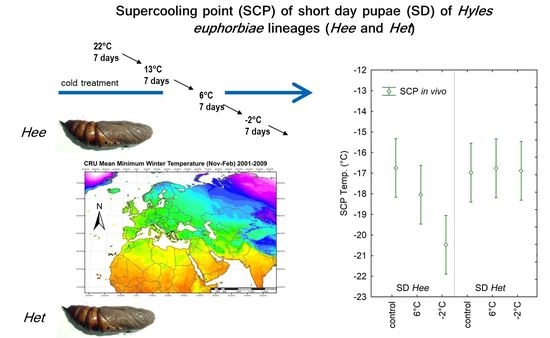
Super Cooling Point Phenotypes and Cold Resistance in Hyles euphorbiae Hawk Moths from Different Climate Zones
Abstract
1. Introduction
2. Materials and Methods
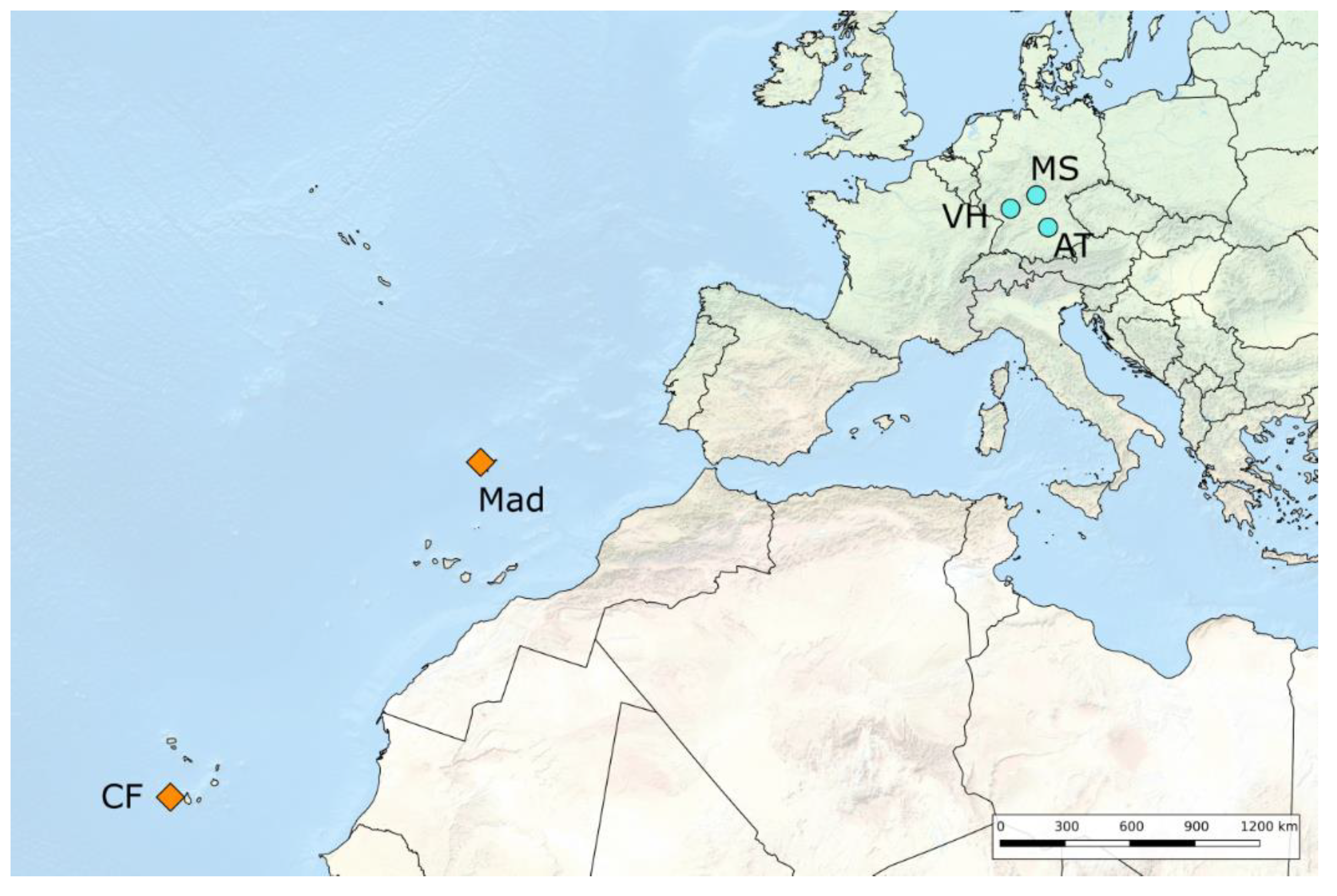
2.1. Sampling
2.2. Larval Breeding
- Long day (LD): 16.5 h light duration at 25 °C and 7.5 h darkness at 23 °C, with a ramp time between light/dark of 1 h.
- Short day (SD): For Het, 11 h light at 25 °C and 13 h darkness at 23 °C, ramp time 1 h, and for Hee, 13 h light at 25 °C and 11 h darkness at 23 °C with 1 h ramp time was needed to ensure larval prosperity (corresponding with natural September daylight lengths in Western African and Central European latitudes, respectively).
