Aquatic Organisms Research with DNA Barcodes
Abstract
1. Introduction
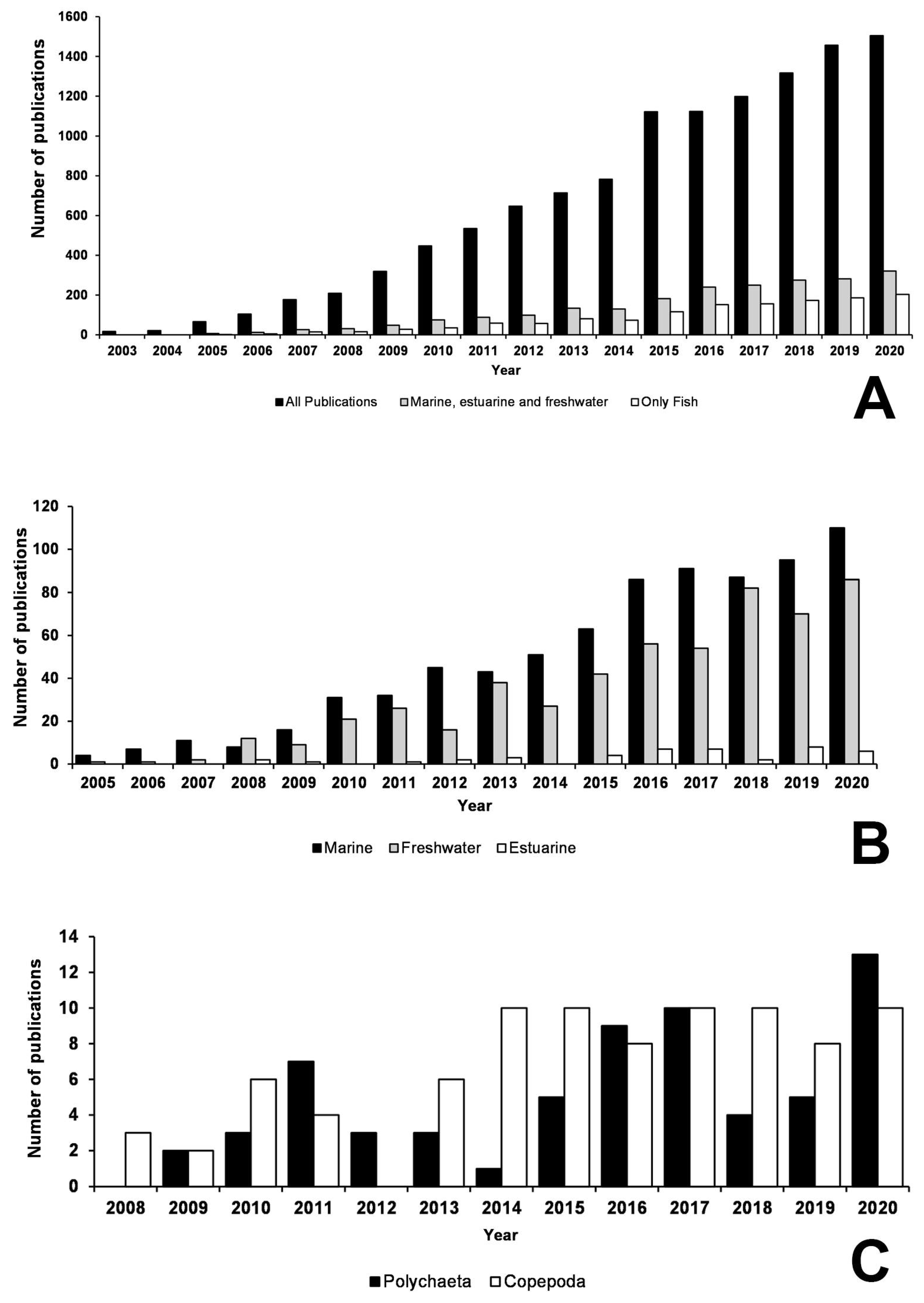
2. Progress in Aquatic DNA Barcoding Studies
3. Species Discovery
4. Integrative Taxonomy
5. Applications
6. Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; Dewaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. B Boil. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Hubert, N.; Hanner, R.; Holm, E.; Mandrak, N.E.; Taylor, E.; Burridge, M.; Watkinson, D.; Dumont, P.; Curry, A.; Bentzen, P.; et al. Identifying Canadian Freshwater Fishes through DNA Barcodes. PLoS ONE 2008, 3, e2490. [Google Scholar] [CrossRef]
- Kerr, K.C.R.; Stoeckle, M.Y.; Dove, C.J.; Weigt, L.A.; Francis, C.M.; Hebert, P.D.N. Comprehensive DNA barcode coverage of North American birds. Mol. Ecol. Notes 2007, 7, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Dewaard, J.R.; Zakharov, E.V.; Prosser, S.W.J.; Sones, J.E.; McKeown, J.T.A.; Mantle, B.; La Salle, J. A DNA ‘Barcode Blitz’: Rapid Digitization and Sequencing of a Natural History Collection. PLoS ONE 2013, 8, e68535. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Schmid-Egger, C.; Moriniere, J.; Haszprunar, G.; Hebert, P.D.N. DNA Barcoding Largely Supports 250 Years of Classical Taxonomy: Identifications for Central European Bees (Hymenoptera, Apoidea Partim). Mol. Ecol. Resour. 2015, 15, 985–1000. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, M.T.; Wild, R.; Elliot, M.; Fujisawa, T.; Balke, M.; Inward, D.J.; Lees, D.C.; Ranaivosolo, R.; Eggleton, P.; Barraclough, T.; et al. Accelerated Species Inventory on Madagascar Using Coalescent-Based Models of Species Delineation. Syst. Biol. 2009, 58, 298–311. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P. A DNA-Based Registry for All Animal Species: The Barcode Index Number (BIN) System. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef]
- Kekkonen, M.; Hebert, P.D.N. DNA Barcode-Based Delineation of Putative Species: Efficient Start for Taxonomic Workflows. Mol. Ecol. Resour. 2014, 14, 706–715. [Google Scholar] [CrossRef]
- Hebert, P.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. USA 2004, 101, 14812–14817. [Google Scholar] [CrossRef]
- Smith, M.A.; Rodriguez, J.J.; Whitfield, J.B.; Deans, A.; Janzen, D.H.; Hallwachs, W.; Hebert, P. Extreme diversity of tropical parasitoid wasps exposed by iterative integration of natural history, DNA barcoding, morphology, and collections. Proc. Natl. Acad. Sci. USA 2008, 105, 12359–12364. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. Bold: The Barcode of Life Data System (www.Barcodinglife.Org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Bucklin, A.; Steinke, D.; Blanco-Bercial, L. Barcoding of Marine Metazoa. Annu. Rev. Mar. Sci. 2011, 3, 471–508. [Google Scholar] [CrossRef]
- Trivedi, S.; Aloufi, A.A.; Ansari, A.A.; Ghosh, S.K. Role of DNA barcoding in marine biodiversity assessment and conservation: An update. Saudi J. Biol. Sci. 2016, 23, 161–171. [Google Scholar] [CrossRef]
- Mauchline, J.; Blaxter, J.H.S.; Southward, A.J.; Tyler, P.A. The Biology of Calanoid Copepods—Introduction. In Advances in Marine Biology; Academic Press: San Diego, CA, USA, 1998; Volume 33, Reprint, Not in File. [Google Scholar]
- Bucklin, A.; Ortman, B.D.; Jennings, R.M.; Nigro, L.M.; Sweetman, C.J.; Copley, N.; Sutton, T.; Wiebe, P.A. “Rosetta Stone” for metazoan zooplankton: DNA barcode analysis of species diversity of the Sargasso Sea (Northwest Atlantic Ocean). Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 2234–2247. [Google Scholar] [CrossRef]
- Hirai, J.; Shimode, S.; Tsuda, A.; Hirai, J.; Shimode, S.; Tsuda, A. Evaluation of ITS2-28S as a molecular marker for identification of calanoid copepods in the subtropical western North Pacific. J. Plankton Res. 2013, 35, 644–656. [Google Scholar] [CrossRef]
- Karanovic, I.; Huyen, P.T.M.; Yoo, H.; Nakao, Y.; Tsukagoshi, A. Shell and Appendages Variability in Two Allopatric Ostracod Species Seen through the Light of Molecular Data. Contrib. Zool. 2020, 89, 247–269. [Google Scholar] [CrossRef]
- Jeffery, N.W.; Elías-Gutiérrez, M.; Adamowicz, S.J. Species Diversity and Phylogeographical Affinities of the Branchiopoda (Crustacea) of Churchill, Manitoba, Canada. PLoS ONE 2011, 6, e18364. [Google Scholar] [CrossRef]
- Rowe, C.L.; Adamowicz, S.J.; Hebert, P. Three new cryptic species of the freshwater zooplankton genus Holopedium (Crustacea: Branchiopoda: Ctenopoda), revealed by genetic methods. Zootaxa 2007, 1656, 1–50. [Google Scholar] [CrossRef]
- Elías-Gutiérrez, M.; Valdez-Moreno, M.; Topan, J.; Young, M.R.; Cohuo-Colli, J.A. Improved protocols to accelerate the assembly of DNA barcode reference libraries for freshwater zooplankton. Ecol. Evol. 2018, 8, 3002–3018. [Google Scholar] [CrossRef]
- Wattier, R.; Mamos, T.; Copilaş-Ciocianu, D.; Jelić, M.; Ollivier, A.; Chaumot, A.; Danger, M.; Felten, V.; Piscart, C.; Žganec, K.; et al. Continental-scale patterns of hyper-cryptic diversity within the freshwater model taxon Gammarus fossarum (Crustacea, Amphipoda). Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Galimberti, A.; Assandri, G.; Maggioni, D.; Ramazzotti, F.; Baroni, D.; Bazzi, G.; Chiandetti, I.; Corso, A.; Ferri, V.; Galuppi, M.; et al. Italian Odonates in the Pandora’s Box: A Comprehensive DNA Barcoding Inventory Shows Taxonomic Warnings at the Holarctic Scale. Mol. Ecol. Resour. 2021, 21, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Denys, G.P.J.; Dettai, A.; Persat, H.; Hautecoeur, M.; Keith, P. Morphological and Molecular Evidence of Three Species of Pikes Esox spp. (Actinopterygii, Esocidae) in France, Including the Description of a New Species. C. R. Biol. 2014, 337, 521–534. [Google Scholar] [CrossRef]
