Abstract
A novel Gram-negative, aerobic, motile, lemon-yellow-colored, and non-spore-forming rod-shaped bacterium designated strain NZ-12BT was isolated in February 2021 from a sponge species (Crateromorpha) collected at the southern Kermadec Ridge, Pacific Ocean, New Zealand. Comparative 16S rRNA gene-based analyses indicated that strain NZ-12BT shared 98.58%, 96.44%, 96.23%, and 94.78% 16S rRNA sequence similarity to Alteriqipengyuania lutimaris S-5T, Qipengyuania pelagi UST081027-248T, Qipengyuania citreus RE35F/1T, and Alteriqipengyuania halimionae CPA5T, respectively. The major respiratory quinone was ubiquinone-10(Q-10). The polar lipid profile of NZ-12BT was composed of diphosphatidylglycerol, phosphatidylethanolamine, phosphatidyl-N-methyl-ethanolamine, phosphatidylcholine, sphingoglycolipid, phosphatidylglycerol, one unknown polar lipid, three unknown phospholipids, and three unknown glycolipids. The major fatty acids of strain NZ-12BT were C18:1ω12t, C16:0, C17:1ω6c, and C14:02-OH. Carotenoids were present. Genome mining analysis revealed a biosynthetic gene cluster encoding for the terpene biosynthesis. Pairwise ANI and dDDH values of strain NZ-12BT and closely related phylogenetic neighbors were below the threshold values of 95% and 70%, respectively. The DNA G+C content was 65.4 mol% (by genome). Based on data obtained by a polyphasic approach, type strain NZ-12BT (=DSM 112810T = NCCB 100841T) represents a novel species of the genus Alteriqipengyuania, for which the name Alteriqipengyuania abyssalis sp. nov. is proposed.
1. Introduction
The development of new antibiotics that are both safe and effective against resistant microbes is critical in combating the growing issue of antibiotic resistance [1,2]. Natural products produced by marine microbes have proven to be a valuable source of clinically important compounds. The deep sea, representing ocean depths below 1000 m, signifies a marginally scrutinized habitat sheltering novel and yet to be discovered microorganisms. The deep sea extreme environments have equipped its microbiota with unique physiological mechanisms, a capacity to form diverse metabolites with unique structures, and a copious range of functions [3]. During the course of the study aimed at the isolation of novel bioactive compounds from deep-sea sponge-associated bacteria, we encountered a strain designated NZ-12BT. The isolate was shown to be closely related to Alteriqipengyuania lutimaris S-5T, a member of the family Erythrobacteraceae based on comparative 16S rRNA gene analyses. The taxonomy of the family Erythrobacteraceae was recently revised using genome-based methods, resulting in the creation of 11 new genera within the family [4]. Creation of novel genera has resulted in the placement of Erythrobacter lutimaris into a novel genus Alteriqipengyuania. Genus Alteriqipengyuania, a member of the family Erythrobacteraceae [5], belongs to the class Alphaproteobacteria, with the description of Alteriqipengyuania lutimaris as the type species [6]. At the time of writing this paper, the genus Alteriqipengyuania comprises two species with validly published names: A. halimionae CECT 9130T and A. lutimaris CECT 8624T https://lpsn.dsmz.de/genus/alteriqipengyuania (accessed on 4 August 2021) [7]. Members of the family Erythrobacteraceae have been isolated from marine organisms [8], marine cyanobacterial mat, seawater, marine and mangrove sediments [9,10,11], solar saltern [12], ice core [13], and estuary environments [14]. The members of the family Erythrobacteraceae are Gram-negative, rod or pleomorphic coccoid-shaped, pink-, red-, orange-, or yellow-pigmented, and aerobic chemoorganotrophs [15]. Identification of novel microbial species with potential industrial relevance from extreme environments provides a framework for the improvement of sequence databases and may lead to the identification of new biotechnologically relevant entities. The present work aimed to determine the taxonomic position of NZ-12BT using a polyphasic characterization approach, including the determinations of chemotaxonomic and other phenotypic properties, a detailed phylogenetic investigation based on 16S rRNA gene sequences, and genetic analyses of genome data.
2. Materials and Methods
2.1. Isolation, Cultivation and Maintenance of Bacteria
The sponge species (Crateromorpha) was collected in February 2017 during a sample collection expedition from a depth of 1165 m below the ocean surface at the southern Kermadec ridge (35° 60/N, 178° 85/W), Pacific Ocean, New Zealand. The sponge sample for bacterial isolation was washed with sterile artificial seawater (ASW) to remove the loosely bound bacterial cells and debris. Approximately 1 cm3 of the sponge tissue sample was cut with a sterile scalpel, ground using a mortar and pestle, and immediately transferred to 9 mL of sterile artificial seawater. Several dilutions (from 10−2 to 10−6) were prepared and 0.1 mL was spread on sea water glutamate (SWG) media containing artificial sea-water (3.9% (w/v) of sea salt from ATI Coral Ocean), 0.1% sodium glutamate, solidified with 1.6% agar (Difco) [16]. Filter sterilized cycloheximide (50 mg/L) was added to the media after autoclaving to inhibit fungal growth. Agar plates were incubated at 30 °C and routinely checked for observed growth under a stereomicroscope for 2–4 weeks. The strain NZ-12BT was purified by repeated streaking on marine agar (MA, Difco). The purified strain was preserved in marine broth 2216 (MB, Difco laboratories, BD, USA) with 50% (v/v) glycerol at −80 °C.
Unless otherwise specified, all characteristics described hereafter are based on cultures incubated on marine agar (MA) or marine broth (MB) for three days at 30 °C. Type strains Q. pelagi NRRL 59511T and Q. citreus DSM 14432T were obtained from the northern regional research lab, USA, and the German collection of microorganisms and cell cultures DSMZ, respectively.