2.3. Cooling Treatments of the Pupae
2.4. Experimental Design: SCP and Mortality Tests
2.5. In Vitro Calorimetric Hemolymph SCP Analyses
2.6. In Vivo SCP of Entire Pupae
3. Results
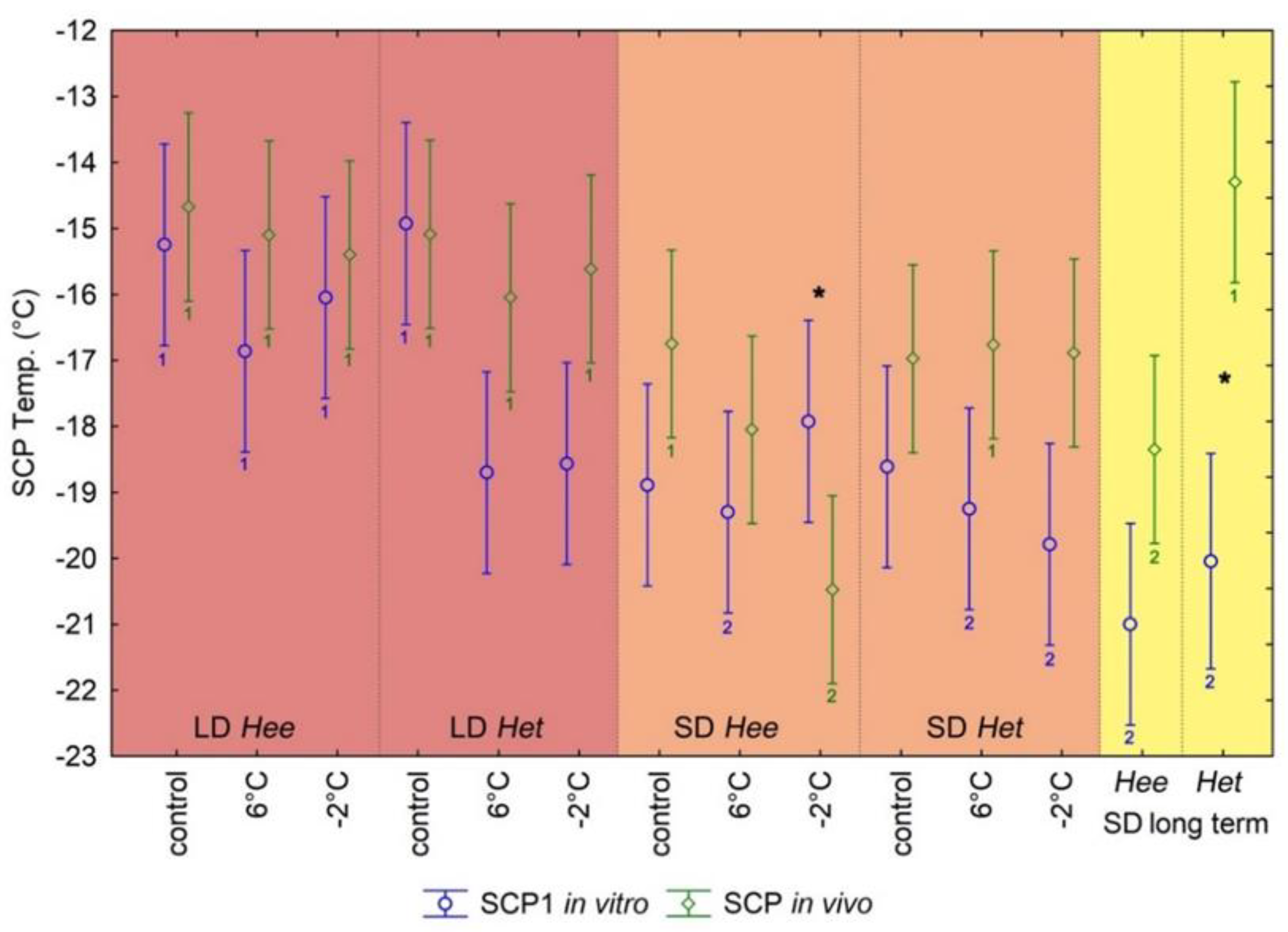
3.1. In Vitro Calorimetric Hemolymph SCP Analyses
3.2. In Vivo SCP Measurements on Entire Pupae
3.3. Mortality Tests
4. Discussion
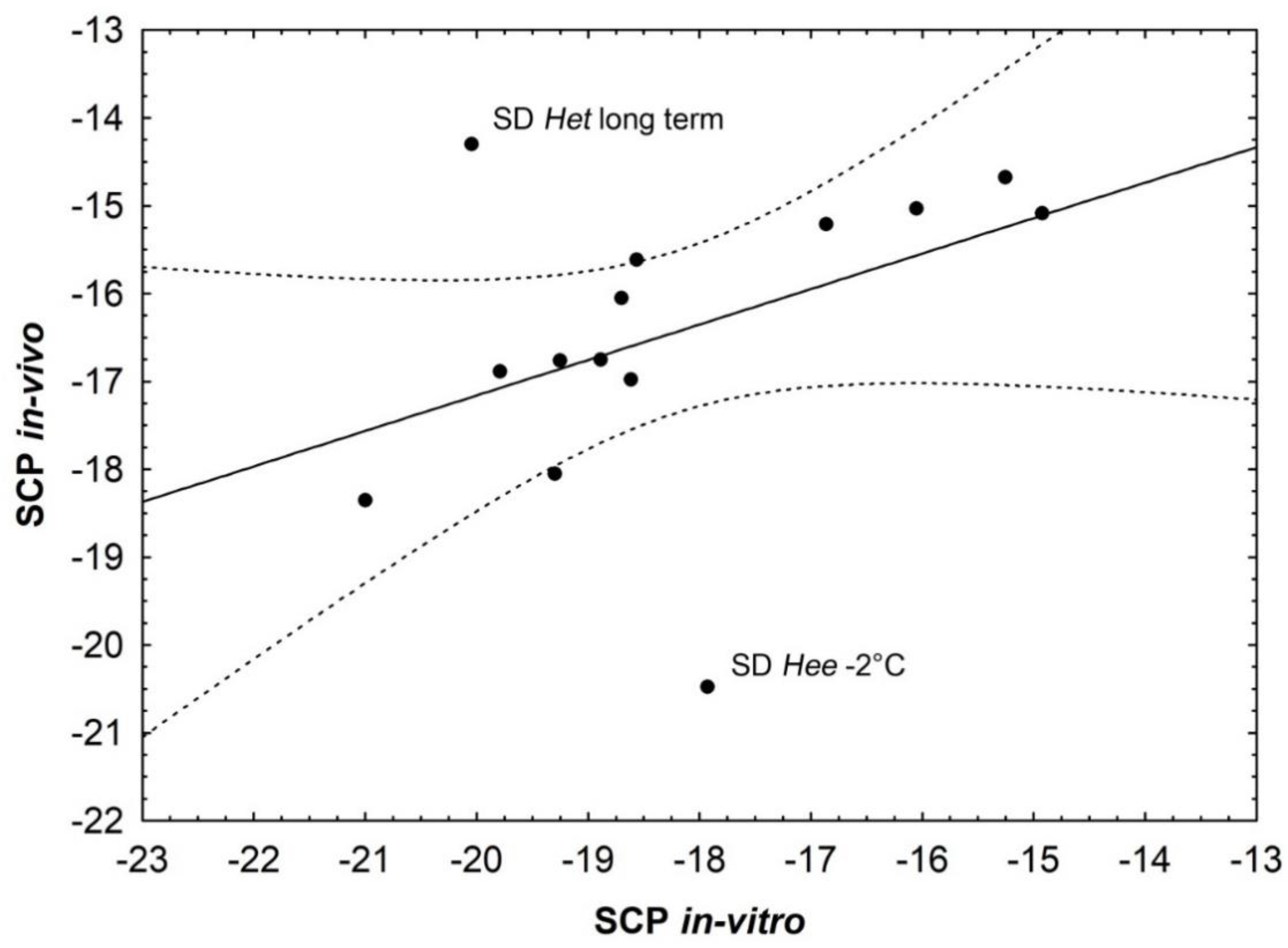
4.1. In Vitro and In Vivo SCP Analyses
4.2. Mortality Tests and Survival Strategies in Pupa Stage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| In-Vitro | In-Vivo | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ecotype | Day Length | Treatment | ID Sample | Sex | Mass (g) | SCP | Ecotype | Day Length | Treatment | ID Sample | Sex | Mass (g) | SCP |
| Hee | LD | control | VH31 | f | 2.34 | −15.9 | Hee | LD | control | VH015 | m | 1.588 | −14.7 |
| Hee | LD | control | VH32 | f | 2.443 | −15.5 | Hee | LD | control | VH035 | m | 2.707 | −15.2 |
| Hee | LD | control | VH26 | m | 2.028 | −14.9 | Hee | LD | control | VH041 | f | 1.961 | −16.2 |
| Hee | LD | control | VH27 | m | 2.273 | −15.4 | Hee | LD | control | SA001 | m | 1.077 | −15 |
| Hee | LD | control | VH23 | m | 1.657 | −14.9 | Hee | LD | control | SA002 | f | 2.533 | −14.7 |
| Hee | LD | control | VH49 | f | 2.298 | −15.3 | Hee | LD | control | SA003 | m | 2.011 | −16.3 |
| Hee | LD | control | VH16 | m | 2.305 | −14.8 | Hee | LD | control | SA004 | f | 1.943 | −7.3 |
| Hee | LD | control | VH19 | m | 1.923 | −15.3 | Hee | LD | control | SA005 | m | 1.959 | −18 |
| Hee | LD | control | VH03 | m | 2.76 | −15.3 | Hee | LD | 6 °C | VH021 | f | 2.117 | −16.7 |
| Hee | LD | 6 °C | VH28 | m | 2.512 | −16.2 | Hee | LD | 6 °C | VH030 | f | 2.262 | −16.1 |
| Hee | LD | 6 °C | VH39 | m | 2.393 | −18.4 | Hee | LD | 6 °C | VH036 | f | 2.207 | −8.2 |
| Hee | LD | 6 °C | VH42 | f | 2.206 | −14.6 | Hee | LD | 6 °C | VH038 | f | 2.373 | −16.5 |
| Hee | LD | 6 °C | VH43 | f | 2.38 | −15.4 | Hee | LD | 6 °C | VH040 | f | 2.766 | −15.1 |
| Hee | LD | 6 °C | VH29 | m | 2.006 | −16.7 | Hee | LD | 6 °C | VH045 | f | 2.673 | −17.2 |
| Hee | LD | 6 °C | VH08 | f | 2.644 | −18.6 | Hee | LD | 6 °C | VH001 | m | 2.644 | −15.8 |
| Hee | LD | 6 °C | VH09 | f | 2.517 | −15.1 | Hee | LD | 6 °C | VH012 | m | 1.729 | −15.2 |
| Hee | LD | 6 °C | VH14 | f | 2.655 | −19.9 | Hee | LD | 6 °C | VH024 | m | 1.942 | −16.1 |
| Hee | LD | −2 °C | VH82 | f | 2.528 | −13.2 | Hee | LD | −2 °C | VH046 | f | 2.21 | −15.4 |
| Hee | LD | −2 °C | VH83 | f | 1.939 | −15.6 | Hee | LD | −2 °C | VH047 | f | 2.301 | −10.6 |
| Hee | LD | −2 °C | VH90 | f | 2.282 | −15 | Hee | LD | −2 °C | VH052 | f | 2.071 | −13.7 |
| Hee | LD | −2 °C | VH80 | f | 2.423 | −16.5 | Hee | LD | −2 °C | VH055 | f | 2.474 | −15.1 |
| Hee | LD | −2 °C | VH76 | f | 2.6 | −15.7 | Hee | LD | −2 °C | VH056 | f | 2.206 | −16.3 |
| Hee | LD | −2 °C | VH78 | m | 1.988 | −17.3 | Hee | LD | −2 °C | VH070 | f | 1.215 | −18.1 |
| Hee | LD | −2 °C | VH79 | m | 1.729 | −17.7 | Hee | LD | −2 °C | VH060 | m | 2.622 | −17.2 |