- Denys, G.P.J.; Dettai, A.; Persat, H.; Daszkiewicz, P.; Hautecoeur, M.; Keith, P. Revision of Phoxinus in France with the Description of Two New Species (Teleostei, Leuciscidae). Cybium 2020, 44, 205–237. [Google Scholar]
- Buj, I.; Šanda, R.; Zogaris, S.; Freyhof, J.; Geiger, M.F.; Vukic, J. Cryptic diversity in Telestes pleurobipunctatus (Actinopterygii; Leuciscidae) as a consequence of historical biogeography in the Ionian Freshwater Ecoregion (Greece, Albania). Hydrobiology 2019, 835, 147–163. [Google Scholar] [CrossRef]
- Epitashvili, G.; Geiger, M.; Astrin, J.J.; Herder, F.; Japoshvili, B.; Mumladze, L. Towards retrieving the Promethean treasure: A first molecular assessment of the freshwater fish diversity of Georgia. Biodivers. Data J. 2020, 8, e57862. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Moreno, M.; Vásquez-Yeomans, L.; Elías-Gutiérrez, M.; Ivanova, N.V.; Hebert, P. Using DNA barcodes to connect adults and early life stages of marine fishes from the Yucatan Peninsula, Mexico: Potential in fisheries management. Mar. Freshw. Res. 2010, 61, 655–671. [Google Scholar] [CrossRef]
- Valdez-Moreno, M.; Ivanova, N.V.; Elías-Gutiérrez, M.; Contreras-Balderas, S.; Hebert, P.D.N. DNA Barcodes in Freshwater Fishes from Mexico and Guatemala. J. Fish Biol. 2007. submitted. [Google Scholar]
- Leyva-Cruz, E.; Vásquez-Yeomans, L.; Carrillo, L.; Valdez-Moreno, M. Identifying pelagic fish eggs in the southeast Yucatan Peninsula using DNA barcodes. Genome 2016, 59, 1117–1129. [Google Scholar] [CrossRef]
- Vásquez-Yeomans, L.; Carrillo, L.; Morales, S.; Malca, E.; Morris, J.A.; Schultz, T.; Lamkin, J.T. First larval record of Pterois volitans (Pisces: Scorpaenidae) collected from the ichthyoplankton in the Atlantic. Biol. Invasions 2011, 13, 2635–2640. [Google Scholar] [CrossRef]
- Victor, B.C.; Vasquez-Yeomans, L.; Valdez-Moreno, M.; Wilk, L.; Jones, D.L.; Lara, M.R.; Caldow, C.; Shivji, M. The larval, juvenile, and adult stages of the Caribbean goby, Coryphopterus kuna (Teleostei: Gobiidae): A reef fish with a pelagic larval duration longer than the post-settlement lifespan. Zootaxa 2010, 2346, 53–61. [Google Scholar] [CrossRef]
- Victor, B.C. Hypoplectrus floridae n. sp. and Hypoplectrus ecosur n. sp., Two New Barred Hamlets from the Gulf of Mexico (Pisces: Serranidae): More Than 3% Different in COI MtDNA Sequence from the Caribbean Hypoplectrus Species Flock. J. Ocean Sci. Found. 2012, 5, 2–19. [Google Scholar]
- Ahern, A.; Gómez-Gutiérrez, J.; Aburto-Oropeza, O.; Saldierna-Martínez, R.; Johnson, A.F.; Harada, A.; Sánchez-Uvera, A.; Erisman, B.; Arvizú, D.C.; Burton, R. DNA sequencing of fish eggs and larvae reveals high species diversity and seasonal changes in spawning activity in the southeastern Gulf of California. Mar. Ecol. Prog. Ser. 2018, 592, 159–179. [Google Scholar] [CrossRef]
- Bearez, P.; Dettai, A.; Gomon, M.F. Polylepion russelli (Labridae), a Trans-Indo-Pacific Species. Cybium 2013, 37, 305–306. [Google Scholar]
- Victor, B.C.; Alfaro, M.E.; Sorenson, L. Rediscovery of Sagittalarva inornata n. gen., n. comb. (Gilbert, 1890) (Perciformes: Labridae), a long-lost deepwater fish from the eastern Pacific Ocean: A case study of a forensic approach to taxonomy using DNA barcoding. Zootaxa 2013, 3669, 551–570. [Google Scholar] [CrossRef] [PubMed]
- Mejía, O.; León-Romero, Y.; Soto-Galera, E. DNA barcoding of the ichthyofauna of Pánuco–Tamesí complex: Evidence for taxonomic conflicts in some groups. Mitochondrial DNA 2012, 23, 471–476. [Google Scholar] [CrossRef]
- Venegas, R.D.L.P.; Hueter, R.; Cano, J.G.; Tyminski, J.; Remolina, J.G.; Maslanka, M.; Ormos, A.; Weigt, L.; Carlson, B.; Dove, A. An Unprecedented Aggregation of Whale Sharks, Rhincodon typus, in Mexican Coastal Waters of the Caribbean Sea. PLoS ONE 2011, 6, e18994. [Google Scholar] [CrossRef]
- Nogueira, A.F.; Oliveira, C.; Langeani, F.; Netto-Ferreira, A.L. Overlooked biodiversity of mitochondrial lineages in Hemiodus (Ostariophysi, Characiformes). Zool. Scr. 2021, 50, 337–351. [Google Scholar] [CrossRef]
- Adelir-Alves, J.; Spier, D.; Gerum, H.L.N.; Machado, L.F.; Spach, H.L.; Boza, B.R.; Oliveira, C. Plectorhinchus macrolepis (Actinopterygii: Haemulidae) in the western Atlantic Ocean. J. Fish Biol. 2019, 95, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Arruda, P.S.S.; Ferreira, D.C.; Oliveira, C.; Venere, P.C. DNA Barcoding Reveals High Levels of Divergence among Mitochondrial Lineages of Brycon (Characiformes, Bryconidae). Genes 2019, 10, 639. [Google Scholar] [CrossRef] [PubMed]
- Caires, R.A.; Santos, W.C.R.d.; Machado, L.; Oliveira, C.; Cerqueira, N.; Rotundo, M.M.; Oliveira, C.; Marceniuk, A.P. The Tonkin Weakfish, Cynoscion similis (Sciaenidae, Perciformes), an Endemic Species of the Amazonas-Orinoco Plume. Acta Amaz. 2019, 49, 197–207. [Google Scholar] [CrossRef]
- Carvalho, C.O.; Marceniuk, A.P.; Oliveira, C.; Wosiacki, W.B. Integrative Taxonomy of the Species Complex Haemulon steindachneri (Jordan and Gilbert, 1882) (Eupercaria; Haemulidae) with a Description of a New Species from the Western Atlantic. Zoology 2020, 141, 125782. [Google Scholar] [CrossRef]
- de Queiroz, L.J.; Cardoso, Y.P.; Jacot-Des-Combes, C.; Bahechar, I.A.; Lucena, C.A.; Py-Daniel, L.R.; Soares, L.M.S.; Nylinder, S.; Oliveira, C.; Parente, T.E.; et al. Evolutionary units delimitation and continental multilocus phylogeny of the hyperdiverse catfish genus Hypostomus. Mol. Phylogenetics Evol. 2020, 145, 106711. [Google Scholar] [CrossRef]
