Molecular Survey of Pathogens in Wild Amazon Parrot Nestlings: Implications for Conservation
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piacentini, V.Q.; Aleixo, A.; Agne, C.E.; Mauricio, G.N.; Pacheco, J.F.; Bravo, G.A.; Brito, G.R.R.; Naka, N.L.; Olmos, F.; Posso, S.; et al. Annotated checklist of the birds of Brazil by the Brazilian Ornithological Records Committee. Rev. Bras. Ornitol. 2015, 23, 91–298. [Google Scholar] [CrossRef]
- IUCN—International Union for Conservation of Nature. Red List of Threatened Species, Version 2010-2; 2020. Available online: http://www.iucnredlist.org (accessed on 15 March 2021).
- Seixas, G.H.F.; Mourão, G. Communal roosts of the Blue-fronted Amazons (Amazona aestiva) in a large tropical wetland: Are they of different types? PLoS ONE. 2018, 13, e0204824. [Google Scholar] [CrossRef]
- CITES—Convention on International Trade in Endangered Species of Wild Fauna and Flora. CITES Trade Database—1975–2018. 2018. Available online: https://trade.cites.org/ (accessed on 15 March 2021).
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 2000, 28, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Berkunsky, I.; Quillfeldt, P.; Brightsmith, D.J.; Abbud, M.C.; Aguilar, J.M.R.E.; Alemán-Zelaya, U.; Aramburú, R.M.; Arce Arias, A.; Balas McNab, R.; Balsby, T.J.S.; et al. Current threats faced by Neotropical parrot populations. Biol. Cons. 2017, 214, 278–287. [Google Scholar] [CrossRef]
- Marini, M.A.; Garcia, F.I. Bird conservation in Brazil. Cons. Biol. 2005, 19, 665–671. [Google Scholar] [CrossRef]
- Phalen, D.N. The globalization of parrot viruses as the result of the trade in wild caught parrots and its potential conservation implications. In Proceedings of the Wildlife Diseases Association International Conference, Sunshine Coast, Australia, 26–30 July 2015; p. 24. [Google Scholar]
- Gaskin, J.M.; Robbins, C.M.; Jacobson, E.R. An explosive outbreak of Pacheco’s parrot disease and preliminary experimental findings. In Proceedings of the American Association of Zoo Veterinarians, Knoxville, TN, USA, 7–9 November 2019; pp. 365–374. [Google Scholar]
- Raso, T.F.; Godoy, S.N.; Milanelo, L.; Souza, C.A.I.; Matushima, E.R.; Araújo Júnior, J.P.; Pinto, A.A. An outbreak of chlamydiosis in captive Blue-fronted Amazon parrots (Amazona aestiva) in Brazil. J. Zoo Wildl. Med. 2004, 35, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Faulkes, C.G.; Greenwood, A.G.; Jones, C.G.; Kaiser, P.; Lyne, O.D.; Black, S.A.; Chowrimootoo, A.; Groombridge, J.J. Tracking viral evolution during a disease outbreak: The rapid and complete selective sweep of a circovirus in the endangered Echo parakeet. J. Virol. 2012, 86, 5221–5229. [Google Scholar] [CrossRef]
- Olsen, G.H.; Crosta, L.; Gartrell, B.D.; Marsh, P.M.; Stringfield, C.E. Conservation of avian species. In Current Therapy in Avian Medicine and Surgery; Speer, B.L., Ed.; Elsevier: St.Louis, MO, USA, 2016; pp. 719–748. [Google Scholar]
- Raso, T.F.; Teixeira, R.H.F.; Carrasco, A.O.T.; Araújo, J.P., Jr.; Pinto, A.A. Chlamydophila psittaci infections in hyacinth macaws (Anodohynchus hyacinthinus) confiscated in Brazil. J. Zoo Wildl. Med. 2013, 44, 169–172. [Google Scholar] [CrossRef]
- Luppi, M.M.; Luiz, A.P.M.F.; Coelho, F.M.; Malta, M.C.C.; Preis, I.S.; Ecco, R.; Fonseca, F.G.; Resende, M. Identification and isolation of psittacid herpesvirus from psittacids in Brazil. Vet. Microbiol. 2011, 154, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Esteves, F.C.B.; Marín, S.Y.; Resende, M.; Silva, A.S.G.; Coelho, H.L.G.; Barbosa, M.B.; D’Aparecida, N.S.D.; Resende, J.S.; Torres, A.C.D.; Martins, N.R.S. Avian pox in native captive psittacines, Brazil. Emerg. Infect. Dis. 2015, 23, 154–156. [Google Scholar] [CrossRef]
- Werther, K.; Raso, T.F.; Durigon, E.L.; Latimer, K.S.; Campagnoli, R.P. Psittacine Beak and Feather Disease in Brazil: Case report. Braz. J. Poult. Sci. 1999, 1, 85–88. [Google Scholar]
- Araújo, A.V.; Andery, D.A.; Ferreira, F.C., Jr.; Ortiz, M.C.; Marques, M.V.R.; Marin, S.Y.; Vilela, D.A.R.; Resende, J.S.; Resende, M.; Donatti, R.V.; et al. Molecular diagnosis of beak and feather disease in native psittacine species. Brazil. J. Poult. Sci. 2015, 17, 451–458. [Google Scholar] [CrossRef]
- Azevedo, N.P. Detecção de bornavírus, poliomavírus e circovírus em amostras biológicas, utilizando PCR e RT-PCR, de psitacídeos com diferentes aspectos clínicos. Master’s Thesis, University of São Paulo, São Paulo, 2014. Available online: https://teses.usp.br/teses/disponiveis/10/10133/tde-07102014-152516/pt-br.php (accessed on 8 January 2021).
