, 238–241. [Google Scholar] [CrossRef]
- Verlaque, M.; Mrannock, P.M.; Komatsu, T.; Villalard-Bohnsack, M.; Marston, M. The genus Grateloupia C. Agarch (Halymeniaceae, Rhodophyta) in the Thau Lagoon (France, Mediterranean): A case study of marine plurispecific introductions. Phycologia 2005, 44, 477–496. [Google Scholar] [CrossRef]
- Burel, T.; Le Duff, M.; Ar Gall, E. Updated check-list of the seaweeds of the French coasts, Channel and Atlantic Ocean. An aod. Les Cahiers Naturalistes de l’Observatoire Marin Brest. 2019, pp. 1–38, 1 Figure, 2 Tables. Available online: https://www-iuem.univ-brest.fr/observatoire/l-observatoire/ressources/cahiers-naturalistes/AnAod_2019_VII_1_pp_1_38.pdf (accessed on 18 July 2021).
- Yang, M.Y.; Han, E.G.; Kim, M.S. Molecular identification of Grateloupia elliptica and G. lanceolata (Rhodophyta) inferred from plastid rbcL and mitochondrial COI genes sequence data. Genes Genom. 2013, 35, 239–246. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, S.Y.; Nelson, W. Symphyocladia lithophila sp. nov. (Rhodomelaceae, Ceramiales), a new Korean red algal species based on morphology and rbcL sequences. Bot. Mar. 2010, 53, 233–241. [Google Scholar] [CrossRef]
- Saunders, G.W. A DNA barcode examination of the red algal family Dumontiaceae in Canadian waters reveals substantial cryptic species diversity. 1. The foliose Dilsea-Neodilsea complex and Weeksia. Botany 2008, 86, 773–789. [Google Scholar] [CrossRef]
- McCarthy, C. Chromas Version 1.45; School of Health Science, Griffith University: Southport, Australia, 1998. [Google Scholar]