- Ferrette, B.L.D.S.; Domingues, R.R.; Ussami, L.H.F.; Moraes, L.; Magalhães, C.D.O.; De Amorim, A.F.; Hilsdorf, A.W.S.; Oliveira, C.; Foresti, F.; Mendonça, F.F. DNA-based species identification of shark finning seizures in Southwest Atlantic: Implications for wildlife trade surveillance and law enforcement. Biodivers. Conserv. 2019, 28, 4007–4025. [Google Scholar] [CrossRef]
- Mateussi, N.T.B.; Melo, B.F.; Oliveira, C. Molecular Delimitation and Taxonomic Revision of the Wimple Piranhacatoprion (Characiformes: Serrasalmidae) with the Description of a New Species. J. Fish Biol. 2020, 97, 668–685. [Google Scholar] [CrossRef]
- Mattox, G.M.T.; Souza, C.S.; Toledo-Piza, M.; Britz, R.; Oliveira, C. A New Miniature Species of Priocharax (Teleostei: Characiformes: Characidae) from the Rio Madeira Drainage, Brazil, with Comments on the Adipose Fin in Characiforms. Vertebr. Zool. 2020, 70, 417–433. [Google Scholar]
- Hashimoto, S.; Py-Daniel, L.H.R.; Batista, J.S. A molecular assessment of species diversity in Tympanopleura and Ageneiosus catfishes (Auchenipteridae: Siluriformes). J. Fish Biol. 2020, 96, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.H.G.; Pazian, M.; Hanner, R.; Foresti, F.; Oliveira, C. DNA barcoding reveals hidden diversity in the Neotropical freshwater fish Piabina argentea (Characiformes: Characidae) from the Upper Paraná Basin of Brazil. Mitochondrial DNA 2011, 22, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Castro Paz, F.P.; Batista, J.D.; Porto, J.I.R. DNA Barcodes of Rosy Tetras and Allied Species (Characiformes: Characidae: Hyphessobrycon) from the Brazilian Amazon Basin. PLoS ONE 2014, 9, e98603. [Google Scholar] [CrossRef]
- Benzaquem, D.C.; Oliveira, C.; Batista, J.D.S.; Zuanon, J.; Porto, J.I.R. DNA Barcoding in Pencilfishes (Lebiasinidae: Nannostomus) Reveals Cryptic Diversity across the Brazilian Amazon. PLoS ONE 2015, 10, e0112217. [Google Scholar] [CrossRef]
- Rossini, B.C.; Oliveira, C.A.M.; de Melo, F.A.G.; Bertaco, V.D.; de Astarloa, J.M.D.; Rosso, J.J.; Foresti, F.; Oliveira, C. Highlighting Astyanax Species Diversity through DNA Barcoding. PLoS ONE 2016, 11, e0167203. [Google Scholar] [CrossRef]
- Machado, V.N.; Collins, R.A.; Ota, R.P.; Andrade, M.C.; Farias, I.; Hrbek, T. One thousand DNA barcodes of piranhas and pacus reveal geographic structure and unrecognised diversity in the Amazon. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Carvalho, A.P.C.; Collins, R.A.; Martinez, J.G.; Farias, I.P.; Hrbek, T. From Shallow to Deep Divergences: Mixed Messages from Amazon Basin Cichlids. Hydrobiologia 2019, 832, 317–329. [Google Scholar] [CrossRef]
- Hubert, N.; Hadiaty, R.K.; Paradis, E.; Pouyaud, L. Cryptic Diversity in Indo-Australian Rainbowfishes Revealed by DNA Barcoding: Implications for Conservation in a Biodiversity Hotspot Candidate. PLoS ONE 2012, 7, e40627. [Google Scholar] [CrossRef]
- Keith, P.; Hadiaty, R.; Hubert, N.; Busson, F.; Lord, C. Three New Species of Lentipes from Indonesia (Gobiidae). Cybium 2014, 38, 133–146. [Google Scholar]
- Lim, H.; Abidin, M.Z.; Pulungan, C.P.; de Bruyn, M.; Nor, S.A.M. DNA Barcoding Reveals High Cryptic Diversity of the Freshwater Halfbeak Genus Hemirhamphodon from Sundaland. PLoS ONE 2016, 11, e0163596. [Google Scholar] [CrossRef]
- Beck, S.V.; Carvalho, G.R.; Barlow, A.; Rüber, L.; Tan, H.H.; Nugroho, E.; Wowor, D.; Nor, S.A.M.; Herder, F.; Muchlisin, Z.A.; et al. Plio-Pleistocene phylogeography of the Southeast Asian Blue Panchax killifish, Aplocheilus panchax. PLoS ONE 2017, 12, e0179557. [Google Scholar] [CrossRef]
- Dahruddin, H.; Hutama, A.; Busson, F.; Sauri, S.; Hanner, R.; Keith, P.; Hadiaty, R.; Hubert, N. Data from: Revisiting the ichthyodiversity of Java and Bali through DNA barcodes: Taxonomic coverage, identification accuracy, cryptic diversity and identification of exotic species. Mol. Ecol. Resour. 2017, 17, 288–299. [Google Scholar] [CrossRef]
- Hutama, A.; Dahruddin, H.; Busson, F.; Sauri, S.; Keith, P.; Hadiaty, R.K.; Hanner, R.; Suryobroto, B.; Hubert, N. Identifying spatially concordant evolutionary significant units across multiple species through DNA barcodes: Application to the conservation genetics of the freshwater fishes of Java and Bali. Glob. Ecol. Conserv. 2017, 12, 170–187. [Google Scholar] [CrossRef]
- Conte-Grand, C.; Britz, R.; Dahanukar, N.; Raghavan, R.; Pethiyagoda, R.; Tan, H.H.; Hadiaty, R.K.; Yaakob, N.S.; Rüber, L. Barcoding snakeheads (Teleostei, Channidae) revisited: Discovering greater species diversity and resolving perpetuated taxonomic confusions. PLoS ONE 2017, 12, e0184017. [Google Scholar] [CrossRef]
- Farhana, S.N.; Muchlisin, Z.A.; Duong, T.Y.; Tanyaros, S.; Page, L.M.; Zhao, Y.H.; Adamson, E.A.S.; Khaironizam, M.Z.; de Bruyn, M.; Azizah, M.N.S. Exploring Hidden Diversity in Southeast Asia’s Dermogenys spp. (Beloniformes: Zenarchopteridae) through DNA Barcoding. Sci. Rep. 2018, 8, 1–11. [Google Scholar]
- Shen, Y.; Hubert, N.; Huang, Y.; Wang, X.; Gan, X.; Peng, Z.; He, S. DNA barcoding the ichthyofauna of the Yangtze River: Insights from the molecular inventory of a mega-diverse temperate fauna. Mol. Ecol. Resour. 2019, 19, 1278–1291. [Google Scholar] [CrossRef]
- Hubert, N.; Lumbantobing, D.; Sholihah, A.; Dahruddin, H.; Delrieu-Trottin, E.; Busson, F.; Sauri, S.; Hadiaty, R.; Keith, P. Revisiting Species Boundaries and Distribution Ranges of Nemacheilus spp. (Cypriniformes: Nemacheilidae) and Rasbora spp. (Cypriniformes: Cyprinidae) in Java, Bali and Lombok through DNA Barcodes: Implications for Conservation in a Biodiversity Hotspot. Conserv. Genet. 2019, 20, 517–529. [Google Scholar] [CrossRef]
- Sholihah, A.; Delrieu-Trottin, E.; Condamine, F.L.; Wowor, D.; Rüber, L.; Pouyaud, L.; Agnèse, J.-F.; Hubert, N. Impact of Pleistocene Eustatic Fluctuations on Evolutionary Dynamics in Southeast Asian Biodiversity Hotspots. Syst. Biol. 2021, syab006. [Google Scholar] [CrossRef] [PubMed]