| Hee | LD | −2 °C | VH87 | m | 2.138 | −17.4 | Hee | LD | −2 °C | VH066 | m | 2.053 | −16.8 |
| Het | LD | control | CF37 | m | 2.478 | −16.3 | Hee | LD | −2 °C | VH077 | m | 2.27 | −12.1 |
| Het | LD | control | CF65 | f | 2.143 | −16.2 | Het | LD | control | CF005 | f | 2.707 | −16 |
| Het | LD | control | CF80 | f | 2.776 | −13.6 | Het | LD | control | CF009 | f | 2.06 | −15.1 |
| Het | LD | control | CF91 | f | 1.867 | −15.2 | Het | LD | control | CF017 | f | 2.398 | −14.7 |
| Het | LD | control | CF76 | m | 1.369 | −15.3 | Het | LD | control | CF026 | f | 2.292 | −15.7 |
| Het | LD | control | CF83 | m | 2.189 | −13.4 | Het | LD | control | CF011 | m | 1.938 | −13 |
| Het | LD | control | CF90 | m | 2.217 | −15.5 | Het | LD | control | CF014 | m | 2.196 | −15.9 |
| Het | LD | control | CF92 | m | 1.671 | −13.9 | Het | LD | control | CF025 | m | 2.319 | −16 |
| Het | LD | 6 °C | CF66 | f | 1.971 | −21.2 | Het | LD | control | CF055 | m | 2.115 | −14.3 |
| Het | LD | 6 °C | CF74 | f | 1.982 | −20.8 | Het | LD | 6 °C | CF006 | f | 2.378 | −13.9 |
| Het | LD | 6 °C | CF78 | f | 2.685 | −17.3 | Het | LD | 6 °C | CF020 | f | 2.491 | −16.9 |
| Het | LD | 6 °C | CF46 | m | 1.906 | −22 | Het | LD | 6 °C | CF021 | f | 1.935 | −16.7 |
| Het | LD | 6 °C | CF69 | m | 2.4 | −18.1 | Het | LD | 6 °C | CF030 | f | 2.085 | −17.2 |
| Het | LD | 6 °C | CF72 | m | 2.033 | −17.9 | Het | LD | 6 °C | CF034 | f | 2.933 | −15.5 |
| Het | LD | 6 °C | CF36 | m | 1.604 | −15.6 | Het | LD | 6 °C | CF039 | f | 2.137 | −16.2 |
| Het | LD | 6 °C | CF64 | f | 2.18 | −16.7 | Het | LD | 6 °C | CF003 | m | 1.622 | −16 |
| Het | LD | −2 °C | CF82 | f | 2.503 | −17.4 | Het | LD | 6 °C | CF033 | m | 2.262 | −16 |
| Het | LD | −2 °C | CF86 | f | 2.846 | −17.9 | Het | LD | −2 °C | CF040 | n.a. | n.a. | −13.6 |
| Het | LD | −2 °C | CF87 | f | 2.479 | −17.9 | Het | LD | −2 °C | CF043 | n.a. | n.a. | −14.3 |
| Het | LD | −2 °C | CF88 | f | 2.428 | −21.2 | Het | LD | −2 °C | CF044 | n.a. | n.a. | −15 |
| Het | LD | −2 °C | CF93 | f | 2.944 | −22.5 | Het | LD | −2 °C | CF052 | n.a. | n.a. | −17.6 |
| Het | LD | −2 °C | CF08 | m | 1.492 | −17.7 | Het | LD | −2 °C | CF054 | n.a. | n.a. | −16.8 |
| Het | LD | −2 °C | CF81 | m | 1.768 | −18.9 | Het | LD | −2 °C | CF041 | n.a. | n.a. | −15.8 |
| Het | LD | −2 °C | CF31 | f | 1.91 | −15 | Het | LD | −2 °C | CF045 | n.a. | n.a. | −15.7 |
| Hee | SD | control | AT008 | m | 1.677 | −15.8 | Het | LD | −2 °C | CF067 | n.a. | n.a. | −16.1 |
| Hee | SD | control | AT010 | m | 1.612 | −19.2 | Hee | SD | control | AT32 | m | 1.819 | −17.6 |
| Hee | SD | control | AT012 | m | 1.714 | −19.4 | Hee | SD | control | AT34 | m | 1.922 | −18.1 |
| Hee | SD | control | AT024 | f | 2.203 | −22.7 | Hee | SD | control | AT36 | m | 1.73 | −15.7 |
| Hee | SD | control | MS032 | m | 2.011 | −19.3 | Hee | SD | control | AT51 | f | 2.372 | −18.4 |
| Hee | SD | control | MS036 | f | 1.874 | −11.1 | Hee | SD | control | AT52 | f | 1.8 | −16.6 |
| Hee | SD | control | MS039 | f | 2.324 | −23.8 | Hee | SD | control | MS33 | m | 1.613 | −18.5 |
