Predation and Scavenging in the City: A Review of Spatio-Temporal Trends in Research
Abstract
1. Introduction
2. Materials and Methods
2.1. Systematic Literature Review
2.2. Data Extraction
2.3. Statistical Analyses
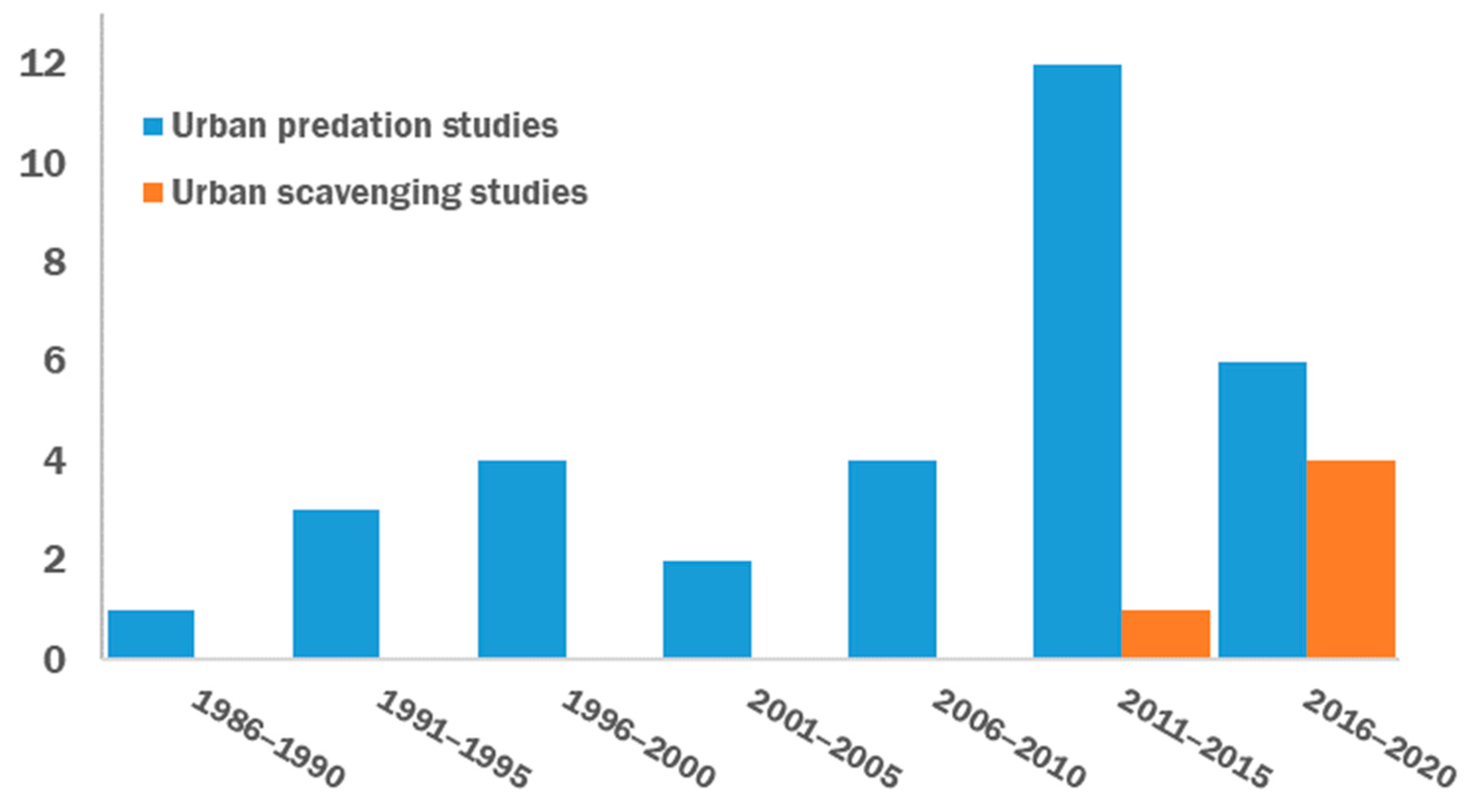
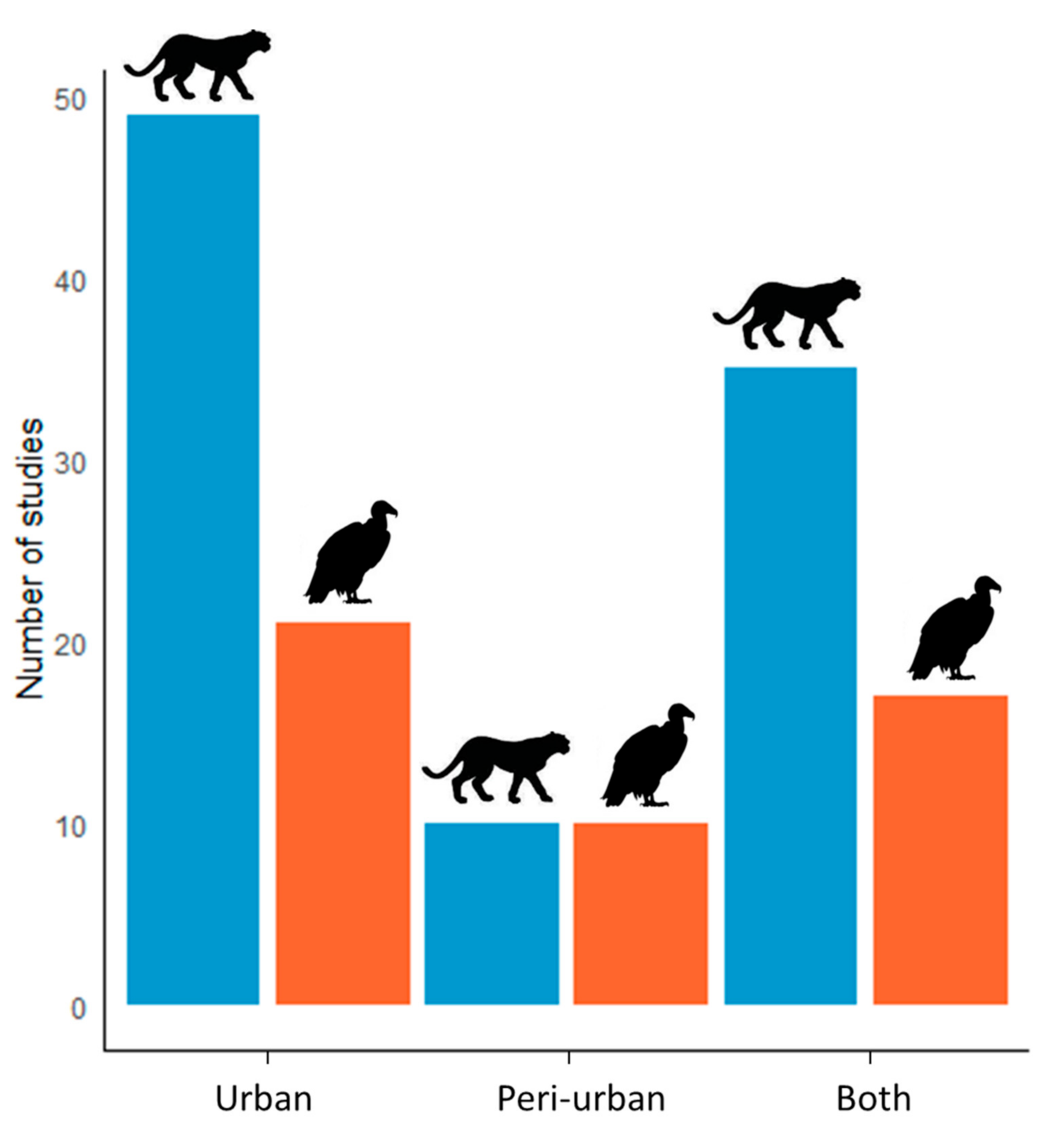
3. Results
4. Discussion
4.1. Temporal Trends of Predation/Scavenging Studies in Urban Ecology
4.2. The Bias Extant in Urban Predation and Scavenging Studies across Regions
4.3. Predator and Scavenger Species in Urban Environments
4.4. Conservation Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dearborn, D.C.; Kark, S. Motivations for conserving urban biodiversity. Conserv. Biol. 2010, 24, 432–440. [Google Scholar] [CrossRef]
- Aronson, M.F.; La Sorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, N.S.; Cilliers, S.; Clarkson, B.; et al. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B 2014, 281, 20133330. [Google Scholar] [CrossRef]
- Braaker, S.; Ghazoul, J.; Obrist, M.K.; Moretti, M. Habitat connectivity shapes urban arthropod communities: The key role of green roofs. Ecology 2014, 95, 1010–1021. [Google Scholar] [CrossRef]
- Threlfall, C.G.; Walker, K.; Williams, N.S.; Hahs, A.K.; Mata, L.; Stork, N.; Livesley, S.J. The conservation value of urban green space habitats for Australian native bee communities. Biol. Conserv. 2015, 187, 240–248. [Google Scholar] [CrossRef]
- Clergeau, P.; Croci, S.; Jokimäki, J.; Kaisanlahti-Jokimäki, M.L.; Dinetti, M. Avifauna homogenisation by urbanisation: Analysis at different European latitudes. Biol. Conserv. 2006, 127, 336–344. [Google Scholar] [CrossRef]
- Ortega-Álvarez, R.; MacGregor-Fors, I. Living in the big city: Effects of urban land-use on bird community structure, diversity, and composition. Landsc. Urban Plan. 2009, 90, 189–195. [Google Scholar] [CrossRef]
- Faeth, S.H.; Bang, C.; Saari, S. Urban biodiversity: Patterns and mechanisms. Ann. N. Y. Acad. Sci. 2011, 1223, 69–81. [Google Scholar] [CrossRef]
- Rebolo-Ifrán, N.; Tella, J.L.; Carrete, M. Urban conservation hotspots: Predation release allows the grassland-specialist burrowing owl to perform better in the city. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Recio, M.R.; Payne, K.; Seddon, P.J. Emblematic forest dwellers reintroduced into cities: Resource selection by translocated juvenile kaka. Curr. Zool. 2016, 62, 15–22. [Google Scholar] [CrossRef]
- Luna, Á.; Romero-Vidal, P.; Hiraldo, F.; Tella, J.L. Cities may save some threatened species but not their ecological functions. PeerJ 2018, 6, e4908. [Google Scholar] [CrossRef]
- Soanes, K.; Lentini, P.E. When cities are the last chance for saving species. Front. Ecol. Environ. 2019, 17, 225–231. [Google Scholar] [CrossRef]
- Thomas, J.P.; Jung, T.S. Life in a northern town: Rural villages in the boreal forest are islands of habitat for an endangered bat. Ecosphere 2019, 10, e02563. [Google Scholar] [CrossRef]
- Kinzig, A.P.; Warren, P.; Martin, C.; Hope, D.; Katti, M. The effects of human socioeconomic status and cultural characteristics on urban patterns of biodiversity. Ecol. Soc. 2005, 10, 1–13. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Aronson, M.F.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; MacIvor, J.S. Biodiversity in the city: Fundamental questions for understanding the ecology of urban green spaces for biodiversity conservation. BioScience 2017, 67, 799–807. [Google Scholar] [CrossRef]
- Cardoso, G.C.; Klingbeil, B.T.; La Sorte, F.A.; Lepczyk, C.A.; Fink, D.; Flather, C.H. Exposure to noise pollution across North American passerines supports the noise filter hypothesis. Glob. Ecol. Biogeogr. 2020, 29, 1430–1434. [Google Scholar] [CrossRef]