- Sholihah, A.; Delrieu-Trottin, E.; Sukmono, T.; Dahruddin, H.; Risdawati, R.; Elvyra, R.; Wibowo, A.; Kustiati, K.; Busson, F.; Sauri, S.; et al. Disentangling the taxonomy of the subfamily Rasborinae (Cypriniformes, Danionidae) in Sundaland using DNA barcodes. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Delrieu-Trottin, E.; Durand, J.; Limmon, G.; Sukmono, T.; Sugeha, H.Y.; Chen, W.-J.; Busson, F.; Borsa, P.; Dahruddin, H.; Sauri, S.; et al. Biodiversity inventory of the grey mullets (Actinopterygii: Mugilidae) of the Indo-Australian Archipelago through the iterative use of DNA-based species delimitation and specimen assignment methods. Evol. Appl. 2020, 13, 1451–1467. [Google Scholar] [CrossRef]
- Rüber, L.; Tan, H.H.; Britz, R. Snakehead (Teleostei: Channidae) diversity and the Eastern Himalaya biodiversity hotspot. J. Zool. Syst. Evol. Res. 2019, 58, 356–386. [Google Scholar] [CrossRef]
- Chakona, A.; Kadye, W.T.; Bere, T.; Mazungula, D.N.; Vreven, E. Evidence of hidden diversity and taxonomic conflicts in five stream fishes from the Eastern Zimbabwe Highlands freshwater ecoregion. ZooKeys 2018, 768, 69–95. [Google Scholar] [CrossRef] [PubMed]
- Iyiola, O.A.; Nneji, L.M.; Mustapha, M.K.; Nzeh, C.G.; Oladipo, S.; Nneji, I.C.; Okeyoyin, A.O.; Nwani, C.D.; Ugwumba, O.A.; Ugwumba, A.A.A.; et al. DNA barcoding of economically important freshwater fish species from north-central Nigeria uncovers cryptic diversity. Ecol. Evol. 2018, 8, 6932–6951. [Google Scholar] [CrossRef]
- Sonet, G.; Snoeks, J.; Nagy, Z.T.; Vreven, E.; Boden, G.; Breman, F.C.; Decru, E.; Hanssens, M.; Zamba, A.I.; Jordaens, K.; et al. DNA barcoding fishes from the Congo and the Lower Guinean provinces: Assembling a reference library for poorly inventoried fauna. Mol. Ecol. Resour. 2018, 19, 728–743. [Google Scholar] [CrossRef]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D.N. DNA Barcoding Australia’s Fish Species. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef]
- Ward, R.D.; Holmes, B.H.; White, W.; Last, P.R. DNA barcoding Australasian chondrichthyans: Results and potential uses in conservation. Mar. Freshw. Res. 2008, 59, 57–71. [Google Scholar] [CrossRef]
- Hubert, N.; Paradis, E.; Bruggemann, H.; Planes, S. Community assembly and diversification in Indo-Pacific coral reef fishes. Ecol. Evol. 2011, 1, 229–277. [Google Scholar] [CrossRef]
- Hubert, N.; Meyer, C.P.; Bruggemann, H.J.; Guérin, F.; Komeno, R.J.L.; Espiau, B.; Causse, R.; Williams, J.T.; Planes, S. Cryptic Diversity in Indo-Pacific Coral-Reef Fishes Revealed by DNA-Barcoding Provides New Support to the Centre-of-Overlap Hypothesis. PLoS ONE 2012, 7, e28987. [Google Scholar] [CrossRef]
- Jaafar, T.N.A.M.; Taylor, M.I.; Nor, S.A.M.; de Bruyn, M.; Carvalho, G.R. DNA Barcoding Reveals Cryptic Diversity within Commercially Exploited Indo-Malay Carangidae (Teleosteii: Perciformes). PLoS ONE 2012, 7, e49623. [Google Scholar] [CrossRef] [PubMed]
- Winterbottom, R.; Hanner, R.H.; Burridge, M.; Zur, M. A cornucopia of cryptic species—A DNA barcode analysis of the gobiid fish genus Trimma (Percomorpha, Gobiiformes). ZooKeys 2014, 381, 79–111. [Google Scholar] [CrossRef] [PubMed]
- Durand, J.-D.; Hubert, N.; Shen, K.-N.; Borsa, P. DNA barcoding grey mullets. Rev. Fish Biol. Fish. 2017, 27, 233–243. [Google Scholar] [CrossRef]
- Delrieu-Trottin, E.; Williams, J.T.; Pitassy, D.; Driskell, A.; Hubert, N.; Viviani, J.; Cribb, T.; Espiau, B.; Galzin, R.; Kulbicki, M.; et al. A DNA barcode reference library of French Polynesian shore fishes. Sci. Data 2019, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Steinke, D.; Zemlak, T.S.; Hebert, P. Barcoding Nemo: DNA-Based Identifications for the Ornamental Fish Trade. PLoS ONE 2009, 4, e6300. [Google Scholar] [CrossRef] [PubMed]
- Skelton, P.H.; Swartz, E.R. Walking the tightrope: Trends in African freshwater systematic ichthyology. J. Fish Biol. 2011, 79, 1413–1435. [Google Scholar] [CrossRef]
- Adeoba, M.I.; Tesfamichael, S.G.; Yessoufou, K. Preserving the tree of life of the fish family Cyprinidae in Africa in the face of the ongoing extinction crisis. Genome 2019, 62, 170–182. [Google Scholar] [CrossRef]
- Montes-Ortiz, L.; Elías-Gutiérrez, M. Water Mite Diversity (Acariformes: Prostigmata: Parasitengonina: Hydrachnidiae) from Karst Ecosystems in Southern of Mexico: A Barcoding Approach. Diversity 2020, 12, 329. [Google Scholar] [CrossRef]
- De León, L.F.; Cornejo, A.; Gavilán, R.G.; Aguilar, C. Hidden biodiversity in Neotropical streams: DNA barcoding uncovers high endemicity of freshwater macroinvertebrates at small spatial scales. PLoS ONE 2020, 15, e0231683. [Google Scholar] [CrossRef] [PubMed]
- García-Morales, A.E.; Domínguez-Domínguez, O. Cryptic species within the rotifer Lecane bulla (Rotifera: Monogononta: Lecanidae) from North America based on molecular species delimitation. Rev. Mex. Biodivers. 2020, 91, 913116. [Google Scholar] [CrossRef]
- Gischler, E.; Gibson, M.A.; Oschmann, W. Giant Holocene Freshwater Microbialites, Laguna Bacalar, Quintana Roo, Mexico. Sedimentology 2008, 55, 1293–1309. [Google Scholar] [CrossRef]
- Elías-Gutiérrez, M.; Montes-Ortiz, L. Present Day Kwnoledge on Diversity of Freshwater Zooplancton (Invertebrates) of the Yucatan Peninsula, Using Integrated Taxonomy. Teor. Y Prax. 2018, 14, 31–48. [Google Scholar]
- Montes-Ortiz, L.; Elías-Gutiérrez, M. Faunistic survey of the zooplankton community in an oligotrophic sinkhole, Cenote Azul (Quintana Roo, Mexico), using different sampling methods, and documented with DNA barcodes. J. Limnol. 2018, 77, 428–440. [Google Scholar] [CrossRef]