- Raso, T.F.; Seixas, G.H.F.; Guedes, N.M.R.; Pinto, A.A. Chlamydophila psittaci in free-living Blue-fronted Amazon parrots (Amazona aestiva) and Hyacinth macaws (Anodorhynchus hyacinthinus) in the Pantanal of Mato Grosso do Sul, Brazil. Vet. Microbiol. 2006, 117, 235–241. [Google Scholar] [CrossRef]
- Ribas, J.M.; Sipinski, E.A.B.; Serafini, P.P.; Ferreira, V.L.; Raso, T.F.; Pinto, A.A. Chlamydophila psittaci assessment in threatened Red-tailed Amazon (Amazona brasiliensis) parrots in Paraná, Brazil. Ornithologia 2014, 6, 144–147. [Google Scholar]
- Vaz, F.F.; Serafini, P.P.; Locatelli-Dittrich, R.; Meurer, R.; Durigon, E.L.; Araújo, J.; Thomazelli, L.M.; Ometto, T.; Spinski, E.A.B.; Sezerban, R.M.; et al. Survey of pathogens in threatened wild red-tailed Amazon parrot (Amazona brasiliensis) nestlings in Rasa Island, Brazil. Brazil J. Microbiol. 2017, 48, 747–753. [Google Scholar] [CrossRef]
- Ehricht, R.; Slickers, P.; Goellner, S.; Hotzel, H.; Sachse, K. Optimized DNA microarray assay allows detection and genotyping of single PCR-amplifiable target copies. Mol. Cell Probes. 2006, 20, 60–63. [Google Scholar] [CrossRef]
- Tomaszewski, E.K.; Kaleta, E.F.; Phalen, D.N. Molecular phylogeny of the psittacid herpesviruses causing Pacheco’s disease: Correlation of genotype and phenotypic expression. J. Virol. 2003, 77, 11260–11267. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.H.; Lee, K.H. Application of the polymerase chain reaction for the diagnosis of fowl poxvirus infection. J. Virol. Meth. 1997, 63, 113–119. [Google Scholar] [CrossRef]
- Ypelaar, I.; Bassami, M.R.; Wilcox, G.E.; Raidal, S.R. A universal polymerase chain reaction for the detection of psittacine beak and feather disease virus. Vet. Microbiol. 1999, 16, 141–148. [Google Scholar] [CrossRef]
