A Primer on Spider Assemblages in Levantine Caves: The Neglected Subterranean Habitats of the Levant—A Biodiversity Mine
Abstract
1. Introduction
1.1. The Levant and Its Caves
1.2. Spider and Other Arachnid Assemblages in Caves
2. Materials and Methods
2.1. Field Surveys
2.2. Statistical Analysis
3. Results
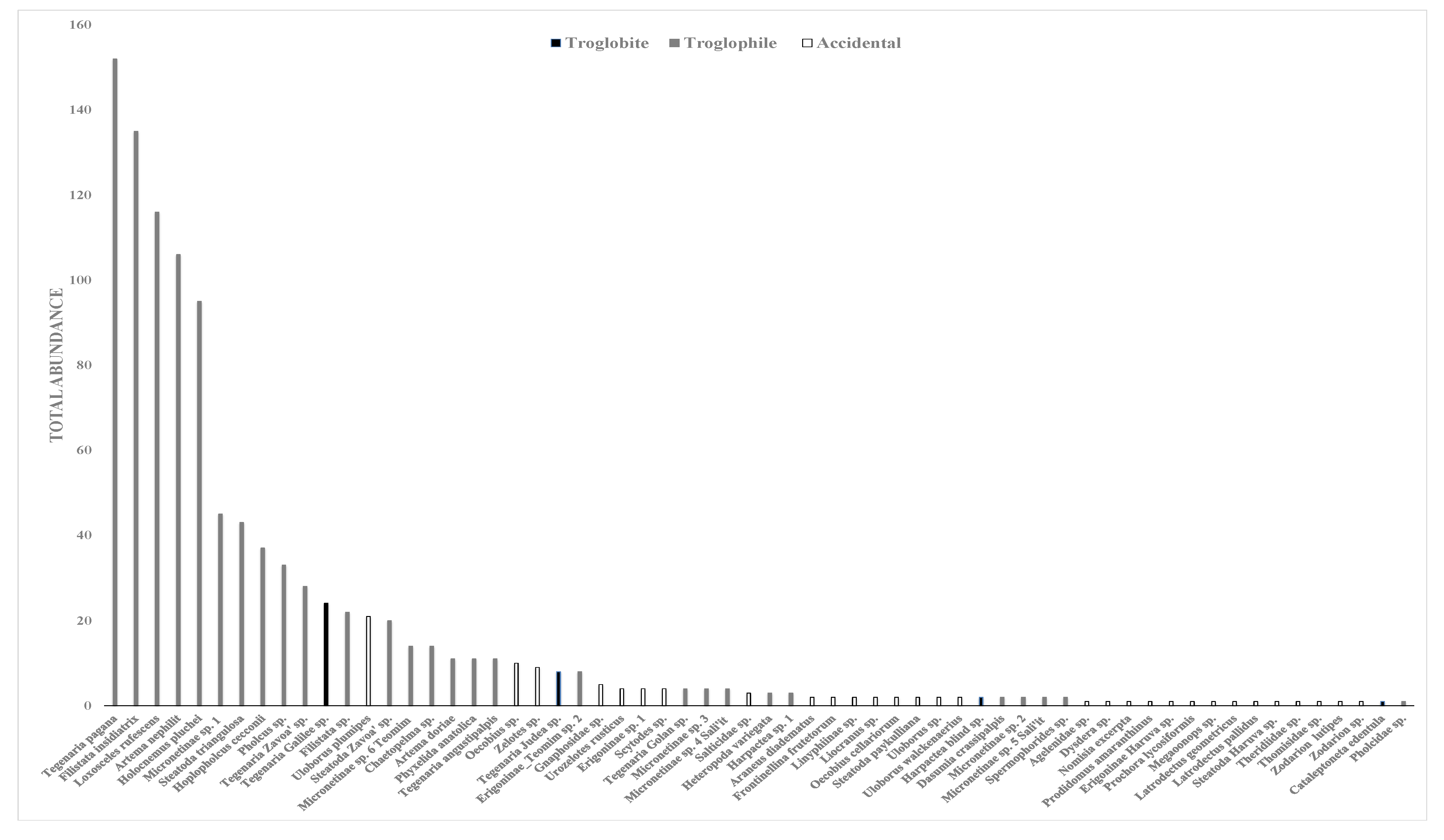
3.1. Cave Arachnofauna from Field Surveys and Additional Visits to Caves
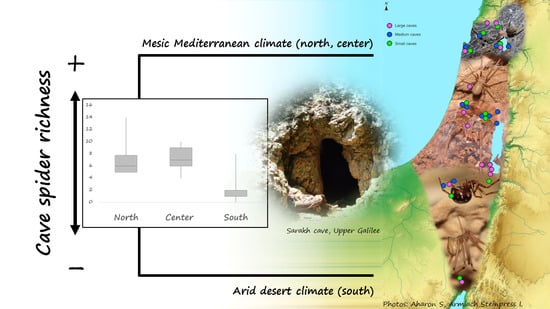
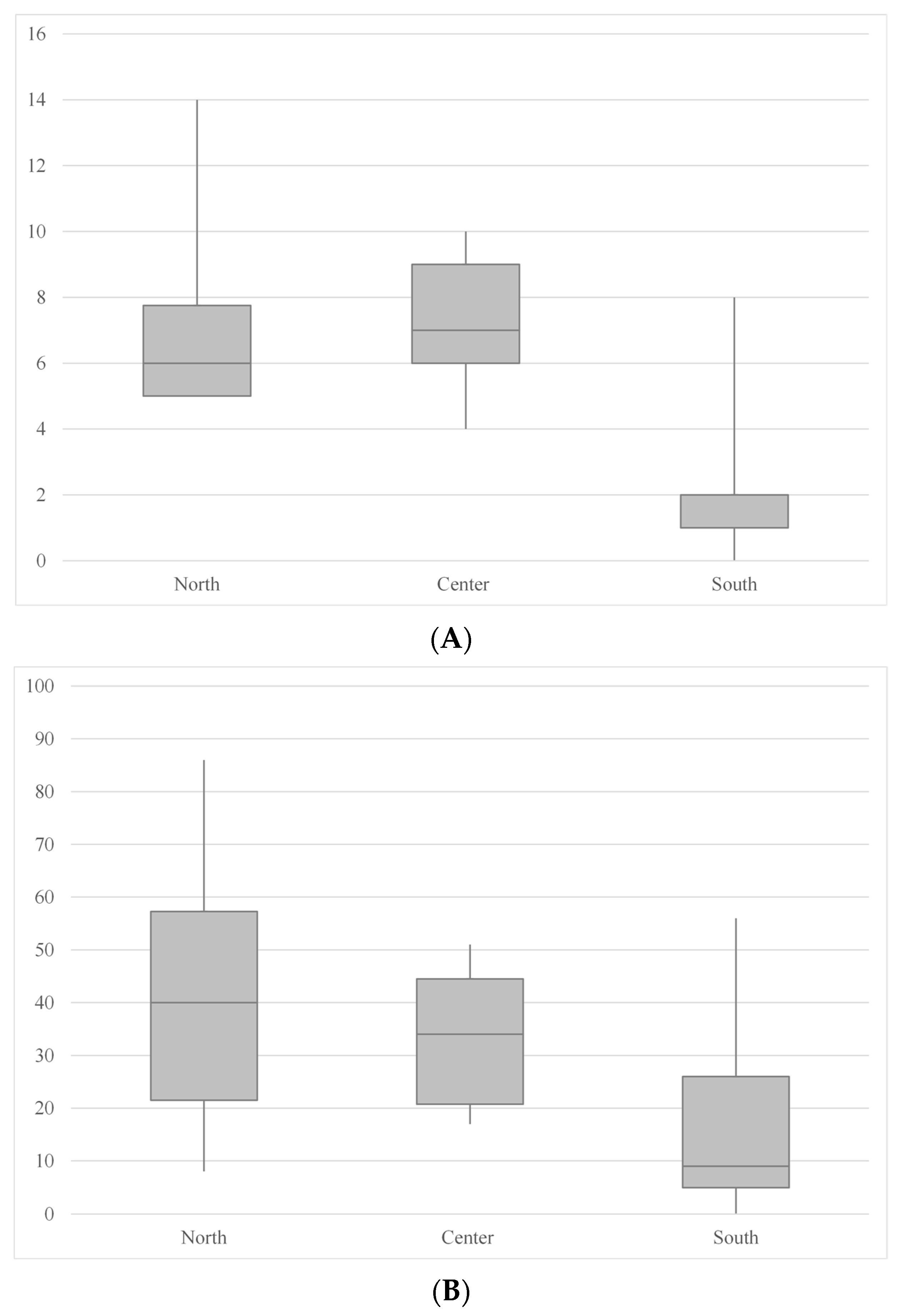
3.2. Cave Geographic Region, Bat Presence and Spider Richness and Abundance
3.3. Troglobites and Troglophiles
3.4. Spider Foraging Guilds
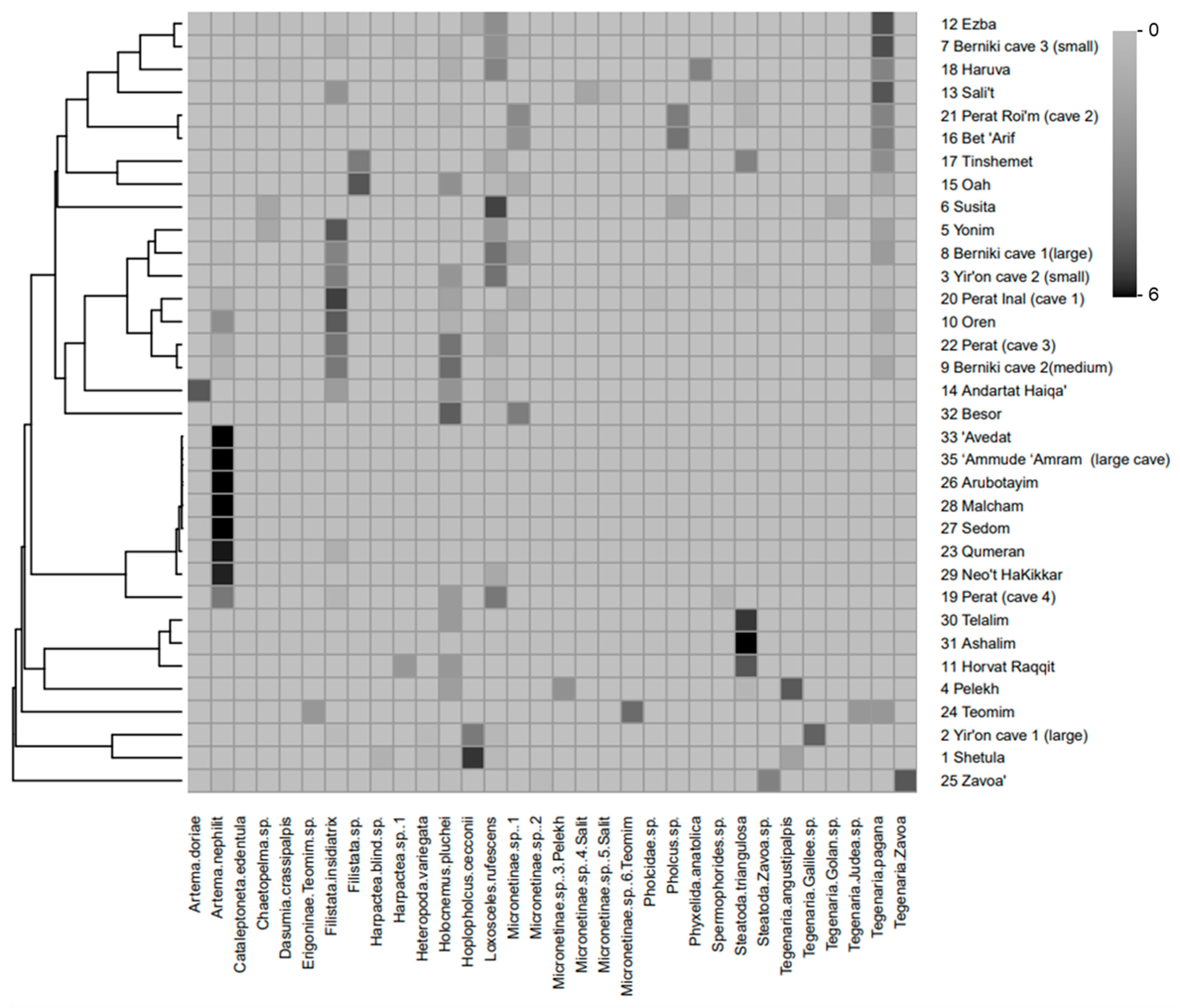
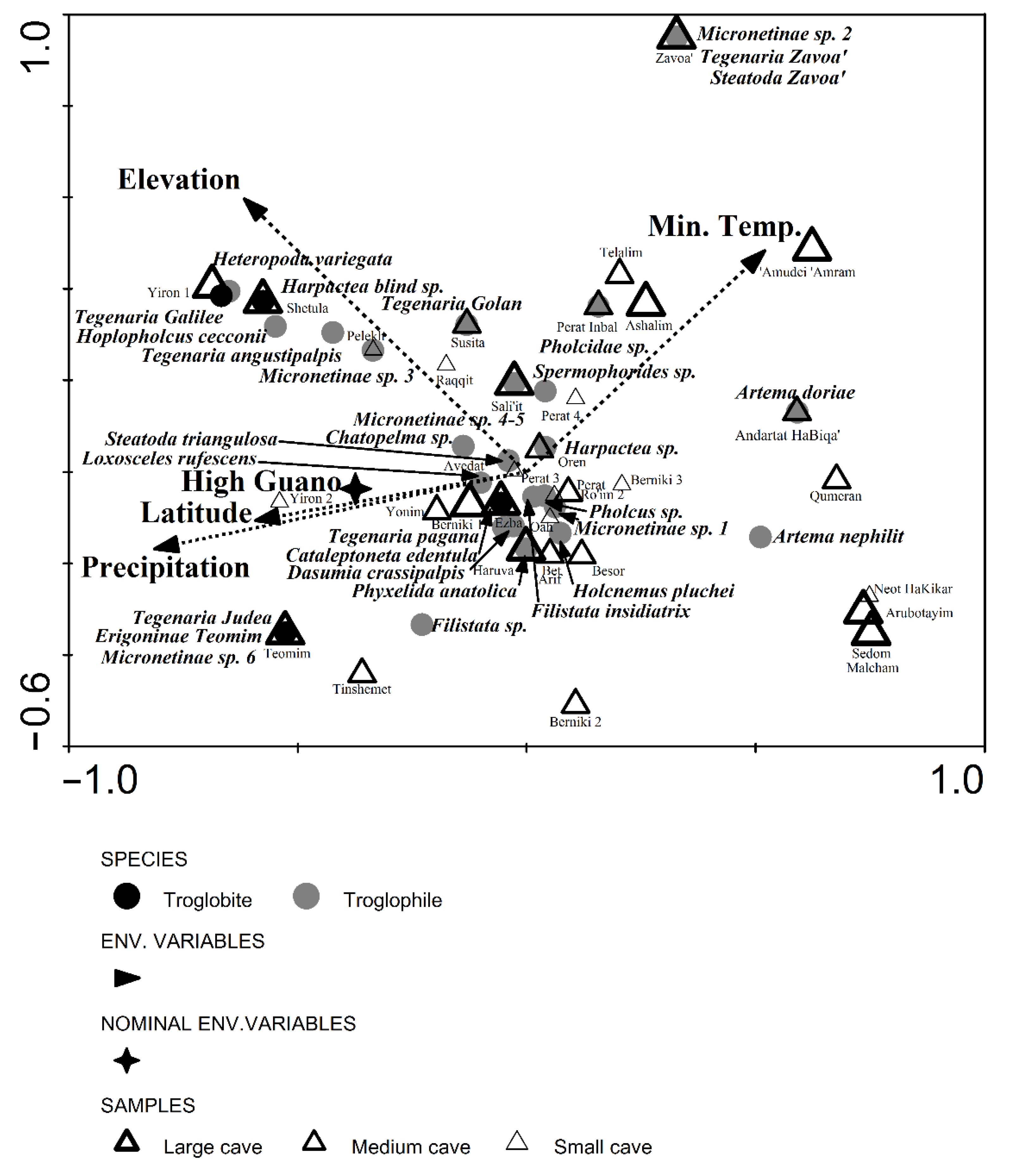
3.5. Levantine Cave Spider Assemblages: Similarities and Environmental Variables from Field Survey
4. Discussion
4.1. Levantine Cave Arachnofauna
4.2. Troglobites and Troglophiles
4.3. Foraging Guilds
4.4. Species-Pool, Regions, and Bat Inhabitance
4.5. Levantine Cave Spider Assemblages
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
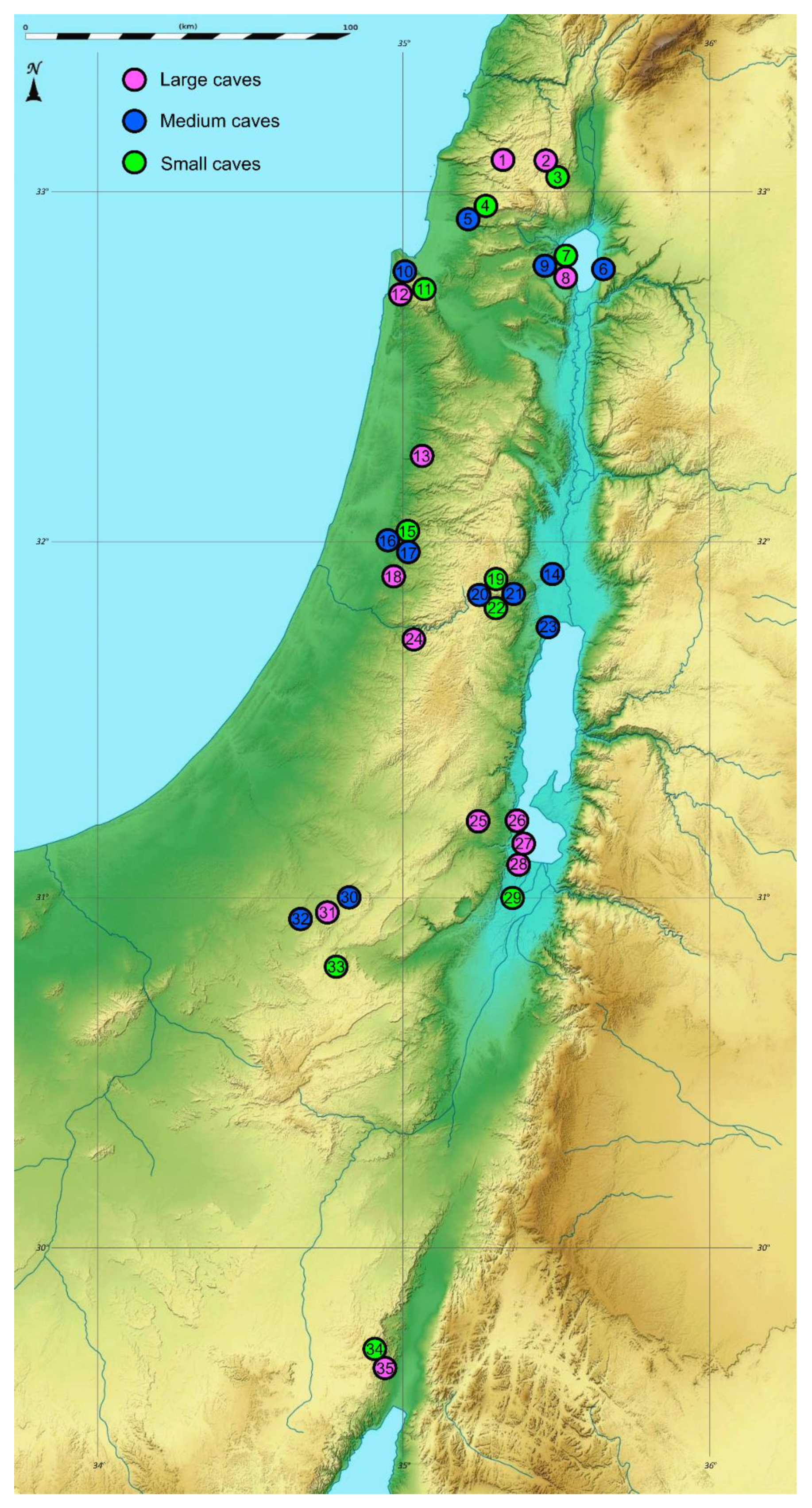
| District | Geographic Region | Cave | N | E | Elevation | Climate Type | Precipitation (mm) | Cave Minimum Temp. (E, T, D) | Lithology Category | Lithology Geological Age | Cave Size | Length Estimate | Bat | Guano Level | Spider Abundance, Richness |