- Perry, E.; Velazquez-Oliman, G.; Marin, L. The Hydrogeochemistry of the Karst Aquifer System of the Northern Yucatan Peninsula, Mexico. Int. Geol. Rev. 2002, 44, 191–221. [Google Scholar] [CrossRef]
- von Rintelen, K.; von Rintelen, T.; Glaubrecht, M. Molecular Phylogeny and Diversification of Freshwater Shrimps (Decapoda, Atyidae, Caridina) from Ancient Lake Poso (Sulawesi, Indonesia)—The Importance of Being Colourful. Mol. Phylogenetics Evol. 2007, 45, 1033–1041. [Google Scholar] [CrossRef]
- Castelin, M.; De Mazancourt, V.; Marquet, G.; Zimmerman, G.; Keith, P. Genetic and morphological evidence for cryptic species in Macrobrachium australe and resurrection of M. ustulatum (Crustacea, Palaemonidae). Eur. J. Taxon. 2017, 289, 1–27. [Google Scholar] [CrossRef]
- de Mazancourt, V.; Klotz, W.; Marquet, G.; Mos, B.; Rogers, D.C.; Keith, P. The complex study of complexes: The first well-supported phylogeny of two species complexes within genus Caridina (Decapoda: Caridea: Atyidae) sheds light on evolution, biogeography, and habitat. Mol. Phylogenetics Evol. 2019, 131, 164–180. [Google Scholar] [CrossRef]
- Hernawati, R.; Nurhaman, U.; Busson, F.; Suryobroto, B.; Hanner, R.; Keith, P.; Wowor, D.; Hubert, N. Exploring community assembly among Javanese and Balinese freshwater shrimps (Atyidae, Palaemonidae) through DNA barcodes. Hydrobiology 2019, 847, 647–663. [Google Scholar] [CrossRef]
- Garibian, P.G.; Neretina, A.N.; Taylor, D.J.; Kotov, A.A. Partial Revision of the Neustonic Genus Scapholeberis Schoedler, 1858 (Crustacea: Cladocera): Decoding of the Barcoding.Results. PeerJ 2020, 8, e10410. [Google Scholar] [CrossRef]
- Yamamoto, A.; Makino, W.; Urabe, J. The taxonomic position of Asian Holopedium (Crustacea: Cladocera) confirmed by morphological and genetic analyses. Limnology 2019, 21, 97–106. [Google Scholar] [CrossRef]
- Liu, Y.; Fend, S.V.; Martinsson, S.; Erséus, C. Extensive cryptic diversity in the cosmopolitan sludge worm Limnodrilus hoffmeisteri (Clitellata, Naididae). Org. Divers. Evol. 2017, 17, 477–495. [Google Scholar] [CrossRef]
- Chuluunbat, S.; Morse, J.C.; Boldbaatar, S. Caddisflies of Mongolia: Distribution and diversity. Zoosymposia 2016, 10, 96–116. [Google Scholar] [CrossRef]
- Wu, R.-W.; Liu, Y.-T.; Wang, S.; Liu, X.-J.; Zanatta, D.; Roe, K.J.; Song, X.-L.; An, C.-T.; Wu, X.-P. Testing the utility of DNA barcodes and a preliminary phylogenetic framework for Chinese freshwater mussels (Bivalvia: Unionidae) from the middle and lower Yangtze River. PLoS ONE 2018, 13, e0200956. [Google Scholar] [CrossRef] [PubMed]
- Makino, W.; Machida, R.J.; Okitsu, J.; Usio, N. Underestimated species diversity and hidden habitat preference in Moina (Crustacea, Cladocera) revealed by integrative taxonomy. Hydrobiology 2020, 847, 857–878. [Google Scholar] [CrossRef]
- Locke, S.A.; Al-Nasiri, F.S.; Caffara, M.; Drago, F.; Kalbe, M.; Lapierre, A.R.; McLaughlin, J.D.; Nie, P.; Overstreet, R.M.; Souza, G.T.; et al. Diversity, specificity and speciation in larval Diplostomidae (Platyhelminthes: Digenea) in the eyes of freshwater fish, as revealed by DNA barcodes. Int. J. Parasitol. 2015, 45, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Locke, S.A.; Caffara, M.; Barčák, D.; Sonko, P.; Tedesco, P.; Fioravanti, M.L.; Li, W. A new species of Clinostomum Leidy, 1856 in East Asia based on genomic and morphological data. Parasitol. Res. 2019, 118, 3253–3265. [Google Scholar] [CrossRef]
- Ng, T.H.; Annate, S.; Jeratthitikul, E.; Sutcharit, C.; Limpanont, Y.; Panha, S. Disappearing Apple Snails (Caenogastropoda: Ampullariidae) of Thailand: A Comprehensive Update of Their Taxonomic Status and Distribution. J. Molluscan Stud. 2020, 86, 290–305. [Google Scholar] [CrossRef]
- Vikhrev, I.V.; Konopleva, E.S.; Gofarov, M.Y.; Kondakov, A.V.; Chapurina, Y.E.; Bolotov, I.N. A Tropical Biodiversity Hotspot under the New Threat: Discovery and DNA Barcoding of the Invasive Chinese Pond Mussel Sinanodonta Woodiana in Myanmar. Trop. Conserv. Sci. 2017, 10, 1–11. [Google Scholar] [CrossRef]
- Lima, F.D.; Berbel-Filho, W.M.; Leite, T.S.; Rosas, C.; Lima, S.M.Q. Occurrence of Octopus insularis Leite and Haimovici, 2008 in the Tropical Northwestern Atlantic and implications of species misidentification to octopus fisheries management. Mar. Biodivers. 2017, 47, 723–734. [Google Scholar] [CrossRef]
- Rosas-Luis, R.; Badillo, M.D.L.J.; Elena, L.M.; Morillo-Velarde, P.S. Food and feeding habits of Octopus insularis in the Veracruz Reef System National Park and confirmation of its presence in the southwest Gulf of Mexico. Mar. Ecol. 2019, 40, e12535. [Google Scholar] [CrossRef]
- Caffara, M.; Locke, S.A.; Echi, P.C.; Halajian, A.; Benini, D.; Luus-Powell, W.J.; Tavakol, S.; Fioravanti, M.L. A morphological and molecular study of Clinostomid metacercariae from African fish with a redescription of Clinostomum tilapiae. Parasitology 2017, 144, 1519–1529. [Google Scholar] [CrossRef]
- Chibwana, F.D.; Blasco-Costa, I.; Georgieva, S.; Hosea, K.M.; Nkwengulila, G.; Scholz, T.; Kostadinova, A. A first insight into the barcodes for African diplostomids (Digenea: Diplostomidae): Brain parasites in Clarias gariepinus (Siluriformes: Clariidae). Infect. Genet. Evol. 2013, 17, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Laidemitt, M.R.; Brant, S.V.; Mutuku, M.W.; Mkoji, G.M.; Loker, E.S. The diverse echinostomes from East Africa: With a focus on species that use Biomphalaria and Bulinus as intermediate hosts. Acta Trop. 2019, 193, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Webster, B.; Webster, J.P.; Gouvras, A.; Garba, A.; Lamine, M.S.; Diaw, O.T.; Seye, M.M.; Tchuenté, L.-A.T.; Simoonga, C.; Mubila, L.; et al. DNA ‘barcoding’ of Schistosoma mansoni across sub-Saharan Africa supports substantial within locality diversity and geographical separation of genotypes. Acta Trop. 2013, 128, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Stothard, J.R.; Ameri, H.; Khamis, I.S.; Blair, L.; Nyandindi, U.S.; Kane, R.A.; Johnston, D.A.; Webster, B.; Rollinson, D. Parasitological and malacological surveys reveal urogenital schistosomiasis on Mafia Island, Tanzania to be an imported infection. Acta Trop. 2013, 128, 326–333. [Google Scholar] [CrossRef]