2.2. Morphological, Cultural and Physiological Characterization
Morphological observations were carried out on MA 2216 medium (Difco laboratories, BD, USA) incubated at 30 °C over the course of a week. Cell morphology was determined using phase-contrast microscopy and electron microscopy (SEM and negative staining TEM, Zeiss, Oberkochen, Germany). Scanning electron microscopy was performed as previously described with slight modifications [17]. In brief, bacteria were fixed in medium with subsequent addition of aldehydes (30 min in 2% glutaraldehyde final concentration, followed by 30 min in 5% formaldehyde final concentration). Dehydration was undertaken in a gradient series of acetone, followed by critical point drying and sputter coating with gold palladium. A Zeiss Merlin field emission SEM was used for image acquisition at various magnifications and a 25:75 ratio of Everhart–Thornley and Inlens detector. For transmission electron microscopy, a thin carbon film was floated on a sample drop, taken off with a grid, washed twice, and negatively stained with 4% uranyl acetate before analysis with a Zeiss Libra 120 at various magnifications.
Gram staining was performed using a Gram stain kit (Sigma-Aldrich, CA, USA) according to the manufacturer’s instructions. The color of the culture was determined by comparison with RAL color code 1005 (Deutsches Institut für Gütesicherung und Kennzeichnung e.V.—Reichs-Ausschuss für Lieferbedingungen). The activities of oxidase and catalase were determined with N, N-dimethyl-p-phenylenediamine dihydrochloride and 3% (v/v) H2O2 solutions, respectively. Growth under anaerobic conditions was tested on MA in an anaerobic chamber at 30 °C using the Anaerocult A system, following the manufacturer’s instructions. Motility was determined using the wet mount slide and hanging drop methods [18]. Growth at different temperatures (4, 15, 20, 25, 30, 37, 44 °C) was determined on MA medium. Growth at pH 3–11 at intervals of one unit pH was examined in MB (pH was confirmed after autoclaving). The requirement for and tolerance to various salt concentrations were tested on 1.6% agar medium containing (per liter) 5.9 g MgCl2, 3.24 g Na2SO4, 1.8 g CaCl2, 0.55 g KCl, 5.0 g peptone, 0.1 g ferric citrate, and 1.0 g yeast extract (pH 7.6) in the presence of 2.5, 5.0, 7.5, and 10% (w/v) NaCl [19].
Enzyme activities and assimilation of substrates were tested by employing API ZYM, API 20E, and API 20NE strips (Biomerieux, Marcy l’Etoile, France) and the Biolog Gen Microplate system according to the manufacturer’s instructions. Carbon sources (arabinose, cellulose, fructose, inositol, mannitol, raffinose, rhamnose, sucrose, and xylose) utilization was carried out in MA medium with the addition of 1% filter-sterilized carbon sources in a micro-dilution well plate. For the antibiotic resistance test, pre-cooled agar was supplemented with filter-sterilized antibiotics with the final concentration adjusted to 50 µg ml−1 except for oxytetracycline, ampicillin, chloramphenicol, and hygromycin. The antibiotics used were polymyxin (50 µg/mL), gentamicin (50 µg/mL), oxytetracyclin (10 µg/mL), ampicillin (100 µg/mL), chloramphenicol (30 µg/mL), spectinomycin (50 µg/mL), kanamycin (50 µg/mL), cephalosporin (50 µg/mL), fusidic acid (50 µg/mL), bacitracin (50 µg/mL), thiostrepton (50 µg/mL), trimethoprim (50 µg/mL), and hygromycin (150 µg/mL) [20].
2.3. 16S rRNA Gene Sequence and Phylogenetic Analysis
Molecular taxonomic characterization was performed using 16S rRNA gene sequence analysis. Strain NZ-12BT was grown in MB medium for three days at 30 °C. Genomic DNA was extracted using the protocol of the Nucleospin Microbial DNA kit (Macherey Nagel). The 16S rRNA gene was amplified using the primers F27 (5′-AGAGTTTGATCMTGGCTCAG-3′) and R1492 (5′-GGTTACCTTGTTACGACTT-3′). The PCR product was purified using the Nucleospin Gel and PCR clean-up kit (Macherey Nagel) following analysis of the PCR product by gel electrophoresis using a 0.8% (w/v) agarose gel at 80 V for 30 min. Primers F1100 (5′-CAACGAGCGCAACCC-3′), R1100 (5′-GGGTTGCGCTCGTTG-3′), and R518 (5′-CGTATTACCGCGGCTGCTGG-3′), in addition to the primers used for primary PCR, were applied for sequencing of the 16S rRNA gene using a 370XL automatic sequencer from Applied Biosystems (ABI). The acquired sequences were assembled using the Bioedit program (version 7.0.5.3) [21] (http://www.mbio.ncsu.edu/BioEdit/bioedit.html) (accessed on 25 July 2021). The consensus sequences were produced using the cap contig assembly function of the Bioedit sequence alignment editor version 7.0.5.3 [20]. Pairwise sequence similarities were calculated using the database of 16S rRNA gene sequences from the EZBioCloud server (https://www.EZBioCloud.net) (accessed on 25 July 2021). Phylogenies were inferred by the GGDC web server [22] available at http://ggdc.dsmz.de/ (accessed on 25 July 2021) using the DSMZ phylogenomics pipeline [23] adapted to single genes. A multiple sequence alignment was created with MUSCLE [24]. Maximum likelihood (ML) and maximum parsimony (MP) trees were inferred from the alignment with RAxML [25] and TNT [26], respectively. For ML, rapid bootstrapping in conjunction with the autoMRE bootstopping criterion [27] and subsequent search for the best tree was used for MP; 1000 bootstrapping replicates were used in conjunction with tree-bisection-and-reconnection branch swapping and ten random sequence addition replicates. The sequences were checked for a compositional bias using the Χ2 test as implemented in PAUP. The tree was also inferred using the neighbor joining algorithm from MEGA X [28]. The topologies of the inferred tree were examined by bootstrap analyses based on 1000 replicates. The 16S rRNA gene sequence of strain NZ-12BT was deposited at Genbank under accession code MZ569434.