| Hee | SD | control | AT025 | f | 1.756 | −19.8 | Hee | SD | control | MS43 | f | 2.173 | −11 |
| Hee | SD | 6 °C | AT016 | f | 2.134 | −20.6 | Hee | SD | control | MS46 | f | 1.933 | −18.1 |
| Hee | SD | 6 °C | AT018 | m | 1.634 | −14.5 | Hee | SD | 6 °C | VH003 | m | 1.633 | −15.5 |
| Hee | SD | 6 °C | AT022 | m | 1.948 | −20.6 | Hee | SD | 6 °C | VH004 | f | 2.392 | −16.8 |
| Hee | SD | 6 °C | MS005 | m | 2.374 | −20.5 | Hee | SD | 6 °C | VH006 | f | 3.068 | −18.9 |
| Hee | SD | 6 °C | MS010 | f | 2.432 | −17.9 | Hee | SD | 6 °C | VH007 | m | 2.184 | −18.1 |
| Hee | SD | 6 °C | MS014 | f | 1.753 | −21.5 | Hee | SD | 6 °C | VH013 | m | 2.015 | −18.4 |
| Hee | SD | 6 °C | AT017 | f | 2.197 | −19 | Hee | SD | 6 °C | VH015 | m | 2.513 | −19.2 |
| Hee | SD | 6 °C | AT023 | m | 1.711 | −19.8 | Hee | SD | 6 °C | VH017 | f | 2.401 | −18.4 |
| Hee | SD | −2 °C | AT028 | m | 2.396 | −18.2 | Hee | SD | 6 °C | VH020 | f | 2.152 | −19.1 |
| Hee | SD | −2 °C | AT029 | m | 1.985 | −17.2 | Hee | SD | −2 °C | MS19 | f | 2.514 | −18.1 |
| Hee | SD | −2 °C | AT039 | f | 2.259 | −17.1 | Hee | SD | −2 °C | VH021 | m | 2.366 | −18.7 |
| Hee | SD | −2 °C | AT062 | f | 2.307 | −20.8 | Hee | SD | −2 °C | VH023 | f | 2.46 | −20.9 |
| Hee | SD | −2 °C | MS007 | m | 2.189 | −20.2 | Hee | SD | −2 °C | VH029 | m | 1.625 | −22.6 |
| Hee | SD | −2 °C | MS015 | f | 2.499 | −17.2 | Hee | SD | −2 °C | VH030 | m | 1.992 | −22.8 |
| Hee | SD | −2 °C | MS016 | f | 2.472 | −17.4 | Hee | SD | −2 °C | VH035 | f | 2.559 | −20.1 |
| Hee | SD | −2 °C | AT042 | f | 1.968 | −15.3 | Hee | SD | −2 °C | VH036 | m | 2.611 | −22.2 |
| Het | SD | control | Mad143 | f | 2.599 | −18.8 | Hee | SD | −2 °C | VH039 | f | 2.033 | −18.4 |
| Het | SD | control | Mad073 | f | 3.08 | −19.2 | Het | SD | control | CF099 | f | 1.912 | −16.6 |
| Het | SD | control | Mad029 | m | 2.549 | −17.3 | Het | SD | control | CF103 | f | 1.626 | −17.1 |
| Het | SD | control | Mad003 | m | 2.704 | −16.8 | Het | SD | control | CF037 | m | 1.956 | −15.2 |
| Het | SD | control | Mad072 | f | 2.452 | −20.1 | Het | SD | control | CF077 | m | 2.195 | −17.6 |
| Het | SD | control | Mad014 | m | 2.675 | −17.5 | Het | SD | control | CF004 | f | 0.996 | −14.7 |
| Het | SD | control | Mad074 | m | 2.224 | −19 | Het | SD | control | CF009 | f | 2.061 | −19.2 |
| Het | SD | control | Mad001 | m | 2.186 | −20.2 | Het | SD | control | CF013 | m | 1.232 | −17.9 |
| Het | SD | 6 °C | CF024 | f | 2.148 | −19.6 | Het | SD | control | CF018 | f | 1.791 | −17.5 |
| Het | SD | 6 °C | CF028 | f | 2.1 | −20.9 | Het | SD | 6 °C | CF017 | f | 2.397 | −16.7 |
| Het | SD | 6 °C | CF029 | f | 2.265 | −19.9 | Het | SD | 6 °C | CF019 | f | 1.853 | −17.5 |
| Het | SD | 6 °C | CF023 | m | 1.646 | −22.3 | Het | SD | 6 °C | CF020 | f | 2.166 | −14.2 |
| Het | SD | 6 °C | CF027 | m | 2.207 | −16.1 | Het | SD | 6 °C | CF022 | f | 2.03 | −19.1 |
| Het | SD | 6 °C | CF030 | m | 2.207 | −16.3 | Het | SD | 6 °C | CF001 | m | 2.107 | −16.5 |
| Het | SD | 6 °C | CF033 | f | 2.036 | −17.8 | Het | SD | 6 °C | CF005 | m | 2.333 | −16.3 |