- Gaston, K.J.; Visser, M.E.; Hölker, F. The biological impacts of artificial light at night: The research challenge. Philos. Trans. R. Soc. B 2015, 370, 20140133. [Google Scholar] [CrossRef]
- Ciach, M.; Fröhlich, A. Habitat preferences of the Syrian Woodpecker Dendrocopos syriacus in urban environments: An ambiguous effect of pollution. Bird Study 2013, 60, 491–499. [Google Scholar] [CrossRef]
- Foltz, S.L.; Ross, A.E.; Laing, B.T.; Rock, R.P.; Battle, K.E.; Moore, I.T. Get off my lawn: Increased aggression in urban song sparrows is related to resource availability. Behav. Ecol. 2015, 26, 1548–1557. [Google Scholar] [CrossRef]
- Kark, S.; Iwaniuk, A.; Schalimtzek, A.; Banker, E. Living in the city: Can anyone become an ’urban exploiter’? J. Biogeogr. 2007, 34, 638–651. [Google Scholar] [CrossRef]
- Møller, A.P. Successful city dwellers: A comparative study of the ecological characteristics of urban birds in the Western Palearctic. Oecologia 2009, 159, 849–858. [Google Scholar] [CrossRef]
- Evans, K.L.; Chamberlain, D.E.; Hatchwell, B.J.; Gregory, R.D.; Gaston, K.J. What makes an urban bird? Glob. Chang. Biol. 2011, 7, 32–44. [Google Scholar] [CrossRef]
- Leveau, L.M. Bird traits in urban–rural gradients: How many functional groups are there? J. Ornithol. 2013, 154, 655–662. [Google Scholar] [CrossRef]
- Baker, P.J.; Ansell, R.J.; Dodds, P.A.A.; Webber, C.E.; Harris, S. Factors affecting the distribution of small mammals in an urban area. Mammal Rev. 2003, 33, 95–100. [Google Scholar] [CrossRef]
- Santini, L.; González-Suárez, M.; Russo, D.; Gonzalez-Voyer, A.; von Hardenberg, A.; Ancillotto, L. One strategy does not fit all: Determinants of urban adaptation in mammals. Ecol. Lett. 2019, 22, 365–376. [Google Scholar] [CrossRef]
- Lowry, H.; Lill, A.; Wong, B.B. Behavioural responses of wildlife to urban environments. Biol. Rev. 2013, 88, 537–549. [Google Scholar] [CrossRef]
- Murray, M.; Cembrowski, A.; Latham, A.D.M.; Lukasik, V.M.; Pruss, S.; St Clair, C.C. Greater consumption of protein-poor anthropogenic food by urban relative to rural coyotes increases diet breadth and potential for human–wildlife conflict. Ecography 2015, 38, 1235–1242. [Google Scholar] [CrossRef]
- de Araujo, G.M.; Peres, C.A.; Baccaro, F.B.; Guerta, R.S. Urban waste disposal explains the distribution of Black Vultures (Coragyps atratus) in an Amazonian metropolis: Management implications for birdstrikes and urban planning. PeerJ 2018, 6, e5491. [Google Scholar] [CrossRef]
- Houston, D.C.; Mee, A.; McGrady, M. Why do condors and vultures eat junk?: The implications for conservation. J. Raptor Res. 2007, 41, 235–238. [Google Scholar] [CrossRef]
- Oro, D.; Genovart, M.; Tavecchia, G.; Fowler, M.S.; Martínez-Abraín, A. Ecological and evolutionary implications of food subsidies from humans. Ecol. Lett. 2013, 16, 1501–1514. [Google Scholar] [CrossRef]
- Plaza, P.I.; Lambertucci, S.A. How are garbage dumps impacting vertebrate demography, health, and conservation? Glob. Ecol. Conserv. 2017, 12, 9–20. [Google Scholar] [CrossRef]
- Hindmarch, S.; Elliott, J.E. When owls go to town: The diet of urban Barred Owls. J. Raptor. Res. 2015, 49, 66–74. [Google Scholar] [CrossRef]
- McPherson, S.C.; Brown, M.; Downs, C.T. Diet of the crowned eagle (Stephanoaetus coronatus) in an urban landscape: Potential for human-wildlife conflict? Urban Ecosyst. 2016, 19, 383–396. [Google Scholar] [CrossRef]
- Bateman, P.W.; Fleming, P.A. Big city life: Carnivores in urban environments. J. Zool. 2012, 287, 1–23. [Google Scholar] [CrossRef]
