The Introduction of the Asian Red Algae Melanothamnus japonicus (Harvey) Díaz-Tapia & Maggs in Peru as a Means to Adopt Management Strategies to Reduce Invasive Non-Indigenous Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Analysis
2.2. Phylogenetic Analysis
3. Results
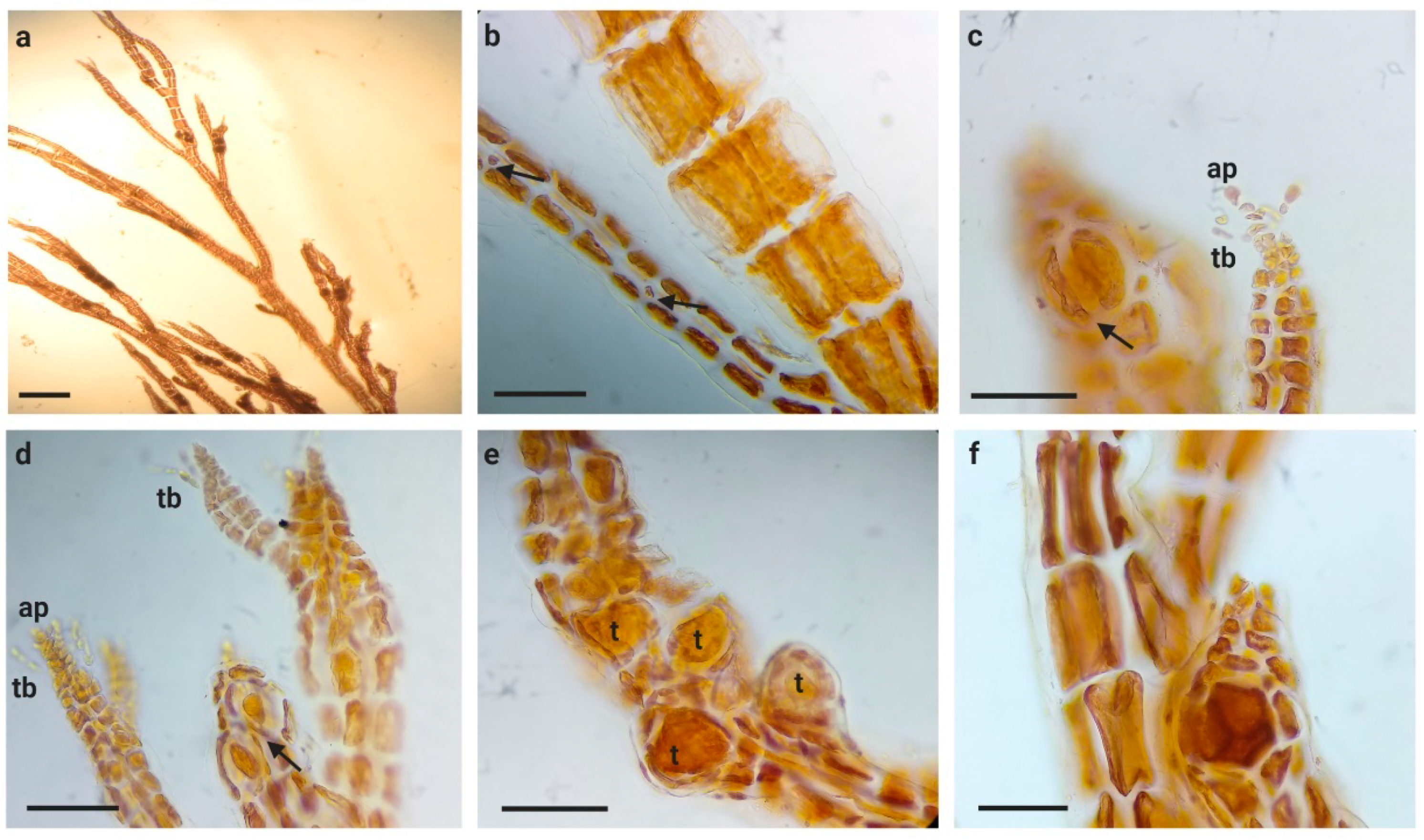
3.1. Morphological Observations
3.2. Molecular Phylogeny
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNEP. United Nations Environment Programme. Invasive Alien Speceis—A Growing Threat in Regional Seas Alien Species. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/13623/invasive_alien_brochure.pdf?sequence=1&%3BisAllowed= (accessed on 18 December 2020).