- Yang, M.Y.; Kim, M.S. Taxonomy of Grateloupia (Halymeniales, Rhodophyta) by DNA barcode marker analysis and a description of Pachymeiopsis volvita sp. nov. J. Appl. Phycol. 2015, 27, 1373–1384. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver. 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Res. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 1997, 147, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Freshwater, D.W.; Tudor, K.; O’Shaughnessy, K.; Wysor, B. DNA barcoding in the red algal order Gelidiales: Comparison of COI with rbcL and verification of the “barcoding gap”. Cryptogam Algol. 2010, 31, 435–449. [Google Scholar]
- Kitamura, A.; Takano, O.; Takata, H.; Omete, H. Late Pliocene-early Pleistocene paleoceanographic evolution of the Sea of Japan. Palaeogeogr Palaeoclim. Palaeoecol. 2001, 172, 81–98. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.-M.; Uwai, S.; Yu, S.-H.; Komatsu, T.; Ajisaka, T.; Duan, D.-L. Phylogeographic heterogeneity of the brown macroalga Sargassum horneri (Fucaceae) in the northwestern Pacific in relation to late Pleistocene glaciation and tectonic configurations. Mol. Ecol. 2011, 20, 3894–3909. [Google Scholar] [CrossRef]
- Hu, Z.-M.; Kantachumpoo, A.; Liu, R.-Y.; Sun, Z.-M.; Yao, J.-T.; Komatsu, T.; Uwai, S.; Duan, D.-L. A late Pleistocene marine glacial refugium in the south-west of Hainan Island, China: Phylogeographical insights from the brown alga Sargassum polycystum. J. Biogeogra 2018, 45, 355–366. [Google Scholar] [CrossRef]
- Wang, P. Response of western Pacific marginal seas to glacial cycles: Paleoceanographic and sedimentological features. Mar. Geol. 1999, 156, 5–39. [Google Scholar] [CrossRef]