- Newsome, S.D.; Garbe, H.M.; Wilson, E.C.; Gehrt, S.D. Individual variation in anthropogenic resource use in an urban carnivore. Oecologia 2015, 178, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Bildstein, K.L.; Therrien, J.F. Urban birds of prey: A lengthy history of human-raptor cohabitation. In Urban Raptors; Island Press: Washington, DC, USA, 2018; pp. 3–17. [Google Scholar]
- Contesse, P.; Hegglin, D.; Gloor, S.; Bontadina, F.; Deplazes, P. The diet of urban foxes (Vulpes vulpes) and the availability of anthropogenic food in the city of Zurich, Switzerland. Mamm. Biol. 2004, 69, 81–95. [Google Scholar] [CrossRef]
- Kristan, W.B., III; Boarman, W.I.; Crayon, J.J. Diet composition of common ravens across the urban-wildland interface of the West Mojave Desert. Wildl. Soc. Bull. 2004, 32, 244–253. [Google Scholar] [CrossRef]
- Allen, B.L.; Carmelito, E.; Amos, M.; Goullet, M.S.; Allen, L.R.; Speed, J.; Leung, L.K.P. Diet of dingoes and other wild dogs in peri-urban areas of north-eastern Australia. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Morgan, S.A.; Hansen, C.M.; Ross, J.G.; Hickling, G.J.; Ogilvie, S.C.; Paterson, A.M. Urban cat (Felis catus) movement and predation activity associated with a wetland reserve in New Zealand. Wild. Res. 2009, 36, 574–580. [Google Scholar] [CrossRef]
- Henriques, M.; Granadeiro, J.P.; Hamilton Monteiro, A.N.; Lecoq, M.; Cardoso, P.; Regalla, A.; Catry, P. Not in wilderness: African vulture strongholds remain in areas with high human density. PLoS ONE 2018, 13, e0190594. [Google Scholar] [CrossRef]
- Larson, R.N.; Morin, D.J.; Wierzbowska, I.A.; Crooks, K.R. Food habits of coyotes, gray foxes, and bobcats in a coastal southern California urban landscape. West. N. Am. Nat. 2015, 75, 339–347. [Google Scholar] [CrossRef]
- Méndez, A.; Montalvo, T.; Aymí, R.; Carmona, M.; Figuerola, J.; Navarro, J. Adapting to urban ecosystems: Unravelling the foraging ecology of an opportunistic predator living in cities. Urban Ecosyst. 2020, 23, 1117–1126. [Google Scholar] [CrossRef]
- Sims, V.; Evans, K.L.; Newson, S.E.; Tratalos, J.A.; Gaston, K.J. Avian assemblage structure and domestic cat densities in urban environments. Divers. Distrib. 2008, 14, 387–399. [Google Scholar] [CrossRef]
- Drewitt, E.J.; Dixon, N. Diet and prey selection of urban-dwelling Peregrine Falcons in southwest England. Br. Birds 2008, 101, 58. [Google Scholar]
- Braczkowski, A.R.; O’Bryan, C.J.; Stringer, M.J.; Watson, J.E.; Possingham, H.P.; Beyer, H.L. Leopards provide public health benefits in Mumbai, India. Front. Ecol. Environ. 2018, 16, 176–182. [Google Scholar] [CrossRef]
- Shochat, E.; Warren, P.S.; Faeth, S.H.; McIntyre, N.E.; Hope, D. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. 2006, 21, 186–191. [Google Scholar] [CrossRef]
- Rodewald, A.D.; Kearns, L.J.; Shustack, D.P. Anthropogenic resource subsidies decouple predator–prey relationships. Ecol. App. 2011, 21, 936–943. [Google Scholar] [CrossRef]
- Fischer, J.D.; Cleeton, S.H.; Lyons, T.P.; Miller, J.R. Urbanization and the predation paradox: The role of trophic dynamics in structuring vertebrate communities. Bioscience 2012, 62, 809–818. [Google Scholar] [CrossRef]
- Inger, R.; Cox, D.T.; Per, E.; Norton, B.A.; Gaston, K.J. Ecological role of vertebrate scavengers in urban ecosystems in the UK. Ecol. Evol. 2016, 6, 7015–7023. [Google Scholar] [CrossRef]
- Schwartz, A.L.; Williams, H.F.; Chadwick, E.; Thomas, R.J.; Perkins, S.E. Roadkill scavenging behaviour in an urban environment. J. Urban Ecol. 2018, 4, juy006. [Google Scholar] [CrossRef]
- Plaza, P.I.; Lambertucci, S.A. More massive but potentially less healthy: Black vultures feeding in rubbish dumps differed in clinical and biochemical parameters with wild feeding birds. PeerJ 2018, 6, e4645. [Google Scholar] [CrossRef]