- ICES. Code of Practice on the Introductions and Transfers of Marine Organisms 2005. Technical Report. Available online: https://www.nobanis.org/globalassets/ices-code-of-practice.pdf (accessed on 18 November 2020).
- Genovesi, P.; Shine, C. European strategy on invasive alien species. In Convention on the Conservation of European Wildlife and Natural Habitats, 1st ed.; Council of Europe Publishing: Strasbourg, France, 2004; 68p. [Google Scholar]
- IUCN. Guidelines for the Prevention of Biodiversity Loss Caused by Alien Invasive Species. Prepared by the IUCN/SSC Invasive Species Specialist Group (ISSG) and Approved by the 51st Meeting of the IUCN Council, Gland, Switzerlan, February 2000. Available online: https://portals.iucn.org/library/efiles/documents/Rep-2000-052.pdf (accessed on 18 November 2020).
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef]
- Pierucci, A.; De La Fuente, G.; Cannas, R.; Chiantore, M. A new record of the invasive seaweed Caulerpa cylindracea Sonder in the South Adriatic Sea. Heliyon 2019, 5, e02449. [Google Scholar] [CrossRef]
- Dijkstra, J.A.; Harris, L.G.; Mello, K.; Litterer, A.; Wells, C.; Ware, C. Invasive seaweeds transform habitat structure and increase biodiversity of associated species. J. Ecol. 2017, 105, 1668–1678. [Google Scholar] [CrossRef]
- Kim, M.-S.; Yang, E.C.; Mansilla, A.; Boo, S.M. Recent introduction of Polysiphonia morrowii (Ceramiales, Rhodophyta) to Punta Arenas, Chile. Bot. Mar. 2004, 47, 389–394. [Google Scholar] [CrossRef]
- World Register of Introduced Marine Species. Available online: http://www.marinespecies.org/introduced/ (accessed on 18 November 2020).
- Manghisi, A.; Armeli-Minicante, S.; Bertuccio, C.; Morabito, M.; Torricelli, P.; Genovese, G. A cryptic alien seaweed spreading in Mediterranean coastal lagoons. Transit. Waters Bull. 2011, 5, 1–7. [Google Scholar] [CrossRef]
- Piñeiro-Corbeira, C.; Verbruggen, H.; Díaz-Tapia, P. Molecular survey of the red algal family Rhodomelaceae (Ceramiales, Rhodophyta) in Australia reveals new introduced species. Environ. Boil. Fishes 2020, 32, 2535–2547. [Google Scholar] [CrossRef]
- Bolton, J.J.; Andreakis, N.; Anderson, R.J. Molecular evidence for three separate cryptic introductions of the red seaweedAsparagopsis(Bonnemaisoniales, Rhodophyta) in South Africa. Afr. J. Mar. Sci. 2011, 33, 263–271. [Google Scholar] [CrossRef]
- Morais, P.; Reichard, M. Cryptic invasions: A review. Sci. Total. Environ. 2018, 613, 1438–1448. [Google Scholar] [CrossRef]
- Williams, S.L.; Smith, J.E. A Global Review of the Distribution, Taxonomy, and Impacts of Introduced Seaweeds. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 327–359. [Google Scholar] [CrossRef]
- McIvor, L.; Maggs, C.A.; Provan, J.; Stanhope, M.J. rbcL sequences reveal multiple cryptic introductions of the Japanese red alga Polysiphonia harveyi. Mol. Ecol. 2001, 10, 911–919. [Google Scholar] [CrossRef]
- Galil, B.S.; Occhipinti-Ambrogi, A.; Gollasch, S. Chapter 4. Biodiversity impacts of species introductions via marine vessels. In Maritime Traffic Effects on Biodiversity in the Mediterranean Sea. Review of Impacts, Priority Areas and Mitigation Measures, 1st ed.; Abdulla, A., Linden, O., Eds.; IUCN Centre for Mediterranean Cooperation: Malaga, Spain, 2008; Volume 1, pp. 118–150. [Google Scholar]