| North | Upper Galilee | 1 Shetula (P) | 33.0873 | 35.3169 | 690 | Mediterenaean | 840.4 | 11.5, 14.5, 15.5 | Carbonate rocks | Cenomanian | Large | 150 | Insectivorous | Low | 21, 6 |
| 2 Yir’on (cave 1) (P) | 33.0679 | 35.4665 | 528 | 716.4 | 16.5, 13, 20 | Eocene | Large | 150 | Insectivorous | High | 49, 5 | ||||
| 3 Yir’on (cave 2) (P) | 33.0672 | 35.4672 | 541 | 716.4 | 8 | Small | 10 | without | Zero | 26, 8 | |||||
| 4 Pelekh (P) | 32.9324 | 35.238 | 488 | 648.5 | 12.5 | Turonian | Small | 5 | without | Zero | 16, 5 | ||||
| 5 Yonim (P) | 32.9236 | 35.2168 | 216 | 648.5 | 12.5, 12.5 | Medium | 50 | without | Zero | 61, 14 | |||||
| Golan | 6 Susita (P) | 32.7793 | 35.6577 | 70 | 382.5 | 21,21 | Basalt | Pliocene | Medium | 50 | Insectivorous | Low | 31, 5 | ||
| Lower Galilee | 7 Berniki (cave 3) (P) | 32.7775 | 35.5401 | −102 | 455 | 17 | Carbonate rocks | Turonian | Small | 5 | without | Zero | 23, 6 | ||
| 8 Berniki (cave 1) (P) | 32.7775 | 35.5401 | −102 | 455 | 19.5, 17, 20.5 | Large | 480 | Insectivorous | High | 53, 6 | |||||
| 9 Berniki (cave 2) (P) | 32.7768 | 35.5413 | −166 | 455 | 12.5, 12.5 | Medium | 15 | without | Zero | 86, 11 | |||||
| Karmel | 10 Oren (P) | 32.7144 | 34.9749 | 73 | 611.2 | 16.5, 15.5 | Medium | 36 | without | Zero | 55, 5 | ||||
| 11 Horvat Raqqit (P) | 32.7128 | 35.0123 | 355 | 611.2 | 14.5 | Cenomanian | Small | 3 | without | Zero | 8, 5 | ||||