- Lambertucci, S.A.; Speziale, K.L.; Rogers, T.E.; Morales, J.M. How do roads affect the habitat use of an assemblage of scavenging raptors? Biodivers. Conserv. 2009, 18, 2063–2074. [Google Scholar] [CrossRef]
- Arrondo, E.; Sanz-Aguilar, A.; Pérez-García, J.M.; Cortés-Avizanda, A.; Sánchez-Zapata, J.A.; Donázar, J.A. Landscape anthropization shapes the survival of a top avian scavenger. Biodivers. Conserv. 2020, 29, 1411–1425. [Google Scholar] [CrossRef]
- Ogada, D.L.; Keesing, F.; Virani, M.Z. Dropping dead: Causes and consequences of vulture population declines worldwide. Ann. N. Y. Acad. Sci. 2012, 1249, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Buechley, E.R.; McGrady, M.J.; Çoban, E.; Şekercioğlu, Ç.H. Satellite tracking a wide-ranging endangered vulture species to target conservation actions in the Middle East and East Africa. Biodivers. Conserv. 2018, 27, 2293–2310. [Google Scholar] [CrossRef]
- Novaes, W.G.; Abreu, T.L.D.S.; Guerta, R.S. Assessing vulture translocation as a management tool to mitigate airport bird strikes. Hum.-Wildl. Interact. 2020, 14, 19. [Google Scholar]
- Parra, J.; Tellería, J.L. The increase in the Spanish population of Griffon Vulture Gyps fulvus during 1989–1999: Effects of food and nest site availability. Bird Conserv. Int. 2004, 14, 33–41. [Google Scholar] [CrossRef]
- Moleón, M.; Sanchez-Zapata, J.A.; Margalida, A.; Carrete, M.; Owen-Smith, N.; Donazar, J.A. Humans and scavengers: The evolution of interactions and ecosystem services. BioScience 2014, 64, 394–403. [Google Scholar] [CrossRef]
- McDonnell, M.J.; Niemelä, J. The history of urban ecology. Urban Ecol. 2011, 9, 34–49. [Google Scholar]
- Magle, S.B.; Hunt, V.M.; Vernon, M.; Crooks, K.R. Urban wildlife research: Past, present, and future. Biol. Conserv. 2012, 155, 23–32. [Google Scholar] [CrossRef]
- McPhearson, T.; Pickett, S.T.; Grimm, N.B.; Niemelä, J.; Alberti, M.; Elmqvist, T.; Qureshi, S. Advancing urban ecology toward a science of cities. BioScience 2016, 66, 198–212. [Google Scholar] [CrossRef]
- DeVault, T.L.; Rhodes, O.E., Jr.; Shivik, J.A. Scavenging by vertebrates: Behavioral, ecological, and evolutionary perspectives on an important energy transfer pathway in terrestrial ecosystems. Oikos 2003, 102, 225–234. [Google Scholar]
- Cincotta, R.P.; Wisnewski, J.; Engelman, R. Human population in the biodiversity hotspots. Nature 2000, 404, 990–992. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, C.J.; Sodhi, N.S.; Brook, B.W. Tropical turmoil: A biodiversity tragedy in progress. Front. Ecol. Environ. 2009, 7, 79–87. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Woodcock, P.; Macura, B.; Collins, A. Making literature reviews more reliable through application of lessons from systematic reviews. Conserv. Biol. 2015, 29, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Lozano, J.; Olszańska, A.; Morales-Reyes, Z.; Castro, A.A.; Malo, A.F.; Moleón, M.; Sánchez-Zapata, J.A.; Cortés-Avizanda, A.; von Wehrden, H.; Dorresteijn, I.; et al. Human-carnivore relations: A systematic review. Biol. Conserv. 2019, 237, 480–492. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Bayliss, H.R. Shades of grey: Two forms of grey literature important for reviews in conservation. Biol. Conserv. 2015, 191, 827–829. [Google Scholar] [CrossRef]
- Paez, A. Gray literature: An important resource in systematic reviews. J. Evid. Based. Med. 2017, 10, 233–240. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial Ecoregions of the World: A New Map of Life on Earth: A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. BioScience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Wilman, H.; Belmaker, J.; Simpson, J.; de la Rosa, C.; Rivadeneira, M.M.; Jetz, W. EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals: Ecological Archives E095-178. Ecology 2014, 95, 2027. [Google Scholar] [CrossRef]