- Cheang, C.C.; Chu, K.H.; Ang, P.O., Jr. Phylogeography of the marine macroalga Sargassum hemiphyllum (Phaeophyceae, Heterokontophyta) in northwestern Pacific. Mol. Ecol. 2010, 19, 2933–2948. [Google Scholar] [CrossRef]
- Grant, W.S.; Bowen, B.W. Shallow population histories in deep evolutionary lineages of marine fishes: Insights from sardines and anchovies and lessons for conservation. J. Hered 1998, 89, 415–426. [Google Scholar] [CrossRef]
- Avise, J.C. Phylogeography, the History and Formation of Species; Harvard University Press: Cambridge, UK, 2000; pp. 1–447. [Google Scholar]
- Posada, D.; Crandall, K.A. Intraspecific gene genealogies: Trees grafting into networks. Trends Ecol. Evol. 2001, 16, 37–45. [Google Scholar] [CrossRef]
- Matocq, M.; Villablanca, F. Low genetic diversity in an endangered species: Recent or historic pattern? Biol. Conserv. 2001, 98, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Benzie, J.A.H. Population genetic structure in penaeid prawns. Aquat. Res. 2000, 31, 95–119. [Google Scholar] [CrossRef]
- Stamatis, C.; Triantafyllidis, A.; Moutou, K.A.; Mamuris, Z. Mitochondrial DNA variation in Northeast Atlantic and Mediterranean populations of Norway lobster, Nephrops norvegicus. Mol. Ecol. 2004, 13, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Hoarau, G.; Coyer, J.A.; Veldsink, J.H.; Stam, W.T.; Olsen, J.L. Glacial refugia and recolonization pathways in the brown seaweed Fucus serratus. Mol. Ecol. 2007, 16, 3606–3616. [Google Scholar] [CrossRef]