- Alcántar-Escalera, F.A.; García-Varela, M.; Vázquez-Domínguez, E.; de León, G.P. Using DNA Barcoding to Link Cystacanths and Adults of the Acanthocephalan Polymorphus brevis in Central Mexico. Mol. Ecol. Resour. 2013, 13, 1116–1124. [Google Scholar]
- Lawton, S.P.; Allan, F.; Hayes, P.M.; Smit, N.J. DNA barcoding of the medically important freshwater snail Physa acuta reveals multiple invasion events into Africa. Acta Trop. 2018, 188, 86–92. [Google Scholar] [CrossRef]
- Angyal, D.; Balázs, G.; Krízsik, V.; Herczeg, G.; Fehér, Z. Molecular and morphological divergence in a stygobiont gastropod lineage (Truncatelloidea, Moitessieriidae, Paladilhiopsis) within an isolated karstic area in the Mecsek Mountains (Hungary). J. Zool. Syst. Evol. Res. 2018, 56, 493–504. [Google Scholar] [CrossRef]
- Elderkin, C.L.; Clewing, C.; Ndeo, O.W.; Albrecht, C. Molecular Phylogeny and DNA Barcoding Confirm Cryptic Species in the African Freshwater Oyster Etheria elliptica Lamarck, 1807 (Bivalvia: Etheriidae). Biol. J. Linn. Soc. 2016, 118, 369–381. [Google Scholar] [CrossRef]
- Carr, C.M.; Hardy, S.M.; Brown, T.M.; Macdonald, T.A.; Hebert, P. A Tri-Oceanic Perspective: DNA Barcoding Reveals Geographic Structure and Cryptic Diversity in Canadian Polychaetes. PLoS ONE 2011, 6, e22232. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Guerrero, T.F.; Carrera-Parra, L.F. Redescription of Alitta succinea (Leuckart, 1847) and reinstatement of A. acutifolia (Ehlers, 1901) n. comb. based upon morphological and molecular data (Polychaeta: Nereididae). Zootaxa 2015, 3919, 157–178. [Google Scholar] [CrossRef]
- Salazar-Silva, P.; Carrera-Parra, L.F. Revision of Lepidonopsis humilis (Augener, 1922) and description of L. barnichae sp. nov. (Annelida: Polychaeta: Polynoidae) based upon morphological and molecular characters. Zootaxa 2014, 3790, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Parra, L.F.; Salazar-Vallejo, S.I. Redescriptions of Eunice filamentosa and E. denticulata and Description of E. tovarae n. sp. (Polychaeta: Eunicidae), Highlighted with Morphological and Molecular Data. Zootaxa 2011, 2880, 51–64. [Google Scholar] [CrossRef]
- Keith, P.; Dahruddin, H.; Limmon, G.; Hubert, N. A New Species of Schismatogobius (Teleostei: Gobiidae) from Halmahera (Indonesia). Cybium 2018, 42, 195–200. [Google Scholar]
- Keith, P.; Lord, C.; Darhuddin, H.; Limmon, G.; Sukmono, T.; Hadiaty, R.; Hubert, N. Schismatogobius (Gobiidae) from Indonesia, with Description of Four New Species. Cybium 2017, 41, 195–211. [Google Scholar]
- Gutiérrez-Aguirre, M.A.; Cervantes-Martínez, A.; Elías-Gutiérrez, M.; Lugo-Vázquez, A. Remarks on Mastigodiaptomus (Calanoida: Diaptomidae) from Mexico using integrative taxonomy, with a key of identification and three new species. PeerJ 2020, 8, e8416. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Sossa, C.; Buitron-Caicedo, L.; Elías-Gutiérrez, M. A new species of Scapholeberis Schoedler, 1858 (Anomopoda: Daphniidae: Scapholeberinae) from the Colombian Amazon basin highlighted by DNA barcodes and morphology. PeerJ 2020, 8, e9989. [Google Scholar] [CrossRef]
- Elías-Gutiérrez, M.; Valdez-Moreno, M. A New Cryptic Species of Leberis Smirnov, 1989 (Crustacea, Cladocera, Chydoridae) from the Mexican Semi-Desert Region, Highlighted by DNA Barcoding. Hidrobiologica 2008, 18, 63–74. [Google Scholar]
- Keith, P.; Mennesson, M.I.; Sauri, S.; Busson, F.; Delrieu-Trottin, E.; Limmon, G.; Dahruddin, H.; Hubert, N. Giuris (Teleostei: Eleotridae) from Indonesia, with Description of a New Species. Cybium 2020, 44, 317–329. [Google Scholar]
- Escobar L, M.D.; Ota, R.P.; Machado-Allison, A.; Andrade-López, J.; Farias, I.P.; Hrbek, T. A new species of Piaractus (Characiformes: Serrasalmidae) from the Orinoco Basin with a redescription of Piaractus brachypomus. J. Fish Biol. 2019, 95, 411–427. [Google Scholar] [CrossRef]
- Ota, R.P.; Machado, V.N.; Andrade, M.C.; Collins, R.A.; Farias, I.P.; Hrbek, T. Integrative Taxonomy Reveals a New Species of Pacu (Characiformes: Serrasalmidae: Myloplus) from the Brazilian Amazon. Neotrop. Ichthyol. 2020, 18. [Google Scholar] [CrossRef]
- Bekker, E.I.; Karabanov, D.; Galimov, Y.R.; Kotov, A.A. DNA Barcoding Reveals High Cryptic Diversity in the North Eurasian Moina Species (Crustacea: Cladocera). PLoS ONE 2016, 11, e0161737. [Google Scholar] [CrossRef]
- Elías-Gutiérrez, M.; Juracka, P.J.; Montoliu-Elena, L.; Miracle, M.R.; Petrusek, A.; Korinek, V. Who Is Moina micrura? Redescription of One of the Most Confusing Cladocerans from Terra Typica, Based on Integrative Taxonomy. Limnetica 2019, 38, 227–252. [Google Scholar]
- Quiroz-Vázquez, P.; Elías-Gutiérrez, M. A New Species of the Freshwater Cladoceran Genus Scapholeberis Schoedler, 1858 (Cladocera: Anomopoda) from the Semidesert Northern Mexico, Highlighted by DNA Barcoding. Zootaxa 2009, 2236, 50–64. [Google Scholar] [CrossRef]
- Mercado-Salas, N.F.; Khodami, S.; Kihara, T.C.; Elías-Gutiérrez, M.; Arbizu, P.M. Genetic Structure and Distributional Patterns of the Genus Mastigodiaptomus (Copepoda) in Mexico, with the Description of a New Species from the Yucatan Peninsula. Arthropod Syst. Phylogeny 2018, 76, 487–507. [Google Scholar]