| 12 Ezba’ (P) | 32.7118 | 34.9747 | 120 | 611.2 | 14.5, 15.5, 18 | Turonian | Large | 52 | Frugivorous | High | 58, 7 | ||||
| Central Israel and Palestine | Northern Samaria | 13 Sal’it (P) | 32.2454 | 35.0456 | 254 | 620.4 | 17, 18, 19.5 | Cenomanian | Large | 108 | without | Zero | 26, 6 | ||
| Central Jordan Valley | 14 Andartat HaBiqa’ * (E) | 32.0524 | 35.4589 | −184 | Hyper-arid | 19.9 | 19.5, 25.5 | Turonian | Medium | 23.5 | Insectivorous | Medium | 31, 10 | ||
| Western Samaria | 15 Oah (P) | 32.0053 | 34.9722 | 123 | Mediterenaean | 569 | 16 | Small | 10 | without | Zero | 19, 7 | |||
| 16 Bet ‘A’rif (P) | 32.0026 | 34.9642 | 95 | 569 | 15.5, 18, 18 | Medium | 45 | without | Zero | 42, 7 | |||||
| 17 Tinshemet (P) | 31.9994 | 34.9681 | 100 | 569 | 15.5 | Medium | 43 | Frugivorous | High | 51, 6 | |||||
| HaShfela | 18 Haruva (P) | 31.9133 | 34.9607 | 180 | 537.8 | 14.5, 16, 18 | Chalk | ConiacianCampanian | Large | 100 | without | Zero | 43, 9 | ||
| Northern Judean Desert | 19 Perat Southern Slope (cave 4) * (PE) | 31.8334 | 35.3054 | 295 | Semi-arid | 250 | 15 | Carbonate rocks | Turonian | Small | 10 | without | Zero | 17, 6 | |
| 20 Perat ‘Inbal (cave 1) * (PE) | 31.8332 | 35.3019 | 314 | 250 | 17, 16.5 | Medium | 40 | without | Zero | 37, 9 | |||||
| 21 Perat Ro’im (cave 2) * (PE) | 31.8325 | 35.313 | 238 | 250 | 13.5, 16.5 | Medium | 25 | Insectivorous | Low | 45, 9 | |||||
