Secondary Serpentine Forests of Poland as a Refuge for Vascular Flora
Abstract
1. Introduction
2. Materials and Methods
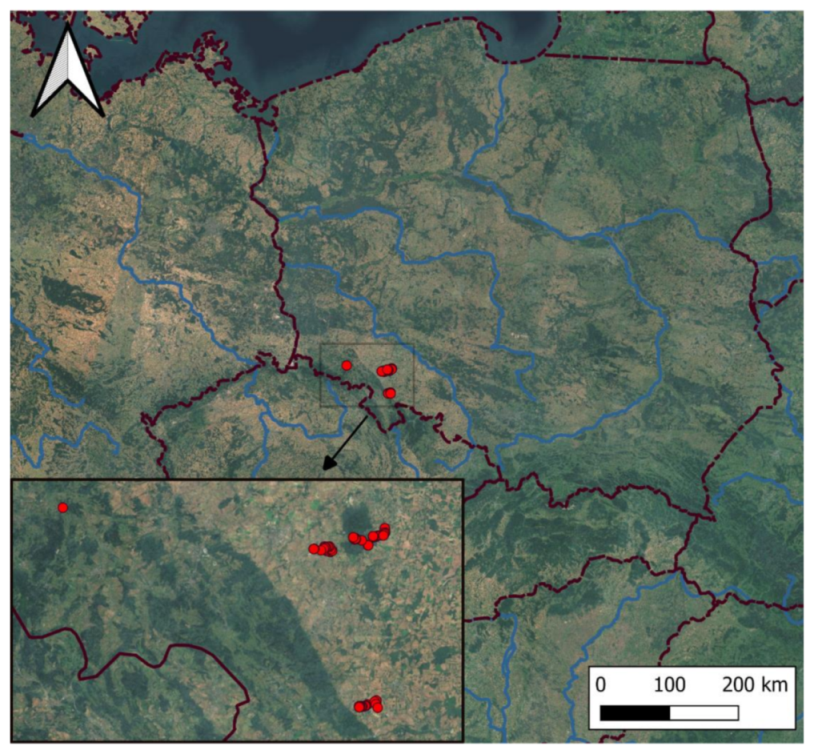
2.1. Study Area
2.2. Field Sampling and Nomenclature
2.3. Differentiation and Diversity Measures of Studied Communities
2.4. Analysis of Species of Interest Relationships
3. Results
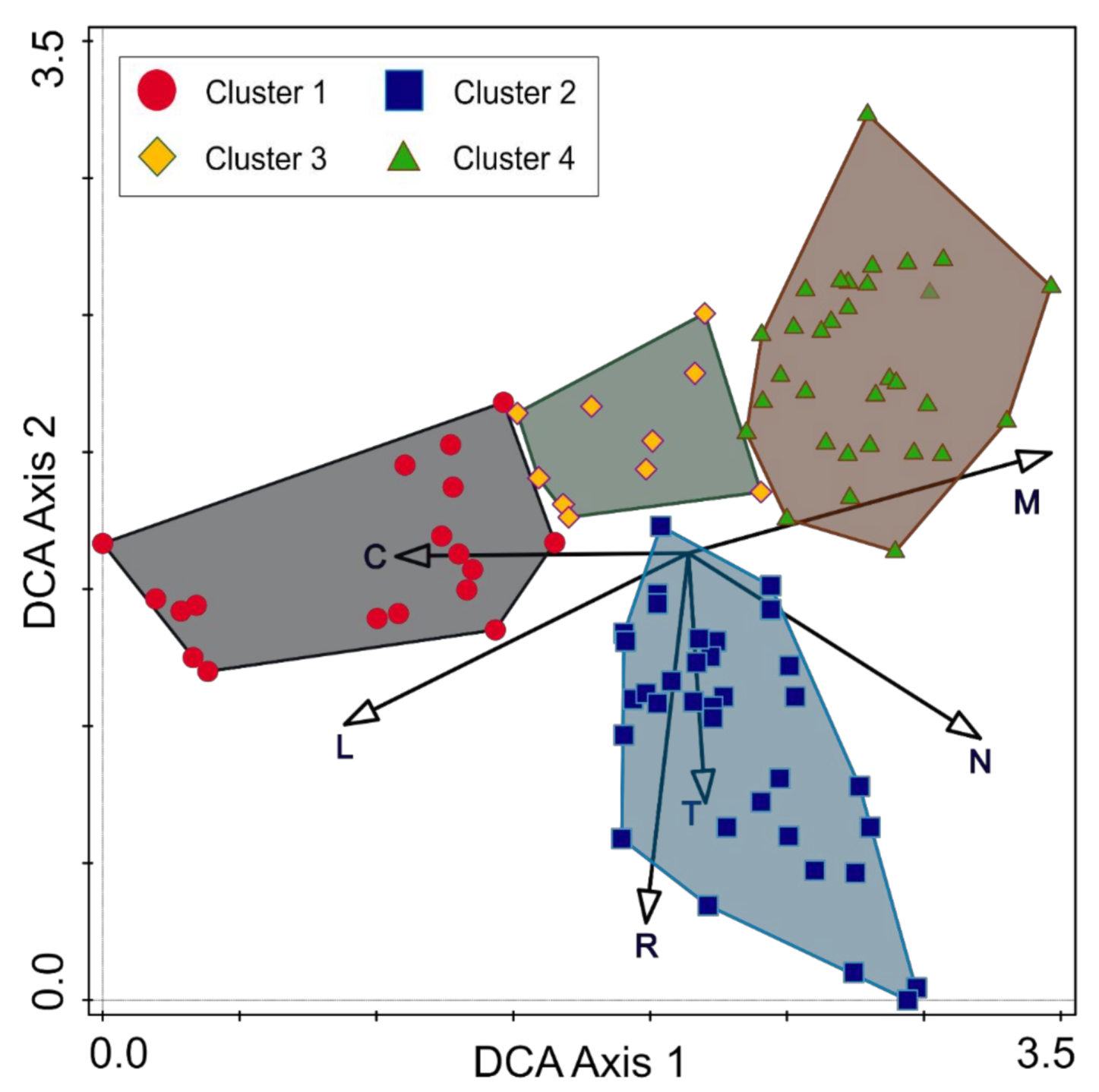
3.1. Differentiation of Forest Communities
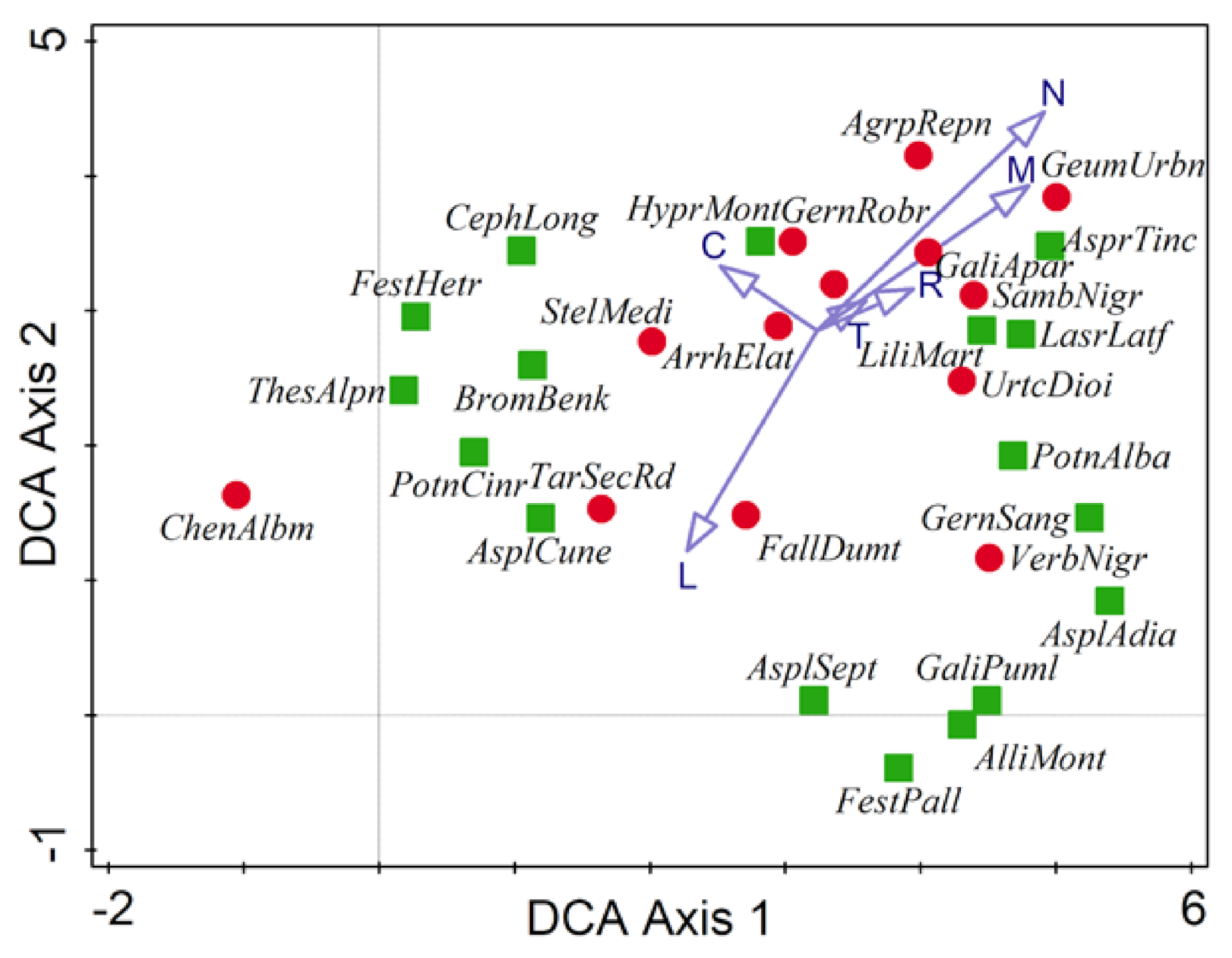
3.2. Species of Interest in Studied Forest Communities
3.3. Main Environmental Gradients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leuschner, C.; Ellenberg, H. Ecology of Central European Forests: Vegetation Ecology of Central Europe; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Parviainen, J. Virgin and natural forests in the temperate zone of Europe. Snow Landsc. Res. 2005, 79, 9–18. [Google Scholar]
- Sabatini, F.M.; Burrascano, S.; Keeton, W.S.; Levers, C.; Lindner, M.; Pötzschner, F.; Verkerk, P.J.; Bauhus, J.; Buchwald, E.; Chaskovsky, O.; et al. Where are Europe’s last primary forests? Divers. Distrib. 2018, 24, 1426–1439. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [PubMed]
- Perring, M.P.; Diekmann, M.; Midolo, G.; Schellenberger Costa, D.; Bernhardt-Römermann, M.; Otto, J.C.J.; Gilliam, F.S.; Hedwall, P.-O.; Nordin, A.; Dirnböck, T.; et al. Understanding context dependency in the response of forest understorey plant communities to nitrogen deposition. Environ. Pollut. 2018, 242, 1787–1799. [Google Scholar] [CrossRef]
- Staude, I.R.; Waller, D.M.; Bernhardt-Römermann, M.; Bjorkman, A.D.; Brunet, J.; De Frenne, P.; Hédl, R.; Jandt, U.; Lenoir, J.; Máliš, F.; et al. Replacements of small- by large-ranged species scale up to diversity loss in Europe’s temperate forest biome. Nat. Ecol. Evol. 2020, 4, 802–808. [Google Scholar] [CrossRef]
- Paillet, Y.; Bergès, L.; Hjältén, J.; Odor, P.; Avon, C.; Bernhardt-Römermann, M.; Bijlsma, R.-J.; De Bruyn, L.; Fuhr, M.; Grandin, U.; et al. Biodiversity differences between managed and unmanaged forests: Meta-analysis of species richness in Europe. Conserv. Biol. 2010, 24, 101–112. [Google Scholar] [CrossRef]
- Lelli, C.; Bruun, H.H.; Chiarucci, A.; Donati, D.; Frascaroli, F.; Fritz, Ö.; Goldberg, I.; Nascimbene, J.; Tøttrup, A.P.; Rahbek, C.; et al. Biodiversity response to forest structure and management: Comparing species richness, conservation relevant species and functional diversity as metrics in forest conservation. Ecol. Manag. 2019, 432, 707–717. [Google Scholar] [CrossRef]
- Bertrand, R.; Lenoir, J.; Piedallu, C.; Riofrío-Dillon, G.; de Ruffray, P.; Vidal, C.; Pierrat, J.-C.; Gégout, J.-C. Changes in plant community composition lag behind climate warming in lowland forests. Nature 2011, 479, 517–520. [Google Scholar] [CrossRef]