- Mineur, F.; Johnson, M.P.; Maggs, C.A. Macroalgal Introductions by Hull Fouling on Recreational Vessels: Seaweeds and Sailors. Environ. Manag. 2008, 42, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.A.; Buosi, A.; Juhmani, A.-S.F.; Sfriso, A. Shellfish import and hull fouling as vectors for new red algal introductions in the Venice Lagoon. Estuar. Coast. Shelf Sci. 2018, 215, 30–38. [Google Scholar] [CrossRef]
- Cianciola, E.N.; Popolizio, T.R.; Schneider, C.W.; Lane, C.E. Using Molecular-Assisted Alpha Taxonomy to Better Understand Red Algal Biodiversity in Bermuda. Diversity 2010, 2, 946–958. [Google Scholar] [CrossRef]
- Rindi, F.; Gavio, B.; Díaz-Tapia, P.; Di Camillo, C.G.; Romagnoli, T. Long-term changes in the benthic macroalgal flora of a coastal area affected by urban impacts (Conero Riviera, Mediterranean Sea). Biodivers. Conserv. 2020, 29, 2275–2295. [Google Scholar] [CrossRef]
- Sfriso, A. Invasion of alien macroalgae in the Venice Lagoon, a pest or a resource? Aquat. Invasions 2020, 15, 245–270. [Google Scholar] [CrossRef]
- Savoie, A.M.; Saunders, G.W. Evidence for the introduction of the Asian red algaNeosiphonia japonicaand its introgression withNeosiphonia harveyi(Ceramiales, Rhodophyta) in the Northwest Atlantic. Mol. Ecol. 2015, 24, 5927–5937. [Google Scholar] [CrossRef]
- Bustamante, D.E.; Won, B.Y.; Cho, T.O. Neosiphonia ramirezii sp. nov. (Rhodomelaceae, Rhodophyta) from Peru. ALGAE 2013, 28, 73–82. [Google Scholar] [CrossRef][Green Version]
- Bustamante, D.E.; Won, B.Y.; Ramírez, M.E.; Cho, T.O. Neosiphonia peruviensis sp. nov. (Rhodomelaceae, Rhodophyta) from the Pacific coast of South America. Bot. Mar. 2012, 55, 359. [Google Scholar] [CrossRef]
- Avila-Peltroche, J.; Padilla-Vallejos, J. The seaweed resources of Peru. Bot. Mar. 2020, 63, 381–394. [Google Scholar] [CrossRef]
- Ramírez, M.E.; Santelices, B. Catálogo de las Algas Marinas Bentónicas de la Costa Temperada del Pacífico de Sudamérica; Pontificia Universidad Católica de Chile: Santiago, Chile, 1991. [Google Scholar]
- Calderon, M.S.; Boo, S.M. A new genus Phyllophorella gen. nov. (Phyllophoraceae, Rhodophyta) from central Peru, including Phyllophorella peruviana comb. nov., Phyllophorella Humboldtiana sp. nov., and Phyllophorella Limaensis sp. nov. Bot. Mar. 2016, 59, 339–352. [Google Scholar] [CrossRef]
- Calderon, M.S.; Boo, S.M. Phylogeny of Phyllophoraceae (Rhodophyta, Gigartinales) reveals Asterfilopsis gen. nov. from the Southern Hemisphere. Phycologia 2016, 55, 543–554. [Google Scholar] [CrossRef]
- Calderon, M.S.; Boo, S.M. The Phyllophoraceae (Gigartinales, Rhodophyta) from Peru with descriptions of Acletoa tarazonae gen. & sp. nov. and Gymnogongrus caespitosus sp. nov. Phycologia 2017, 56, 686–696. [Google Scholar] [CrossRef]
- Bustamante, D.E.; Won, B.Y.; Lindstrom, S.C.; Cho, T.O. The new genusSymphyocladiella gen. nov. (Ceramiales, Rhodophyta) based onS. bartlingiana comb. nov. from the Pacific Ocean. Phycologia 2019, 58, 9–17. [Google Scholar] [CrossRef]
- Ramírez, M.E.; Contreras-Porcia, L.; Guillemin, M.-L.; Brodie, J.; Valdivia, C.; Flores-Molina, M.R.; Núñez, A.; Contador, C.B.; Lovazzano, C. Pyropia orbicularis sp. nov. (Rhodophyta, Bangiaceae) based on a population previously known as Porphyra columbina from the central coast of Chile. Phytotaxa 2014, 158, 133. [Google Scholar] [CrossRef]