- Sachse, K.; Laroucau, K.; Vorimore, F.; Magnino, S.; Feige, J.; Müller, W.; Kube, S.; Hotzel, H.; Schubert, E.; Slickers, S.; et al. DNA microarray-based genotyping of Chlamydophila psittaci strains from culture and clinical samples. Vet. Microbiol. 2009, 135, 22–30. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Regnard, G.L.; Boyes, R.S.; Martin, R.O.; Hitzeroth, I.I.; Rybicki, E.P. Beak and feather disease viruses circulating in Cape parrots (Poicepahlus robustus) in South Africa. Arch. Virol. 2015, 160, 47–54. [Google Scholar] [CrossRef]
- Fogell, D.J.; Martin, R.O.; Bunbury, N.; Lawson, B.; Sells, J.; McKeand, A.M. Trade and conservation implications of new beak and feather disease virus detection in native and introduced parrots. Cons Biol. 2018, 32, 1325–1335. [Google Scholar] [CrossRef]
- Deem, S.L.; Noss, A.J.; Cuellar, R.L.; Karesh, W.B. Health evaluation of free-ranging and captive blue-fronted amazon parrots (Amazona aestiva) in the Gran Chaco, Bolivia. J. Zoo Wildl. Med. 2005, 36, 598–605. [Google Scholar] [CrossRef]
- Gilardi, K.V.K.; Lowentime, L.J.; Gilardi, J.D.; Munn, C.A.A. A survey for selected viral, chlamydial, and parasitic diseases in wild dusky-headed parakeets (Aratinga weddelli) and tui parakeets (Brotogeris sanctithomae) in Peru. J. Wildl. Dis. 1995, 31, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Raso, T.F.; Almeida, M.A.B.; Santos, E.; Bercini, M.; Barbosa, M. Genetic characterization of Chlamydophila psittaci from monk parakeet (Myiopsitta monachus). In Proceedings of the Congresso Latinoamericano de Microbiologia, Santos, Brazil, 28–31 October 2012; p. 152. [Google Scholar]