- Zellweger, F.; De Frenne, P.; Lenoir, J.; Vangansbeke, P.; Verheyen, K.; Bernhardt-Römermann, M.; Baeten, L.; Hédl, R.; Berki, I.; Brunet, J.; et al. Forest microclimate dynamics drive plant responses to warming. Science 2020, 368, 772–775. [Google Scholar] [CrossRef]
- Bernhardt-Römermann, M.; Baeten, L.; Craven, D.; De Frenne, P.; Hédl, R.; Lenoir, J.; Bert, D.; Brunet, J.; Chudomelová, M.; Decocq, G.; et al. Drivers of temporal changes in temperate forest plant diversity vary across spatial scales. Glob. Chang. Biol. 2015, 21, 3726–3737. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Proctor, J.; Reeves, R.D. The vegetation of ultramafic (serpentine) soils. In Proceedings of the First International Conference on Serpentine Ecology, University of California, Davis, CA, USA, 19–22 June 1991. [Google Scholar]
- Proctor, J.; Woodell, S.R.J. The plant ecology of serpentine: I. Serpentine vegetation of England and Scotland. J. Ecol. 1971, 59, 375–395. [Google Scholar] [CrossRef]
- Mota, J.; Merlo, E.; Martínez-Hernández, F.; Mendoza-Fernández, A.J.; Pérez-García, F.J.; Salmerón-Sánchez, E. Plants on rich-magnesium dolomite barrens: A global phenomenon. Biology 2021, 10, 38. [Google Scholar] [CrossRef]
- Kazakou, E.; Dimitrakopoulos, P.G.; Baker, A.J.M.; Reeves, R.D.; Troumbis, A.Y. Hypotheses, mechanisms and trade-offs of tolerance and adaptation to serpentine soils: From species to ecosystem level. Biol. Rev. Camb. Philos. Soc. 2008, 83, 495–508. [Google Scholar] [CrossRef]
- Anacker, B.L. The nature of serpentine endemism. Am. J. Bot. 2014, 101, 219–224. [Google Scholar] [CrossRef]
- Chiarucci, A.; De Dominicis, V. Effects of pine plantations on ultramafic vegetation of central Italy. Isr. J. Plant Sci. 1995, 43, 7–20. [Google Scholar] [CrossRef]
- Chiarucci, A.; Robinson, B.H.; Bonini, I.; Petit, D.; Brooks, R.R.; De Dominicis, V. Vegetation of Tuscan ultramafic soils in relation to edaphic and physical factors. Folia Geobot. 1998, 33, 113–131. [Google Scholar] [CrossRef]
- Żołnierz, L. Zbiorowiska trawiaste występujące na dolnośląskich serpentynitach—Wybrane aspekty ekologii. [Grassland on serpentines in Lower Silesia (SW Poland)—Some aspects of their ecology]. Wydawnictwo Uniwersytetu Przyrodniczego We Wroclawiu 2007, 555, 251. (In Polish) [Google Scholar]
- Reczyńska, K. Diversity and Ecology of Oak Forests in SW Poland (Sudetes Mts.). Phytocoenologia 2015, 45, 85–105. [Google Scholar] [CrossRef]
- Szymura, T.H. How does recent vegetation reflect previous systems of forest management. Pol. J. Ecol. 2012, 60, 859–862. [Google Scholar]
- Müllerová, J.; Hédl, R.; Szabó, P. Coppice abandonment and its implications for species diversity in forest vegetation. Ecol. Manag. 2015, 343, 88–100. [Google Scholar] [CrossRef]
- Becker, T.; Spanka, J.; Schröder, L.; Leuschner, C. Forty years of vegetation change in former coppice-with-standards woodlands as a result of management change and N deposition. Appl. Veg. Sci. 2017, 20, 304–313. [Google Scholar] [CrossRef]
