Effects of Continuous Cropping of Codonopsis tangshen on Rhizospheric Soil Bacterial Community as Determined by Pyrosequencing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiment and Soil Sampling
2.2. Measurement of Yield and Lobetyolin Content
2.3. Soil Chemical Characteristics
2.4. DNA Extraction, PCR and 454 Pyrosequencing
2.5. Operational Taxonomic Unit (OTU)-Based Sequence Analysis
2.6. Statistical Analyses
3. Results
3.1. Yield and Lobetyolin Content
3.2. Soil Chemical Properties
3.3. Bacterial Community Composition
3.4. Alpha Diversity Indices
3.5. Bacterial Community Structure
3.6. Shifts in Soil Bacterial Community Composition
3.7. Effects of Soil Chemical Properties on Bacterial Community Abundance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, B.B.; Zhang, J.H. Compatibility of Codonopsis Radix with Different Herbs. Chin. J. Exp. Tradit. Med. Formulae 2016. [Google Scholar] [CrossRef]
- Zhao, W.O.; Pang, L.; Dong, N.; Yang, S. LC-ESI-MS/MS analysis and pharmacokinetics of heterophyllin B, a cyclic octapeptide from Pseudostellaria heterophylla in rat plasma. Biomed. Chromatogr. BMC 2015, 29, 1693–1699. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, Y.N.; Fan, Q.; Han, Q.Q.; Paré, P.W.; Xu, R.; Wang, Y.Q.; Wang, S.M.; Zhang, J.L. Improved Growth and Metabolite Accumulation in Codonopsis pilosula (Franch.) Nannf. by Inoculation of Bacillus amyloliquefaciens GB03. J. Agric. Food Chem. 2016, 64, 8103. [Google Scholar] [CrossRef]
- Wang, Z.T.; Ng, T.B.; Yeung, H.W.; Xu, G.J. Immunomodulatory effect of a polysaccharide-enriched preparation of Codonopsis pilosula roots. Gen. Pharmacol. Vasc. Syst. 1996, 27, 1347–1350. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, L.; Chu, L.; Yang, Y.; Li, Z.; Azeem, S.; Zhang, Z.; Fang, C.; Lin, W. Interaction of Pseudostellaria heterophylla with Fusarium oxysporum f.sp. heterophylla mediated by its root exudates in a consecutive monoculture system. Sci. Rep. 2015, 5, 8197. [Google Scholar] [CrossRef]