2.4. Chemotaxonomy
Freeze-dried cells were used for chemotaxonomic analyses of respiratory quinones, polar lipids, and fatty acids. Cells of strain NZ-12BT, Q. citreus RE35F/1T, and Q. pelagi UST081027-248T were grown in MB for three days at 30 °C, harvested, and washed three times with sterile phosphate buffer saline prior to lyophilization.
Polar lipids were extracted in accordance with the procedure described by [29]. Polar lipids were separated by two-dimensional thin-layer chromatography (2D-TLC) on silica gel and five TLC plates (10 × 10) were used per isolate. Beginning with 10 µL lipid extracts spotted on each plate, the solvent mixture used for the first dimension was chloroform/methanol/water (65:25:4 by vol.), and chloroform/methanol/acetic acid/water (80:12:15:4 by vol.) for the second dimension. Various reagents were used for detection of polar lipids with differing functional groups [18]. The first plate was stained with phosphomolybdic acid to detect all polar lipids. The second plate was stained with ninhydrin to detect aminolipids, followed by staining of the same plate with molybdenum blue to detect phospholipids. The third plate was stained with α-naphthol to detect sugar-containing lipid [18]. The fourth and fifth plates were stained with anisaldehyde and dragendorff’s reagents, respectively. Once stained, plates were heated at 100 to 120 °C to visualize polar lipids, other than the plate stained with molybdenum blue and dragendorff’s reagent, which was developed at room temperature.
The presence or absence of carotenoids was examined by extraction using a ternary solvent system of hexane/methanol/water mixture in a ratio of 2:1:1, respectively [30], and subsequent analysis by HR-ESI-MS (high-resolution electrospray ionization mass spectrometry). High-resolution electron spray ionization mass spectrometry (HR-ESI-MS) data were recorded on a Maxis ESI-TOF-MS spectrometer (Bruker, Bremen, Germany) equipped with an Agilent 1260 series RP-HPLC system using an Acquity C18 column 2.1 × 50 mm, 1.7 µm. Solvent A was water + 0.1 formic acid and solvent B was acetonitrile + 0.1 formic acid. The gradient system was 5% B for 0.5 min, increasing to 100% B in 19.5 min and holding for 5 min at 100% B with the flow rate of 0.6 mL/min. Respiratory quinones were extracted according to the method of Minnikin et al. (1984) and were analyzed by high-performance liquid chromatography with some modifications of mobile phase and flow rate [31]. Solvent A (35% isopropanol) and solvent B (65% acetonitrile) were used as isocratic condition mobile phase with a flow rate of 0.3 mL/min. Fatty acids methyl esters (FAME) analysis was conducted using 6890N gas chromatography equipped with FID (flame ionization detector). Separation of the fatty acids’ methyl esters was carried out with a Macherey Nagel Optima 5 column (5% phenyl, 95% dimethylpolysiloxane, 50 m length; 0.32 mm inner diameter, 0.25 µm film thickness). Individual fatty acid methyl esters were identified by their retention time compared with standards (in-house reference standard).
2.5. Genome Analysis
Genomic DNA used for sequencing was extracted from axenic cultures grown for three days on MA at 30 °C using the protocol of the Nucleospin microbial DNA kit according to the manufacturer’s instructions. Genome sequencing was carried out at the Sequencing facility (HZI, Braunschweig, Germany) on the Illumina MiSeq (300 bp, paired-end reads) using the NexteraXT protocol for library preparation followed by de novo assembly using a unicycler genome assembler [32]. Automated genome annotation was carried out using the NCBI Prokaryotic Genome Annotation Pipeline PGAP [33]. The whole-genome sequence of strain NZ-12BT was deposited at DDBJ/ENA/GenBank under the accession JAHWXP000000000. Draft genome assembly was analyzed using ContEst16S (www.ezbiocloud.net/tools/contest16s) (accessed on 19 August 2021) [34] to verify that the genome was uncontaminated. The DNA G+C content (mol%) was determined based on whole-genome data. The genomic sequence was also uploaded to the RAST (Rapid Annotation using Subsystem Technology) server (https://rast.nmpdr. org/) (accessed on 21 August 2021) [35] and the antiSMASH (antibiotics and secondary metabolite analysis shell) server (https://antismash.secondarymetabolites.org/) (accessed on 21 August 2021) [36,37] for metabolic reconstruction analysis and prediction of secondary metabolite gene clusters, respectively.
The phylogenomic tree was created based on the whole-genome sequence of NZ-12BT and its closest phylogenetic hits using the type (strain) genome server (TYGS) (https://tygs.dsmz.de/) (accessed on 25 August 2021). For the phylogenomic inference, all pairwise comparisons among the set of genomes were conducted using GBDP and accurate intergenomic distances inferred under the algorithm ‘trimming’ and distance formula d5 [22]. One hundred distance replicates were calculated each. In silico DNA–DNA hybridization estimates were calculated using the Genome-to Genome Distance Calculator (GGDC; version 2.1) developed by Meier-Kolthoff et al. [38]. Pairwise average nucleotide identity (ANI) values between genome sequences of the closest phylogenetic relatives were calculated using the EzBioCloud server (www.ezbiocloud.net/tools/ani) (accessed on 21 August 2021) [39].