- Buckley, P. Coppice restoration and conservation: A European perspective. J. For. Res. 2020, 25, 125–133. [Google Scholar] [CrossRef]
- European Commission DG Environment. Interpretation Manual of European Union Habitats, Version EUR 28; European Commission, DG-ENV: Brussels, Belgium, 2013. [Google Scholar]
- Novák, P.; Willner, W.; Zukal, D.; Kollár, J.; Roleček, J.; Świerkosz, K.; Ewald, J.; Wohlgemuth, T.; Csiky, J.; Onyshchenko, V.; et al. Oak-Hornbeam forests of Central Europe: A formalized classification and syntaxonomic revision. Preslia 2020, 92, 1–34. [Google Scholar] [CrossRef]
- Zelený, D. Asplenio cuneifolii-Pinetum sylvestris Pišta ex Husová in Husová et al. 2002. In Vegetation of the Czech Republic 4. Forest and Scrub Vegetation; Chytrý, M., Ed.; Academia Praha: Prague, Czech Republic, 2013; pp. 401–404. ISBN 9788020022998. [Google Scholar]
- Reczyńska, K.; Pech, P.; Swierkosz, K. Phytosociological analysis of natural and artificial pine forests of the class Vaccinio-Piceetea Br.-Bl. in Br.-Bl. et al. 1939 in the Sudetes and their foreland (Bohemian Massif, Central Europe). Forests 2021, 12, 98. [Google Scholar] [CrossRef]
- Schmid, M.; Pautasso, M.; Holdenrieder, O. Ecological consequences of douglas fir (Pseudotsuga menziesii) cultivation in Europe. Eur. J. Res. 2014, 133, 13–29. [Google Scholar] [CrossRef]
- Chmura, D. The spread and role of the invasive alien tree Quercus rubra (L.) in novel forest ecosystems in Central Europe. Trees Livelihoods 2020, 11, 586. [Google Scholar] [CrossRef]
- Vítková, M.; Müllerová, J.; Sádlo, J.; Pergl, J.; Pyšek, P. Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe. Ecol. Manag. 2017, 384, 287–302. [Google Scholar] [CrossRef]
- Šibíková, M.; Jarolímek, I.; Hegedüšová, K.; Májeková, J.; Mikulová, K.; Slabejová, D.; Škodová, I.; Zaliberová, M.; Medvecká, J. Effect of planting alien Robinia pseudoacacia trees on homogenization of Central European forest vegetation. Sci. Total Environ. 2019, 687, 1164–1175. [Google Scholar] [CrossRef]
- Tokarska-Guzik, B.; Dajdok, Z.; Zając, M.; Zając, A.; Urbisz, A.; Danielewicz, W.; Hołdyński, C. Rośliny Obcego Pochodzenia W Polsce Ze Szczególnym Uwzględnieniem Gatunków Inwazyjnych; Generalna Dyrekcja Ochrony Środowiska: Warszawa, Poland, 2012; ISBN 9788362940349. [Google Scholar]
- Spiecker, H. Silvicultural management in maintaining biodiversity and resistance of forests in Europe-temperate zone. J. Environ. Manag. 2003, 67, 55–65. [Google Scholar] [CrossRef]
- Mueller-Dombois, D.; Ellenberg, H. Aims and Methods of Vegetation Ecology; Reprint; The Blackburn Press: Caldwell, NJ, USA, 2002; ISBN 9781930665736. [Google Scholar]
- Hennekens, S.M.; Schaminée, J.H.J. TURBOVEG, a comprehensive data base management system for vegetation data. J. Veg. Sci. 2001, 12, 589–591. [Google Scholar] [CrossRef]
- The Euro+ Med PlantBase-the Information Resource for Euro-Mediterranean Plant Diversity. Available online: http://ww2.bgbm.org/EuroPlusMed/ (accessed on 1 April 2021).