- Roderick, G.K. Geographic structure of insect populations: Gene flow, phylogeography, and their uses. Annu. Rev. Entomol. 1996, 41, 325–352. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.E.; Grave, S.D.; Daniels, S.R. Phylogeographic patterning among two codistributed shrimp species (Crustacea: Decapoda: Palaemonidae) reveals high levels of connectivity across biogeographic regions along the South African coast. PLoS ONE 2017, 12, e0173356. [Google Scholar] [CrossRef]
- Han, I.S.; Lee, J.-S. Change the annual amplitude of sea surface temperature due to climate change in a recent decade around the Korean Peninsula. Korean Soc. Mar. Environ. Saf. 2020, 26, 233–241. [Google Scholar]
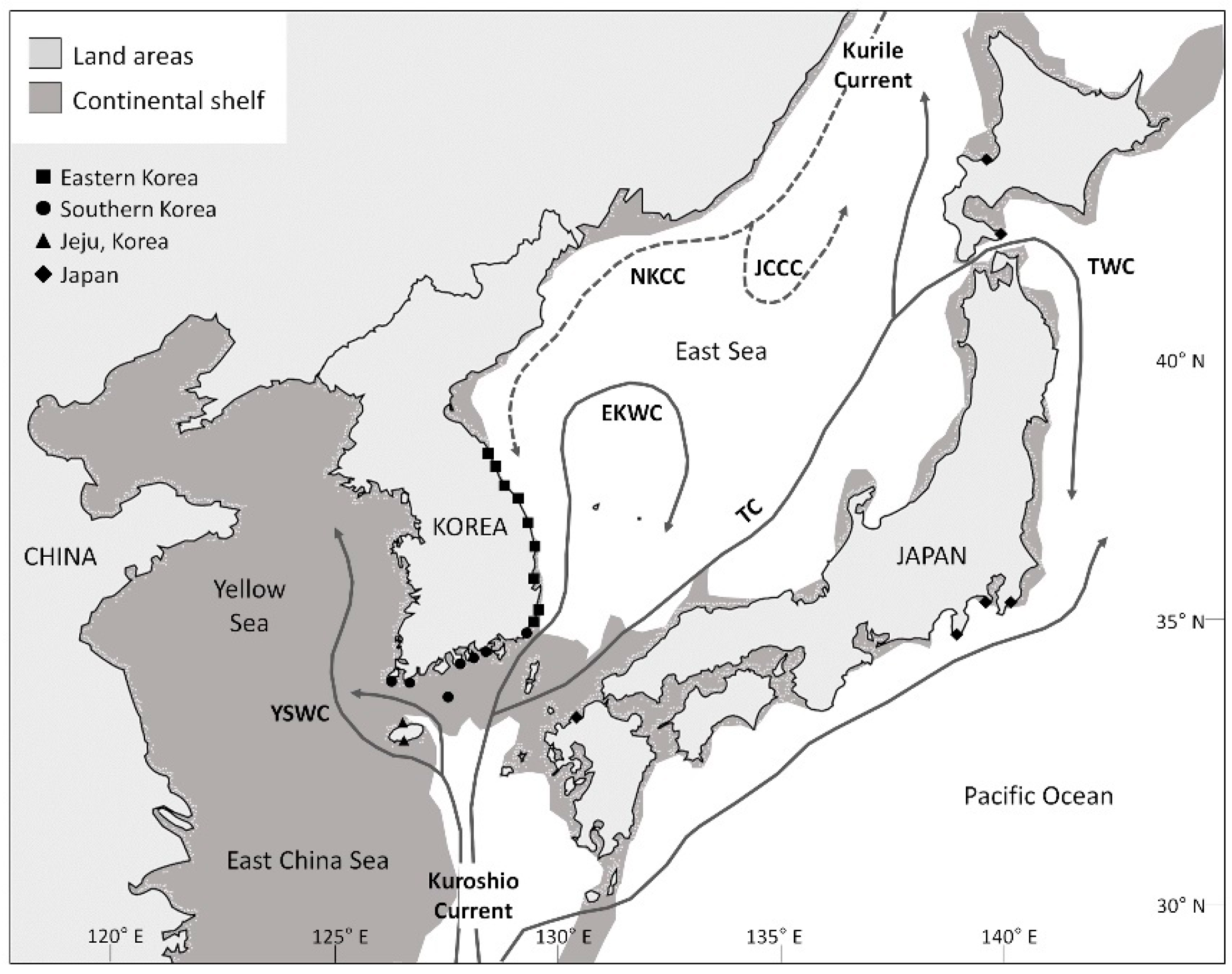
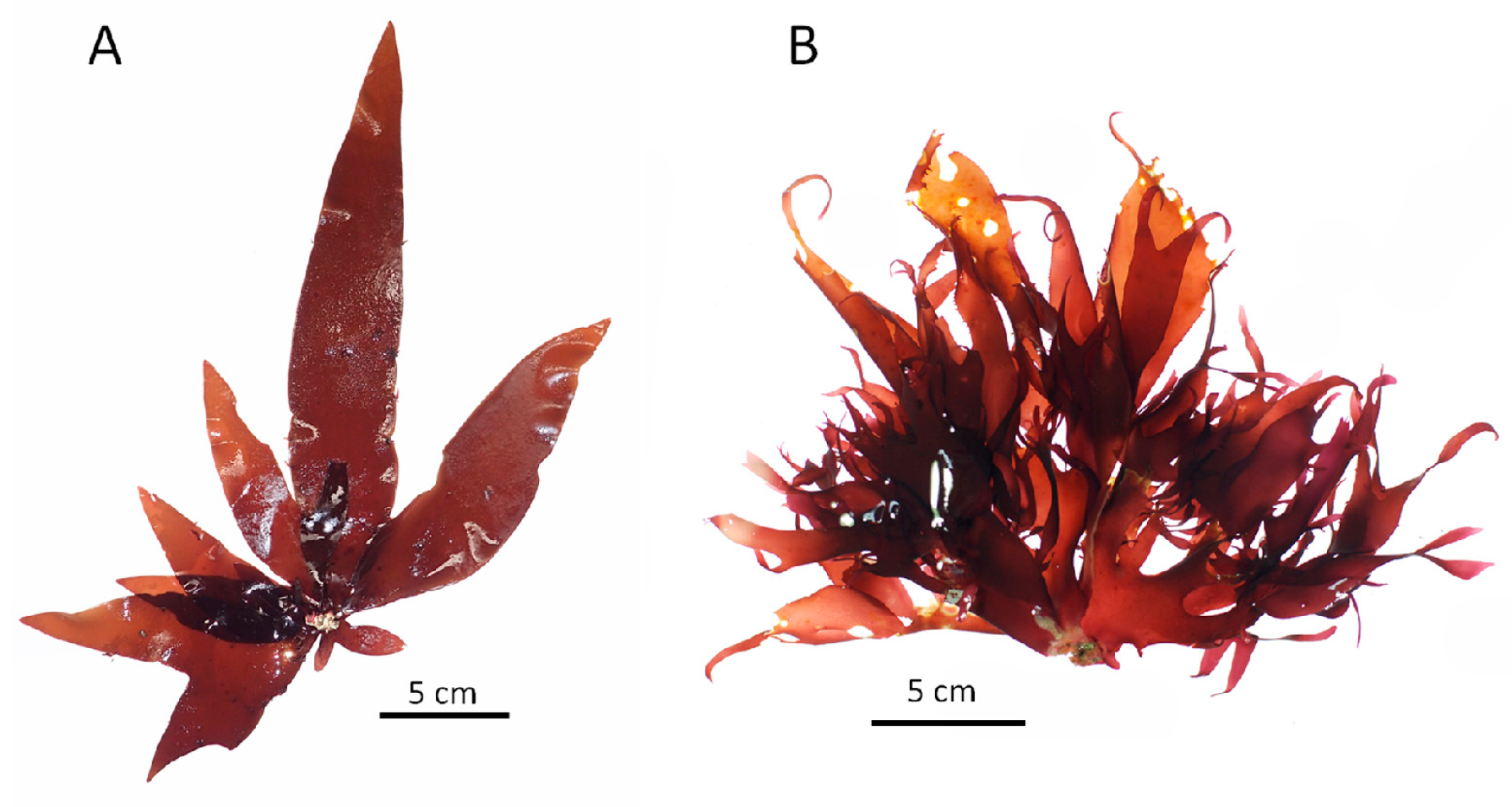
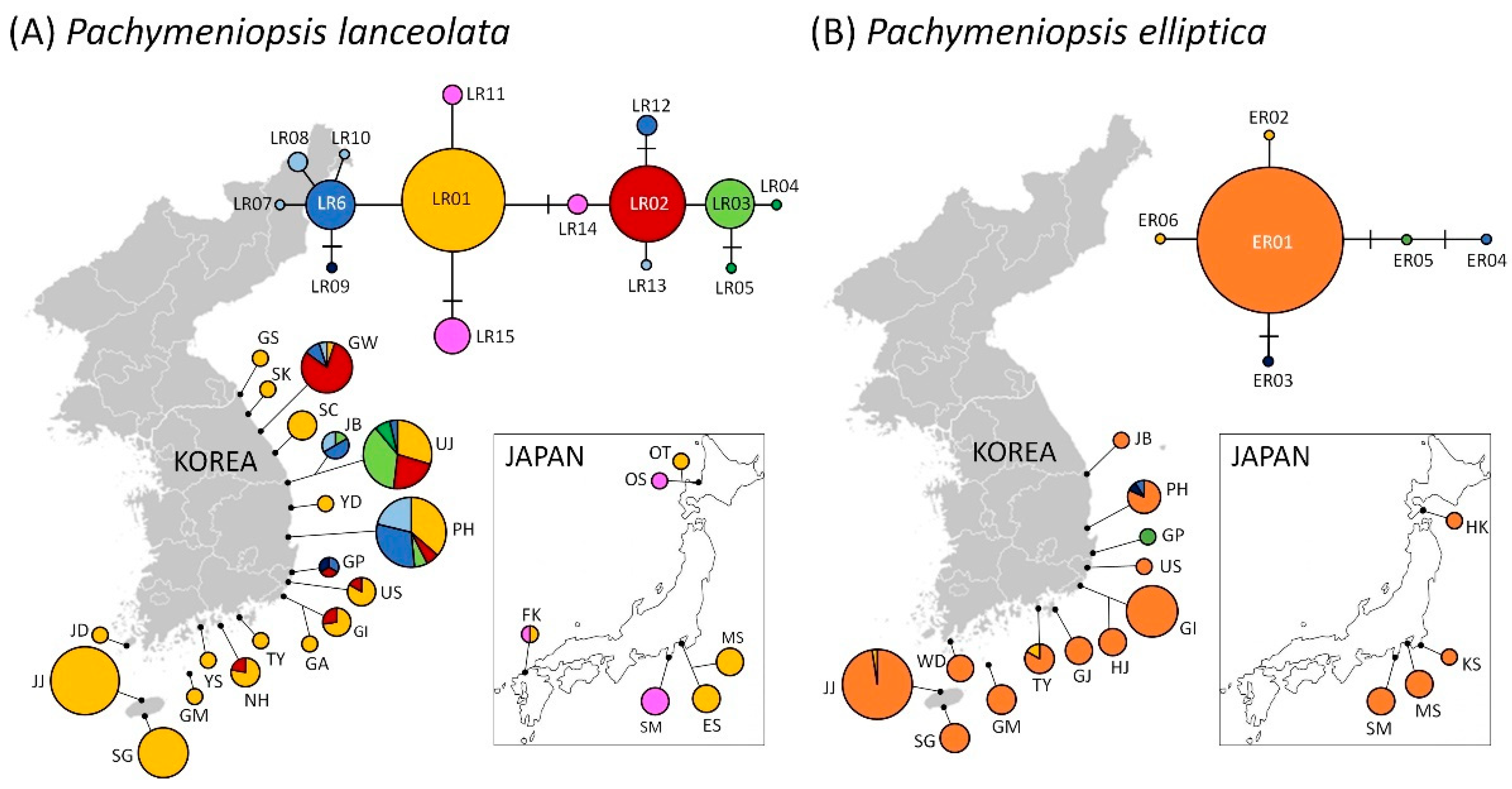
| Region | Pachymeniopsis lanceolata | Pachymeniopsis elliptica | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Nh | h | π | Tajima’s D | Fu’s Fs | N | Nh | h | π | Tajima’s D | Fu’s Fs | |
| Total | 215 | 15 | 0.624 | 0.00143 | −0.6827 | −3.9598 * | 138 | 6 | 0.071 | 0.00012 | − | − |
| Korea | 191 | 10 | 0.609 | 0.00143 | −0.4063 | −1.9044 | 128 | 6 | 0.076 | 0.00013 | − | − |
| Eastern Korea | 127 | 12 | 0.752 | 0.00179 | −0.0801 | −1.4365 | 38 | 4 | 0.153 | 0.00034 | − | − |
| Southern Korea | 17 | 2 | 0.308 | 0.00077 | − | − | 37 | 2 | 0.054 | 0.00005 | − | − |
| Jeju | 47 | 1 | − | − | − | − | 53 | 2 | 0.037 | 0.00003 | − | − |
| Japan | 24 | 4 | 0.634 | 0.00103 | 0.4288 | 0.7110 | 10 | 1 | − | − | − | − |
| Source of Variation | d.f. 1 | Percentage of Variation | Φ Statistic | p-Value |
|---|---|---|---|---|
| P. lanceolata | ||||
| Among groups | 3 | 15.25 | FCT = 0.1525 | 0.01369 |