- Gutiérrez-Aguirre, M.A.; Cervantes-Martínez, A. A new species of Mastigodiaptomus Light, 1939 from Mexico, with notes of species diversity of the genus (Copepoda, Calanoida, Diaptomidae). ZooKeys 2016, 637, 61–79. [Google Scholar] [CrossRef][Green Version]
- Pegg, G.G.; Sinclair, B.; Briskey, L.; Aspden, W.J. MtDNA barcode identification of fish larvae in the southern Great Barrier Reef—Australia. Sci. Mar. 2006, 70, 7–12. [Google Scholar] [CrossRef]
- Hubert, N.; Delrieu-Trottin, E.; Irisson, J.-O.; Meyer, C.; Planes, S. Identifying coral reef fish larvae through DNA barcoding: A test case with the families Acanthuridae and Holocentridae. Mol. Phylogenetics Evol. 2010, 55, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Hubert, N.; Espiau, B.; Meyer, C.; Planes, S. Identifying the ichthyoplankton of a coral reef using DNA barcodes. Mol. Ecol. Resour. 2015, 15, 57–67. [Google Scholar] [CrossRef]
- Ko, H.-L.; Wang, Y.-T.; Chiu, T.-S.; Lee, M.-A.; Leu, M.-Y.; Chang, K.-Z.; Chen, W.-Y.; Shao, K.-T. Evaluating the Accuracy of Morphological Identification of Larval Fishes by Applying DNA Barcoding. PLoS ONE 2013, 8, e53451. [Google Scholar] [CrossRef]
- Azmir, I.A.; Esa, Y.; Amin, S.M.N.; Yasin, I.S.M.; Yusof, F.Z.M.; Azmir, I.A.; Esa, Y.; Amin, S.M.N.; Yasin, I.S.M.; Yusof, F.Z.M. Identification of larval fish in mangrove areas of Peninsular Malaysia using morphology and DNA barcoding methods. J. Appl. Ichthyol. 2017, 33, 998–1006. [Google Scholar] [CrossRef]
- Collet, A.; Durand, J.-D.; Desmarais, E.; Cerqueira, F.; Cantinelli, T.; Valade, P.; Ponton, D. DNA barcoding post-larvae can improve the knowledge about fish biodiversity: An example from La Reunion, SW Indian Ocean. Mitochondrial DNA Part A 2018, 29, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Mariac, C.; Vigouroux, Y.; Duponchelle, F.; García-Dávila, C.; Nunez, J.; Desmarais, E.; Renno, J. Metabarcoding by capture using a single COI probe (MCSP) to identify and quantify fish species in ichthyoplankton swarms. PLoS ONE 2018, 13, e0202976. [Google Scholar] [CrossRef] [PubMed]
- Steinke, D.; Connell, A.D.; Hebert, P.D. Linking adults and immatures of South African marine fishes. Genome 2016, 59, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jacobus, L.M.; DeWalt, R.E.; Adamowicz, S.J.; Hebert, P.D.N. Ephemeroptera, Plecoptera, and Trichoptera fauna of Churchill (Manitoba, Canada): Insights into biodiversity patterns from DNA barcoding. J. N. Am. Benthol. Soc. 2010, 29, 814–837. [Google Scholar] [CrossRef]
- Carew, M.E.; Pettigrove, V.; Cox, R.L.; Hoffmann, A.A. DNA identification of urban Tanytarsini chironomids (Diptera: Chironomidae). J. N. Am. Benthol. Soc. 2007, 26, 587–600. [Google Scholar] [CrossRef]
- Kusche, H.; Hanel, R. Consumers of mislabeled tropical fish exhibit increased risks of ciguatera intoxication: A report on substitution patterns in fish imported at Frankfurt Airport, Germany. Food Control 2021, 121, 107647. [Google Scholar] [CrossRef]
- Wong, E.H.K.; Hanner, R. DNA Barcoding Detects Market Substitution in North American Seafood. Food Res. Int. 2008, 41, 828–837. [Google Scholar] [CrossRef]
- Sarmiento-Camacho, S.; Valdez-Moreno, M. DNA barcode identification of commercial fish sold in Mexican markets. Genome 2018, 61, 457–466. [Google Scholar] [CrossRef]
- Chang, C.H.; Lin, H.Y.; Ren, Q.; Lin, Y.S.; Shao, K.T. DNA Barcode Identification of Fish Products in Taiwan: Government-Commissioned Authentication Cases. Food Control 2016, 66, 38–43. [Google Scholar] [CrossRef]
- Günther, B.; Raupach, M.J.; Knebelsberger, T. Full-length and mini-length DNA barcoding for the identification of seafood commercially traded in Germany. Food Control 2017, 73, 922–929. [Google Scholar] [CrossRef]
- Christiansen, H.; Fournier, N.; Hellemans, B.; Volckaert, F.A. Seafood substitution and mislabeling in Brussels’ restaurants and canteens. Food Control 2018, 85, 66–75. [Google Scholar] [CrossRef]
- Vandamme, S.G.; Griffiths, A.M.; Taylor, S.-A.; Di Muri, C.; Hankard, E.A.; Towne, J.A.; Watson, M.; Mariani, S. Sushi barcoding in the UK: Another kettle of fish. PeerJ 2016, 4, e1891. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, D.-M.; Baillie, C.; Mariani, S. Generic names and mislabeling conceal high species diversity in global fisheries markets. Conserv. Lett. 2018, 11, e12573. [Google Scholar] [CrossRef]
- Delpiani, G.; Delpiani, S.; Antoni, M.D.; Ale, M.C.; Fischer, L.; Lucifora, L.; de Astarloa, J.D. Are we sure we eat what we buy? Fish mislabelling in Buenos Aires province, the largest sea food market in Argentina. Fish. Res. 2020, 221, 105373. [Google Scholar] [CrossRef]
- Valdez-Moreno, M.; Quintal-Lizama, C.; Gómez-Lozano, R.; García-Rivas, M.D.C. Monitoring an Alien Invasion: DNA Barcoding and the Identification of Lionfish and Their Prey on Coral Reefs of the Mexican Caribbean. PLoS ONE 2012, 7, e36636. [Google Scholar] [CrossRef]
- Valdez-Moreno, M.; Ivanova, N.V.; Elías-Gutiérrez, M.; Pedersen, S.L.; Bessonov, K.; Hebert, P.D.N. Using eDNA to biomonitor the fish community in a tropical oligotrophic lake. PLoS ONE 2019, 14, e0215505. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Braukmann, T.W.A.; Prosser, S.W.J.; Ratnasingham, S.; Dewaard, J.R.; Ivanova, N.V.; Janzen, D.H.; Hallwachs, W.; Naik, S.; Sones, J.E.; et al. A Sequel to Sanger: Amplicon sequencing that scales. BMC Genom. 2018, 19, 219. [Google Scholar] [CrossRef] [PubMed]