- Ochyra, R.; Żarnowiec, J.; Bednarek-Ochyra, H. Census catalogue of Polish Mosses. Biodiversity of Poland. Kraków Inst. Bot. Pas 2003, 3, 372. [Google Scholar]
- Mucina, L.; Bültmann, H.; Dierßen, K. Vegetation of Europe: Hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl. Veg. Sci. 2016, 19, 3–264. [Google Scholar] [CrossRef]
- Roleček, J.; Tichý, L.; Zelený, D.; Chytrý, M. Modified TWINSPAN classification in which the hierarchy respects cluster heterogeneity. J. Veg. Sci. 2009, 20, 596–602. [Google Scholar] [CrossRef]
- Tichý, L. JUICE, software for vegetation classification. J. Veg. Sci. 2002, 13, 451–453. [Google Scholar] [CrossRef]
- Fischer, H.S. On the combination of species cover values from different vegetation layers. Appl. Veg. Sci. 2015, 18, 169–170. [Google Scholar] [CrossRef]
- Tichý, L.; Chytrý, M. Statistical determination of diagnostic species for site groups of unequal size. J. Veg. Sci. 2006, 17, 809–818. [Google Scholar] [CrossRef]
- Willner, W.; Tichý, L.; Chytrý, M. Effects of different fidelity measures and contexts on the determination of diagnostic species. J. Veg. Sci. 2009, 20, 130–137. [Google Scholar] [CrossRef]
- Dengler, J.; Berg, C.; Jansen, F. New ideas for modern phytosociological monographs. Ann. Bot. 2005, 5, 195–213. [Google Scholar]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, W.; Werner, W.; Paulien, D. Zeigerwerte von Pflanzen in Mitteleuropa. Scr. Geobot. 1991, 18, 1–258. [Google Scholar]
- Berg, C.; Welk, E.; Jäger, E.J. Revising Ellenberg’s indicator values for continentality based on global vascular plant species distribution. Appl. Veg. Sci. 2017, 20, 482–493. [Google Scholar] [CrossRef]
- Kaźmierczakowa, R.; Bloch-Orłowska, J.; Celka, Z.; Cwener, A.; Dajdok, Z.; Michalska-Hejduk, D.; Pawlikowski, P.; Szczęśniak, E.; Ziarnek, K. Polska Czerwona Lista Paprotników I Roślin Kwiatowych; Instytut Ochrony Przyrody PAN: Kraków, Poland, 2016; ISBN 9788361191889. [Google Scholar]
- Kącki, Z.; Dajdok, Z.; Ewa, S. Czerwona lista roślin naczyniowych Dolnego Śląska. In Zagrożone Gatunki Flory Naczyniowej Dolnego Śląska; Kącki, Z., Ed.; Instytut Biologii Roślin, Uniwersytet Wrocławski, Polskie Towarzystwo Przyjaciół Przyrody “Pro Natura”: Wrocław, Poland, 2003; pp. 9–65. ISBN 9788391962602. [Google Scholar]
- Vojík, M.; Boublík, K. Fear of the dark: Decline in plant diversity and invasion of alien species due to increased tree canopy density and eutrophication in lowland woodlands. Plant Ecol. 2018, 219, 749–758. [Google Scholar] [CrossRef]
- ter Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012.