- Wu, L.; Wang, H.; Zhang, Z.; Lin, R.; Lin, W. Comparative metaproteomic analysis on consecutively Rehmannia glutinosa-monocultured rhizosphere soil. PLoS ONE 2011, 6, e20611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.F.; Song, L.X.; Xia, X.J.; Mao, W.H.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Plant-Soil Feedbacks and Soil Sickness: From Mechanisms to Application in Agriculture. J. Chem. Ecol. 2013, 39, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Raza, W.; Yang, X.; Jiang, H.; Huang, Q.; Xu, Y.; Liu, X.; Wei, R.; Shen, Q. Control of Fusarium wilt disease of cucumber plants with the application of a bioorganic fertilizer. Biol. Fertil. Soils 2008, 44, 1073. [Google Scholar] [CrossRef]
- Harleen, K.; Rajwant, K.; Surinder, K.; Baldwin, I.T. Taking ecological function seriously: Soil microbial communities can obviate allelopathic effects of released metabolites. PLoS ONE 2009, 4, e4700. [Google Scholar]
- el Zahar Haichar, F.; Marol, C.; Berge, O.; Rangel-Castro, J.I.; Prosser, J.I.; Balesdent, J.; Heulin, T.; Achouak, W. Plant host habitat and root exudates shape soil bacterial community structure. ISME J. 2008, 2, 1221–1230. [Google Scholar] [CrossRef]
- Trivedi, P.; He, Z.; Van Nostrand, J.D.; Albrigo, G.; Zhou, J.; Wang, N. Huanglongbing alters the structure and functional diversity of microbial communities associated with citrus rhizosphere. ISME J. 2012, 6, 363–383. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.J.; Bending, G.D.; Chandler, D.; Hilton, S.; Mills, P. Meeting the demand for crop production: The challenge of yield decline in crops grown in short rotations. Biol. Rev. 2012, 87, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Bramley, R.; Ellis, N.; Nable, R.O.; Garside, A.L. Changes in soil chemical properties under long-term sugar cane monoculture and their possible role in sugar yield decline. Aust. J. Soil Res. 1996, 34, 967. [Google Scholar] [CrossRef]