| Het | SD | 6 °C | CF016 | m | 2.182 | −21.1 | Het | SD | 6 °C | CF010 | m | 2.216 | −16.9 |
| Het | SD | −2 °C | CF097 | f | 2.696 | −17.8 | Het | SD | 6 °C | CF013 | m | 1.847 | −16.9 |
| Het | SD | −2 °C | CF098 | f | 2.286 | −22.9 | Het | SD | −2 °C | CF041 | f | 2.762 | −20.7 |
| Het | SD | −2 °C | CF101 | f | 2.217 | −16.5 | Het | SD | −2 °C | CF053 | f | 2.575 | −18.7 |
| Het | SD | −2 °C | CF102 | f | 2.21 | −19.9 | Het | SD | −2 °C | CF057 | f | 2.532 | −16.7 |
| Het | SD | −2 °C | CF064 | m | 2.253 | −20.8 | Het | SD | −2 °C | CF075 | f | 2.338 | −16.4 |
| Het | SD | −2 °C | CF070 | m | 2.287 | −18.2 | Het | SD | −2 °C | CF093 | m | 1.755 | −18.6 |
| Het | SD | −2 °C | CF104 | m | 1.814 | −18.5 | Het | SD | −2 °C | CF039 | m | 1.91 | −11.3 |
| Het | SD | −2 °C | CF069 | m | 1.636 | −23.7 | Het | SD | −2 °C | CF051 | m | 2.389 | −16.6 |
| Hee | SD | long term | MS31 | f | 2.273 | −19.5 | Het | SD | −2 °C | CF096 | m | 2.019 | −16.1 |
| Hee | SD | long term | AT53 | f | 2.267 | −20.7 | Hee | SD | long term | MS25 | f | 2.228 | −17.4 |
| Hee | SD | long term | AT58 | f | 2.467 | −22.4 | Hee | SD | long term | MS26 | f | 1.781 | −17 |
| Hee | SD | long term | AT47 | f | 1.838 | −23.6 | Hee | SD | long term | MS28 | f | 2.237 | −17.1 |
| Hee | SD | long term | AT59 | m | 1.793 | −21.3 | Hee | SD | long term | AT30 | f | 2.002 | −20.4 |
| Hee | SD | long term | AT60 | m | 1.641 | −20.8 | Hee | SD | long term | MS24 | f | 2.571 | −19.1 |
| Hee | SD | long term | MS11 | m | 2.204 | −22.1 | Hee | SD | long term | MS48 | m | 1.539 | −16.6 |
| Hee | SD | long term | MS30 | f | 1.75 | −17.6 | Hee | SD | long term | AT31 | m | 1.618 | −17.6 |
| Het | SD | long term | Mad077 | f | 2.757 | −22.6 | Hee | SD | long term | AT43 | m | 1.766 | −21.6 |
| Het | SD | long term | Mad060 | f | 2.144 | −16.6 | Het | SD | long term | Mad082 | f | 2.328 | −14.3 |
| Het | SD | long term | Mad103 | f | 2.972 | −24.3 | Het | SD | long term | Mad059 | f | 2.408 | −11.8 |
| Het | SD | long term | Mad114 | m | 1.922 | −17.7 | Het | SD | long term | Mad098 | f | 3.154 | −15.9 |
| Het | SD | long term | Mad079 | m | 2.399 | −21.4 | Het | SD | long term | Mad030 | m | 2.653 | −14.2 |
| Het | SD | long term | Mad017 | m | 2.471 | −18 | Het | SD | long term | Mad051 | m | 2.723 | −14.3 |
| Het | SD | long term | Mad087 | m | 2.082 | −19.7 | Het | SD | long term | Mad126 | m | 2.113 | −12.7 |
| Het | SD | long term | Mad041 | m | 2.773 | −16.9 | |||||||
References
- Mende, M.B.; Bartel, M.; Hundsdoerfer, A.K. A comprehensive phylogeography of the Hyles euphorbiae complex (Lepidoptera: Sphingidae) indicates a “glacial refuge belt”. Sci. Rep. 2016, 6, 29527. [Google Scholar] [CrossRef]
- Hundsdoerfer, A.K.; Lee, K.M.; Kitching, I.J.; Mutanen, M. Genome-wide SNP Data Reveal an Overestimation of Species Diversity in a Group of Hawkmoths. Genome Biol. Evol. 2019, 11, 136–2150. [Google Scholar] [CrossRef] [PubMed]