- Carboni, M.; Thuiller, W.; Izzi, F.; Acosta, A. Disentangling the relative effects of environmental versus human factors on the abundance of native and alien plant species in mediterranean sandy shores. Divers. Distrib. 2010, 16, 537–546. [Google Scholar] [CrossRef]
- Fois, M.; Podda, L.; Médail, F.; Bacchetta, G. Endemic and alien vascular plant diversity in the small mediterranean islands of Sardinia: Drivers and implications for their conservation. Biol. Conserv. 2020, 244, 108519. [Google Scholar] [CrossRef]
- Roleček, J.; Vild, O.; Sladký, J.; Řepka, R. Habitat requirements of endangered species in a former coppice of high conservation value. Folia Geobot. 2017, 52, 59–69. [Google Scholar] [CrossRef]
- Roleček, J.; Řepka, R. Formerly coppiced old growth stands act as refugia of threatened biodiversity in a managed steppic oak forest. Ecol. Manag. 2020, 472, 118245. [Google Scholar] [CrossRef]
- Berg, A.; Ehnstrom, B.; Gustafsson, L.; Hallingback, T.; Jonsell, M.; Weslien, J. Threatened plant, animal, and fungus species in Swedish forests: Distribution and habitat associations. Conserv. Biol. 1994, 8, 718–731. [Google Scholar] [CrossRef]
- Kapusta, P.; Szarek-Łukaszewska, G.; Jędrzejczyk-Korycińska, M.; Zagórna, M. Do Heavy-metal grassland species survive under a scots pine canopy during early stages of secondary succession? Folia Geobot. 2015, 50, 317–329. [Google Scholar] [CrossRef]
- Łazarski, G. Expansion of cold-adapted orchid Goodyera repens (Orchidaceae) in times of global warming—Report from southern Poland. Pol. J. Ecol. 2021, 68, 313–322. [Google Scholar] [CrossRef]
- Zielińska, K.M.; Kiedrzyński, M.; Grzyl, A.; Tomczyk, P.P. Anthropogenic sites maintain the last individuals during the rapid decline of the lowland refugium of the alpine-arctic plant Pulsatilla vernalis (L.) Mill. Pak. J. Bot. 2018, 50, 211–215. [Google Scholar]
- Chiarucci, A. Species diversity in plant communities on ultramafic soils in relation to pine afforestation. J. Veg. Sci. 1996, 7, 57–62. [Google Scholar] [CrossRef]
- Hédl, R.; Kopecký, M.; Komárek, J. Half a century of succession in a temperate oakwood: From species-rich community to mesic forest. Divers. Distrib. 2010, 16, 267–276. [Google Scholar] [CrossRef]
- Wagensommer, R.P.; Medagli, P.; Turco, A.; Perrino, E.V. IUCN red list evaluation of the Orchidaceae endemic to Apulia (Italy) and considerations on the application of the IUCN protocol to rare species. Nat. Conserv. Res. 2020, 5, 90–101. [Google Scholar] [CrossRef]
- Pethiyagoda, R.S.; Manamendra-Arachchi, K. Endangered anurans in a novel forest in the highlands of Sri Lanka. Wildl. Res. 2012, 39, 641–648. [Google Scholar] [CrossRef]
- Barbé, M.; Fenton, N.J.; Lavergne, C.; Le Péchon, T.; Baider, C.; Gigord, L.D.B. Changes in lowland dry-forest native and alien plant communities on Réunion Island (Indian Ocean) over 16 years. Botany 2015, 93, 843–857. [Google Scholar] [CrossRef]
- Kowarik, I.; Hiller, A.; Planchuelo, G.; Seitz, B.; von der Lippe, M.; Buchholz, S. Emerging urban forests: Opportunities for promoting the wild side of the urban green infrastructure. Sustain. Sci. Pract. Policy 2019, 11, 6318. [Google Scholar] [CrossRef]
- Powell, K.I.; Chase, J.M.; Knight, T.M. Invasive plants have scale-dependent effects on diversity by altering species-area relationships. Science 2013, 339, 316–318. [Google Scholar] [CrossRef]
- Powell, K.I.; Chase, J.M.; Knight, T.M. A synthesis of plant invasion effects on biodiversity across spatial scales. Am. J. Bot. 2011, 98, 539–548. [Google Scholar] [CrossRef]