- Shine, R.; Harlow, P.S. Ecological traits of commercially harvested water monitors, Varanus salvator, in northern Sumatra. Wildl. Res. 1998, 25, 437–447. [Google Scholar] [CrossRef]
- Guarino, F. Diet of a large carnivorous lizard, Varanus varius. Wildl. Res. 2001, 28, 627–630. [Google Scholar] [CrossRef]
- Guarino, F. Spatial ecology of a large carnivorous lizard, Varanus varius (Squamata: Varanidae). J. Zool. 2002, 258, 449–457. [Google Scholar] [CrossRef]
- Martins, F.I.; De Souza, F.L.; Da Costa, H.T.M. Feeding habits of Phrynops geoffroanus (Chelidae) in an urban river in Central Brazil. Chelonian Conserv. Biol. 2010, 9, 294–297. [Google Scholar] [CrossRef]
- Balakrishna, S.; Batabyal, A.; Thaker, M. Dining in the city: Dietary shifts in Indian rock agamas across an urban–rural landscape. J. Herpetol. 2016, 50, 423–428. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (2ª), Ecological Modelling; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Arnold, T.W. Uninformative parameters and model selection using Akaike’s Information Criterion. J. Wildl. Manag. 2010, 74, 1175–1178. [Google Scholar] [CrossRef]
- Kleiber, C.; Zeileis, A. Applied Econometrics with R; Springer: New York, NY, USA, 2008; ISBN 978-0-387-77316-2. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing (R-3.6.1); R Foundation for StatisticalComputing: Vienna, Austria, 2019. [Google Scholar]
- Sazima, I. Australian Raven (Corvus coronoides) scavenges on all five major vertebrate groups at urban Sydney, Southeast Australia. Trop. Nat. Hist. 2020, 20, 89–94. [Google Scholar]
- Clifton, B.; Jones, D.N. Finding food in a human-dominated environment: Exploring the foraging behaviour of urban Torresian Crows’ Corvus orru’. Aust. Field Ornithol. 2017, 34, 30. [Google Scholar] [CrossRef][Green Version]
- Schlacher, T.A.; Weston, M.A.; Lynn, D.; Schoeman, D.S.; Huijbers, C.M.; Olds, A.D.; Connolly, R.M. Conservation gone to the dogs: When canids rule the beach in small coastal reserves. Biodivers. Conserv. 2015, 24, 493–509. [Google Scholar] [CrossRef]
- Welti, N.; Scherler, P.; Grüebler, M.U. Carcass predictability but not domestic pet introduction affects functional response of scavenger assemblage in urbanized habitats. Funct. Ecol. 2020, 34, 265–275. [Google Scholar] [CrossRef]
- Wu, J. Urban ecology and sustainability: The state-of-the-science and future directions. Landsc. Urban Plan. 2014, 125, 209–221. [Google Scholar] [CrossRef]
- Beasley, J.C.; Olson, Z.H.; DeVault, T.L. Ecological role of vertebrate scavengers. In Carrion Ecology, Evolution and Their Applications, 1st ed.; Eric, B., Jeffery, K.T., Aaron, M.T., Eds.; CRC Press: Cleveland, OH, USA, 2015; pp. 107–127. [Google Scholar]
- King, D. The scientific impact of nations. Nature 2004, 430, 311–316. [Google Scholar] [CrossRef]
- Hoornweg, D.; Bhada-Tata, P. What a waste: A global review of solid waste management. Urban development series; knowledge papers no. 15. World Bank: Washington, DC, USA, 2012. [Google Scholar]
- Mudzengerere, F.H.; Chigwenya, A. Waste Management in Bulawayo city council in Zimbabwe: In search of Sustainable waste Management in the city. J. Sustain. Dev. Afr. 2012, 14, 228–244. [Google Scholar]
- Abay, G.Y.; Bauer, H.; Gebrihiwot, K.; Deckers, J. Peri-urban spotted hyena (Crocuta crocuta) in northern Ethiopia: Diet, economic impact, and abundance. Eur. J. Wildl. Res. 2011, 57, 759–765. [Google Scholar] [CrossRef]