- Miloslavich, P.; Klein, E.; Díaz, J.M.; Hernandez, C.E.; Bigatti, G.; Campos, L.; Artigas, F.; Castillo, J.; Penchaszadeh, P.E.; Neill, P.E.; et al. Marine Biodiversity in the Atlantic and Pacific Coasts of South America: Knowledge and Gaps. PLoS ONE 2011, 6, e14631. [Google Scholar] [CrossRef]
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P.; et al. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 2016, 7, 12485. [Google Scholar] [CrossRef]
- Barrios, J.; Bolaños, J.; López, R. Blanqueamiento de arrecifes coralinos por la invasión de Kappaphycus alvarezii (Rhodo-phyta) en Isla Cubagua, Estado Nueva Esparta, Venezuela. Boletín Inst. Ocean. Venez. 2007, 46, 147–152. [Google Scholar]
- Cebrian, E.; Linares, C.; Marschal, C.; Garrabou, J. Exploring the effects of invasive algae on the persistence of gorgonian populations. Biol. Invasions 2012, 14, 2647–2656. [Google Scholar] [CrossRef]
- Smith, J.E.; Hunter, C.L.; Smith, C.M. Distribution and Reproductive Characteristics of Nonindigenous and Invasive Marine Algae in the Hawaiian Islands. Pac. Sci. 2002, 56, 299–315. [Google Scholar] [CrossRef]
- Produce. Information Obtained by Requesting “Access to the Information”, an Online Service Implemented by the Min-Isterio de la Producción from the Peruvian Government. Available online: https://www.gob.pe/produce (accessed on 12 December 2020).
- Aroni, E. Global Fishing Watch Holds Workshops with Authorities in Peru’s Three Main Fishing Ports. Available online: https://globalfishingwatch.org/fisheries/workshops-peru-ports/#:~:text=Global Fishing Watch holds workshops with au-thorities in Peru’s three main fishing ports,-By Eloy Aroni&text=Global Fishing Watch recently met,Paita%2C Chimbote and Callao (accessed on 18 November 2020).
- Xu, J.; Wickramarathne, T.; Grey, E.; Steinhaeuser, K.; Keller, R.; Drake, J.; Chawla, N.; Lodge, D.M. Patterns of ship-borne species spread: A clustering approach for risk assessment and management of non-indigenous species spread. arXiv 2014, arXiv:1401.5407. [Google Scholar]
- Kützing, F.T. Phycologia Generalis: Oder, Anatomie, Physiologie und Systemkunde der Tange/Bearb. von Friedrich Traugott Kützing. Mit 80 Farbig Gedruckten Tafeln, Gezeichnet und Gravirt vom Verfasser; Milner and Sowerby: London, UK, 1843; p. 143. [Google Scholar]
- Yoon, H.Y. A Taxonomic study of genus Polysiphonia (Rhodophyta) from Korea. Algae 1986, 1, 3–86. [Google Scholar]
- Howe, M.A. The marine Algae of Peru; Press of the New era Printing Company: New York, NY, USA, 1914; Volume 15, 330p. [Google Scholar]
- Riosmena-Rodriguez, R.; Siqueiros-Beltrones, D.A. Morphology and distribution of Corallina vancouverensis (Corallinales, Rhodophyta) in Northwest Mexico. Ciencias Mar. 1995, 21, 187–199. [Google Scholar] [CrossRef]
- Walker, R.H.; Brodie, J.; Russell, S.; Irvine, L.M.; Orfanidis, S. Biodiversity of Coralline Algae in the Northeastern Atlantic Including Corallina Caespitosa sp. nov. (corallinoideae, rhodophyta). J. Phycol. 2009, 45, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Calderon, M.; Ramírez, M.E.; Bustamante, D. Notas sobre tres especies de Gigartinaceae (Rhodophyta) del litoral peruano. Rev. Peru. Biol. 2011, 17, 115–121. [Google Scholar] [CrossRef]
- Calderon, M.S.; Boo, G.H.; Boo, S.M. Morphology and phylogeny of Ramirezia osornoensis gen. & sp. nov. and Phyllymenia acletoi sp. nov. (Halymeniales, Rhodophyta) from South America. Phycologia 2014, 53, 23–36. [Google Scholar] [CrossRef]