- Valone, T.J.; Weyers, D.P. Invasion intensity influences scale-dependent effects of an exotic species on native plant diversity. Sci. Rep. 2019, 9, 18769. [Google Scholar] [CrossRef]
- Rédei, T.; Csecserits, A.; Lhotsky, B.; Barabás, S.; Kröel-Dulay, G.; Ónodi, G.; Botta-Dukát, Z. Plantation forests cannot support the richness of forest specialist plants in the forest-steppe zone. Ecol. Manag. 2020, 461, 117964. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. The State of the World’s Forests 2020: Forests, Biodiversity and People; Food & Agriculture Organization: Rome, Italy, 2020; ISBN 9789251324196. [Google Scholar]
- Turner, I.M.; Wong, Y.K.; Chew, P.T.; Ibrahim, A.B. Tree species richness in primary and old secondary tropical forest in Singapore. Biodivers. Conserv. 1997, 6, 537–543. [Google Scholar] [CrossRef]
- Coelho, G.C.; da Silva Rigo, M.; Libardoni, J.B.; de Oliveira, R.; Benvenuti-Ferreira, G. Understory structure in two successional stages of a semi-deciduous seasonal forest remnant of Southern Brazil. Biota Neotrop. 2011, 11, 63–74. [Google Scholar] [CrossRef][Green Version]
- Ren, H.; Zeng, S.; Li, L.; Zhang, Q.; Yang, L.; Wang, J.; Wang, Z.; Guo, Q. Reintroduction of Tigridiopalma magnifica, a rare and critically endangered herb endemic to China. Oryx 2012, 46, 391–398. [Google Scholar] [CrossRef]
- Ren, H.; Jian, S.; Chen, Y.; Liu, H.; Zhang, Q.; Liu, N.; Xu, Y.; Luo, J. Distribution, status, and conservation of Camellia changii Ye (Theaceae), a critically endangered plant endemic to Southern China. Oryx 2014, 48, 358–360. [Google Scholar] [CrossRef][Green Version]
- Tamaki, I.; Nomura, K.; Nomura, R.; Tate, C.; Watanabe, C.; Miyakami, Y.; Yabe, Y. Evaluation of a field experiment for the conservation of a Magnolia stellata stand using clear-cutting. Landsc. Ecol. Eng. 2018, 14, 269–276. [Google Scholar] [CrossRef]
- Bonari, G.; Acosta, A.T.R.; Angiolini, C. Mediterranean coastal pine forest stands: Understorey distinctiveness or not? Ecol. Manag. 2017, 391, 19–28. [Google Scholar] [CrossRef]
- Świerkosz, K. Remarks on the main forest and shrub communities of the Langtang Khela Valley (Langtang Range, Central Himalayas, Nepal). Čas. Slez. Muz. Opava (A) 2008, 57, 141–148. [Google Scholar]
- Brockerhoff, E.G.; Ecroyd, C.E.; Leckie, A.C.; Kimberley, M.O. Diversity and succession of adventive and indigenous vascular understorey plants in Pinus radiata plantation forests in New Zealand. Ecol. Manag. 2003, 185, 307–326. [Google Scholar] [CrossRef]
- Qi, D.-H.; Guo, H.-J.; Sheng, C.-Y. Assessment of plant species diversity of ancient tea garden communities in Yunnan, Southwest of China. Agrofor. Syst. 2013, 87, 465–474. [Google Scholar] [CrossRef]
- Prévosto, B.; Kuiters, L.; Bernhardt-Römermann, M.; Dölle, M.; Van Uytvanck, J. Impacts of land abandonment on vegetation: Successional pathways in European habitats. Folia Geobot. 2011, 46, 303–325. [Google Scholar] [CrossRef]
- Wehling, S.; Diekmann, M. Importance of hedgerows as habitat corridors for forest plants in agricultural landscapes. Biol. Conserv. 2009, 142, 2522–2530. [Google Scholar] [CrossRef]
- Weaver, P.L.; Danilo Chinea, J. Secondary subtropical dry forest at the La Tinaja Tract of the Cartagena Lagoon national wildlife refuge, Puerto Rico. Caribb. J. Sci. 2003, 39, 273–285. [Google Scholar]
- Zhu, J.; Mao, Z.; Hu, L.; Zhang, J. Plant diversity of secondary forests in response to anthropogenic disturbance levels in montane regions of Northeastern China. J. For. Res. 2007, 12, 403–416. [Google Scholar] [CrossRef]