- Sol, D.; Gonzalez-Lagos, C.; Moreira, D.; Maspons, J. Urbanisation tolerance and the loss of avian diversity. Ecol. Lett. 2014, 17, 942–950. [Google Scholar] [CrossRef]
- Carrete, M.; Tella, J.L. Behavioral correlations associated with fear of humans differ between rural and urban burrowing owls. Front. Ecol. Evol. 2017, 5, 54. [Google Scholar] [CrossRef]
- Beninde, J.; Veith, M.; Hochkirch, A. Biodiversity in cities needs space: A meta-analysis of factors determining intra-urban biodiversity variation. Ecol. Lett. 2015, 18, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Iserhard, C.A.; Duarte, L.; Seraphim, N.; Freitas, A.V.L. How urbanization affects multiple dimensions of biodiversity in tropical butterfly assemblages. Biodivers. Conserv. 2019, 28, 621–638. [Google Scholar] [CrossRef]
- Huijbers, C.M.; Schlacher, T.A.; Schoeman, D.S.; Weston, M.A.; Connolly, R.M. Urbanisation alters processing of marine carrion on sandy beaches. Landsc Urban Plan. 2013, 119, 1–8. [Google Scholar] [CrossRef]
- Riding, C.S.; Loss, S.R. Factors influencing experimental estimation of scavenger removal and observer detection in bird–window collision surveys. Ecol. Appl. 2018, 28, 2119–2129. [Google Scholar] [CrossRef]
- Kulabtong, S.; Mahaprom, R. Observation on food items of Asian water monitor, Varanus salvator (Laurenti, 1768) (Squamata Varanidae), in urban eco-system, Central Thailand. Biodivers. J. 2014, 6, 695–698. [Google Scholar]
- Rahman, K.M.; Khan, M.M.H.; Rakhimov, I.I. Scavenging Behavior of the Bengal Monitor (Varanus bengalensis) in Jahangirnagar University Campus, Bangladesh. J. Sci. Res. Rep. 2015, 539–550. [Google Scholar] [CrossRef]
- Karanth, K.U.; Gopal, R. An ecology-based policy framework for human-tiger coexistence in India. Conserv. Biol. Ser.-Camb. 2005, 9, 373–387. [Google Scholar]
- Meena, V.; Jhala, Y.V.; Chellam, R.; Pathak, B. Implications of diet composition of Asiatic lions for their conservation. J. Zool. 2011, 284, 60–67. [Google Scholar] [CrossRef]
- Kumbhojkar, S.; Yosef, R.; Kosicki, J.Z.; Kwiatkowska, P.K.; Tryjanowski, P. Dependence of the leopard Panthera pardus fusca in Jaipur, India, on domestic animals. Oryx 2020, 1–7. [Google Scholar] [CrossRef]
- Medina, F.M.; Bonnaud, E.; Vidal, E.; Tershy, B.R.; Zavaleta, E.S.; Josh Donlan, C.; Nogales, M. A global review of the impacts of invasive cats on island endangered vertebrates. Glob. Chang. Biol. 2011, 17, 3503–3510. [Google Scholar] [CrossRef]
- Young, J.K.; Olson, K.A.; Reading, R.P.; Amgalanbaatar, S.; Berger, J. Is wildlife going to the dogs? Impacts of feral and free-roaming dogs on wildlife populations. Bioscience 2011, 61, 125–132. [Google Scholar] [CrossRef]
- Loss, S.R.; Will, T.; Marra, P.P. The impact of free-ranging domestic cats on wildlife of the United States. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Hughes, J.; Macdonald, D.W. A review of the interactions between free-roaming domestic dogs and wildlife. Biol. Conserv. 2013, 157, 341–351. [Google Scholar] [CrossRef]
- Coronel-Arellano, H.; Rocha-Ortega, M.; Gual-Sill, F.; Martínez-Meyer, E.; Ramos-Rendón, A.K.; González-Negrete, M.; Zambrano, L. Raining feral cats and dogs? Implications for the conservation of medium-sized wild mammals in an urban protected area. Urban Ecosyst. 2020, 1–12. [Google Scholar] [CrossRef]
- Mella-Méndez, I.; Flores-Peredo, R.; Bolívar-Cimé, B.; Vázquez-Domínguez, G. Effect of free-ranging dogs and cats on medium-sized wild mammal assemblages in urban protected areas of a Mexican city. Wildl. Res. 2020, 46, 669–678. [Google Scholar] [CrossRef]
- Seymour, C.L.; Simmons, R.E.; Morling, F.; George, S.T.; Peters, K.; O’Riain, M.J. Caught on camera: The impacts of urban domestic cats on wild prey in an African city and neighbouring protected areas. Glob. Ecol. Conserv. 2020, 23, e01198. [Google Scholar] [CrossRef]