3. Results and Discussion
3.1. Morphological and Physiological Characteristics
The strain was isolated from a sponge sample obtained in February 2017 on the southern Kermadec ridge, Pacific Ocean (350° 60/N, 178° 85/W) about 1165 m beneath the ocean surface. Cells of strain NZ-12BT were observed to be Gram-negative, aerobic, catalase and oxidase-positive, motile, straight rods that occur singly and in pairs and were non-spore-forming. Colonies were lemon-yellow-colored, circular, and convex with entire margins (Figure 1). Electron microscopy images of strain NZ-12BT clearly displayed the rod-shaped morphology and presence of a single polar flagellum (Figure 1). Strain NZ-12BT grew at 15–37 °C (optimal growth at 15–30 °C). No growth was shown at 4 and 44 °C. Growth was observed at pH 6–9 (optimum at pH 6). Growth occurs in the presence of 0–7.5% (w/v) NaCl with optimum growth at 2.5% (w/v) NaCl. Apart from marine agar 2216 (Difco), the strain also grew on ASW-CY medium, (Casitone 3.00 g, CaCl2 × 2 H2O 1.36 g, Yeast extract 1.00 g, Agar 16.00 g, distilled water 1000 mL, pH 7.2) supplemented with 39 g artificial sea salts. Antibiotic susceptibility test data indicated that the strain was sensitive to gentamicin, thiostrepton, chloramphenicol, kanamycin, and fusidic acid. However, it was resistant to hygromycin, trimethoprim, spectinomycin, ampicillin, oxytetracyclin, cephalosporin, bacitracin, and polymyxin. Physiological and biochemical properties of strain NZ-12BT according to API ZYM (Table S1 in Supplementary Materials), API 20E (Table S2), and API 20NE (Table S3) strips and the Biolog Gen III Microplate system (Table S4), showed strong activity for alkaline phosphatase, leucine arylamidase, valine arylamidase, cystine arylamidase, trypsin, acid phosphatase, naphthol-AS-BI-phosphohydrolase, α-glucosidase, arginine dihydrolase, and esculin hydrolysis. In contrast, no activity was observed for α-galactosidase, β-galactosidase, β-glucuronidase, β-glucosidase, N-acetyl-β-glucosaminidase, α- mannosidase, α-fucosidase, β-galactosidase (ONPG), ornithine decarboxylase, urease, bromo-succinic acid, or mannose assimilation. Strain NZ-12BT differed from A. lutimaris KCTC 42109T, Q. pelagi JCM 14468T, and Q. citreus DSM 14432T in gelatinase, esculin hydrolysis, and tryptophan deaminase activities. In contrast to Q. pelagi 14468T and Q. citreus 14432T, which reduce nitrate, strain NZ-12BT was negative for nitrate reduction. Strain NZ-12BT was distinguishable from members of genus Alteriqipengyuania by certain properties. Cells of strain NZ-12BT are motile rods, whereas A. lutimaris KCTC 42109T cells are non-motile and coccoid or oval shaped. Strain NZ-12BT was positive for α- glucosidase, whereas A. lutimaris KCTC 42109T and A. halimionae CPA5T were negative for the said property. Strain NZ-12BT was positive for esterase (C4) and arginine dihydrolase, whereas A. lutimaris KCTC 42109T was negative for esterase (C4) and A. halimionae CPA5T was negative for arginine dihydrolase. Strain NZ-12BT was sensitive to gentamicin, whereas A. lutimaris KCTC 42109T was resistant to gentamicin. Strain NZ-12BT showed optimal growth at pH 6, whereas A. lutimaris KCTC 42109T and A. halimionae CPA5T displayed optimum growth at pH above 7. Detailed comparisons of enzymatic activities between strain NZ-12BT and reference strains are shown in (Table S1–S3).

Figure 1.
(a) Growth of strain NZ-12BT on marine agar after three days. (b) Phase contrast microscopy of strain NZ-12BT (bar 50 μm). (c) Transmission electron microscope (TEM) image of negatively stained cells of NZ-12BT showing the presence of a single flagellum (1 μm). (d) Scanning electron micrograph (SEM) of cells grown on marine agar (bar 10 μm) depicting rod-shaped morphology.
3.2. 16S rRNA Gene-Based Phylogenetic Analysis
16S rRNA gene sequence analysis is a primary marker for deducing taxonomic relationships. The almost full-length 16S rRNA gene sequence of strain NZ-12BT (1422 nucleotides) was subjected to comparative analysis. Sequence similarity comparisons of 16S rRNA gene using the EzTaxon database [39] revealed that the strain NZ-12BT was most closely related to A. lutimaris S-5T (98.58%), Q. pelagi UST081027-248T (96.44%), and Q. citreus RE35F/1T (96.23%). Because the closely related genus Alteriqipengyuania contains only two species (A. lutimaris and A. halimionae), comparison of 16S rRNA sequences was also performed with A. halimionae. Strain NZ-12BT showed 94.78% 16S rRNA sequence similarity to A. halimionae CPA5T. Phylogenetic trees based on an almost complete 16S rRNA gene sequence (1422 nucleotides) and inferred from the maximum likelihood (Figure S1 and neighbor joining methods (Figure 2) showed that strain NZ-12BT formed a well-defined tight cluster with A. lutimaris S-5T supported by a high bootstrap value of 100%. Based on the 16S rRNA gene sequence similarities and phylogenetic analysis, A. lutimaris KCTC 42109T, Q. pelagi JCM 14468T, Q. citreus DSM 14432T, and A. halimionae CPA5T were selected as the reference strains for comparative analysis.
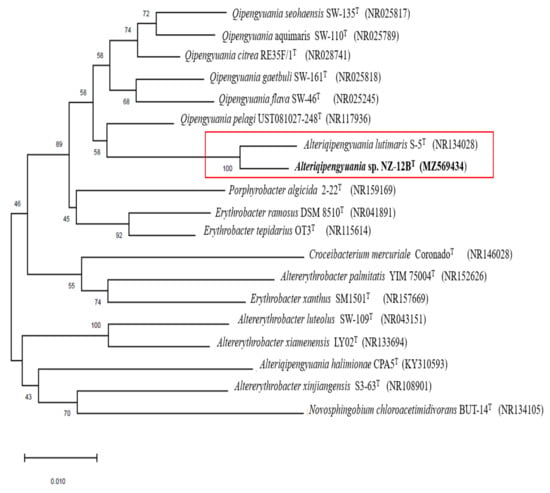
Figure 2.
Neighbor joining phylogenetic tree based on almost complete 16S rRNA gene sequence of NZ-12BT and its most closely related species. The numbers above the branches are bootstrap support. Bar, 0.010 substitution per nucleotide position.