- Wu, L.; Li, Z.; Ji, L.; Khan, M.A.; Huang, W.; Zhang, Z.; Lin, W. Assessment of shifts in microbial community structure and catabolic diversity in response to Rehmannia glutinosa monoculture. Appl. Soil Ecol. 2013, 67, 1–9. [Google Scholar] [CrossRef]
- Wu, L.; Chen, J.; Wu, H.; Wang, J.; Wu, Y.; Lin, S.; Khan, M.U.; Zhang, Z.; Lin, W. Effects of consecutive monoculture of Pseudostellaria heterophyllaon soil fungal community as determined by pyrosequencing. Sci. Rep. 2016, 6, 26601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Yu, G.; Wu, F. Soil phenolics in a continuously monocropped cucumber (Cucumis sativus L.) system and their effects on cucumber seedling growth and soil microbial communities. Eur. J. Soil Sci. 2012, 63, 332–340. [Google Scholar] [CrossRef]
- Li, X.; Ding, C.; Ke, H.; Zhang, T.; Zhang, Y.; Ling, Z.; Yang, Y.; Liu, J.; Wang, X. Soil sickness of peanuts is attributable to modifications in soil microbes induced by peanut root exudates rather than to direct allelopathy. Soil Biol. Biochem. 2014, 78, 149–159. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.G.; Xin, Y.H. High diversity and distinctive community structure of bacteria on glaciers in China revealed by 454 pyrosequencing. Syst. Appl. Microbiol. 2015, 38, 578–585. [Google Scholar] [CrossRef]
- Song, D.; Cheng, X.M.; Long-Yun, L.I.; Zhong, G.Y.; Wang, Z.T. Determination of lobetyolin in root of Codonopsis tangshen from various cultivation areas by high-performance liquid chromatography. Zhongguo Zhong Yao Za Zhi 2008, 33, 2133–2135. [Google Scholar]
- Hedlund, A.; Witter, E.; An, B.X. Assessment of N, P and K management by nutrient balances and flows on peri-urban smallholder farms in southern Vietnam. Eur. J. Agron. 2003, 20, 71–87. [Google Scholar] [CrossRef]