- Hundsdoerfer, A.K.; Kitching, I.J.; Wink, M. A molecular phylogeny of the hawkmoth genus Hyles (Lepidoptera: Sphingidae, Macroglossinae). Mol. Phyl. Evol. 2005, 35, 442–458. [Google Scholar] [CrossRef] [PubMed]
- Hundsdoerfer, A.K.; Rubinoff, D.; Attié, M.; Wink, M.; Kitching, I.J. A revised molecular phylogeny of the globally distributed hawkmoth genus Hyles (Lepidoptera: Sphingidae), based on mitochondrial and nuclear DNA sequences. Mol. Phyl. Evol. 2009, 52, 852–865. [Google Scholar] [CrossRef]
- Hundsdoerfer, A.K.; Mende, M.B.; Harbich, H.; Pittaway, A.R.; Kitching, I.J. Larval pattern morphotypes in the Western Palaearctic Hyles euphorbiae complex (Lepidoptera: Sphingidae: Macroglossinae). Insect Syst. Evol. 2011, 42, 41–86. [Google Scholar]
- Hundsdoerfer, A.K.; Mende, M.B.; Kitching, I.J.; Cordellier, M. Taxonomy, phylogeography and climate relations of the Western Palaearctic spurge hawkmoth (Lepidoptera, Sphingidae, Macroglossinae). Zool. Scr. 2011, 40, 403–417. [Google Scholar] [CrossRef]
- Mende, M.B.; Hundsdoerfer, A.K. Mitochondrial lineage sorting in action—Historical biogeography of the Hyles euphorbiae complex (Sphingidae, Lepidoptera) in Italy. BMC Evol. Biol. 2013, 13, 83. [Google Scholar] [CrossRef]
- Ayres, M.P.; Scriber, J.M. Local adaptation to regional climates in Papilio canadensis (Lepidoptera: Papilionidae). Ecol. Monogr. 1994, 64, 465–482. [Google Scholar] [CrossRef]
- Aardema, M.; Scriber, J.; Hellmann, J. Considering local adaptation in issues of lepidopteran conservation—A review and recommendations. Am. Midl. Nat. 2011, 165, 294–303. [Google Scholar] [CrossRef]
- Barth, M.B.; Buchwalder, K.; Kawahara, A.Y.; Zhou, X.; Liu, S.; Krezdorn, N.; Rotter, B.; Horres, R.; Hundsdoerfer, A.K. Functional characterization of the Hyles euphorbiae hawkmoth transcriptome reveals strong expression of phorbol ester detoxification and seasonal cold hardiness genes. Front. Zool. 2018, 15, 20. [Google Scholar] [CrossRef]
- Stuckas, H.; Mende, M.B.; Hundsdoerfer, A.K. Response to cold acclimation in diapause pupae of Hyles euphorbiae (Lepidoptera: Sphingidae): Candidate biomarker identification using proteomics. Insect Mol. Biol. 2014, 23, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Harbich, H. Das photosensible Raupenstadium von Celerio euphorbiae euphorbiae (Lep.: Sphingidae). Entomol. Z. 1976, 86, 233–236. [Google Scholar]
- World Weather & Climate Information. Available online: https://weather-and-climate.com/ (accessed on 23 March 2020).
- De Freina, J. Über Biologie, Morphologie und Taxonomie von Hyles tithymali deserticola (Bartel). Entomol. Z. 1994, 104, 33–60. [Google Scholar]
- Harris, P.; Alex, J. Euphorbia esula L., leafy spurge, and Euphorbia cyparissias L., cypress spurge (Euphorbiaceae). Commonw. Inst. Biol. Contr. Tech. Commun. 1971, 4, 83–88. [Google Scholar]
- Heller, J.; Mochnacka, I. Hyperglycaemic reaction in overwintering pupae. Compt. Rend. Soc. Sci. Let Wroc. 1951, 6, 56–57. [Google Scholar]
- Pittaway, A.R. HYLES Hübner, [1819]. Available online: https://tpittaway.tripod.com/sphinx/h_eup.htm (accessed on 15 March 2020).