| 22 Perat (cave 3) * (PE) | 31.8321 | 35.3083 | 268 | 250 | 13 | Small | 10 | without | Zero | 19, 7 | |||||
| Nortern Dead-Sea Area | 23 Qumeran * (E) | 31.7556 | 35.459 | −308 | Arid | 95.8 | 21, 21 | Cenomanian | Medium | 20 | without | Zero | 30, 4 | ||
| Judean Mountain | 24 Te’omim (P) | 31.7262 | 35.0217 | 375 | Mediterenaean | 509 | 13, 13.5, 14.5 | Large | 134 | Frugivorous | High | 45, 5 | |||
| South | Southern Judean Desert | 25 Zavoa’ (PE) | 31.2086 | 35.2311 | 495 | Arid | 100 | 22, 23, 23.5 | Carbonate rocks | Turonian | Large | 600 | Unknown | Low | 56, 8 |
| Southern Dead-Sea Area | 26 Arubotayim (E) | 31.1016 | 35.39 | −348 | Hyper-arid | 41.1 | 20, 19.5, 19.5 | Salt | Pliocene | Large | 60 | without | Zero | 5, 1 | |
| 27 Sedom (E) | 31.0872 | 35.3958 | −381 | 41.1 | 20, 19.5, 19.5 | Large | 1800 | without | Zero | 28, 1 | |||||
| 28 Malcham (E) | 31.0765 | 35.3971 | −380 | 41.1 | 20, 19.5, 19.5 | Large | 10,000 | without | Zero | 11, 1 | |||||
| 29 Ne’ot HaKikkar (Nezirim Burial cave) (E) | 30.9911 | 35.3465 | −333 | 41.1 | 20.5 | Marlstone | Quaternary | Small | 4 | without | Zero | 5, 2 | |||
| Negev Desert | 30 Telalim (PE) | 30.9734 | 34.7929 | 482 | Arid | 91.4 | 17, 15 | Carbonate rocks | Turonian | Medium | 27 | without | Zero | 7, 2 | |