- Bentonjonesjr, J. Soil testing in the united states. Commun. Soil Sci. Plant. Anal. 1973, 4, 307–322. [Google Scholar]
- Tanja, M.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar]
- Tan, G.Y.; Yang, Z.L.; Yuan, Z.L.; Yang, X. Research advances in continuous cropping obstacle in medicinal plants and its management. J. Northwest A F Univ. 2012, 40, 197–204. [Google Scholar]
- Chen, C.B.; Liu, J.Y.; Wang, Y.Y.; Yan, S.; Xu, S.Q. Allelopathy of Ginseng Rhizosphere and Its Effect on Germination of Seed. J. Jilin Agric. Univ. 2006, 28, 534–541. [Google Scholar]
- Sun, M.; Ye, L.; Zhang, Z. Progress on the Cause of Continuous Cropping Obstacle of Panax Notoginseng and its Countermeasures. J. Mt. Agric. Biol. 2015, 40, 197–204. [Google Scholar]
- Zhang, C.L.; Sun, Q.; Qing, Y.E. Obstacle Effect of Continuous Cropping on Salvia miltiorrhiza Growth. Acta Bot. Boreali-Occident. Sin. 2005, 25, 1029–1034. [Google Scholar]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Heikki, S.L.; Putten, W.H.; Van Der Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Haney, C.H.; Ausubel, F.M. Microbiome. Plant microbiome blueprints. Science 2015, 349, 788–789. [Google Scholar] [CrossRef] [PubMed]
- Heijden, M.G.A.; Van Der Bardgett, R.D.; van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2010, 11, 296–310. [Google Scholar] [CrossRef]
- Santhanam, R.; Luu, V.T.; Weinhold, A.; Goldberg, J.; Oh, Y.; Baldwin, I.T. Native root-associated bacteria rescue a plant from a sudden-wilt disease that emerged during continuous cropping. Proc. Natl. Acad. Sci. USA 2015, 112, E5013. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Ravnskov, S.; Larsen, J.; Nilsson, R.H.; Nicolaisen, M. Soil fungal community structure along a soil health gradient in pea fields examined using deep amplicon sequencing. Soil Biol. Biochem. 2012, 46, 26–32. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, F. Dynamics of the diversity of fungal and Fusarium communities during continuous cropping of cucumber in the greenhouse. FEMS Microbiol. Ecol. 2012, 80, 469–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Wu, X.; Wu, T.; Dai, W.; Liu, H.; Yang, R. Analysis of unculturable bacterial communities in tea orchard soils based on nested PCR-DGGE. World J. Microbiol. Biotechnol. 2012, 28, 1967–1979. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Jiang, Y.; Weng, B.; Lin, W. Variations of rhizosphere bacterial communities in tea (Camellia sinensis L.) continuous cropping soil by high-throughput pyrosequencing approach. J. Appl. Microbiol. 2016, 121, 787–799. [Google Scholar] [CrossRef]
- Heiko, N.; Andrea, T.; Antje, W.; Christiane, W.; Ladislav, H.; Nadine, H.; Ingo, S.N.; Marion, S.; Rolf, D. Pyrosequencing-based assessment of bacterial community structure along different management types in German forest and grassland soils. PLoS ONE 2011, 6, e17000. [Google Scholar]
- Shen, Z.; Wang, D.; Ruan, Y.; Xue, C.; Zhang, J.; Li, R.; Shen, Q. Deep 16S rRNA Pyrosequencing Reveals a Bacterial Community Associated with Banana Fusarium Wilt Disease Suppression Induced by Bio-Organic Fertilizer Application. PLoS ONE 2014, 9, e98420. [Google Scholar] [CrossRef]
- Noah, F.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar]
- Xiong, W.; Zhao, Q.; Zhao, J.; Xun, W.; Li, R.; Zhang, R.; Wu, H.; Shen, Q. Different Continuous Cropping Spans Significantly Affect Microbial Community Membership and Structure in a Vanilla-Grown Soil as Revealed by Deep Pyrosequencing. Microb. Ecol. 2015, 70, 209–218. [Google Scholar] [CrossRef]
- Xiong, W.; Li, Z.; Liu, H.; Xue, C.; Zhang, R.; Wu, H.; Li, R.; Shen, Q. The Effect of Long-Term Continuous Cropping of Black Pepper on Soil Bacterial Communities as Determined by 454 Pyrosequencing. PLoS ONE 2015, 10, e0136946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, M.; Marco, K.; Irene, D.B.; Ester, D.; Menno, V.D.V.; Schneider, J.H.M.; Piceno, Y.M.; Desantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar]