- Calderon, M.S.; Boo, G.H.; Boo, S.M. Neorubra decipiens gen. & comb. nov. and Phyllymenia lancifolia comb. nov. (Halymeniales, Rhodophyta) from South America. Phycologia 2014, 53, 409–422. [Google Scholar] [CrossRef]
- Bustamante, D.E.; Won, B.Y.; Cho, T.O. Morphology and phylogeny of Pterosiphonia dendroidea (Rhodomelaceae, Ceramiales) described as Pterosiphonia tanakae from Japan. Bot. Mar. 2016, 59, 353–361. [Google Scholar] [CrossRef]
- Zuccarello, G.C.; Lokhorst, G.M. Molecular phylogeny of the genus Tribonema (Xanthophyceae) using rbcL gene sequence data: Monophyly of morphologically simple algal species. Phycologia 2005, 44, 384–392. [Google Scholar] [CrossRef]
- Tan, J.; Lim, P.-E.; Phang, S.-M.; Hong, D.D.; Sunarpi, H.; Hurtado, A.Q. Assessment of Four Molecular Markers as Potential DNA Barcodes for Red Algae Kappaphycus Doty and Eucheuma J. Agardh (Solieriaceae, Rhodophyta). PLoS ONE 2012, 7, e52905. [Google Scholar] [CrossRef]
- Samarakoon, T.; Wang, S.Y.; Alford, M.H. Enhancing PCR Amplification of DNA from Recalcitrant Plant Specimens Using a Trehalose-Based Additive. Appl. Plant Sci. 2013, 1, 1200236. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Stoeckle, M.Y.; Kerr, K.C.R. Frequency Matrix Approach Demonstrates High Sequence Quality in Avian BARCODEs and Highlights Cryptic Pseudogenes. PLoS ONE 2012, 7, e43992. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A Rapid Bootstrap Algorithm for the RAxML Web Servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut, A.; Suchard, M.A.; Xie, D.; Drummond, A.J. Tracer v1.6. 2014. Available online: http://beast.bio.ed.ac.uk/Tracer (accessed on 12 March 2021).
- Masuda, M.; Kudo, T.; Kawaguchi, S.; Guiry, M.D. Lectotypification of some marine red algae described by Harvey, W.H. from Japan. Phycol. Res. 1995, 43, 191–202. [Google Scholar] [CrossRef]
- Kudo, T.; Masuda, M. A taxonomic study of Polysiphonia japonica Harvey and P. akkeshiensis Segi (Rhodophyta). Jpn. J. Phycol. 1986, 34, 293–310. [Google Scholar]
- Miura, O. Molecular genetic approaches to elucidate the ecological and evolutionary issues associated with biological invasions. Ecol. Res. 2007, 22, 876–883. [Google Scholar] [CrossRef]
- Smith, D.R. Mutation Rates in Plastid Genomes: They Are Lower than You Might Think. Genome Biol. Evol. 2015, 7, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Hua, J.; Lee, R.W.; Keeling, P.J. Relative rates of evolution among the three genetic compartments of the red alga Porphyra differ from those of green plants and do not correlate with genome architecture. Mol. PhyloGenet. Evol. 2012, 65, 339–344. [Google Scholar] [CrossRef]
- Robba, L.; Russell, S.J.; Barker, G.L.; Brodie, J. Assessing the use of the mitochondrial cox1 marker for use in DNA barcoding of red algae (Rhodophyta). Am. J. Bot. 2006, 93, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Geburzi, J.C.; McCarthy, M.L. How Do They Do It?—Understanding the Success of Marine Invasive Species. In Youmares 8—Oceans Across Boundaries: Learning from Each Other; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 109–124. [Google Scholar]
- Ardura, A.; Borrell, Y.J.; Fernández, S.; Arenales, M.G.; Martínez, J.L.; Garcia-Vazquez, E. Nuisance Algae in Ballast Water Facing International Conventions. Insights from DNA Metabarcoding in Ships Arriving in Bay of Biscay. Water 2020, 12, 2168. [Google Scholar] [CrossRef]