- Zachariasson, K.E. Physiology of cold tolerance in insects. Physiol. Rev. 1985, 65, 799–832. [Google Scholar] [CrossRef]
- Kukal, O.; Serianni, A.S.; Duman, J.G. Glycerol metabolism in a freeze-tolerant arctic insect: An in vivo 13C NMR study. J. Comp. Physiol. B 1988, 158, 175–183. [Google Scholar] [CrossRef]
- Khani, A.; Moharramipour, S.; Barzegar, M.; Naderi-Manesh, H. Comparison of fatty acid composition in total lipid of diapause and non-diapause larvae of Cydia pomonella (Lepidoptera: Tortricidae). Insect Sci. 2007, 14, 125–131. [Google Scholar] [CrossRef]
- Denlinger, D.L. Regulation of diapause. Ann. Rev. Entomol. 2002, 47, 93–122. [Google Scholar] [CrossRef]
- Bale, J.S. Insect cold hardiness: A matter of life and death. Eur. J. Entomol. 1996, 93, 369–382. [Google Scholar]
- Lee, R.E.; Costanzo, J.P.; Mugano, J.A. Regulation of supercooling and ice nucleation in insects. Eur. J. Entomol. 1996, 93, 405–418. [Google Scholar]
- Vanin, S.; Bubacco, L.; Beltramini, M. Seasonal variation of trehalose and glycerol concentrations in winter snow-active insects. CryoLetters 2008, 29, 485–491. [Google Scholar] [PubMed]
- Wharton, D.A. Supercooling and freezing tolerant animals. In Supercooling; Wilson, P., Ed.; InTech: Rijeka, Croatia, 2012; pp. 17–28. [Google Scholar]
- De Freina, J.J. Über Biologie und Morphologie der auf Madeira beheimateten Hyles euphorbiae gecki ssp.n. (Lepidoptera, Sphingidae). Nachr. Bayer Entomol. 1991, 40, 65–72. [Google Scholar]
- Bachmetjev, P.T. Experimentelle entomologische Studien, Band I; Facsimile Publisher: Leipzig, Germany, 1901; 160p. [Google Scholar]
- Wittstadt, H. Wanderfalterbericht für das Jahr 1965. Entomol. Z. 1966, 76, 241–243. [Google Scholar]
- Harbich, H. Erfahrungen bei der Aufzucht von Sphingidenraupen mit einem Kombinationsfutter (Lepidoptera: Sphingidae). Entomol. Z. 1994, 104, 112–117. [Google Scholar]
- Block, W. Cold tolerance of insects and other arthropods. Trans. R. Soc. Lond. B Biol. Sci. 1990, 326, 613–633. [Google Scholar]
- Deswal, R.; Sharma, B. Antifreeze proteins in plants: An overview with an insight into the detection techniques including nanobiotechnology. J. Prot. Proteom. 2014, 5, 89–107. [Google Scholar]
- Carrillo, M.A.; Kaliyan, N.; Cannon, C.A.; Morey, R.V.; Wilcke, W.F. A simple method to adjust cooling rates for supercooling point determination. CryoLetters 2004, 25, 155–160. [Google Scholar]
- Carrillo, M.A.; Cannon, C.A.; Wilcke, W.F.; Morey, R.V.; Kaliyan, N.; Hutchison, W.D. Relationship between supercooling point and mortality at low temperatures in Indian meal moth (Lepidoptera: Pyralidae). J. Econ. Entomol. 2005, 98, 618–625. [Google Scholar] [CrossRef]
- StatSoft Inc. STATISTICA (Data Analysis Software System), Version 8.0. Available online: www.statsoft.com (accessed on 14 February 2008).
- Khani, A.; Moharramipour, S. Cold hardiness and supercooling capacity in the overwintering larvae of the codling moth. Cydia pomonella. J. Insect Sci. 2010, 10, 83. [Google Scholar]
- Sinclair, B.J.; Addo-Bediako, A.; Chown, S.L. Climatic variability and the evolution of insect freeze tolerance. Biol. Rev. 2003, 78, 181–195. [Google Scholar] [CrossRef]
- Bale, J.S.; Hayward, S.A.L. Insect overwintering in a changing climate. J. Exp. Biol. 2010, 213, 980–994. [Google Scholar] [CrossRef] [PubMed]
- Teets, N.M.; Denlinger, D.L. Physiological mechanisms of seasonal and rapid cold-hardening in insects. Physiolog. Entomol. 2013, 38, 105–116. [Google Scholar] [CrossRef]
- Duman, J.G. Animal ice binding (antifreeze) proteins and glycolipids: An overview with emphasis on physiological function. J. Exp. Biol. 2015, 218, 1846–1855. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, M.A.; Cannon, C.A. Supercooling point variability in the Indian meal moth, Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae). J. Stor. Prod. Res. 2005, 41, 556–564. [Google Scholar] [CrossRef]
- Khani, A.; Moharramipour, S. Seasonal change of cold hardiness in the codling moth, Cydia pomonella (Lepidoptera: Tortricidae). Pak. J. Biol. Sci. 2007, 10, 2591–2594. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sømme, L. The physiology of cold hardiness in terrestrial arthropods. Eur. J. Entomol. 1999, 96, 1–10. [Google Scholar]
- Sinclair, B.J. Insect cold tolerance: How many kinds of frozen? Eur. J. Entomol. 1999, 96, 157–164. [Google Scholar]
- Sinclair, B.J.; Vernon, P.; Klok, C.J.; Chown, S.L. Insects at low temperatures: An ecological perspective. Tr. Ecol. Evol. 2003, 18, 257–262. [Google Scholar] [CrossRef]
- Turnock, W.J.; Fields, P.G. Winter climates and cold hardiness in terrestrial insects. Eur. J. Entomol. 2005, 102, 561. [Google Scholar] [CrossRef]
- Goto, S.G.; Numata, H. Insect photoperiodism. In Insect Molecular Biology and Ecology; Hoffmann, K.H., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 217–244. [Google Scholar]
- Pullin, A.S. Physiological relationships between insect diapause and cold tolerance: Coevolution or coincidence. Eur. J. Entomol. 1996, 93, 121–130. [Google Scholar]
- Nylin, S. Induction of diapause and seasonal morphs in butterflies and other insects: Knowns, unknowns and the challenge of integration. Physiol. Entomol. 2013, 38, 96–104. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Insect cold hardiness: Metabolic, gene, and protein adaptation. Can. J. Zool. 2012, 90, 456–475. [Google Scholar] [CrossRef]
- Denlinger, D.L. Relationship between cold hardiness and diapause. In Insects at Low Temperatures; Lee, R.E., Denlinger, D.L., Eds.; Chapman and Hall: New York, NY, USA, 1991; pp. 174–198. [Google Scholar]