| 31 Ashalim (PE) | 30.9434 | 34.7391 | 404 | 91.4 | 17.5, 19.5, 17 | Large | 540 | Insectivorous | Low | 13, 1 | |||||
| 32 Besor (PE) | 30.9415 | 34.6961 | 356 | 91.4 | 12, 16.5 | Medium | 35 | without | Zero | 26, 5 | |||||
| 33 ‘Avedat (Nezirim cave) (PE) | 30.7941 | 34.772 | 601 | 93.3 | 11 | Eocene | Small | 5 | without | Zero | 9, 2 | ||||
| ‘Arava D esert | 34 ‘Ammude ‘Amram (cave 2) (PE) | 29.6518 | 34.9337 | 288 | Hyper-arid | 22.5 | 21 | Sandstone | Cambrian | Small | 5 | without | Zero | 0, 0 | |
| 35 ‘Ammude ‘Amram (cave 1) (PE) | 29.6515 | 34.9336 | 293 | 22.5 | 21, 24, 24 | Large | 80 | Insectivorous | Low | 2, 2 |
- (1)
- Localities in Palestine (West Bank) are marked by asterisk (*). Letters in parentheses after cave name indicate the zoogeographical region: (P)—Palaearctic; (E)—Ethiopian; (PE)—Palaeoeremic (after Por, 1975).
- (2)
- Precipitation data is taken from Israel Meteorological Service (https://ims.gov.il/en/ClimateAtlas, 1 February 2021) for the average annual mean for 1980–2010, from the closest meteorological station for each cave.
- (3)
- Minimum Temperature is the average of the minimum temperature measured by us for each cave for two months (between the first and second visit to each cave) during the survey.
- (4)
- Length was estimated from cave maps when available, or in field, and represent the distance from the cave opening toward the darker region of the cave that we could reach.