- Raso, T.F.; Ferreira, V.L.; TIMM, L.N.; Abreu, M.F.T. Psittacosis domiciliary outbreak associated with monk parakeets (Myiopsitta monachus) in Brazil: Need for surveillance and control. JMM Case Rep. 2014, 1, e003343. [Google Scholar] [CrossRef]
- Pacheco, G.; Bier, O. Epizootie chex les perroquets du Brésil. Relations avec le psittacose. CR Soc. Biol. 1930, 105, 109–111. [Google Scholar]
- Miller, T.D.; Millar, D.L.; Naqi, S.A. Isolation of Pacheco’s disease herpesvirus in Texas. Avian Dis. 1979, 23, 753–756. [Google Scholar] [CrossRef]
- Luppi, M.M.; Luiz, A.P.M.F.; Coelho, F.M.; Ecco, R.; Fonseca, F.G.; Resende, M. Genotypic characterization of psittacid herpesvirus isolates from Brazil. Braz. J. Microbiol. 2016, 47, 217–224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saidenberg, A.B.S.; Zuniga, E.; Melville, P.A.; Salaberry, S.; Benites, N.R. Health-screening protocols for vinaceous amazons (Amazona vinacea) in a reintroduction project. J. Zoo Wildl. Med. 2015, 46, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Murer, L.; Westenhofen, M.; Kommers, G.D.; Furian, T.Q.; Borges, K.A.; Kunert-Filho, H.C.; Streck, A.F.; Lovato, M. Identification and phylogenetic analysis of clade C Avipoxvirus in a fowlpox outbreak in exotic psittacines in southern Brazil. J. Vet. Diagn. Investig. 2018, 30, 946–950. [Google Scholar] [CrossRef]
- Fogell, D.J.; Martin, R.O.; Groombridge, J.J. Beak and feather disease virus in wild and captive parrots: An analysis of geographic and taxonomic distribution and methodological trends. Arch. Virol. 2016, 161, 2059–2074. [Google Scholar] [CrossRef]
- Raidal, S.R.; Peters, A. Psittacine beak and feather disease: Ecology and implications for conservation. Emu—Austral Ornithol. 2018, 118, 80–93. [Google Scholar] [CrossRef]