- Zaiko, A.; Martinez, J.L.; Schmidt-Petersen, J.; Ribicic, D.; Samuiloviene, A.; Garcia-Vazquez, E. Metabarcoding approach for the ballast water surveillance—An advantageous solution or an awkward challenge? Mar. Pollut. Bull. 2015, 92, 25–34. [Google Scholar] [CrossRef]
- Santelices, B.; Aedo, D.; Hoffmann, A. Banks of microscopic forms and survival to darkness of propagules and microscopic stages of macroalgae. Rev. Chil. Hist. Nat. 2002, 75, 547–555. [Google Scholar] [CrossRef]
- Godwin, L.S. Hull Fouling of Maritime Vessels as a Pathway for Marine Species Invasions to the Hawaiian Islands. Biofouling 2003, 19, 123–131. [Google Scholar] [CrossRef]
- Mineur, F.; Johnson, M.P.; Maggs, C.A.; Stegenga, H. Hull fouling on commercial ships as a vector of macroalgal introduction. Mar. Biol. 2006, 151, 1299–1307. [Google Scholar] [CrossRef]
- Gollasch, S. The Importance of Ship Hull Fouling as a Vector of Species Introductions into the North Sea. Biofouling 2002, 18, 105–121. [Google Scholar] [CrossRef]
- Mineur, F.; Belsher, T.; Johnson, M.P.; Maggs, C.A.; Verlaque, M. Experimental assessment of oyster transfers as a vector for macroalgal introductions. Biol. Conserv. 2007, 137, 237–247. [Google Scholar] [CrossRef]
- Raffo, M.P.; Geoffroy, A.; Destombe, C.; Schwindt, E. First record of the invasive red alga Polysiphonia morrowii Harvey (Rhodomelaceae, Rhodophyta) on the Patagonian shores of the Southwestern Atlantic. Bot. Mar. 2014, 57, 21–26. [Google Scholar] [CrossRef]
- IMARPE. Adaptación y reproducción de la ostra japonesa Crassostrea gigas en ambiente controlado. Informe Preliminar. 1995. Available online: http://biblioimarpe.imarpe.gob.pe/bitstream/123456789/892/1/IP13.pdf (accessed on 16 November 2020).
- IMARPE. Informe de las Condiciones Oceanográficas y Biológico-Pesqueras Abril 2020. Available online: http://www.imarpe.pe/imarpe/archivos/informes/imarpe_informe_gti_abril_2020.pdf (accessed on 25 November 2020).
- Seebens, H.; Schwartz, N.; Schupp, P.J.; Blasius, B. Predicting the spread of marine species introduced by global shipping. Proc. Natl. Acad. Sci. USA 2016, 113, 5646–5651. [Google Scholar] [CrossRef]
- Sakai, A.K.; Allendorf, F.W.; Holt, J.S.; Lodge, D.M.; Molofsky, J.; With, K.A.; Baughman, S.; Cabin, R.J.; Cohen, J.E.; Ellstrand, N.C.; et al. The Population Biology of Invasive Species. Annu. Rev. Ecol. Syst. 2001, 32, 305–332. [Google Scholar] [CrossRef]
- Daehler, C.C.; Strong, D.R. Hybridization between introduced smooth cordgrass (Spartina alterniflora; Poaceae) and native California cordgrass (S. foliosa) in San Francisco Bay, California, USA. Am. J. Bot. 1997, 84, 607–611. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Wallentinus, I.; Zenetos, A.; Leppäkoski, E.; Çinar, M.E.; Oztürk, B.; Grabowski, M.; Golani, D.; Cardoso, A.C. Impacts of invasive alien marine species on ecosystem services and biodiversity: A pan-European review. Aquat. Invasions 2014, 9, 391–423. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Thuiller, W.; Miaud, C. Prediction and validation of the potential global distribution of a problematic alien invasive species—The American bullfrog. Divers. Distrib. 2007, 13, 476–485. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, M.D.; Base, A. World-wide electronic publication, National University of Ireland, Galway. Available online: https://www.algaebase.org/search/genus/detail/?genus_id=37461&-session=abv4:AC1F06401b4682AB03gHD18097D0 (accessed on 17 November 2020).