- Rumberger, A.; Merwin, I.A.; Thies, J.E. Microbial community development in the rhizosphere of apple trees at a replant disease site. Soil Biol. Biochem. 2007, 39, 1645–1654. [Google Scholar] [CrossRef]
- Aravind, R.; Kumar, A.; Eapen, S.J.; Ramana, K.V. Endophytic bacterial flora in root and stem tissues of black pepper (Piper nigrum L.) genotype: Isolation, identification and evaluation against Phytophthora capsici. Lett. Appl. Microbiol. 2010, 48, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ding, C.; Zhang, T.; Wang, X. Fungal pathogen accumulation at the expense of plant-beneficial fungi as a consequence of consecutive peanut monoculturing. Soil Biol. Biochem. 2014, 72, 11–18. [Google Scholar] [CrossRef]
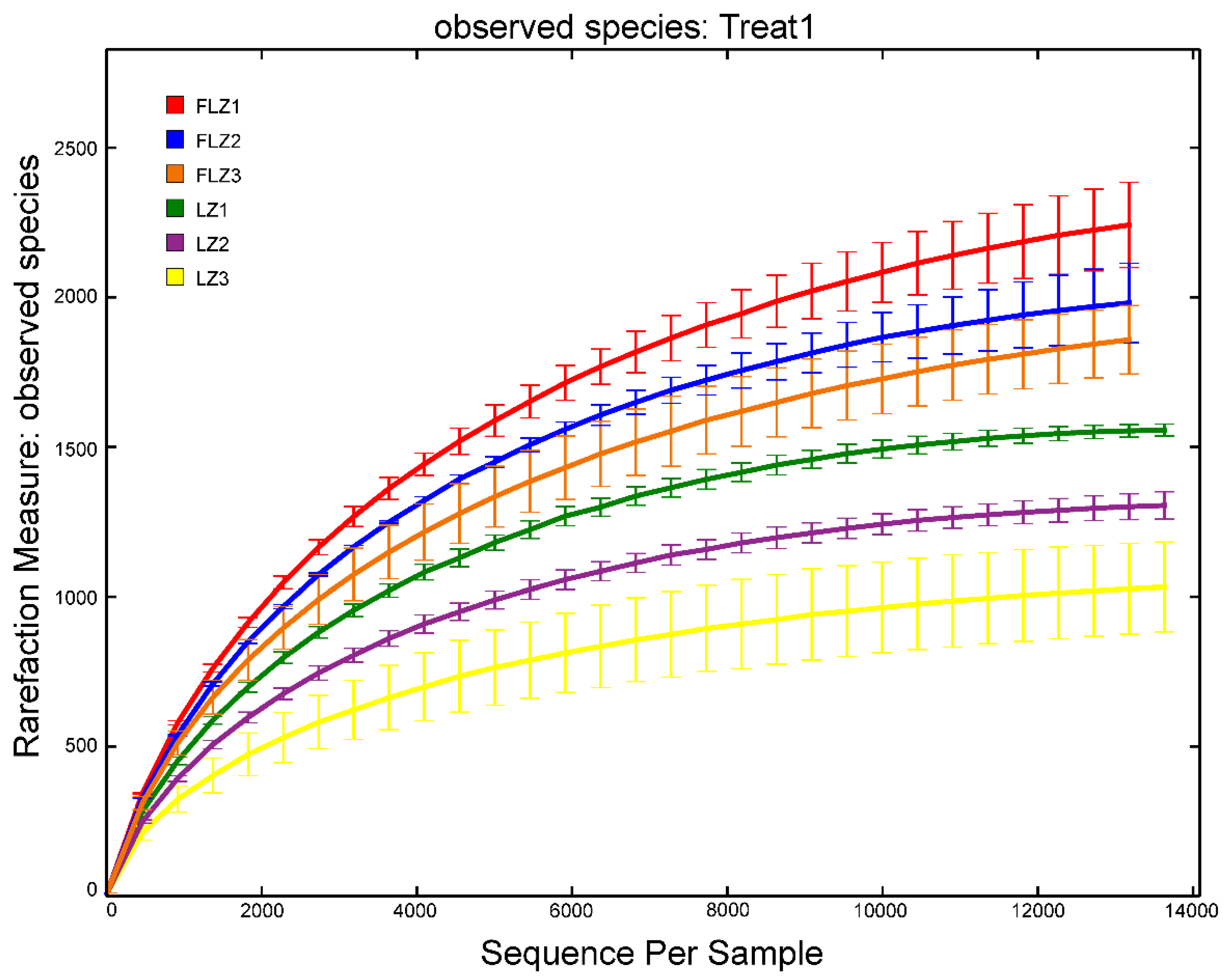
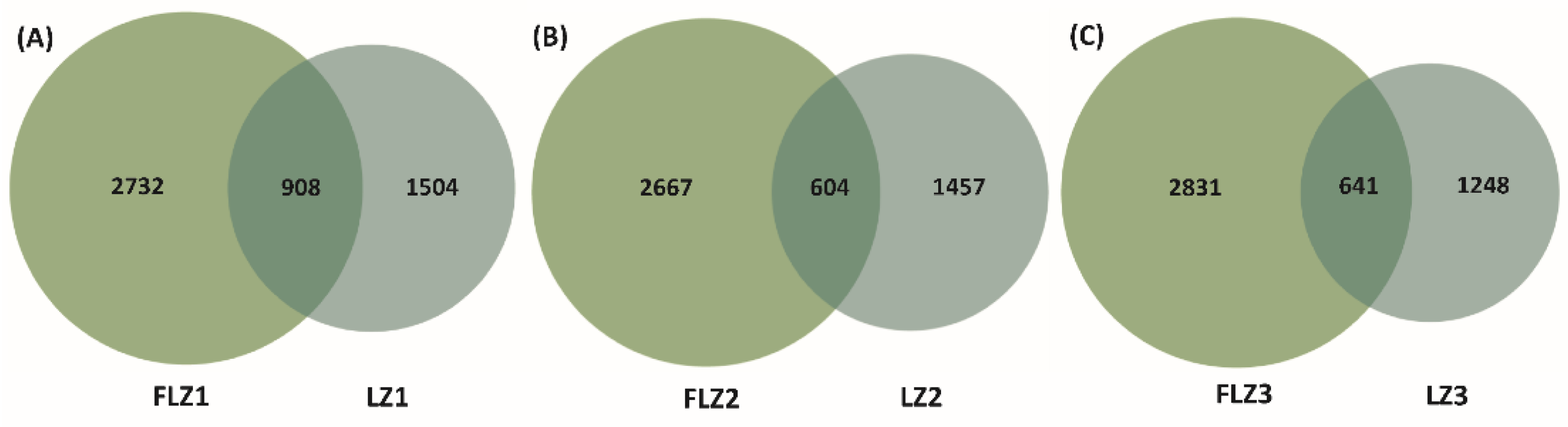
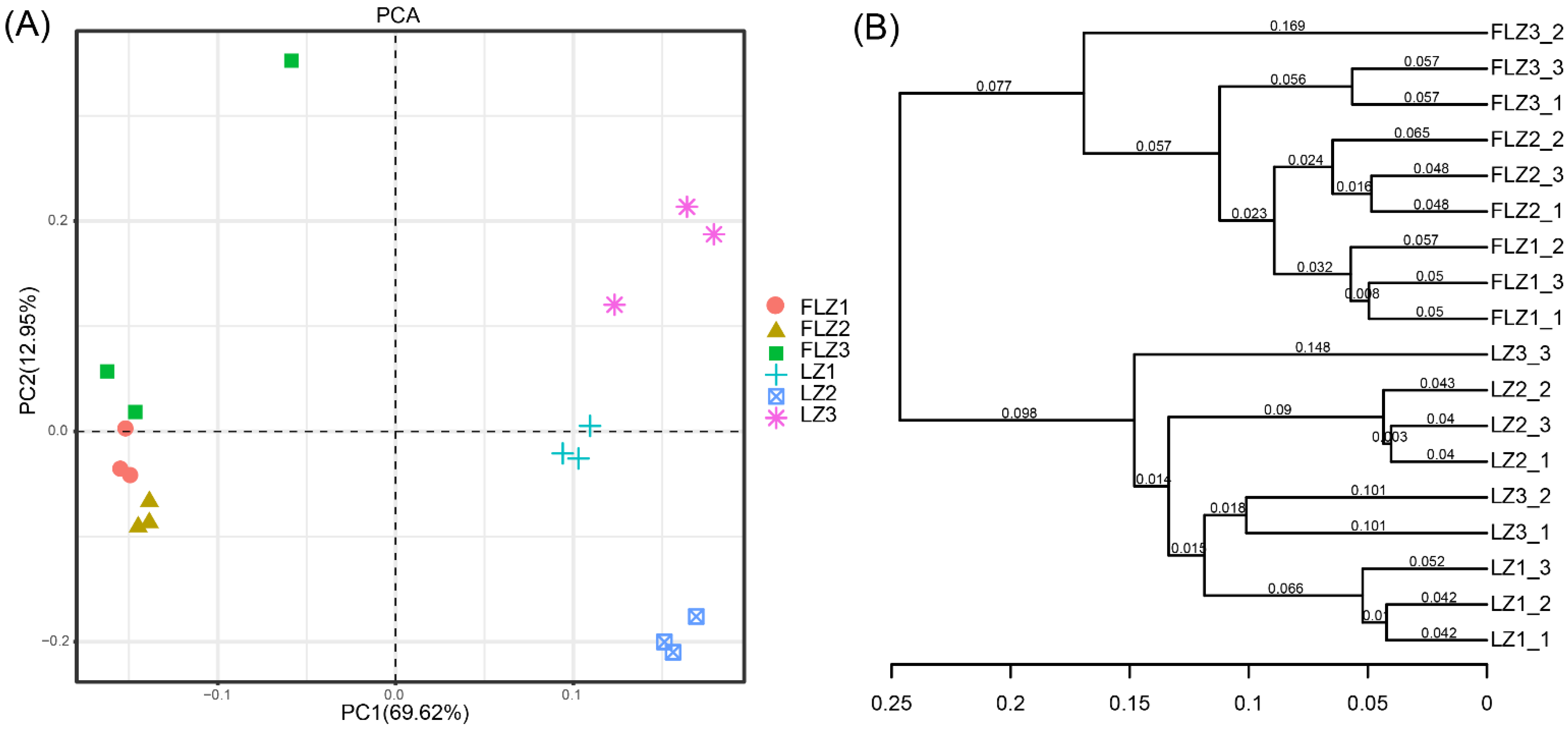
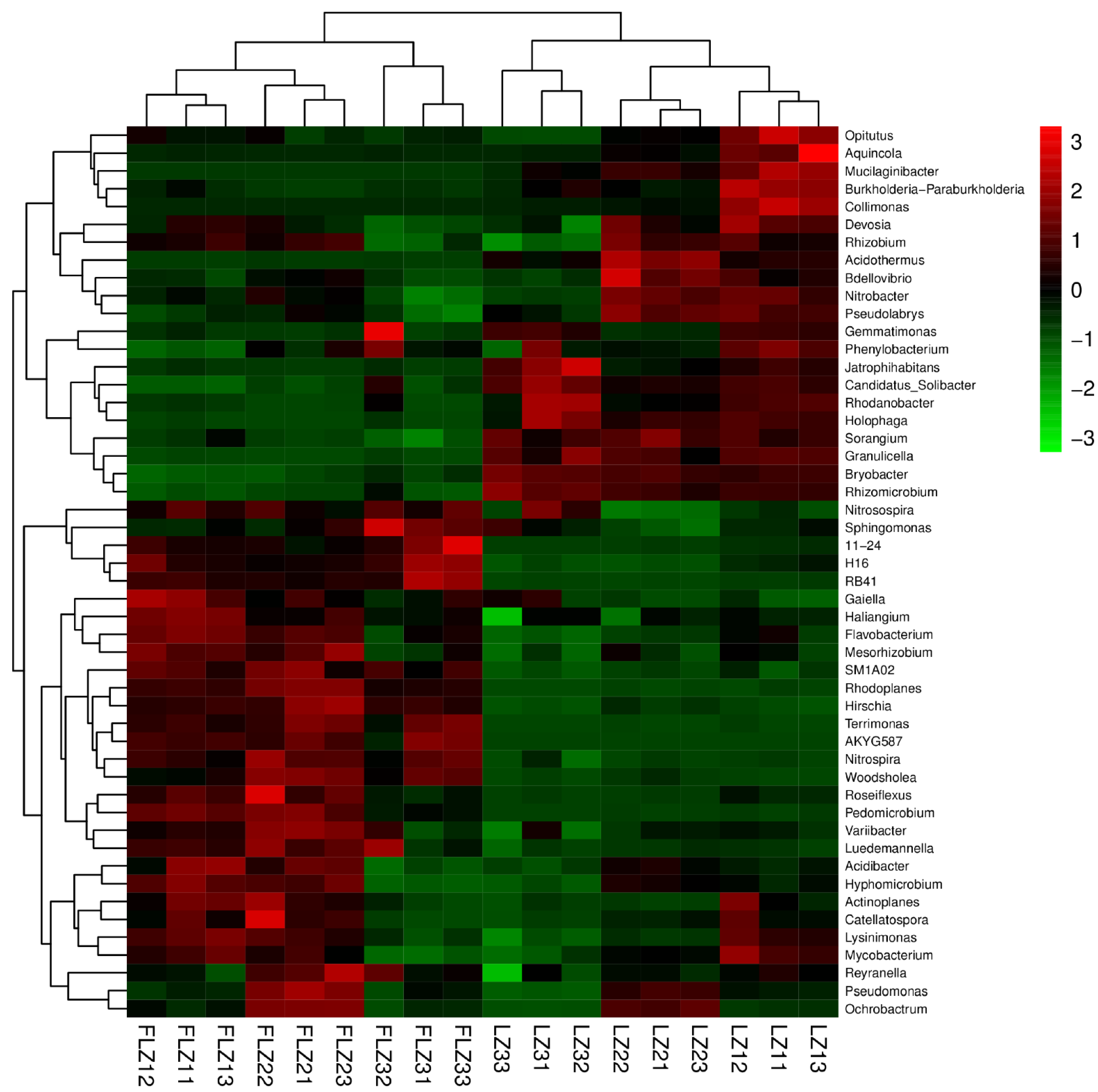


| Treatment | Yield (g/plant, FW) | Lobetyolin Content (mg/g) |
|---|---|---|
| LZ | 667.67 ± 15.20 | 2.74 ± 0.13 |
| FLZ | 740.93 ± 21.40 * | 1.30 ± 0.08 * |
| pH | OMC g/kg | TN % | AN mg/kg | TP % | AP mg/kg | TK % | AK mg/kg | |
|---|---|---|---|---|---|---|---|---|
| LZ1 | 5.64 b | 21.24 a | 0.21 a | 196.07 a | 0.07 a | 51.65 a | 1.91 a | 268.80 a |
| FLZ1 | 6.24 a | 15.85 bc | 0.17 bc | 148.35 b | 0.05 c | 8.22 c | 1.09 b | 104.26 c |
| LZ2 | 5.41 b | 20.06 a | 0.18 b | 111.70 c | 0.06 ab | 35.43 b | 1.85 a | 239.74 abc |
| FLZ2 | 5.37 b | 14.30 c | 0.16 c | 101.55 c | 0.05 c | 6.70 c | 1.87 a | 148.93 abc |
| LZ3 | 5.36 b | 18.45 ab | 0.18 b | 133.34 bc | 0.06 b | 24.74 b | 1.88 a | 258.60 ab |
| FLZ3 | 5.38 b | 14.68 bc | 0.16 c | 125.39 bc | 0.05 c | 5.83 c | 1.87 a | 111.65 bc |
| Treatment | Phylum | Class | Order | Family | Genus |
|---|---|---|---|---|---|
| LZ1 | 1579.66 c | 1553.00 c | 1357.66 d | 919.00 b | 295.00 ab |