References
- Poulson, T.L.; White, W.B. The Cave Environment. Science 1969, 165, 971–981. [Google Scholar] [CrossRef]
- Howarth, F.G. Ecology of Cave Arthropods. Annu. Rev. Entomol. 1983, 28, 365–389. [Google Scholar] [CrossRef]
- Lauritzen, S.-E. Physiography of the Caves. In Cave Ecology; Moldovan, O.T., Kováč, Ľ., Halse, S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 7–21. ISBN 978-3-319-98852-8. [Google Scholar]
- Zobel, M.; Otto, R.; Laanisto, L.; Naranjo-Cigala, A.; Pärtel, M.; Fernández-Palacios, J.M. The Formation of Species Pools: Historical Habitat Abundance Affects Current Local Diversity: Species Pool Formation and Current Diversity. Global Ecol. Biogeogr. 2011, 20, 251–259. [Google Scholar] [CrossRef]
- Trivellone, V.; Bougeard, S.; Giavi, S.; Krebs, P.; Balseiro, D.; Dray, S.; Moretti, M. Factors Shaping Community Assemblages and Species Co-Occurrence of Different Trophic Levels. Ecol. Evol. 2017, 7, 4745–4754. [Google Scholar] [CrossRef]
- Willis, K.J.; Whittaker, R.J. Species Diversity: Scale Matters. Science 2002, 295, 1245–1248. [Google Scholar] [CrossRef]
- Gibert, J.; Deharveng, L. Subterranean Ecosystems: A Truncated Functional Biodiversity. BioScience 2002, 52, 473. [Google Scholar] [CrossRef]
- Mammola, S.; Arnedo, M.A.; Pantini, P.; Piano, E.; Chiappetta, N.; Isaia, M. Ecological Speciation in Darkness? Spatial Niche Partitioning in Sibling Subterranean Spiders (Araneae: Linyphiidae: Troglohyphantes). Invert. Syst. 2018, 32, 1069. [Google Scholar] [CrossRef]
- Mammola, S.; Aharon, S.; Seifan, M.; Lubin, Y.; Gavish-Regev, E. Exploring the Interplay Between Local and Regional Drivers of Distribution of a Subterranean Organism. Diversity 2019, 11, 119. [Google Scholar] [CrossRef]
- Schneider, K.; Christman, M.C.; Fagan, W.F. The Influence of Resource Subsidies on Cave Invertebrates: Results from an Ecosystem-Level Manipulation Experiment. Ecology 2011, 92, 765–776. [Google Scholar] [CrossRef]
- Mammola, S. Finding Answers in the Dark: Caves as Models in Ecology Fifty Years after Poulson and White. Ecography 2019, 42, 1331–1351. [Google Scholar] [CrossRef]
- Howarth, F.G. High-Stress Subterranean Habitats and Evolutionary Change in Cave-Inhabiting Arthropods. Am. Nat. 1993, 142, S65–S77. [Google Scholar] [CrossRef]
- Manenti, R.; Lunghi, E.; Ficetola, G.F. The Distribution of Cave Twilight-Zone Spiders Depends on Microclimatic Features and Trophic Supply. Invertebr. Biol. 2015, 134, 242–251. [Google Scholar] [CrossRef]
- Tobin, B.W.; Hutchins, B.T.; Schwartz, B.F. Spatial and Temporal Changes in Invertebrate Assemblage Structure from the Entrance to Deep-Cave Zone of a Temperate Marble Cave. Int. J. Speleobiol. 2013, 42, 203–221. [Google Scholar] [CrossRef]
- Lunghi, E.; Manenti, R.; Ficetola, G.F. Cave Features, Seasonality and Subterranean Distribution of Non-Obligate Cave Dwellers. PeerJ 2017, 5, e3169. [Google Scholar] [CrossRef]
- Lunghi, E.; Manenti, R.; Ficetola, G.F. Seasonal Variation in Microhabitat of Salamanders: Environmental Variation or Shift of Habitat Selection? PeerJ 2015, 3, e1122. [Google Scholar] [CrossRef]
- Culver, D.C.; Pipan, T. The Biology of Caves and Other Subterranean Habitats, 2nd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2019; ISBN 978-0-19-882076-5. [Google Scholar]
- Trajano, E.; de Carvalho, M.R. Towards a Biologically Meaningful Classification of Subterranean Organisms: A Critical Analysis of the Schiner-Racovitza System from a Historical Perspective, Difficulties of Its Application and Implications for Conservation. Subterr. Biol. 2017, 22, 1–26. [Google Scholar] [CrossRef]
- Avni, Y. Tectonic and Physiographic Settings of the Levant. In Quaternary of the Levant: Environments, Climate Change, and Humans; Bar-Yosef, O., Enzel, Y., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 3–16. ISBN 978-1-107-09046-0. [Google Scholar]
- Frumkin, A. Quaternary Evolution of Caves and Associated Palaeoenvironments of the Southern Levant. In Quaternary of the Levant: Environments, Climate Change, and Humans; Bar-Yosef, O., Enzel, Y., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 135–144. ISBN 978-1-107-09046-0. [Google Scholar]
- Frumkin, A. Interaction between Karst, Water and Agriculture over the Climatic Gradient of Israel. Int. J. Speleol. 1999, 28, 99–110. [Google Scholar] [CrossRef]
- Audra, P.; Palmer, A. The Pattern of Caves: Controls of Epigenic Speleogenesis. Geomorphol. Relief Process. Environ. 2011, 17, 359–378. [Google Scholar] [CrossRef]
- Frumkin, A.; Langford, B.; Lisker, S.; Amrani, A. Hypogenic Karst at the Arabian Platform Margins: Implications for Far-Field Groundwater Systems. GSA Bull. 2017, 129, 1636–1659. [Google Scholar] [CrossRef]
- Frumkin, A.; Naor, R. Formation and Modification of Pit Craters–Example from the Golan Volcanic Plateau, Southern Levant. Z. Geomorphol. 2019, 62, 163–181. [Google Scholar] [CrossRef]