- Mo, X.-X.; Zhu, H.; Zhang, Y.-J.; Ferry Slik, J.W.; Liu, J.-X. Traditional forest management has limited impact on plant diversity and composition in a tropical seasonal rainforest in SW China. Biol. Conserv. 2011, 144, 1832–1840. [Google Scholar] [CrossRef]
- Cano, E.; Veloz, A. Contribution to the knowledge of the plant communities of the Caribbean-Cibensean sector in the Dominican Republic. Acta Bot. Gall. 2012, 159, 201–210. [Google Scholar] [CrossRef]
- Gillespie, G.R.; Ahmad, E.; Elahan, B.; Evans, A.; Ancrenaz, M.; Goossens, B.; Scroggie, M.P. Conservation of amphibians in Borneo: Relative value of secondary tropical forest and non-forest habitats. Biol. Conserv. 2012, 152, 136–144. [Google Scholar] [CrossRef]
- Solé, N.; Alberto, A.; Samba, S.; Santana, A.; de Lima, R.F. New hope for the critically endangered São Tomé Grosbeak Neospiza concolor and an alert to protect Obô Natural Park surroundings. Ostrich 2012, 83, 161–163. [Google Scholar] [CrossRef]
- Martin, T.E.; Blackburn, G.A. Conservation value of secondary forest habitats for endemic birds, a perspective from two widely separated tropical ecosystems. Ecography 2014, 37, 250–260. [Google Scholar] [CrossRef]
- Welford, M.R. The importance of early successional habitats to rare, restricted-range, and endangered birds in the Ecuadorian Andes. Bird Conserv. Int. 2000, 10, 351–359. [Google Scholar] [CrossRef][Green Version]
- Pinotti, B.T.; Pagotto, C.P.; Pardini, R. Wildlife recovery during tropical forest succession: Assessing ecological drivers of community change. Biotropica 2015, 47, 765–774. [Google Scholar] [CrossRef]
- De Luca, J.R.; Pardini, R. Use of early and late successional forest patches by the endangered lowland tapir Tapirus terrestris (Perissodactyla: Tapiridae). Mamm. Biol. 2017, 86, 107–114. [Google Scholar] [CrossRef]
- Zhang, Z.; Zang, R. Diversity and distribution of food plants: Implications for conservation of the critically endangered Hainan Gibbon. Nat. Conserv. 2018, 31, 17–33. [Google Scholar] [CrossRef]
- Dunn, R.R. Recovery of faunal communities during tropical forest regeneration. Conserv. Biol. 2004, 18, 302–309. [Google Scholar] [CrossRef]
- Thompson, M.E.; Donnelly, M.A. Effects of secondary forest succession on amphibians and reptiles: A review and meta-analysis. Copeia 2018, 106, 10–19. [Google Scholar] [CrossRef]
- Acevedo-Charry, O.; Aide, T.M. Recovery of amphibian, reptile, bird and mammal diversity during secondary forest succession in the tropics. Oikos 2019, 128, 1065–1078. [Google Scholar] [CrossRef]
- Barlow, J.; Mestre, L.A.M.; Gardner, T.A.; Peres, C.A. The value of primary, secondary and plantation forests for Amazonian birds. Biol. Conserv. 2007, 136, 212–231. [Google Scholar] [CrossRef]

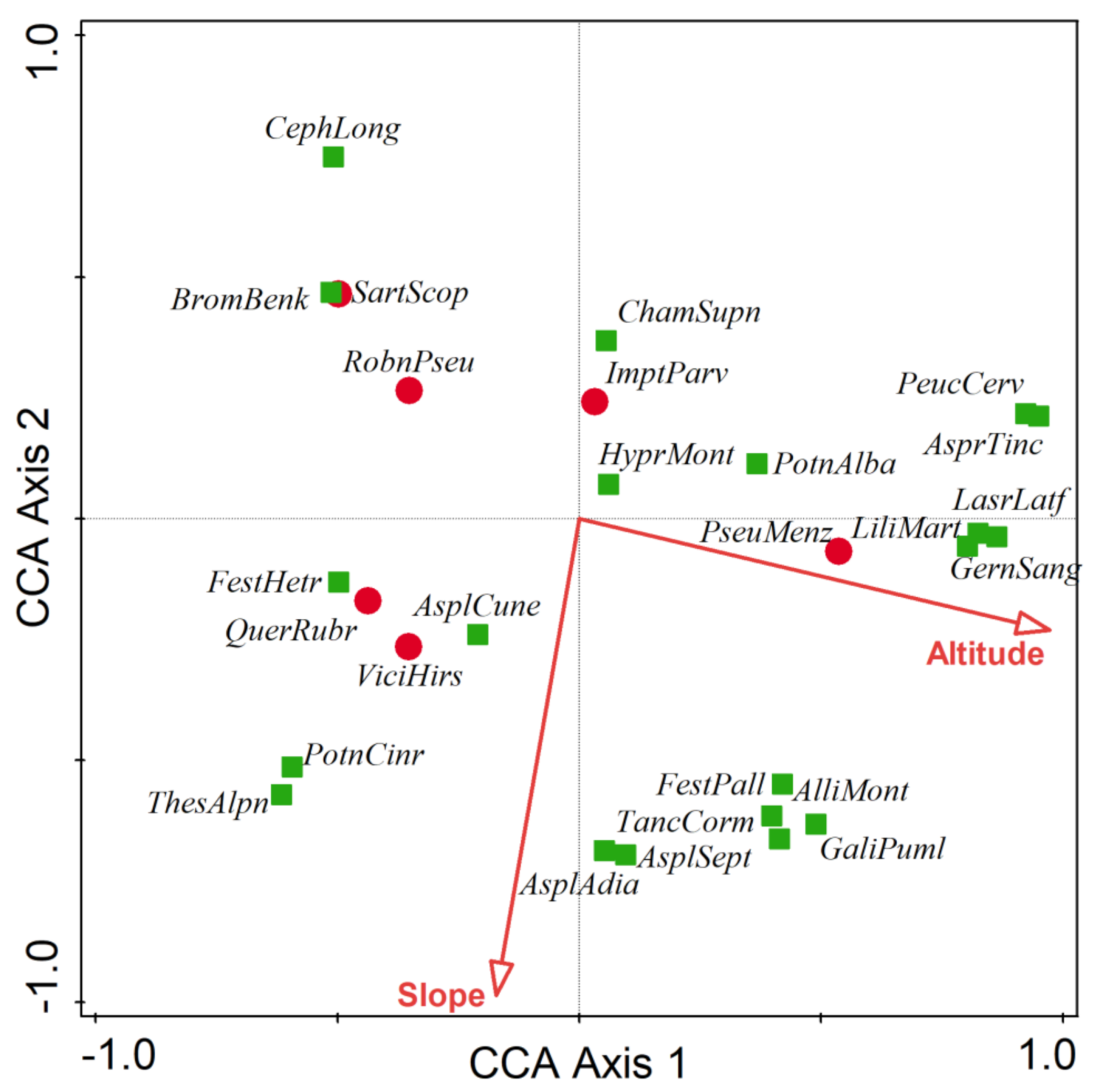
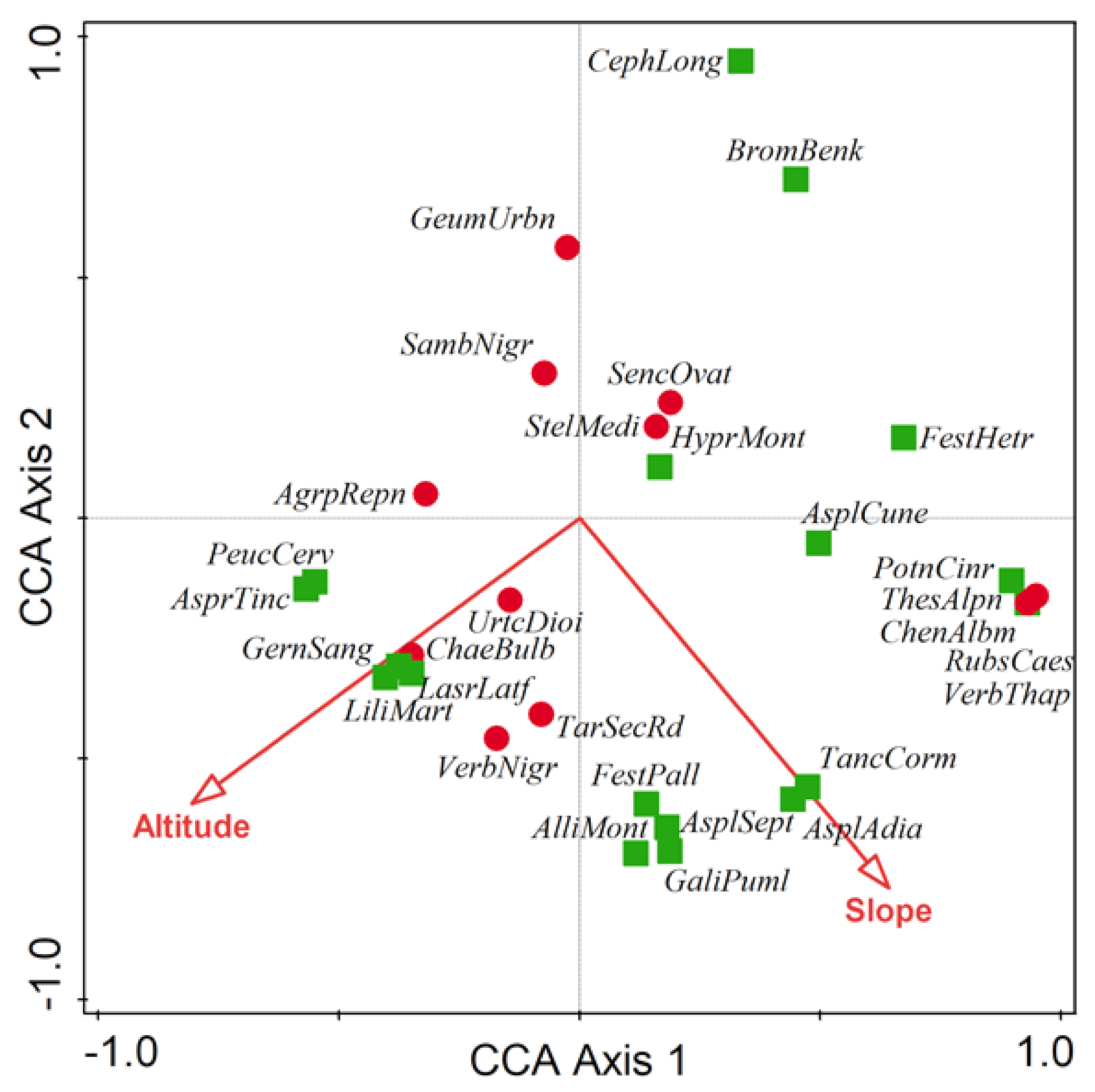
| Forest Community | Mean No. of Species Per Plot | Simpson Index | Shannon–Wiener Index |
|---|---|---|---|
| Ass. Asplenio cuneifolii–Quercetum petraeae | 33.3 | 0.87 | 2.73 |
| Comm. Galium verum–Quercus petraea | 32.8 | 0.83 | 2.62 |
| Comm. Pinus sylvestris–Molinia caerulea | 29.8 | 0.87 | 2.54 |
| Ass. Tilio cordatae–Carpinetum betuli | 23.0 | 0.77 | 2.14 |
| Species Name—with Its Code 1 | Red List of Poland/Silesia Region | Total Number of Sites | Presence of Species in Recognized Cluster (Constancy in %) | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Asplenium cuneifolium—AsplCune | EN/EN | 12 | 50 | 8 | - | - |
| Cephalanthera longifolia—CephLong | VU/VU | 4 | 5 | - | - | 11 |
| Festuca heterophylla—FestHetr | NT/VU | 3 | 6 | 5 | - | 11 |
| Asplenium adiantum-nigrum—AsplAdia | EN/CR | 2 | 6 | 3 | - | - |