3.3. Chemotaxonomy
Analyses of the chemical composition of cell constituents of strain NZ-12BT revealed it possesses chemotaxonomic features that are congruent with those defined for members of the family Erythrobacteraceae [40]. The respiratory quinone profile consisted exclusively of ubiquinone-10 (Q-10), which is in line with the description of the genus Alteriqipengyuania [15]. The polar lipid profile of NZ-12BT was composed of diphosphatidylglycerol, phosphatidylethanolamine, phosphatidyl-N-methyl-ethanolamine, phosphatidylcholine, sphingoglycolipid, phosphatidylglycerol, one unknown polar lipid, three unknown phospholipid, and three unknown glycolipid (Figure 3). The major fatty acids of strain NZ-12BT were C18:1ω12t (41.5%), C16:0 (6.3%), C17:1ω6c (22.8%), and C14:02-OH (5.9%). The fatty acid profile of strain NZ-12BT and its phylogenetically closest members are presented in Table 1. Differences in the percentages of major fatty acids C18:1ω12t, C16:0, and C17:1ω6c distinguish the strain from its close relatives. Ternary solvent extract spectra exhibited a peak at a retention time of 17.80 min with UV absorption at 425 nm and an exact mass of 600.41 (the characteristic spectra of xanthophyll-like carotenoids).

Figure 3.
Polar lipids observed in strain NZ-12BT are DPG: diphosphatidylglycerol; PE: phosphatidylethanolamine; PME: phosphatidyl-N-methyl-ethanolamine: PC: phosphatidylcholine; SGL: sphingoglycolipid; PG: phosphatidylglycerol; L: one unknown polar lipid; PL: three unknown phospholipids, and GL: three unknown glycolipids.

Table 1.
Fatty acid profiles of (1) NZ-12BT and the phylogenetically closest relatives; (2) A. lutimaris S-5T (data from [6]); (3) Q. citreus RE35F/1T; (4) A. halimionae CPA5T (data from [41]); and (5) Q. pelagi UST081027-248T. Values are percentages of the total fatty acids. The bold type represents the major fatty acids. —, Not detected. TR, Trace amount (<0.5%).
3.4. Genome Analysis
The draft assembled genome of strain NZ-12BT was 3,099,119 bp long and consisted of eight contigs with a N50 length of 953,397 bp. The DNA G+C content of strain NZ-12BT was 65.4%, a value in the range reported for members of the genus Alteriqipengyuania, i.e., 63.6–65.5 mol% [4]. The genome contained 3054 coding sequences, 50 tRNA genes, six rRNA operons, and a single tmRNA gene after PGAP annotation. The 16S rRNA gene sequence extracted from the genome sequence was in agreement with the partial sequence determined by the PCR method, indicating that the genome sequence was not contaminated. Analysis using the RAST server revealed that only 30% of the annotated genes were assigned to subsystems (Figure 4). Among the subsystem categories present in the genome, the metabolism of amino acids and derivatives had the highest feature counts (211), followed by protein metabolism, which had 169 feature counts. Thirty-one feature counts were detected for resistance of antibiotic and toxic compounds in the genome of strain 12BT, whereas 29, 40, 15, and 13 feature counts were present in the genomes of Q. pelagi UST081027-248T, Q. citreus RE35F/1T, A. lutimaris S-5T, and A. halimionae CPA5T, respectively, for the said property. 04 feature counts were present in the genome of strain NZ-12BT for isoprenoids for quinones, 21 feature counts for fatty acid metabolism, and three for multidrug resistance efflux pumps, whereas four, 17, and three genes were detected in the genome of A. lutimaris S-5T for quinone biosynthesis, fatty acid metabolism, and multidrug resistance efflux pumps, respectively. The characteristic genes and gene products present in strain NZ-12BT were investigated via RAST analysis (Figure 4). Strain NZ-12BT contained proteins involved in the metabolism of proteins, biosynthesis of amino acids, and metabolism of fatty acids, lipids, isoprenoids, and proteins related to motility, vitamins, pigments, and chemotaxis. With respect to ecology, gene products related to the resistance of antibiotic and toxic compounds such as copper, cobalt, and mercury were present. Underlying genes related to deep-sea survival were deciphered. Strain NZ-12BT contained genes for trehalose biosynthesis, aspartate-semialdehyde dehydrogenase, flagellar biosynthesis, and resistance to antibiotics. Trehalose is a sugar molecule also known as mycose. It is a cryoprotectant for bacterial cells and used as an energy source [42]. Its presence can provide the ability to manage the cold environment in deep-sea environments. Flagellar motility is very important to allow bacteria to move and acquire nutrients in deep-sea environments. Aspartate-semialdehyde dehydrogenase is involved in the biosynthesis of amino acids. Its expression is regulated by high pressure. Thus, asd gene expression, controlled by high pressure in deep-sea bacteria, seems to be one of the striking mechanisms for survival in the deep-sea environment [43] (Table 2). The antiSMASH server predicted one secondary metabolite biosynthetic gene cluster displaying 33% similarity to the terpene biosynthetic gene cluster (Figure S2).

Figure 4.
Subsystem category distribution of strain NZ-12BT based on the RAST annotation server (https://rast.nmpdr.org/) (accessed on 21 August 2021).

Table 2.
Characteristic gene products related to deep-sea survival present in the genome of strain NZ-12BT.
A phylogenomic tree generated with the TYGS server exhibited that the strain NZ-12BT formed a well-supported monophyletic clade with A. lutimaris S-5T, supported by a high bootstrap value of 98% (Figure 5), which confirmed the result obtained from the phylogenetic analysis of the 16S rRNA gene sequences. Through wide genome-based investigation, the taxonomy of the family Erythrobactereacea was recently revised, resulting in the placement of E. lutimaris into a novel genus, Alteriqipengyuania, which is clearly mirrored in the phylogenomic tree. Pairwise ANI values between genome sequence of strain NZ-12BT and the closest phylogenetic relatives A. lutimaris S-5T, Q. pelagi UST081027-248T, Q. citreus RE35F/1T, and A. halimionae CPA5T were 82.1%, 75.1%, 74.03%, and 74.5%. respectively; these values are well below the cut-off value of 95%–96% recommended for species delineation (Table 3). The dDDH values between strain NZ-12BT and strains A. lutimaris S-5T, Q. pelagi UST081027-248T, Q. citreus RE35F/1T, and A. halimionae CPA5T were 19.9%, 20.1%, 19.7%, and 20.3%, respectively, (Table 3) values well below the cut-off value of 70% for the species boundary.