- Sivan, D.; Sisma-Ventura, G.; Greenbaum, N.; Bialik, O.M.; Williams, F.H.; Tamisiea, M.E.; Rohling, E.J.; Frumkin, A.; Avnaim-Katav, S.; Shtienberg, G.; et al. Eastern Mediterranean Sea Levels through the Last Interglacial from a Coastal-Marine Sequence in Northern Israel. Quat. Sci. Rev. 2016, 145, 204–225. [Google Scholar] [CrossRef]
- Frumkin, A.; Fischhendler, I. Morphometry and Distribution of Isolated Caves as a Guide for Phreatic and Confined Paleohydrological Conditions. Geomorphology 2005, 67, 457–471. [Google Scholar] [CrossRef]
- Frumkin, A.; Ben-Tov, D.; Buslov, V.; Langford, B.; Valdman, E.; Yaaran, S. The Southernmost Authigenic Karst Plateau of the Levant. In Proceedings of the 17th International Congress of Speleology, Sydney, Australia, 23–29 July 2017; pp. 13–16. [Google Scholar]
- Frumkin, A.; Langford, B.; Porrat, R. The Judean Desert–The major hypogene cave region of the southern Levant. In Hypogene Karst Regions and Caves of the World; Springer: Berlin/Heidelberg, Germany, 2017; pp. 463–477. [Google Scholar]
- Frumkin, A. Morphology and Development of Salt Caves. Natl. Speleol. Soc. Bull. 1994, 56, 82–95. [Google Scholar]
- Frumkin, A.; Bar-Matthews, M.; Davidovich, U.; Langford, B.; Porat, R.; Ullman, M.; Zissu, B. In-Situ Dating of Ancient Quarries and the Source of Flowstone (‘Calcite-Alabaster’) Artifacts in the Southern Levant. J. Archaeol. Sci. 2014, 41, 749–758. [Google Scholar] [CrossRef]
- Resende, L.P.A.; Bichuette, M.E. Sharing the Space: Coexistence among Terrestrial Predators in Neotropical Caves. J. Nat. Hist. 2016, 50, 2107–2128. [Google Scholar] [CrossRef]
- Mammola, S.; Isaia, M. Spiders in Caves. Proc. R. Soc. B. 2017, 284. [Google Scholar] [CrossRef]
- Mammola, S.; Cardoso, P.; Ribera, C.; Pavlek, M.; Isaia, M. A Synthesis on Cave-Dwelling Spiders in Europe. J. Zool. Syst. Evol. Res. 2018, 56, 301–316. [Google Scholar] [CrossRef]
- Parimuchová, A.; Dušátková, L.P.; Kováč, Ľ.; Macháčková, T.; Slabý, O.; Pekár, S. The Food Web in a Subterranean Ecosystem Is Driven by Intraguild Predation. Sci. Rep. 2021, 11, 4994. [Google Scholar] [CrossRef]
- Reddell, J.R. Spiders and Related Groups. In Encyclopedia Caves; Culver, D.C., White, W.B., Eds.; Elsevier Academic Press: Burlington, MA, USA, 2005; pp. 554–564. [Google Scholar]
- Levy, G. The First Troglobite Scorpion from Israel and a New Chactoid Family (Arachnida: Scorpiones). Zool. Middle East 2007, 40, 91–96. [Google Scholar] [CrossRef]
- Karaman, I. A New Euscorpius Species (Scorpiones: Euscorpiidae) from a Dinaric Cave–the First Record of Troglobite Scorpion in European Fauna. Biol. Serbica 2020, 42, 14–31. [Google Scholar] [CrossRef]
- Aharon, S.; Ballesteros, J.A.; Crawford, A.R.; Friske, K.; Gainett, G.; Langford, B.; Santibáñez-López, C.E.; Ya’aran, S.; Gavish-Regev, E.; Sharma, P.P. The Anatomy of an Unstable Node: A Levantine Relict Precipitates Phylogenomic Dissolution of Higher-Level Relationships of the Armoured Harvestmen (Arachnida: Opiliones: Laniatores). Invert. Syst. 2019. [Google Scholar] [CrossRef]
- Miranda, G.S.; Aharon, S.; Gavish-Regev, E.; Giupponi, A.P.L.; Wizen, G. A New Species of Charinus Simon, 1892 (Arachnida: Amblypygi: Charinidae) from Israel and New Records of C. Ioanniticus (Kritscher, 1959). Eur. J. Taxon. 2016. [Google Scholar] [CrossRef]
- Aharon, S.; Huber, B.A.; Gavish-Regev, E. Daddy-Long-Leg Giants: Revision of the Spider Genus Artema Walckenaer, 1837 (Araneae, Pholcidae). Eur. J. Taxon. 2017. [Google Scholar] [CrossRef]
- Gavish-Regev, E.; Aharon, S.; Armiach, I.; Lubin, Y. Cave Survey Yields a New Spider Family Record for Israel. Aramit 2016, 51, 39–42. [Google Scholar] [CrossRef]
- Cardoso, P.; Pekár, S.; Jocqué, R.; Coddington, J.A. Global Patterns of Guild Composition and Functional Diversity of Spiders. PLoS ONE 2011, 6, e21710. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Ter Braak, C.; Šmilauer, P. CANOCO Reference Manual and User’s Guide to Canoco for Windows. Software for Canonical Community Ordination (Version 4.5); Centre for Biometry: Wageningen, The Netherlands, 2002. [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO, 1st ed.; Cambridge University Press: Cambridge, UK, 2003; ISBN 978-0-521-81409-6. [Google Scholar]
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, M.; et al. Gplots: Various R Programming Tools for Plotting Data. R Package. 2020. Available online: https://github.com/talgalili/gplots (accessed on 18 February 2021).
- Ballesteros, J.A.; Santibáñez López, C.E.; Kováč, Ľ.; Gavish-Regev, E.; Sharma, P.P. Ordered Phylogenomic Subsampling Enables Diagnosis of Systematic Errors in the Placement of the Enigmatic Arachnid Order Palpigradi. Proc. R. Soc. B. 2019, 286. [Google Scholar] [CrossRef] [PubMed]
- Zonstein, S.; Marusik, Y.M. Checklist of the Spiders (Araneae) of Israel. Zootaxa 2013, 3671, 1. [Google Scholar] [CrossRef]
- Nentwig, W.; Blick, T.; Bosmans, R.; Gloor, D.; Hänggi, A.; Kropf, C. Spiders of Europe. Version 02.2021. Available online: https://araneae.nmbe.ch/ (accessed on 18 February 2021).