| Asplenium septentrionale—AsplSept | VU/NT | 3 | - | 8 | - | - |
| Asperula tinctoria—AsprTinc | VU/EN | 1 | - | 3 | - | - |
| Tanacetum corymbosum—TancCorm | VU/VU | 1 | - | 3 | - | - |
| Potentilla alba—PotnAlba | -/NT | 21 | 22 | 38 | 17 | 4 |
| Hypericum montanum—HyprMont | -/NT | 20 | 33 | 27 | 33 | - |
| Lilium martagon—LiliMart | -/LC | 11 | - | 30 | - | - |
| Potentilla cinerea—PotnCinr | -/NT | 9 | 44 | - | - | - |
| Bromus benekenii—BromBenk | -/LC | 5 | 11 | - | - | 4 |
| Festuca pallens—FestPall | -/VU | 3 | - | 8 | - | - |
| Allium montanum—AlliMont | -/VU | 2 | - | 5 | - | - |
| Galium pumilum agg.—GaliPuml | -/LC | 2 | - | 5 | - | - |
| Laserpitium latifolium—LasrLatf | -/VU | 2 | - | 5 | - | - |
| Chamaecytisus supinus—ChamSupn | -/LC | 1 | - | 3 | - | - |
| Thesium alpinum—ThesAlpn | -/EN | 1 | 11 | - | - | - |
| Geranium sanguineum—GernSang | -/VU | 1 | - | 3 | - | - |
| Peucedanum cervaria—PeucCerv | -/NT | 1 | - | 3 | - | - |
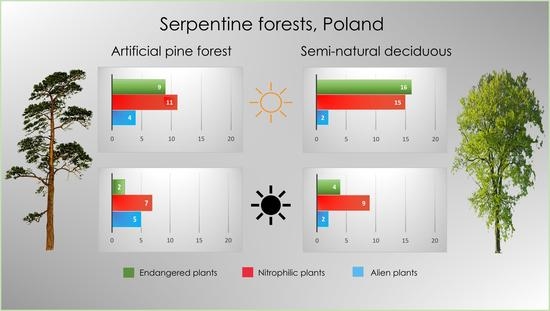
| Total number of rare/endangered species | 9 | 16 | 2 | 4 | ||
| Species Name—with Its Code 1 | Total Number of Sites | Presence of Species in Recognized Cluster (Constancy in %) | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Alien species | |||||
| Impatiens parviflora *—ImptParv | 27 | 6 | 35 | 25 | 36 |
| Robinia pseudoacacia *—RobnPseu | 9 | 50 | - | - | - |
| Pseudotsuga menziesii *—PseuMenz | 7 | - | 3 | 8 | - |
| Quercus rubra *—QuerRubr | 6 | 17 | - | 17 | 4 |
| Vicia hirsuta—ViciHirs | 2 | 11 | - | - | - |
| Sarothamnus scoparius—SartScop | 1 | - | - | 8 | - |
| Amelanchier lamarckii | 1 | - | - | 8 | - |
| Total number of alien species | 4 | 2 | 5 | 2 | |
| Nitrophilic species | |||||
| Stellaria media—StellMedi | 28 | 11 | 46 | 25 | 21 |
| Fallopia dumetorum—FallDumt | 25 | 22 | 54 | 8 | - |
| Agropyron repens—AgrpRepn | 20 | 17 | 41 | - | 7 |
| Galium aparine—GaliApar | 17 | - | 27 | 8 | 21 |
| Geranium robertianum—GernRobr | 15 | 6 | 30 | 17 | 4 |
| Urtica dioica—UrtcDioi | 12 | - | 24 | - | 11 |
| Taraxacum sect. Ruderalia—TarSecRd | 12 | 17 | 24 | - | - |
| Arrhenatherum elatius—ArrhElat | 7 | 17 | 8 | 8 | - |
| Sambucus nigra—SambNigr | 6 | - | 8 | 8 | 7 |
| Senecio ovatus—SencOvat | 6 | 6 | 3 | 25 | 4 |
| Geum urbanum—GeumUrbn | 5 | 6 | 8 | - | 4 |
| Chenopodium album—ChenAlbm | 1 | 6 | - | - | - |
| Chaerophyllum bulbosum—ChaeBulb | 1 | - | 3 | - | - |
| Rubus caesius—RubsCaes | 1 | 6 | - | - | - |
| Verbascum nigrum—VerbNigr | 1 | - | 9 | - | - |
| Verbascum thapsus—VerbThap | 1 | 6 | - | - | - |
| Alliaria petiolata | 1 | - | 3 | - | - |
| Anthriscus sylvestris | 1 | - | 3 | - | - |
| Tanacetum vulgare | 1 | - | - | - | 4 |
| Total number of nitrophilic species | 11 | 15 | 7 | 9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bubel, K.; Reczyńska, K.; Pech, P.; Świerkosz, K. Secondary Serpentine Forests of Poland as a Refuge for Vascular Flora. Diversity 2021, 13, 201. https://doi.org/10.3390/d13050201
Bubel K, Reczyńska K, Pech P, Świerkosz K. Secondary Serpentine Forests of Poland as a Refuge for Vascular Flora. Diversity. 2021; 13(5):201. https://doi.org/10.3390/d13050201
Chicago/Turabian StyleBubel, Karol, Kamila Reczyńska, Paweł Pech, and Krzysztof Świerkosz. 2021. "Secondary Serpentine Forests of Poland as a Refuge for Vascular Flora" Diversity 13, no. 5: 201. https://doi.org/10.3390/d13050201
APA StyleBubel, K., Reczyńska, K., Pech, P., & Świerkosz, K. (2021). Secondary Serpentine Forests of Poland as a Refuge for Vascular Flora. Diversity, 13(5), 201. https://doi.org/10.3390/d13050201