- Global Biodiversity Information Facility. Available online: www.gbif.org. (accessed on 3 November 2010).
- DICAPI. Dirección General de Capitanías y Guardacostas. Informe para sustentar la adhesión del Perú al “Convenio internacional para el control y la gestión del agua de lastre y los sedimentos de los buques, 2004.” Convenio de Agua de Lastre; Ministry of Foreign Affairs: Lima, Peru, 2015; pp. 30–104.
- Castilla, J.C.; Neill, P.E. Marine Bioinvasions in the Southeastern Pacific: Status, Ecology, Economic Impacts, Conservation and Management. In Biogeography of Mycorrhizal Symbiosis; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2008; Volume 204, pp. 439–457. [Google Scholar]
- Smith, D.R.; Arrigo, K.R.; Alderkamp, A.-C.; Allen, A.E. Massive difference in synonymous substitution rates among mitochondrial, plastid, and nuclear genes of Phaeocystis algae. Mol. Phylogenet. Evol. 2014, 71, 36–40. [Google Scholar] [CrossRef]
- Yow, Y.-Y.; Lim, P.-E.; Phang, S.-M. Assessing the use of mitochondrial cox1 gene and cox2-3 spacer for genetic diversity study of Malaysian Gracilaria changii (Gracilariaceae, Rhodophyta) from Peninsular Malaysia. Environ. Boil. Fishes 2013, 25, 831–838. [Google Scholar] [CrossRef]
- International Convention for the Control and Management of Ships’ Ballast Water and Sediments (BWM). Available online: https://www.imo.org/en/About/Conventions/Pages/International-Convention-for-the-Control-and-Management-of-Ships%27-Ballast-Water-and-Sediments-(BWM).aspx (accessed on 23 December 2020).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Velásquez, J.J.; Reyes-Flores, L.E.; Yzásiga-Barrera, C.; Zelada-Mázmela, E. The Introduction of the Asian Red Algae Melanothamnus japonicus (Harvey) Díaz-Tapia & Maggs in Peru as a Means to Adopt Management Strategies to Reduce Invasive Non-Indigenous Species. Diversity 2021, 13, 176. https://doi.org/10.3390/d13050176
Sánchez-Velásquez JJ, Reyes-Flores LE, Yzásiga-Barrera C, Zelada-Mázmela E. The Introduction of the Asian Red Algae Melanothamnus japonicus (Harvey) Díaz-Tapia & Maggs in Peru as a Means to Adopt Management Strategies to Reduce Invasive Non-Indigenous Species. Diversity. 2021; 13(5):176. https://doi.org/10.3390/d13050176
Chicago/Turabian StyleSánchez-Velásquez, Julissa J., Lorenzo E. Reyes-Flores, Carmen Yzásiga-Barrera, and Eliana Zelada-Mázmela. 2021. "The Introduction of the Asian Red Algae Melanothamnus japonicus (Harvey) Díaz-Tapia & Maggs in Peru as a Means to Adopt Management Strategies to Reduce Invasive Non-Indigenous Species" Diversity 13, no. 5: 176. https://doi.org/10.3390/d13050176
APA StyleSánchez-Velásquez, J. J., Reyes-Flores, L. E., Yzásiga-Barrera, C., & Zelada-Mázmela, E. (2021). The Introduction of the Asian Red Algae Melanothamnus japonicus (Harvey) Díaz-Tapia & Maggs in Peru as a Means to Adopt Management Strategies to Reduce Invasive Non-Indigenous Species. Diversity, 13(5), 176. https://doi.org/10.3390/d13050176