Figure 5.
Tree inferred with FastME 2.1.6.1 [44] from GBDP distances calculated from genome sequences of strain NZ-12BT. The branch lengths are scaled in terms of GBDP distance formula d5. The numbers above branches are GBDP pseudo-bootstrap support values >60% from 100 replications. The tree was rooted at the midpoint.

Table 3.
ANI and dDDH values of NZ-12BT and its closely related phylogenetic strains.
Detailed differential properties that distinguish strain NZ-12BT from its closely related phylogenetic neighbors are given in Table 4. In view of these combined morphological, chemotaxonomic, and phylogenetic analyses, strain NZ-12BT warrants classification in the genus Alteriqipengyuania. The level of DNA–DNA relatedness and phenotypic characteristics confirmed that the strain constitutes a separate species. Therefore, based on the data presented, we propose to include strain NZ-12BT in the genus Alteriqipengyuania as Alteriqipengyuania abyssalis sp. nov.

Table 4.
Differential characteristics of strain NZ-12BT and closely related type strains.
4. Emended Description of the Genus Alteriqipengyuania
The description of the genus is as emended by Xu et al. (2020), with the exception that the major fatty acids (>10%) were C17:1 ω6c and C18:1 ω12t. The major polar lipids include phosphatidylethanolamine and phosphatidyl-N-methyl-ethanolamine. Positive for α- glucosidase.
5. Description of Alteriqipengyuania abyssalis sp. nov.
A. abyssalis (a.bys’sa.lis. L. n. abyssus, an abyss, deep-sea abyssalis) pertaining to the abyssal depths of the ocean. Cells are Gram-negative, aerobic, motile, and rod-shaped. Colonies on MA are smooth, circular, and opaque with entire margins, yellow-colored and 1.0–1.2 mm in diameter at 30 °C for three days. Flagellum present. Carotenoids produced. Growth occurs at 15–37 °C (optimum 15–30 °C), at pH 6–9 (optimum pH 6) and in 0–7.5% NaCl (optimum 2.5% (w/v) NaCl). Positive for oxidase and catalase activity. Nitrate is not reduced. Production of H2S, indole and acetoin are negative. The enzyme reactions for alkaline phosphatase, leucine arylamidase, valine arylamidase, cystine arylamidase, trypsin, acid phosphatase, naphthol-AS-BI-phosphohydrolase, α- glucosidase, arginine dihydrolase, esculin hydrolysis are positive. Negative for α-galactosidase, β-galactosidase, β-glucuronidase, β-glucosidase, N-acetyl-β-glucosaminidase, α- mannosidase, α-fucosidase, β-galactosidase (ONPG), ornithine decarboxylase and urease. Dextrin, turanose, α-D-lactose, β-methyl-D-glucoside, D-salicin, N-acetyl-β-d-mannosamine, N-acetyl-D-galactosamine, N-acetylneuraminic acid, D-galactose, D-fucose, L-fucose, D-arabitol, glycerol, D-fructose-6-PO4, L-arginine, L-aspartic acid, L-glutamic acid, L-pyroglutamic acid, lincomycin, D-galacturonic acid, L-galactonic acid lactone, D-gluconic acid, D-glucuronic acid, glucuronamide, mucic acid, quinic acid, D-saccharic acid, D-lactic acid methyl ester, L-lactic acid, α-keto-glutaric acid, L-malic acid, Tween 40, β-hydroxy-d, L-butyric acid and acetic acid can be used as sole carbon sources. The chief fatty acids of strain NZ-12BT are C18:1ω12t, C16:0, C17:1ω6c, and C14:02-OH. The polar lipid profile of NZ-12BT is composed of diphosphatidylglycerol, phosphatidylethanolamine, phosphatidyl-N-methyl-ethanolamine, phosphatidylcholine, sphingoglycolipid, phosphatidylglycerol, one unknown polar lipid, three unknown phospholipids, and three unknown glycolipids. The respiratory quinone is ubiquinone-10 (Q-10). The G+C content is 65.4%.
The type strain NZ-12BT (=DSM 112810; =NCCB 100841T) was isolated from a sponge sample. The GenBank/EMBL/DDBJ accession numbers for the 16S rRNA gene sequence and whole-genome shotgun project of strain NZ-12BT are MZ569434 and JAHWXP000000000, respectively.
6. Conclusions
Cultural, morphological, and chemotaxonomic markers, in addition to phylogenomic tree analysis, revealed that strain NZ-12BT belongs to the genus Alteriqipengyuania. The strain could be distinguished from the closely related type strains by several striking characteristics. Based on study using the polyphasic approach, we propose to include strain NZ-12BT in the genus Alteriqipengyuania as Alteriqipengyuania abyssalis sp. nov.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/d13120670/s1, Table S1: Physiological and biochemical characteristics of NZ-12BT and reference strains on API ZYM strip system. Table S2: Physiological and biochemical characteristics of NZ-12BT and reference strains on API 20E strip system. Table S3: Physiological and biochemical characteristics of NZ-12BT and reference strains on API20NE strip system. Table S4: Physiological and biochemical characteristics of NZ-12BT on Biolog Gen III Microplate. Figure S1: Maximum-likelihood phylogenetic tree based on almost complete 16S rRNA gene sequence of strain NZ-12BT and its most closely related species Figure S2: Predicted secondary metabolite gene cluster for strain NZ-12BT identified by analysis of the NZ-12BT genome sequence with the bioinformatics tool antiSMASH 5.0.