- Markandya, A.; Taylor, T.; Longo, A.; Murty, M.N.; Murty, S.; Dhavala, K. Counting the cost of vulture decline—an appraisal of the human health and other benefits of vultures in India. Ecol. Econ. 2008, 67, 194–204. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Haman, K.H.; Sizemore, G.C.; Farmer, C. Quantifying the presence of feral cat colonies and Toxoplasma gondii in relation to bird conservation areas on O’ahu, Hawai’i. Conserv. Sci. Pract. 2020, 2, e179. [Google Scholar] [CrossRef]
- Seto, K.C.; Güneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. USA 2012, 109, 16083–16088. [Google Scholar] [CrossRef]
- Chen, G.; Li, X.; Liu, X.; Chen, Y.; Liang, X.; Leng, J.; Xu, X.; Liao, W.; Qiu, Y.; Wu, Q.; et al. Global projections of future urban land expansion under shared socioeconomic pathways. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
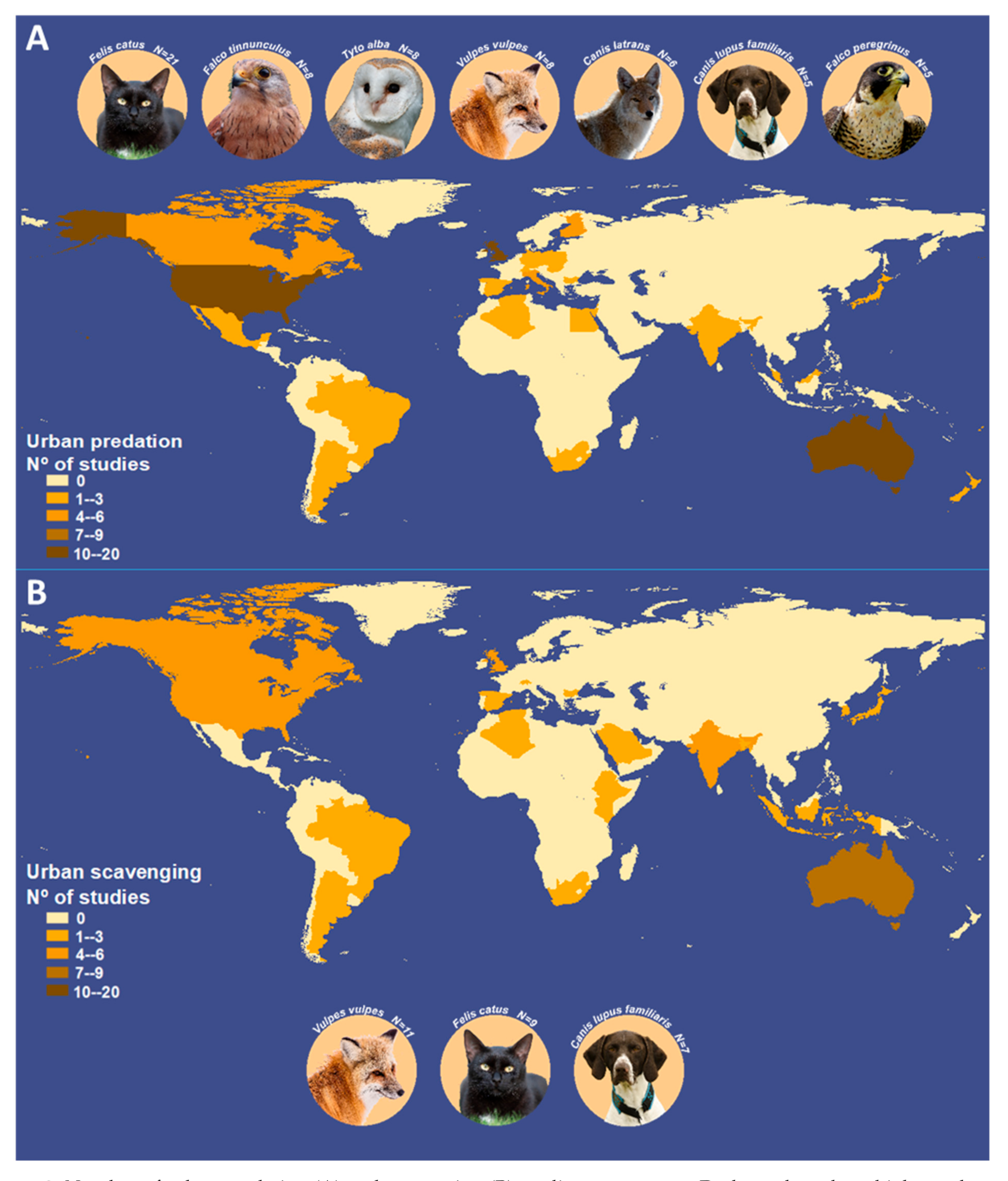
| Species | N | Species | N | Species | N |
|---|---|---|---|---|---|
| Felis catus | 21 | Buteo lineatus | 1 | Sciurus carolinensis | 1 |
| Falco tinnunculus | 8 | Buteo platypterus | 1 | Sciurus niger | 1 |
| Tyto alba | 8 | Dacelo novaeguineae | 1 | Sciurus vulgaris | 1 |
| Vulpes vulpes | 8 | Dumetella carolinensis | 1 | Tamias striatus | 1 |
| Canis familiaris | 5 | Falco columbarius | 1 | Tamiasciurus hudsonicus | 1 |
| Canis latrans | 6 | Falco sparverius | 1 | Vulpes macrotis mutica | 1 |
| Falco peregrinus | 5 | Gralina cyanoleuca | 1 | Chaetophractus villosus | 1 |
| Accipiter cooperii | 4 | Gymnorhina tibicen | 1 | Geranoetus polyosoma | 1 |
| Strix aluco | 4 | Ictinia plumbea | 1 | Athene brama | 1 |
| Martes foina | 4 | Larus delawarensis | 1 | Athene cunicularia | 1 |
| Rattus rattus | 4 | Larus marinus | 1 | Bubo ascalaphus | 1 |
| Corvus brachyrhynchos | 3 | Larus michahellis | 1 | Bubo virginianus | 1 |
| Cyanocitta cristata | 3 | Leucophaeus atricilla | 1 | Strix varia | 1 |
| Asio otus | 3 | Manorina inelanocephala | 1 | Conepatus chinga | 1 |
| Athene noctua | 3 | Passer domesticus | 1 | Pseudalopex gymnocercus | 1 |
| Procyon lotor | 3 | Pica pica | 1 | Antechinus stuartii | 1 |
| Canis lupus dingo | 2 | Quiscalus quiscula | 1 | Genetta tigrina | 1 |
| Accipiter gentilis | 2 | Stephanoaetus coronatus | 1 | Glaucomys volans | 1 |
| Falco femoralis | 2 | Sturmis vulgaris | 1 | Mephitis mephitis | 1 |
| Larus argentatus | 2 | Troglodytes aedon | 1 | Mustela itatsi | 1 |
| Molothrus ater | 2 | Zosterops lateralis | 1 | Puma concolor | 1 |
| Rupornis magnirostris | 2 | Corvus cornix | 1 | Dasypus hybridus | 1 |
| Strepera graculina | 2 | Chroicocephalus ridibundus | 1 | Leopardus colocolo | 1 |
| Ninox strenua | 2 | Corvus corax | 1 | Galictis cuja | 1 |
| Didelphis virginiana | 2 | Perisoreus infaustus | 1 | Pantherophis emoryi | 1 |
| Pseudocheirus peregrinus | 2 | Garrulus glandarius | 1 | Philodryas olfersii | 1 |
| Elaphe obsoleta lindheimerii | 2 | Dendrocopos major | 1 | Phrynops geoffroanus | 1 |
| Accipiter nisus | 1 | Corvus corone | 1 | Psammophilus dorsalis | 1 |
| Acridotheres tristis | 1 | Corvus frugilegus | 1 | Pseudonaja affinis | 1 |
| Aphelocoma californica | 1 | Corvus monedula | 1 | Thamnophis sirtalis | 1 |
| Aquila verreauxii | 1 | Caracara plancus | 1 | Paraphimophis rusticus | 1 |
| Buteo jamaicensis | 1 | Circus buffoni | 1 | Erythrolamprus poecilogyrus | 1 |
| Geranoaetus melanoleucus | 1 | Circus cinereus | 1 | Xenodon dorbignyi | 1 |
| Parabuteo unicinctus | 1 |
| Species | N | Species | N | Species | N |
|---|---|---|---|---|---|
| Vulpes vulpes | 11 | Cyanocitta cristata | 1 | Haliastur sphenurus | 2 |