| FLZ1 | 2344.33 a | 2318.33 a | 2000.00 a | 1176.66 a | 341.66 a |
| LZ2 | 1376.33 c | 1350.66 c | 1128.33 ab | 763.66 c | 255.33 bc |
| FLZ2 | 2061.33 b | 2036.66 b | 1746.00 c | 1061.33 a | 348.00 a |
| LZ3 | 1101.33 d | 1079.33 d | 903.00 e | 588.33 d | 171.33 d |
| FLZ3 | 1956.00 b | 1926.00 b | 1600.33 b | 927.66 b | 229.66 c |
| Treatment | Observed Species | Chao1 | ACE | Simpson | Shannon |
|---|---|---|---|---|---|
| LZ1 | 1833.66 ab | 2033.79 a | 2051.47 ab | 0.9958 a | 9.42 a |
| FLZ1 | 2084.66 a | 2220.55 a | 2303.82 a | 0.9966 a | 9.74 a |
| LZ2 | 1377.00 bc | 1459.92 ab | 1472.48 ab | 0.9906 b | 8.55 b |
| FLZ2 | 2061.66 a | 2232.22 a | 2319.05 a | 0.9974 a | 9.82 a |
| LZ3 | 1097.66 c | 1185.78 b | 1233.78 b | 0.9880 b | 7.99 b |
| FLZ3 | 1952.33 a | 2164.32 a | 2278.98 a | 0.9963 a | 9.56 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; He, Y.; Zhou, W.; Ai, L.; Liu, H.; Chen, L.; Xie, Y. Effects of Continuous Cropping of Codonopsis tangshen on Rhizospheric Soil Bacterial Community as Determined by Pyrosequencing. Diversity 2021, 13, 317. https://doi.org/10.3390/d13070317
Zhang M, He Y, Zhou W, Ai L, Liu H, Chen L, Xie Y. Effects of Continuous Cropping of Codonopsis tangshen on Rhizospheric Soil Bacterial Community as Determined by Pyrosequencing. Diversity. 2021; 13(7):317. https://doi.org/10.3390/d13070317
Chicago/Turabian StyleZhang, Meide, Yinsheng He, Wuxian Zhou, Lunqiang Ai, Haihua Liu, Liang Chen, and Yan Xie. 2021. "Effects of Continuous Cropping of Codonopsis tangshen on Rhizospheric Soil Bacterial Community as Determined by Pyrosequencing" Diversity 13, no. 7: 317. https://doi.org/10.3390/d13070317
APA StyleZhang, M., He, Y., Zhou, W., Ai, L., Liu, H., Chen, L., & Xie, Y. (2021). Effects of Continuous Cropping of Codonopsis tangshen on Rhizospheric Soil Bacterial Community as Determined by Pyrosequencing. Diversity, 13(7), 317. https://doi.org/10.3390/d13070317