Author Contributions
Conceptualization, S.T.; performed experiments, data analyses, manuscript drafting. C.R.; edited manuscript. M.M.; performed electron microscopy. J.W.; supervision, edited manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Strain NZ-12BT is deposited at the German collection of microorganisms and cell cultures (DSMZ) and the Netherlands culture collection of bacteria (NCCB) under accession no. 112810 and 100841 respectively.
Acknowledgments
The authors thanks Stephanie Schulz for excellent technical assistance, Aileen Gollasch for recording the GC data and Ina Schleicher for electron microscopy sample preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations the Review on Antimicrobial Resistance; Government of the United Kingdom: London, UK, 2016.
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the Sustainable Discovery and Development of New Antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Tortorella, E.; Tedesco, P.; Esposito, F.P.; January, G.G.; Fani, R.; Jaspars, M.; de Pascale, D. Antibiotics from Deep-Sea Microorganisms: Current Discoveries and Perspectives. Mar. Drugs 2018, 16, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Sun, C.; Fang, C.; Oren, A.; Xu, X.W. Genomic-Based Taxonomic Classification of the Family Erythrobacteraceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 4470–4495. [Google Scholar] [CrossRef] [PubMed]
- Shiba, T.; Simidu, U. Erythrobacter Longus Gen. Nov., Sp. Nov., an Aerobic Bacterium Which Contains Bacteriochlorophyll A. Int. J. Syst. Bacteriol. 1982, 32, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.-T.; Park, S.; Lee, J.-S.; Yoon, J.-H. Erythrobacter lutimaris sp. nov., isolated from a tidal flat sediment. Int. J. Syst. Evol. Microbiol. 2014, 64 Pt 12, 4184–4190. [Google Scholar] [CrossRef] [PubMed]
- Parte, A.C. LPSN—List of Prokaryotic Names with Standing in Nomenclature (Bacterio.Net), 20 Years On. Int. J. Syst. Evol. Microbiol. 2018, 68, 1825–1829. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Lin, B.; Xu, L.; Li, G.; Wu, C.J.; Luo, L. Erythrobacter spongiae sp. nov., isolated from marine sponge. Int. J. Syst. Evol. Microbiol. 2019, 69, 1111–1116. [Google Scholar] [CrossRef]
- Denner, E.B.M.; Vybiral, D.; Koblízek, M.; Kämpfer, P.; Busse, H.-J.; Velimirov, B. Erythrobacter citreus sp. nov., a yellow-pigmented bacterium that lacks bacteriochlorophyll a, isolated from the western mediterranean sea. Int. J. Syst. Evol. Microbiol. 2002, 52, 1655–1661. [Google Scholar] [CrossRef] [Green Version]
- Lei, X.; Zhang, H.; Chen, Y.; Li, Y.; Chen, Z.; Lai, Q.; Zhang, J.; Zheng, W.; Xu, H.; Zheng, T. Erythrobacter luteus sp. nov., isolated from mangrove sediment. Int. J. Syst. Evol. Microbiol. 2015, 65, 2472–2478. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.; Wu, Y.-H.; Sun, C.; Wang, H.; Cheng, H.; Meng, F.-X.; Wang, C.-S.; Xu, X.-W. Erythrobacter zhengii sp. nov., a bacterium isolated from deep-sea sediment. Int. J. Syst. Evol. Microbiol. 2018, 69, 241–248. [Google Scholar] [CrossRef]
- Subhash, Y.; Tushar, L.; Sasikala, C.; Ramana, C.V.; Ch Ramana, C.V. Erythrobacter odishensis sp. nov. and Pontibacter odishensis sp. nov. isolated from dry soil of a solar saltern. Int. J. Syst. Evol. Microbiol. 2013, 63, 4524–4532. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Liu, Y.; Wang, N.; Xu, B.; Liu, K.; Shen, L.; Gu, Z.; Guo, B.; Zhou, Y.; Liu, H. Erythrobacter arachoides sp. nov., isolated from ice core. Int. J. Syst. Evol. Microbiol. 2017, 67, 4235–4239. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Won, S.M.; Yoon, J.H. Erythrobacter marisflavi sp. nov., isolated from estuary water. Int. J. Syst. Evol. Microbiol. 2019, 69, 2696–2702. [Google Scholar] [CrossRef]
- Tonon, L.A.C.; Moreira, A.P.B.; Thompson, F. The Family Erythrobacteraceae. In The Prokaryotes: Alphaproteobacteria and Betaproteobacteria; Springer: Berlin/Heidelberg, Germany, 2014; Volume 9783642301, pp. 213–235. [Google Scholar] [CrossRef]
- Hosoya, S.; Arunpairojana, V.; Suwannachart, C.; Kanjana-Opas, A.; Yokota, A. Aureispira marina gen. nov., sp. nov., a gliding, arachidonic acid-containing bacterium isolated from the southern coastline of thailand. Int. J. Syst. Evol. Microbiol. 2006, 56, 2931–2935. [Google Scholar] [CrossRef] [Green Version]
- Landwehr, W.; Kämpfer, P.; Glaeser, S.P.; Rückert, C.; Kalinowski, J.; Blom, J.; Goesmann, A.; Mack, M.; Schumann, P.; Atasayar, E.; et al. Taxonomic Analyses of Members of the Streptomyces cinnabarinus Cluster, Description of Streptomyces cinnabarigriseus sp. nov. and Streptomyces davaonensis sp. nov. Int. J. Syst. Evol. Microbiol. 2018, 68, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.R.; Correa, H.; Haltli, B.A.; Kerr, R.G. Fulvivirga aurantia sp. nov. and Xanthovirga aplysinae gen. nov., sp. nov., marine bacteria isolated from the sponge Aplysina fistularis, and Emended Description of the Genus Fulvivirga. Int. J. Syst. Evol. Microbiol. 2020, 70, 2766–2781. [Google Scholar] [CrossRef]