| Among populations within groups | 32 | 32.74 | FSC = 0.3861 | <0.00001 |
| Within populations | 176 | 52.02 | FST = 0.4798 | <0.00001 |
| P. elliptica | ||||
| Among groups | 3 | −7.54 | FCT = −0.0756 | 0.41349 |
| Among populations within groups | 20 | 34.85 | FSC = 0.3244 | 0.00293 |
| Within populations | 108 | 72.69 | FST = 0.2733 | 0.00684 |
| Eastern Korea | Southern Korea | Jeju | Japan | |
|---|---|---|---|---|
| Eastern Korea | ‒ | 0.0232 | 0.0356 * | −0.0304 |
| Southern Korea | 0.0834 * | ‒ | 0.0015 | −0.0489 |
| Jeju | 0.2604 * | 0.2597 * | ‒ | 0.3541 * |
| Japan | 0.1670 * | 0.0940 * | 0.3541 * | −0.0508 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.Y.; Kim, S.Y.; Kim, M.S. Population Genetic Structure and Phylogeography of Co-Distributed Pachymeniopsis Species (Rhodophyta) along the Coast of Korea and Japan. Diversity 2021, 13, 336. https://doi.org/10.3390/d13080336
Yang MY, Kim SY, Kim MS. Population Genetic Structure and Phylogeography of Co-Distributed Pachymeniopsis Species (Rhodophyta) along the Coast of Korea and Japan. Diversity. 2021; 13(8):336. https://doi.org/10.3390/d13080336
Chicago/Turabian StyleYang, Mi Yeon, Su Yeon Kim, and Myung Sook Kim. 2021. "Population Genetic Structure and Phylogeography of Co-Distributed Pachymeniopsis Species (Rhodophyta) along the Coast of Korea and Japan" Diversity 13, no. 8: 336. https://doi.org/10.3390/d13080336
APA StyleYang, M. Y., Kim, S. Y., & Kim, M. S. (2021). Population Genetic Structure and Phylogeography of Co-Distributed Pachymeniopsis Species (Rhodophyta) along the Coast of Korea and Japan. Diversity, 13(8), 336. https://doi.org/10.3390/d13080336