- Hahn, D.A.; Denlinger, D.L. Energetics of insect Diapause. Annu. Rev. Entomol. 2011, 56, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Atapour, M.; Moharramipour, S. Changes of cold hardiness, supercooling capacity, and major cryoprotectants in overwintering larvae of Chilo suppressalis (Lepidoptera: Pyralidae). Environ. Entomol. 2009, 38, 260–265. [Google Scholar] [CrossRef]
- Soudi, S.H.; Moharramipour, S. Cold tolerance and supercooling capacity in overwintering adults of elm leaf beetle Xanthogaleruca luteola (Coleoptera: Chrysomelidae). Environ. Entomol. 2011, 40, 1546–1553. [Google Scholar] [CrossRef]
- Bemani, M.; Izadi, H.; Mahdian, K.; Khani, A. Study on the physiology of diapause, cold hardiness and supercooling point of overwintering pupae of the pistachio fruit hull borer, Arimania comaroffi. J. Insect Physiol. 2012, 58, 897–902. [Google Scholar] [CrossRef]
- Williams, C.M.; Nicolai, A.; Ferguson, L.V.; Bernards, M.A.; Hellmann, J.J.; Sinclair, B.J. Cold hardiness and deacclimation of overwintering Papilio zelicaon pupae. Comp. Biochem. Physiol. A Mol. Int. Physiol. 2014, 178, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Eizaguirre, M.; López, C.; Asín, L.; Albajes, R. Thermoperiodism, photoperiodism and sensitive stage in the diapause induction of Sesamia nonagrioides (Lepidoptera: Noctuidae). J. Insect Physiol. 1994, 40, 113–119. [Google Scholar] [CrossRef]
- Gomi, T. Geographic variation in critical photoperiod for diapause induction and its temperature dependence in Hyphantria cunea Drury (Lepidoptera: Arctiidae). Oecologia 1997, 111, 160–165. [Google Scholar] [CrossRef]
- Gill, H.M.; Goyal, G.; Chahil, G. Insect Diapause: A Review. J. Agric. Sci. Technol. 2017, 7, 454–473. [Google Scholar]
- Wang, X.P.; Yang, Q.S.; Dalin, P.; Zhou, X.M.; Luo, Z.W.; Lei, C.L. Geographic variation in photoperiodic diapause induction and diapause intensity in Sericinus montelus (Lepidoptera: Papilionidae). Insect Sci. 2012, 19, 295–302. [Google Scholar] [CrossRef]
- Chen, Y.S.; Chen, C.; He, H.M.; Xia, Q.W.; Xue, F.S. Geographic variation in diapause induction and termination of the cotton bollworm, Helicoverpa armigera Hübner (Lepidoptera: Noctuidae). J. Insect Physiol. 2013, 59, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.F.; Valella, P.; Thivierge, G.; Aardema, M.L.; Scriber, J.M. The role of latitudinal, genetic and temperature variation in the induction of diapause of Papilio glaucus (Lepidoptera: Papilionidae). Insect Sci. 2018, 25, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.J.; Mou, F.C.; Zhu, X.F.; Xue, F.S. Diapause induction, maintenance and termination in the rice stem borer Chilo suppressalis (Walker). J. Insect Physiol. 2010, 56, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Klockmann, M.; Fischer, K. Strong reduction in diapause survival under warm and humid overwintering conditions in a temperate-zone butterfly. Popul. Ecol. 2019, 61, 150–159. [Google Scholar] [CrossRef]
- Pittaway, A.R. The Hawkmoths of the Western Palaearctic; Harley Books: Colchester, UK, 1993; p. 240. [Google Scholar]
| Locality Code | Locality Name | GPS Coordinates | Climatic Zone and Ecotype | Date of Sampling | Sampled Stage | Collectors |
|---|---|---|---|---|---|---|
| VH | Viernheimer Heide, Hessen, Germany | 49°33′ N, 8°32′ E | Temperate Hee | 2014 | adult female | B. Barth and R. Schidlowski |
| CF | Cabeça Fundão, Fogo island, Cape Verde | 14°54′ N, 24°20′ W | Subtropical Het | 2014 | larvae | B. Barth |
| AT | Altmühlthal, Bavaria, Germany | 48°54′ N, 11°06′ E | Temperate Hee | 2015 | larvae | L. Langer |
| MS | Münnerstadt, Bavaria, Germany | 50°14′ N, 10°10′ E | Temperate Hee | 2015 | larvae | H. Harbich |
| Mad | Madeira, Madeira Archipelago, Portugal | 32°45′ N, 17°00′ W | Subtropical Het | 2015 | larvae | G. and B. Richter |
| Hee | Het | |||
|---|---|---|---|---|
| Treatment (Total N = 225) | LD | SD | LD | SD |
| SCP control | 17 | 16 | 16 | 16 |
| 6 °C | 17 | 16 | 16 | 16 |
| −2 °C | 17 | 16 | 16 | 16 |
| LTC | - | 16 | - | 14 |
| MT (survived) | ||||
| control | 17 (17, 100%) | 20 (17, 85%) | 20 (20, 100%) | 17 (10, 59%) |
| control nondiapause/diapause | 17/0 | 3/14 (3 < 3 months) | 20/0 | 3/7 (1 < 3 months) |
| −10 °C | - | 10 (9, 90%) | - | 8 (5, 63%) |
| −10 °C nondiapause/diapause | - | 0/9 | - | 0/5 |
| Effect | SCP In Vitro | SCP In Vivo | ||
|---|---|---|---|---|
| F | Significance | F | Significance | |
| Ecotype | 4.55 | * | 1.26 | n.s. |
| Day length | 26.45 | *** | 32.09 | *** |
| Cooling group | 4.91 | ** | 2.43 | n.s. |
| Within Hee | ||||
| Day length | 17.57 | *** | 25.05 | *** |
| Cooling group | 1.22 | n.s. | 2.91 | n.s. |
| Within Het | ||||
| Day length | 9.35 | ** | 7.80 | ** |
| Cooling group | 6.70 | ** | 0.22 | n.s. |
| Within SD | ||||
| Ecotype | 0.06 | n.s. | 20.02 | *** |
| Cooling group | 1.66 | n.s. | 4.30 | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daneck, H.; Barth, M.B.; Geck, M.; Hundsdoerfer, A.K. Super Cooling Point Phenotypes and Cold Resistance in Hyles euphorbiae Hawk Moths from Different Climate Zones. Diversity 2021, 13, 207. https://doi.org/10.3390/d13050207
Daneck H, Barth MB, Geck M, Hundsdoerfer AK. Super Cooling Point Phenotypes and Cold Resistance in Hyles euphorbiae Hawk Moths from Different Climate Zones. Diversity. 2021; 13(5):207. https://doi.org/10.3390/d13050207
Chicago/Turabian StyleDaneck, Hana, Matthias Benjamin Barth, Martin Geck, and Anna K. Hundsdoerfer. 2021. "Super Cooling Point Phenotypes and Cold Resistance in Hyles euphorbiae Hawk Moths from Different Climate Zones" Diversity 13, no. 5: 207. https://doi.org/10.3390/d13050207
APA StyleDaneck, H., Barth, M. B., Geck, M., & Hundsdoerfer, A. K. (2021). Super Cooling Point Phenotypes and Cold Resistance in Hyles euphorbiae Hawk Moths from Different Climate Zones. Diversity, 13(5), 207. https://doi.org/10.3390/d13050207