- Kostanjšek, R.; Kuntner, M. BioPortal–Katalog–Araneae Sloveniae. Available online: http://www.bioportal.si/katalog/araneae.php (accessed on 18 February 2021).
- Kostanjšek, R.; Kuntner, M. Araneae Sloveniae: A National Spider Species Checklist. ZooKeys 2015, 474, 1–91. [Google Scholar] [CrossRef]
- Massa, M.; Planas, E.; Ribera, C. The Mediterranean as a Melting Pot: Phylogeography of Loxosceles Rufescens (Sicariidae) in the Mediterranean Basin. PLoS ONE 2018, 13, e0210093. [Google Scholar] [CrossRef]
- Cardoso, P. Diversity and Community Assembly Patterns of Epigean vs. Troglobiont Spiders in the Iberian Peninsula. Int. J. Speleol. 2012, 41, 83–94. [Google Scholar] [CrossRef]
- Frumkin, A.; Aharon, S.; Davidovich, U.; Langford, B.; Negev, Y.; Ullman, M.; Vaks, A.; Ya‘aran, S.; Zissu, B. Old and Recent Processes in a Warm and Humid Desert Hypogene Cave: ‘A’Rak Na‘asane, Israel. Int. J. Speleol. 2018, 47, 307–321. [Google Scholar] [CrossRef]
- Vaks, A.; Bar-Matthews, M.; Ayalon, A.; Schilman, B.; Gilmour, M.; Hawkesworth, C.J.; Frumkin, A.; Kaufman, A.; Matthews, A. Paleoclimate Reconstruction Based on the Timing of Speleothem Growth and Oxygen and Carbon Isotope Composition in a Cave Located in the Rain Shadow in Israel. Quat. Res. 2003, 59, 182–193. [Google Scholar] [CrossRef]
- Ferreira, R.L.; Martins, R.P. Trophic Structure and Natural History of Bat Guano Invertebrate Communities, with Special Reference to Brazilian Caves. Trop. Zool. 1999, 12, 231–252. [Google Scholar] [CrossRef]
- Ferreira, R.; Martins, R.; Prous, X. Structure of Bat Guano Communities in a Dry Brazilian Cave. Trop. Zool. 2007, 20, 55–74. [Google Scholar]






| South (11 Caves) | Center (12 Caves) | North (12 Caves) | |
|---|---|---|---|
| Spider species richness (minimum, median, maximum; mean) | 0 1, 2, 8 2; 2.27 | 4 3, 7, 10 4; 7.08 | 5 5, 6, 14 6; 6.91 |
| Spider abundance (minimum, median, maximum; mean) | 0 1, 9, 56 2; 14.73 | 17 7, 34, 51 8; 33.75 | 8 5, 40, 86 9; 40.58 |
| Ambush Hunters | Specialists and Other Hunters | Sensing Web | Sheet or Space Web | Orb Web | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Df | Wald | p-Value | Wald | p-Value | Wald | p-Value | Wald | p-Value | Wald | p-Value | ||
| Model 1 | Geographic region | 2 | 2.144 | 0.342 | 2.963 | 0.227 | 0.706 | 0.703 | 4.440 | 0.109 | 1.733 | 0.420 |
| Bat presence | 1 | 0.024 | 0.877 | 0.394 | 0.530 | 0.018 | 0.892 | 0.065 | 0.798 | 0.001 | 0.979 | |
| Model 2 | Geographic region | 2 | 2.047 | 0.359 | 4.146 | 0.126 | 0.314 | 0.855 | 3.776 | 0.151 | 1.729 | 0.421 |
| Guano amount | 3 | 0.586 | 0.900 | 3.021 | 0.388 | 0.609 | 0.894 | 0.277 | 0.964 | 0.339 | 0.953 | |
| Eigenvalues | Species-Environment Correlations | Cumulative Percentage Variance | Sum of All Eigenvalues | Sum of All Canonical Eigenvalues | ||
|---|---|---|---|---|---|---|
| of Species Data | of Species—Environment Relation | |||||
| Axis 1 | 0.642 | 0.917 | 8.9 | 31 | 7.195 | 2.070 |
| Axis 2 | 0.589 | 0.831 | 17.1 | 59.5 | ||
| Axis 3 | 0.448 | 0.839 | 23.3 | 81.1 | ||
| Axis 4 | 0.214 | 0.725 | 26.3 | 91.4 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavish-Regev, E.; Aharon, S.; Armiach Steinpress, I.; Seifan, M.; Lubin, Y. A Primer on Spider Assemblages in Levantine Caves: The Neglected Subterranean Habitats of the Levant—A Biodiversity Mine. Diversity 2021, 13, 179. https://doi.org/10.3390/d13050179
Gavish-Regev E, Aharon S, Armiach Steinpress I, Seifan M, Lubin Y. A Primer on Spider Assemblages in Levantine Caves: The Neglected Subterranean Habitats of the Levant—A Biodiversity Mine. Diversity. 2021; 13(5):179. https://doi.org/10.3390/d13050179
Chicago/Turabian StyleGavish-Regev, Efrat, Shlomi Aharon, Igor Armiach Steinpress, Merav Seifan, and Yael Lubin. 2021. "A Primer on Spider Assemblages in Levantine Caves: The Neglected Subterranean Habitats of the Levant—A Biodiversity Mine" Diversity 13, no. 5: 179. https://doi.org/10.3390/d13050179
APA StyleGavish-Regev, E., Aharon, S., Armiach Steinpress, I., Seifan, M., & Lubin, Y. (2021). A Primer on Spider Assemblages in Levantine Caves: The Neglected Subterranean Habitats of the Levant—A Biodiversity Mine. Diversity, 13(5), 179. https://doi.org/10.3390/d13050179