- Pomerantz, A.; Penafiel, N.; Arteaga, A.; Bustamante, L.; Pichardo, F.; Coloma, L.A.; Barrio-Amoros, C.L.; Salazar-Valenzuela, D.; Prost, S. Real-Time DNA Barcoding in a Rainforest Using Nanopore Sequencing: Opportunities for Rapid Biodiversity Assessments and Local Capacity Building. Gigascience 2018, 7, giy033. [Google Scholar] [CrossRef]
- Srivathsan, A.; Baloglu, B.; Wang, W.; Tan, W.X.; Bertrand, D.; Ng, A.H.Q.; Boey, E.J.H.; Koh, J.J.Y.; Nagarajan, N.; Meier, R. A Minion-Based Pipeline for Fast and Cost-Effective DNA Barcoding. Mol. Ecol. Resour. 2018, 18, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Berry, T.E.; Osterrieder, S.K.; Murray, D.C.; Coghlan, M.L.; Richardson, A.J.; Grealy, A.K.; Stat, M.; Bejder, L.; Bunce, M. DNA metabarcoding for diet analysis and biodiversity: A case study using the endangered Australian sea lion (Neophoca cinerea). Ecol. Evol. 2017, 7, 5435–5453. [Google Scholar] [CrossRef]
- Suter, L.; Polanowski, A.; Clarke, L.J.; Kitchener, J.; Deagle, B.E. Capturing open ocean biodiversity: Comparing environmental DNA metabarcoding to the continuous plankton recorder. Mol. Ecol. 2020. [Google Scholar] [CrossRef]
- Zamora-Terol, S.; Novotny, A.; Winder, M. Reconstructing Marine Plankton Food Web Interactions Using DNA Metabarcoding. Mol. Ecol. 2020, 29, 3380–3395. [Google Scholar] [CrossRef]
- Mauffrey, F.; Cordier, T.; Apothéloz-Perret-Gentil, L.; Cermakova, K.; Merzi, T.; Delefosse, M.; Blanc, P.; Pawlowski, J. Benthic monitoring of oil and gas offshore platforms in the North Sea using environmental DNA metabarcoding. Mol. Ecol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Elbrecht, V.; Vamos, E.E.; Meissner, K.; Aroviita, J.; Leese, F. Assessing strengths and weaknesses of DNA metabarcoding-based macroinvertebrate identification for routine stream monitoring. Methods Ecol. Evol. 2017, 8, 1265–1275. [Google Scholar] [CrossRef]
- Andújar, C.; Arribas, P.; Yu, D.W.; Vogler, A.P.; Emerson, B.C. Why the COI barcode should be the community DNA metabarcode for the metazoa. Mol. Ecol. 2018, 27, 3968–3975. [Google Scholar] [CrossRef]
- Deiner, K.; Bik, H.M.; Mächler, E.; Seymour, M.; Lacoursière-Roussel, A.; Altermatt, F.; Creer, S.; Bista, I.; Lodge, D.M.; De Vere, N.; et al. Environmental DNA metabarcoding: Transforming how we survey animal and plant communities. Mol. Ecol. 2017, 26, 5872–5895. [Google Scholar] [CrossRef]
- Rees, H.C.; Maddison, B.C.; Middleditch, D.J.; Patmore, J.R.M.; Gough, K.C. Review the Detection of Aquatic Animal Species Using Environmental DNA—A Review of eDNA as a Survey Tool in Ecology. J. Appl. Ecol. 2014, 51, 1450–1459. [Google Scholar] [CrossRef]
- Valentini, A.; Taberlet, P.; Miaud, C.; Civade, R.; Herder, J.; Thomsen, P.F.; Bellemain, E.; Besnard, A.; Coissac, E.; Boyer, F.; et al. Data from: Next-generation monitoring of aquatic biodiversity using environmental DNA metabarcoding. Mol. Ecol. 2016, 25, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.A.; Bakker, J.; Wangensteen, O.S.; Soto, A.Z.; Corrigan, L.; Sims, D.W.; Genner, M.J.; Mariani, S. Non-specific amplification compromises environmental DNA metabarcoding with COI. Methods Ecol. Evol. 2019, 10, 1985–2001. [Google Scholar] [CrossRef]
- Cordier, T.; Esling, P.; Lejzerowicz, F.; Visco, J.A.; Ouadahi, A.; Martins, C.I.M.; Cedhagen, T.; Pawlowski, J. Predicting the Ecological Quality Status of Marine Environments from eDNA Metabarcoding Data Using Supervised Machine Learning. Environ. Sci. Technol. 2017, 51, 9118–9126. [Google Scholar] [CrossRef]
- Mächler, E.; Walser, J.; Altermatt, F. Decision-making and best practices for taxonomy-free environmental DNA metabarcoding in biomonitoring using Hill numbers. Mol. Ecol. 2020. [Google Scholar] [CrossRef]
- Van Der Walt, K.; Mäkinen, T.; Swartz, E.; Weyl, O. DNA barcoding of South Africa’s ornamental freshwater fish—Are the names reliable? Afr. J. Aquat. Sci. 2017, 42, 155–160. [Google Scholar] [CrossRef]
- Collins, R.A.; Armstrong, K.; Meier, R.; Yi, Y.; Brown, S.; Cruickshank, R.H.; Keeling, S.; Johnston, C. Barcoding and Border Biosecurity: Identifying Cyprinid Fishes in the Aquarium Trade. PLoS ONE 2012, 7, e28381. [Google Scholar] [CrossRef] [PubMed]
- Curry, C.J.; Gibson, J.F.; Shokralla, S.; Hajibabaei, M.; Baird, D.J. Identifying North American freshwater invertebrates using DNA barcodes: Are existing COI sequence libraries fit for purpose? Freshw. Sci. 2018, 37, 178–189. [Google Scholar] [CrossRef]
- Jo, H.; Gim, J.A.; Jeong, K.S.; Kim, H.S.; Joo, G.J. Application of DNA Barcoding for Identification of Freshwater Carnivorous Fish Diets: Is Number of Prey Items Dependent on Size Class for Micropterus Salmoides? Ecol. Evol. 2014, 4, 219–229. [Google Scholar] [CrossRef]
- Jo, H.; Ventura, M.; Vidal, N.; Gim, J.S.; Buchaca, T.; Barmuta, L.A.; Jeppesen, E.; Joo, G.J. Discovering Hidden Biodiversity: The Use of Complementary Monitoring of Fish Diet Based on DNA Barcoding in Freshwater Ecosystems. Ecol. Evol. 2016, 6, 219–232. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elías-Gutiérrez, M.; Hubert, N.; Collins, R.A.; Andrade-Sossa, C. Aquatic Organisms Research with DNA Barcodes. Diversity 2021, 13, 306. https://doi.org/10.3390/d13070306
Elías-Gutiérrez M, Hubert N, Collins RA, Andrade-Sossa C. Aquatic Organisms Research with DNA Barcodes. Diversity. 2021; 13(7):306. https://doi.org/10.3390/d13070306
Chicago/Turabian StyleElías-Gutiérrez, Manuel, Nicolas Hubert, Rupert A. Collins, and Camilo Andrade-Sossa. 2021. "Aquatic Organisms Research with DNA Barcodes" Diversity 13, no. 7: 306. https://doi.org/10.3390/d13070306
APA StyleElías-Gutiérrez, M., Hubert, N., Collins, R. A., & Andrade-Sossa, C. (2021). Aquatic Organisms Research with DNA Barcodes. Diversity, 13(7), 306. https://doi.org/10.3390/d13070306