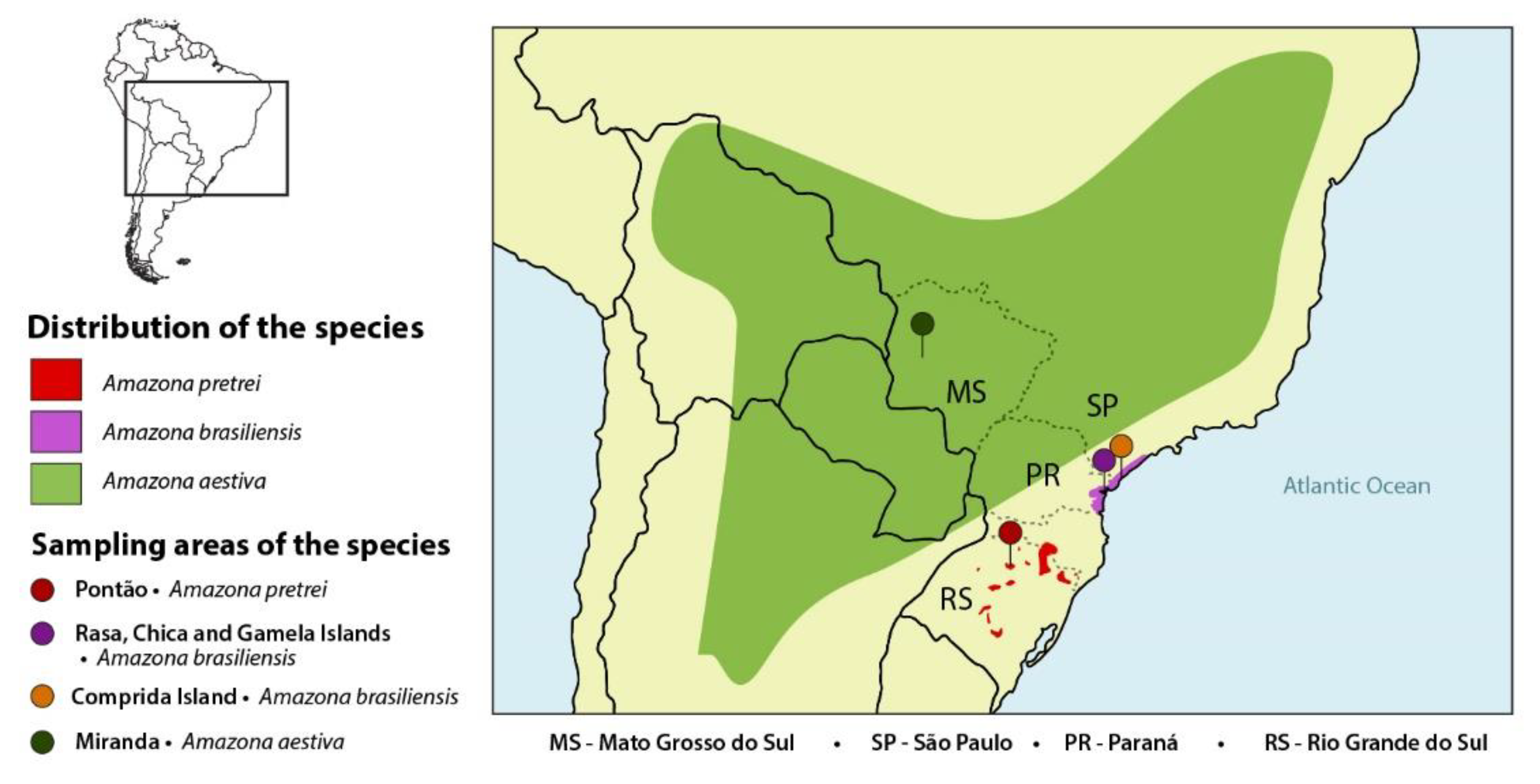
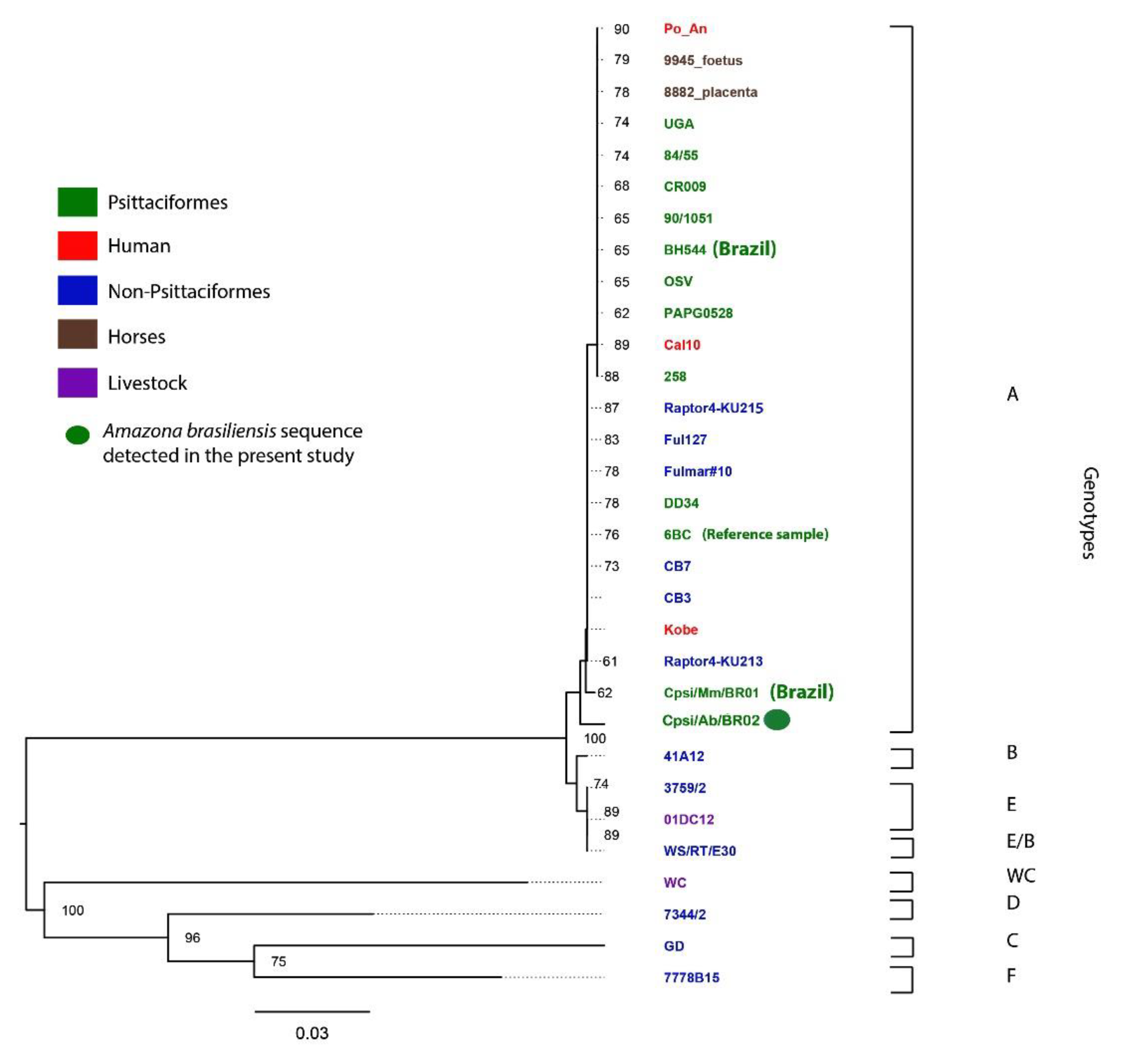
| Amazon Species | State | 2013/ 2014 | 2015 | 2016 | 2017 | 2018 | Total Samples (Swab and/or Blood) | Number of Birds Sampled for Selected Virus **/Positive (%) | Number of Chlamydia Positive/Total Birds (%) |
|---|---|---|---|---|---|---|---|---|---|
| A. pretrei | RS | 0 | 0 | 4 | 0 | 0 | 8 | 4 (0%) | 0/4 (0%) |
| A. brasiliensis | PR/SP | 74 * | 0 | 28 # | 15 | 21 | 230 | 138 (0%) | 2/80 (2.5%) |
| A. aestiva | MS | 17 | 17 | 23 | 3 | 0 | 89 | 63 (0%) | 3/63 (4.8%) |
| Total | 327 | 205 (0%) | 5/147 (3.4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaz, F.F.; Sipinski, E.A.B.; Seixas, G.H.F.; Prestes, N.P.; Martinez, J.; Raso, T.F. Molecular Survey of Pathogens in Wild Amazon Parrot Nestlings: Implications for Conservation. Diversity 2021, 13, 272. https://doi.org/10.3390/d13060272
Vaz FF, Sipinski EAB, Seixas GHF, Prestes NP, Martinez J, Raso TF. Molecular Survey of Pathogens in Wild Amazon Parrot Nestlings: Implications for Conservation. Diversity. 2021; 13(6):272. https://doi.org/10.3390/d13060272
Chicago/Turabian StyleVaz, Frederico Fontanelli, Elenise Angelotti Bastos Sipinski, Gláucia Helena Fernandes Seixas, Nêmora Pauletti Prestes, Jaime Martinez, and Tânia Freitas Raso. 2021. "Molecular Survey of Pathogens in Wild Amazon Parrot Nestlings: Implications for Conservation" Diversity 13, no. 6: 272. https://doi.org/10.3390/d13060272
APA StyleVaz, F. F., Sipinski, E. A. B., Seixas, G. H. F., Prestes, N. P., Martinez, J., & Raso, T. F. (2021). Molecular Survey of Pathogens in Wild Amazon Parrot Nestlings: Implications for Conservation. Diversity, 13(6), 272. https://doi.org/10.3390/d13060272