| Felis catus | 9 | Didelphis virginiana | 1 | Procyon lotor | 2 |
| Canis lupus familiaris | 7 | Erinaceus europaeus | 1 | Varanus varius | 2 |
| Corvus orru | 4 | Geococcyx californianus | 1 | Ciconia ciconia | 1 |
| Milvus milvus | 2 | Haliaeetus leucocephalus | 1 | Corvus coronoides | 1 |
| Canis latrans | 3 | Lanius ludovicianus | 1 | Corvus macrorhynchos | 1 |
| Corvus corone | 3 | Laridae spp. | 1 | Corvus splendens | 1 |
| Crocuta crocuta | 3 | Larus dominicanus | 1 | Varanus bengalensis | 1 |
| Cynictis penicillata | 3 | Larus michahellis | 1 | Varanus salvator | 1 |
| Pica pica | 3 | Larus novaehollandiae | 1 | Pica hudsonia | 1 |
| Rattus spp. | 3 | Larus pacificus | 1 | Scincidae spp. | 1 |
| Chroicocephalus novaehollandiae | 2 | Lutreolina crassicaudata | 1 | Scirus vulgaris | 1 |
| Coragyps atratus | 2 | Martes foina | 1 | Sciurus carolinensis | 1 |
| Corvus brachyrhynchos | 2 | Mephitis mephitis | 1 | Sciurus niger | 1 |
| Galerella sanguinea | 2 | Mustela putorius | 1 | Sus scrofa | 1 |
| Haliaeetus leucogaster | 2 | Necrosyrtes monachus | 1 | Sylvilagus floridanus | 1 |
| Haliastur indus | 2 | Neophron percnopterus | 1 | Tamias striatus | 1 |
| Milvus migrans | 2 | Terrapene carolina | 1 |
| Response Variable | Models | K | AICc | Delta_AICc | AICcWt | Cum.Wt | D2 |
|---|---|---|---|---|---|---|---|
| Urban predation | Carnivore level + Daily rhythm | 3 | 347.804 | 0.000 | 0.798 | 0.798 | 0.27 |
| Carnivore level + Class | 4 | 350.596 | 2.792 | 0.198 | 0.996 | ||
| Daily rhythm | 2 | 358.698 | 10.893 | 0.003 | 0.999 | ||
| Carnivore level + Order + Daily rhythm | 17 | 362.022 | 14.217 | 0.001 | 1.000 | ||
| Carnivore level + Order | 16 | 365.539 | 17.735 | <0.001 | 1.000 | ||
| Carnivore level | 2 | 368.716 | 20.912 | <0.001 | 1.000 | ||
| Order | 15 | 376.378 | 28.574 | <0.001 | 1.000 | ||
| Class | 3 | 377.075 | 29.271 | <0.001 | 1.000 | ||
| NULL | 1 | 387.227 | 39.423 | <0.001 | 1.000 | ||
| Urban scavenging | Carnivore level + Daily rhythm | 3 | 156.082 | 0.000 | 1.000 | 0.802 | 0.29 |
| Carnivore level + Class | 4 | 159.505 | 3.423 | 0.181 | 0.145 | ||
| Daily rhythm | 2 | 161.964 | 5.882 | 0.053 | 0.042 | ||
| Carnivore level + Order | 2 | 164.850 | 8.768 | 0.012 | 0.010 | ||
| Carnivore level | 1 | 171.257 | 15.176 | 0.001 | <0.001 | ||
| Order | 3 | 172.044 | 15.962 | <0.001 | <0.001 | ||
| Class | 18 | 203.664 | 47.582 | <0.001 | <0.001 | ||
| NULL | 19 | 209.212 | 53.131 | <0.001 | <0.001 |
| Models | Explanatory Variables | Estimate | Std. Error | DF | D2 |
|---|---|---|---|---|---|
| Urban predation | (Intercept) | −0.079 | 0.161 | 98 | 0.27 |
| Carnivore level | 0.007 | 0.002 | |||
| Nocturnal | 0.719 | 0.148 | |||
| Urban scavenging | (Intercept) | −0.018 | 0.275 | 42 | 0.29 |
| Carnivore level | 0.010 | 0.003 | |||
| Nocturnal | 0.612 | 0.207 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna, Á.; Romero-Vidal, P.; Arrondo, E. Predation and Scavenging in the City: A Review of Spatio-Temporal Trends in Research. Diversity 2021, 13, 46. https://doi.org/10.3390/d13020046
Luna Á, Romero-Vidal P, Arrondo E. Predation and Scavenging in the City: A Review of Spatio-Temporal Trends in Research. Diversity. 2021; 13(2):46. https://doi.org/10.3390/d13020046
Chicago/Turabian StyleLuna, Álvaro, Pedro Romero-Vidal, and Eneko Arrondo. 2021. "Predation and Scavenging in the City: A Review of Spatio-Temporal Trends in Research" Diversity 13, no. 2: 46. https://doi.org/10.3390/d13020046
APA StyleLuna, Á., Romero-Vidal, P., & Arrondo, E. (2021). Predation and Scavenging in the City: A Review of Spatio-Temporal Trends in Research. Diversity, 13(2), 46. https://doi.org/10.3390/d13020046