- Wu, H.X.; Lai, P.Y.; Lee, O.O.; Zhou, X.J.; Miao, L.; Wang, H.; Qian, P.Y. Erythrobacter Pelagi Sp. Nov., a Member of the Family Erythrobacteraceae isolated from the red sea. Int. J. Syst. Evol. Microbiol. 2012, 62, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Mohr, K.I.; Garcia, R.O.; Gerth, K.; Irschik, H.; Müller, R. Sandaracinus Amylolyticus gen. nov., sp. nov., a starch-degrading soil myxobacterium, and description of Sandaracinaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2012, 62, 1191–1198. [Google Scholar] [CrossRef] [Green Version]
- Hall, T. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.P.; Göker, M. Genome Sequence-Based Species Delimitation with Confidence Intervals and Improved Distance Functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef] [Green Version]
- Meier-Kolthoff, J.P.; Hahnke, R.L.; Petersen, J.; Scheuner, C.; Michael, V.; Fiebig, A.; Rohde, C.; Rohde, M.; Fartmann, B.; Goodwin, L.A.; et al. Complete Genome Sequence of DSM 30083T, the Type Strain (U5/41T) of Escherichia coli, and a Proposal for Delineating Subspecies in Microbial Taxonomy. Stand. Genom. Sci. 2014, 9, 2. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Goloboff, P.A.; Farris, J.S.; Nixon, K.C. TNT, a Free Program for Phylogenetic Analysis. Cladistics 2008, 24, 774–786. [Google Scholar] [CrossRef]
- Pattengale, N.D.; Alipour, M.; Bininda-Emonds, O.R.P.; Moret, B.M.E.; Stamatakis, A. How Many Bootstrap Replicates Are Necessary? J. Comput. Biol. 2010, 17, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Minnikin, D.E.; O’Donnell, A.G.; Goodfellow, M.; Alderson, G.; Athalye, M.; Schaal, A.; Parlett, J.H. An Integrated Procedure for the Extraction of Bacterial Isoprenoid Quinones and Polar Lipids. J. Microbiol. Methods 1984, 2, 233–241. [Google Scholar] [CrossRef]
- Bóna-Lovász, J.; Bóna, A.; Ederer, M.; Sawodny, O.; Ghosh, R. A Rapid Method for the Extraction and Analysis of Carotenoids and Other Hydrophobic Substances Suitable for Systems Biology Studies with Photosynthetic Bacteria. Metabolites 2013, 3, 912–930. [Google Scholar] [CrossRef]
- Risdian, C.; Landwehr, W.; Rohde, M.; Schumann, P.; Hahnke, R.L.; Spröer, C.; Bunk, B.; Kämpfer, P.; Schupp, P.J.; Wink, J. Streptomyces bathyalis sp. nov., an actinobacterium isolated from the sponge in a deep sea. Antonie van Leeuwenhoek 2021, 114, 425–435. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [Green Version]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI Prokaryotic Genome Annotation Pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Lee, I.; Chalita, M.; Ha, S.M.; Na, S.I.; Yoon, S.H.; Chun, J. ContEst16S: An Algorithm That Identifies Contaminated Prokaryotic Genomes Using 16S RNA Gene Sequences. Int. J. Syst. Evol. Microbiol. 2017, 67, 2053–2057. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid Annotations Using Subsystems Technology. BMC Genom. 2008, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Medema, M.H.; Blin, K.; Cimermancic, P.; De Jager, V.; Zakrzewski, P.; Fischbach, M.A.; Weber, T.; Takano, E.; Breitling, R. AntiSMASH: Rapid Identification, Annotation and Analysis of Secondary Metabolite Biosynthesis Gene Clusters in Bacterial and Fungal Genome Sequences. Nucleic Acids Res. 2011, 39 (Suppl. 2), W339–W346. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. AntiSMASH 5.0: Updates to the Secondary Metabolite Genome Mining Pipeline. Nucleic Acids Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef] [Green Version]
- Meier-Kolthoff, J.P.; Göker, M.; Spröer, C.; Klenk, H.P. When Should a DDH Experiment Be Mandatory in Microbial Taxonomy? Arch. Microbiol. 2013, 195, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A Taxonomically United Database of 16S RRNA Gene Sequences and Whole-Genome Assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Kosako, Y.; Yabuuchi, E.; Naka, T.; Fujiwara, N.; Kobayashi, K. Proposal of Sphingomonadaceae fam. nov., consisting of Sphingomonas Yabuuchi et Al. 1990, Erythrobacter Shiba and Shimidu 1982, Erythromicrobium Yurkov et Al. 1994, Porphyrobacter Fuerst et Al. 1993, Zymomonas Kluyver and van Niel 1936, and Sandaracinobac. Microbiol. Immunol. 2000, 44, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, C.; Rocha, J.; Martins, R.; Proença, D.N.; Morais, P.V.; Henriques, I.; Alves, A. Altererythrobacter halimionae sp. nov. and Altererythrobacter endophyticus sp. nov., Two Endophytes from the Salt Marsh Plant Halimione portulacoides. Int. J. Syst. Evol. Microbiol. 2017, 67, 3057–3062. [Google Scholar] [CrossRef]
- Kikawada, T.; Saito, A.; Kanamori, Y.; Nakahara, Y.; Iwata, K.I.; Tanaka, D.; Watanabe, M.; Okuda, T. Trehalose Transporter 1, a Facilitated and High-Capacity Trehalose Transporter, Allows Exogenous Trehalose Uptake into Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 11585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, C.; Smorawinska, M.; Li, L.; Horikoshi, K. Comparison of the Gene Expression of Aspartate /2-D-Semialdehyde Dehydrogenase at Elevated Hydrostatic Pressure in Deep-Sea Bacteria 1. J. Biochem. 1997, 121, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Lefort, V.; Desper, R.; Gascuel, O. FastME 2.0: A Comprehensive, Accurate, and Fast Distance-Based Phylogeny Inference Program. Mol. Biol. Evol. 2015, 32, 2798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).